GM-CSF controls the functional balance of effector and regulatory T cell subsets in EAE.
Keywords: multiple sclerosis, FOXP3+ T cells, tolerance, neuroimmunology
Abstract
Previous studies established that GM-CSF-deficient (Csf2-deficient) mice exhibit profound resistance to experimental autoimmune encephalomyelitis. This study addressed whether the resistance of Csf2-deficient mice was a result of a requirement for GM-CSF in controlling the functional balance between effector and regulatory T cell subsets during experimental autoimmune encephalomyelitis. The main observation was that treatment with the anti-CD25 mAb PC61 rendered Csf2-deficient mice fully susceptible to severe, chronic experimental autoimmune encephalomyelitis, with disease incidences and severities equivalent to that of C57BL/6 mice. When both donors and recipients were treated with PC61 in a passive model of experimental autoimmune encephalomyelitis, adoptive transfer of myelin-specific Csf2-deficient T cells into Csf2-deficient recipients resulted in a nonresolving chronic course of severe paralytic experimental autoimmune encephalomyelitis. The peripheral Csf2-deficient T cell repertoire was marked by elevated CD3+ T cell frequencies that reflected substantial accumulations of naïve CD44null-low CD4+ and CD8+ T cells but essentially normal frequencies of CD4+ CD25+ forkhead box P3+ T cells among the CD3+ T cell pool. These findings suggested that Csf2-deficient mice had secondary deficiencies in peripheral T cell sensitization to environmental antigens. In accordance, myelin oligodendrocyte glycoprotein 35–55/CFA-sensitized Csf2-deficient mice exhibited deficient peripheral sensitization to myelin oligodendrocyte glycoprotein, whereas pretreatment of Csf2-deficient mice with PC61 enabled the robust induction of myelin oligodendrocyte glycoprotein-specific T cell responses in the draining lymphatics. In conclusion, the experimental autoimmune encephalomyelitis resistance of Csf2-deficient mice, at least in part, reflects a deficient induction of effector T cell function that cannot surmount normal regulatory T cell barriers. Experimental autoimmune encephalomyelitis effector responses, however, are unleashed upon depletion of regulatory CD25+ T cells.
Introduction
EAE is widely studied as a model to resolve mechanisms underlying the pathogenesis of multiple sclerosis [1–5]. The study of EAE has revealed several molecules that have crucial roles in pathogenesis. For example, genetic deficiencies in GM-CSF [6], IL-6 [7, 8], the IL-23/IL-23R pathway [9], Stat3 [10], or Stat4 [11] confer profound resistance to disease. GM-CSF has attracted substantial attention as a result of the potential for antibody-mediated clinical intervention [12, 13]. The lack of GM-CSF in Csf2−/− mice was associated with the lack of a sustained perivascular infiltration of the CNS during chronic EAE [6, 14]. Overall, GM-CSF was critical for the continued invasion and proliferation of leukocytes in the CNS and ultimately, for the persistence and penetration of inflammatory demyelinating lesions. Administration of exogenous GM-CSF restored EAE susceptibility in Csf2−/− mice [6], and administration of a neutralizing anti-GM-CSF mAb inhibited disease in EAE-susceptible mice [6, 14–16]. The action of the anti-GM-CSF mAb was transient, as mice relapsed and exhibited severe EAE, ∼10 d after cessation of anti-GM-CSF treatment [6, 14]. Active immunization of donor Csf2−/− mice elicited neuroantigen-specific T cells that secreted IFN-γ and IL-17, but these T cells did not mediate the adoptive transfer of EAE [14, 16]. Conversely, adoptive transfer of GM-CSF sufficient effector T cells that were deficient in both IFN-γ and IL-17 caused severe EAE commensurate with WT T cells. Upon entry into the CNS, neuroantigen-specific T cells are known to undergo reactivation when exposed to endogenous myelin antigens on myeloid APCs [17, 18]. These reactivated effector T cells secrete GM-CSF that, in turn, promotes recruitment, activation, and phagocytic activity of myeloid-derived macrophages, DCs, and microglia to facilitate EAE pathogenesis [9, 14–16, 19–21].
Based on these observations, GM-CSF is widely considered to be the signature cytokine of pathogenic effector T cells in EAE. GM-CSF is believed to be one of the few cytokines critical for EAE and is thought to mediate essential, nonredundant functions in the effector phase of EAE. Evidence presented in this study, however, reveals a more complex role of GM-CSF in homeostasis and autoimmune disease. In an active model of EAE, pretreatment of MOG35–55/CFA-sensitized Csf2−/− mice with the anti-CD25 PC61 mAb caused the depletion of CD4+ CD25+ FOXP3+ T cells and unleashed an encephalitogenic immune response that culminated in a chronic course of severe paralytic EAE. In a passive model of EAE, pretreatment of MOG35–55/CFA-sensitized Csf2−/− donors with the PC61 mAb enabled the generation of pathogenic T cells that transferred severe paralytic EAE to Csf2−/− recipients. Additional treatment of Csf2−/− recipients with the anti-CD25 mAb enabled chronic nonresolving EAE in those recipients. The induction of severe paralytic EAE in Csf2−/− mice reveals that GM-CSF, at least in part, controls disease susceptibility in EAE by setting the functional balance of effector and Treg subsets.
MATERIALS AND METHODS
Animals and reagents
C57BL/6, FIG (B6.Cg-Foxp3tm2Tch/J; Stock Number 006772), and 2D2 [C57BL/6-Tg(Tcra2D2, Tcrb2D2, 1Kuch/J); Stock No. 006912] strains were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and were housed and bred in the Department of Comparative Medicine at East Carolina University (Greenville, NC, USA). The GM-CSF-deficient B6.Csf2−/− strain was a generous gift from Dr. Glenn Dranoff [22]. This strain lacked the ability to synthesize GM-CSF and was resistant to EAE. FIG Csf2−/− and 2D2-FIG Csf2−/− strains were obtained by standard breeding techniques. Csf2−/− and FIG Csf2−/− mice were genotyped by PCR amplification of tail-snip DNA. The FIG genotype was determined by use of forward (5′-CAC CTA TGC CAC CCT TAT CC-3′) and reverse (5′-ATT GTG GGT CAA GGG GAA G-3′) primers. The FIG knock-in product was 390 bp, and the WT product was 341 bp. B6.Csf2−/− genotyping was performed via a neomycin-specific primer (5′-AGG CCA CTT GTG TAG CGC CAA GT-3′), a Csf2 common exon 2-specific primer (5′-TCG TCT CTA ACG AGT TCT CCT TCA-3′), and WT exon 3-specific primer (5′-TGC TCG AAT ATC TTC AGG-3′). The expected Csf2−/− mutant WT targets were 600 and 800 bp, respectively. Animal care and use were performed in accordance with approved animal use protocols and guidelines of the East Carolina University Institutional Animal Care and Use Committee (Animal Use Protocols K147b, 156, and 158). 2D2 mice have a MOG-specific, self-reactive T cell repertoire. Routine screening of 2D2 mice was performed by FACS analysis of PBMC by use of antibodies specific for TCR Vβ11 and/or Vα3.2. Synthetic peptide MOG35–55 was comprised of the following sequence: M-E-V-G-W-Y-R-S-P-F-S-R-V-V-H-L-Y-R-N-G-K.
Flow cytometric analyses of PBMC
Splenocytes were processed to a single-cell suspension and washed 3 times in HBSS. PBMCs were collected via the submandibular vein in 130 mM sodium citrate buffer and washed in HBSS with 2% heat-inactivated FBS. Cells were then stained for 1 h at 4°C in the dark with designated cocktails of fluorochrome-conjugated antibodies, including those specific for CD45 (30-F11), CD3 (17A2), CD4 (GK1.5), CD8, (53–6.7), CD19 (6D5), Ly6G (1A8), CD44 (IM7), and I-Ab (AF6-120.1). After staining PBMC, erythrocytes were lysed with 1:10 HBSS for 20 s, followed by addition of 2× PBS. Data were collected by use of a BD LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed by use of FlowJo software. Bar graphs show the mean, and error bars show the sem, unless designated otherwise. Pairwise comparisons between Csf2−/− and WT samples were analyzed by 2-tailed t tests for data that passed normality (Shapiro-Wilk) and equal variance (Brown-Forsythe) tests. Otherwise, data were assessed with a Mann-Whitney rank sum test. In designated experiments, reference "counting" beads were added to samples immediately before flow cytometric analysis (AccuCheck counting beads; Thermo Fisher Scientific, Waltham, MA, USA). The use of reference beads enabled comparisons of cell yield or absolute cell numbers.
Generation, purification, and administration of the mAb
The PC61-5.3 anti-CD25 rat IgG1(λ), Y13-259 anti-v-H-Ras rat IgG1(κ), R4-6A2 anti-IFN-γ rat IgG1(κ), GK1.5 anti-CD4 rat IgG2b(κ), and 53-6.7 anti-CD8 rat IgG2a(κ) hybridomas were used as a source of depleting antibodies or control mAb. Initial preparations of PC61 mAb (anti-CD25 mAb) and Y13-259 mAb (rat IgG1 isotype control) were generous gifts from Dr. Gregory Sempowski (Duke University School of Medicine, Durham, NC, USA). Subsequent PC61 and Y13 mAb preparations were expressed and purified at East Carolina University. The PC61-5.3 and Y13-259 hybridomas were obtained from American Type Culture Collection (Manassas, VA, USA) and were subcloned twice to ensure stability. For all 5 hybridomas, cells were cultured in supplemented DMEM in C2011 hollow fiber cartridges (FiberCell Systems, Frederick, MD, USA). Hybridoma supernatants were clarified at 7200 g, precipitated with 50% ammonium sulfate, and dissolved in PBS. mAb preparations were purified on protein G agarose columns. Antibody was eluted with 200 mM glycine at pH 3.0 and immediately neutralized by 1 M Tris buffer of pH 9.0. IgG fractions were also purified by ultrafiltration on YM100 membranes in Amicon-stirred cell cylinders. The purity of these mAb was verified by SDS-PAGE. Specific activities of all PC61 preparations were determined by staining of murine CD25+ T cells with serial 1/2-log dilutions of the mAb. After washing, PC61-stained T cells were labeled with a PE-conjugated goat anti-rat IgG(H+L) secondary antibody, followed by flow cytometric analysis. As designated for pretreatment experiments, purified mAb were administered at a dose of 250 µg/injection i.p. to mice on d −5 and −3 (or d −4 and −2) unless designated otherwise. Depletion of specific lymphoid subsets was confirmed by flow cytometric analysis of PBMC on d −1 or 0. Active immunization with MOG35–55 in CFA was initiated on d 0.
Induction and assessment of EAE
For active induction of EAE, CFA [4 mg/ml heat-killed Mycobacterium tuberculosis H37Ra (BD Biosciences) in IFA] was mixed 1:1 with MOG35–55 in saline and emulsified by sonication. A total dose of 200 µg MOG35–55/CFA was injected across the lower back via 3 spaced subcutaneous injections (∼0.033 ml/injection) for a total injection volume of 0.1 ml/mouse. Mice also received injections of 200 ng i.p. Ptx (List Biological Laboratories, Campbell, CA, USA) on d 0 and 2. All immunizations were given under isoflurane anesthesia (Abbott Laboratories, Chicago, IL, USA). For passive induction of EAE, draining lymph nodes and spleen were pooled from donor MOG/CFA-immunized mice and were cultured with 1 µM MOG35–55 and 10 ng/ml IL-23 for 3 d. Cultured T cells were washed extensively in HBSS, and 107 cells were injected i.p. into each recipient. Recipients were given 200 ng i.p. Ptx on d 1 and 3. Mice were assessed daily for clinical score and body weight. The following scale was used to score EAE: 0, no disease; 0.5, partial paralysis of tail without ataxia; 1.0, flaccid paralysis of tail or ataxia but not both; 2.0, flaccid paralysis of tail with ataxia or impaired righting reflex; 3.0, partial hindlimb paralysis marked by inability to walk upright but with ambulatory rhythm in both legs; 3.5, same as above but with full paralysis of 1 leg; 4.0, full hindlimb paralysis of both legs; 5.0, total hindlimb paralysis with forelimb involvement or moribund. A score of 5.0 was a humane endpoint for euthanasia.
Cumulative EAE scores were calculated by summing daily scores for each mouse. Maximal scores were calculated as the most severe EAE score for each mouse. Mice that did not exhibit EAE had a score of 0 for the cumulative and maximal scores, and these scores were included in the group average. To calculate percent maximal weight loss, 100% body weight was assigned as the maximal body weight obtained from d 1 through d 10, and daily body weights were calculated for each day after normalization to this 100% value. The minimum body weight was defined as the lowest body weight during the entire course of EAE after normalization to the 100% value. Maximal weight loss was calculated by subtraction of the normalized minimum value from the maximum 100% value. Average daily weight loss was calculated as the average of daily body-weight measurements from d 10 until the end of experiment, subtracted from the 100% maximal body weight. Cumulative and maximal EAE scores were converted to ranked scores and analyzed by nonparametric ANOVA. Weight loss was analyzed by parametric ANOVA. Nonparametric and parametric ANOVA were assessed with a Bonferroni post hoc test. Error bars portraying data for EAE clinical scores and weight loss represent the sem. After humane euthanasia, the spinal column and brain were removed and were fixed in 10% neutral-buffered formalin. Sections were prepared from the spinal cord, brain, brainstem, and cerebellum. Sections were stained with H&E and were imaged with a Leica DFC420C digital camera connected to a Leica DM400B microscope.
RESULTS
Depletion of the CD4+CD25+ T cell subset in Csf2−/− mice restored susceptibility to EAE
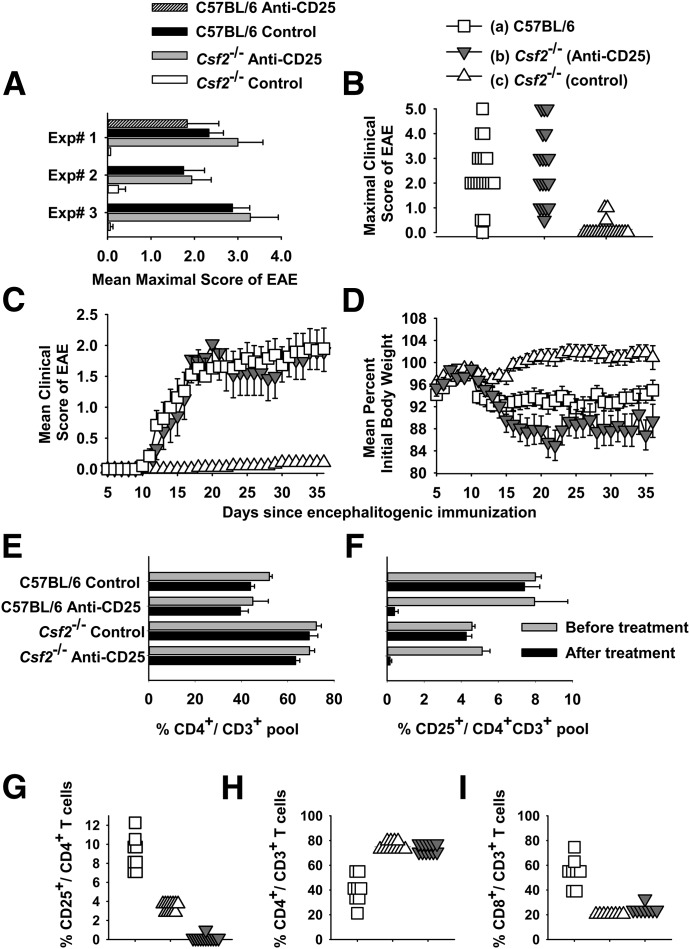
Experiments were performed to assess whether a Treg pathway may contribute to the EAE resistance of Csf2−/− mice. In preliminary experiments, treatment of C57BL/6 mice with the PC61 anti-CD25 mAb purged CD4+ CD25+ Tregs from the blood and spleen for >20 d, followed by a rebound to normal levels by d 40, whereas the Y13 mAb (anti-p21 v-H-Ras rat IgG1 isotype control) had no effect on CD25+ T cell levels (data not shown). Experiments were then performed to assess whether a predominant Treg pathway may contribute to the EAE resistance of Csf2−/− mice (Fig. 1A–D and Table 1). Csf2−/− or C57BL/6 mice were pretreated with 250 µg purified anti-CD25 PC61 mAb on d −4 and −2 to clear the CD4+ CD25+ T cell subset. Mice were immunized with 200 µg MOG35–55 in CFA on d 0 and injected with Ptx (200 ng) i.p. on d 0 and 2, according to the standard EAE induction protocol. In the first experiment, PC61 pretreatment of Csf2−/− mice abrogated the EAE resistance of this strain. However, PC61 had no significant impact on EAE in C57BL/6 mice (Experiment 1 of Fig. 1A and Table 1), most likely because the 200 µg dosage of MOG35–55 overwhelmed any Treg resistance. PC61-pretreated Csf2−/− mice differed qualitatively from control Csf2−/− mice in the mean cumulative score (52.7 vs. 0.0) and mean maximal score (3.0 vs. 0.0), respectively (Table 1). As PC61 did not affect EAE in WT mice, 2 replicate experiments were performed to compare the EAE susceptibility of PC61 pretreated Csf2−/− mice, control Csf2−/− mice, and WT C57BL/6 mice. In both replicate experiments, PC61 pretreatment abrogated the EAE resistance of Csf2−/− mice and enabled severe paralytic disease (Table 1, Experiments 2 and 3). Statistical analysis of the compiled data showed that PC61 pretreatment profoundly affected EAE in Csf2−/− mice, as measured by EAE incidence and both mean cumulative and mean maximal scores (Table 1). Clinical signs of EAE in Csf2−/− mice reflected classic rather than atypical disease manifestations.
Figure 1. The PC61 anti-CD25 mAb enables the active induction of EAE in Csf2−/− mice.
(A–I) C57BL/6 or Csf2−/− mice were or were not pretreated with 250 µg purified PC61 mAb on d −4 and −2 i.p. (A–D) Shown are individual (A) or pooled (B–D) data from Experiments 1–3 of Table 1. Mice were immunized with 200 µg MOG35–55 in CFA subcutaneously on d 0 and injected with Ptx (200 ng) i.p. on days 0 and 2. (A) Mean maximal scores (±sem) are shown for Experiments 1–3 (Csf2−/− with and without PC61, P < 0.02 for all 3 experiments). (B) Frequency analysis of the mean maximal scores are shown for data pooled from Experiments 1–3 for WT mice (n = 19), Csf2−/− mice treated with PC61 (n = 18), and Csf2−/− control mice [n = 19; (c) vs. (b) and (a), P < 0.001]. This description also applies to C and D and G–I. (C and D) The daily mean maximal scores and weight loss are shown for data pooled from Experiments 1–3. Daily mean clinical scores for (a) vs. (c) (d 12 and 13, P < 0.005; d 14–36, P < 0.001) and (b) vs. (c) (d 15 and 16, P < 0.05; d 17–36, P < 0.001) were assessed by nonparametric ANOVA. Daily body weight values for (a) vs. (c) (d 11–13 and d 16–36, P < 0.05) and (b) vs. (c) (d 15, P = 0.008; d 16–36, P < 0.005) were assessed by parametric ANOVA. (E and F) C57BL/6 CD4+ and CD25+ percentages vs. Csf2−/− CD4+ and CD25+ percentages (analyzed groups that were not treated with PC61, P < 0.001). (F) C57BL/6-PC61 and Csf2−/−-PC61 groups on d −4 before treatment vs. d 0 after treatment (n = 3, P < 0.01). (G) All comparisons, P < 0.001. (H and I) (a) vs. (b) and (c), P < 0.001.
TABLE 1.
Pretreatment of Csf2−/− mice with the anti-CD25 PC61 mAb enabled severe paralytic EAE
| Experiment number | Strain | Treatment | Incidence | Mean cumulative score | Median cumulative score | Mean maximal score | Median maximal score |
|---|---|---|---|---|---|---|---|
| 1 | C57BL/6 | Anti-CD25 | 3 of 3 | 39.3 ± 21.0 | 45.0 | 1.8 ± 0.7 | 2.0 |
| C57BL/6 | Saline | 3 of 3 | 34.0 ± 11.9 | 25.5 | 2.3 ± 0.3 | 2.0 | |
| Csf2−/− | Anti-CD25 | 3 of 3 | 52.7 ± 25.7 | 57.5 | 3.0 ± 0.6 | 3.0 | |
| Csf2−/− | Saline | 0 of 3 | 0.0 ± 0.0 | 0.0 | 0.0 ± 0.0 | 0.0 | |
| 2 | C57BL/6 | Saline | 7 of 8 | 19.3 ± 6.6 | 16.8 | 1.8 ± 0.5 | 2.0 |
| Csf2−/− | Anti-CD25 | 8 of 8 | 29.4 ± 9.3 | 17.5 | 1.9 ± 0.4 | 1.5 | |
| Csf2−/− | Saline | 2 of 8 | 2.1 ± 1.6 | 0.0 | 0.3 ± 0.2 | 0.0 | |
| 3 | C57BL/6 | Saline | 8 of 8 | 63.3 ± 8.9 | 51.3 | 2.9 ± 0.4 | 2.5 |
| Csf2−/− | Anti-CD25 | 7 of 7 | 32.5 ± 7.2 | 37.0 | 3.3 ± 0.6 | 3.0 | |
| Csf2−/− | Saline | 1 of 8 | 0.9 ± 0.9 | 0.0 | 0.1 ± 0.1 | 0.0 | |
| 1–3 | (a) C57BL/6 | Saline | 18 of 19 | 46.8 ± 8.6 | 51.0 | 2.3 ± 0.3 | 2.0 |
| (b) Csf2−/− | Anti-CD25 | 18 of 18 | 37.2 ± 6.3 | 31.5 | 2.6 ± 0.4 | 2.5 | |
| (c) Csf2−/− | Saline | 3 of 19 | 1.4 ± 0.8 | 0.0 | 0.1 ± 0.1 | 0.0 | |
| 1–3 | (c) vs. (a) and (b) | P < 0.0001 | P < 0.001 | P < 0.001 | |||
These data are portrayed in Fig. 1. C57BL/6 or Csf2−/− mice were or were not pretreated with 250 µg purified PC61 mAb on d −4 and −2 i.p. Mice were immunized with 200 µg MOG35–55 in CFA on d 0 and injected with Ptx (200 ng) i.p. on d 0 and 2. Shown are the mean/median cumulative and maximal scores (±sem) for 3 independent experiments scored through d 36. Differences in cumulative and maximal EAE scores were assessed by nonparametric ANOVA. Differences in disease incidence were assessed pairwise by a Fisher’s exact test.
The individual maximal scores for each mouse for the 3 combined experiments are shown in Fig. 1B (legend applies to Fig. 1, B–D and G–I). One-half of the PC61-pretreated Csf2−/− mice had severe EAE scores (≥3.0), commensurate with that of control WT mice. In Experiment 3, 3 of 7 Csf2−/− mice in the anti-CD25 pretreatment group reached a humane endpoint (score of 5.0) on d 21, 22, and 28 and were thereafter excluded from scoring. The time course for mean maximal EAE in PC61-pretreated Csf2−/− mice and control WT mice was essentially identical (Fig. 1C; data pooled for Groups a, b, and c from Experiments 1, 2, and 3). Maximal and average weight loss in PC61-treated Csf2−/− mice (18.9% ± 2.6 and 11.0% ± 2.0) also differed significantly from Csf2−/− control mice (7.2% ± 1.4 and −0.4% ± 1.2; parametric ANOVA, P < 0.001, P < 0.001), respectively. These differences are portrayed as a time course in Fig. 1D. The time course for mean body weight showed that the body weight of PC61-pretreated Csf2−/− mice trended below that of WT mice (Fig. 1D). In contrast, control Csf2−/− mice did not exhibit severe EAE and had stable body weights during the entire disease course. Overall, these data indicated that the CD4+ CD25+ Treg subset constituted a major barrier to the induction of EAE in GM-CSF-deficient mice.
For mice shown in Fig. 1A, assessments taken on d −4 (before initiation of treatment with PC61) and d 0 (after treatment) showed only a modest decrease of the global CD4+ pool (Fig. 1E) but a complete PC61-dependent loss of CD25+ CD4+ T cells in the CD4+ T cell pool (Fig. 1F). Unexpectedly, these data also revealed that Csf2−/− mice had higher percentages of CD4+ T cells in the CD3+ T cell pool (Fig. 1E) and lower percentages of CD4+ CD25+ T cells in the CD4+ pool compared with C57BL/6 mice (Fig. 1F), and these strain-dependent differences were independent of PC61 mAb treatment. Experiments with larger cohorts of mice showed that pretreatment with PC61 eliminated CD4+CD25+ T cells from PBMC without affecting circulating levels of CD4+ or CD8+ T cells (Fig. 1G–I). The loss of CD25+ cells from PBMC was a result of cell depletion rather than CD25 down-modulation, as assessed by staining of PBMC with a PE-conjugated anti-rat IgG(H+L) reagent (data not shown). Overall, these data confirmed PC61-mediated elimination of CD25+ T cells and also revealed significant perturbations of T cell subsets in PBMC of Csf2−/− mice.
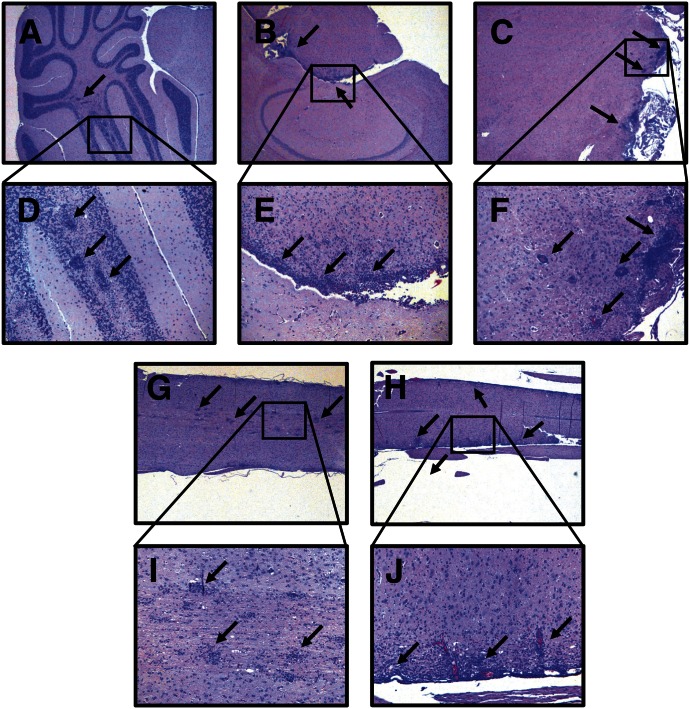
PC61-pretreated Csf2−/− mice that showed severe paralytic EAE (Fig. 1) also had extensive perivascular and periventricular inflammatory lesions in the CNS (Fig. 2). Inflammatory lesions were noted in the cerebellum (Fig. 2A and D), hindbrain (Fig. 2B and E), brainstem (Fig. 2C and F), and spinal cord (Fig. 2G–J). Both periventricular and perivascular lesions were noted throughout in midbrain and hindbrain. In addition to brain involvement, EAE in Csf2−/− mice was associated with numerous focal lesions together with extensive periventricular infiltration in the spinal cord. The images portray 3 representative Csf2−/− mice with full hindlimb paralysis (clinical score = 4.0). Thus, active induction of EAE in PC61-pretreated Csf2−/− mice elicited both clinical and histologic signs of severe EAE.
Figure 2. GM-CSF-deficient Csf2−/− mice pretreated with PC61 exhibited extensive perivascular and periventricular CNS lesions.
Portrayed are representative sections of Csf2−/− mice afflicted with severe paralytic EAE (Fig. 1), including the cerebellum (A and D), hindbrain (B and E), brainstem (C and F), and spinal cord (G and I and H and J). Mononuclear inflammatory lesions are marked by black arrows. Images were acquired at 25× original magnification (A–C, G, and H) or 100× original magnification (D–F, I, and J).
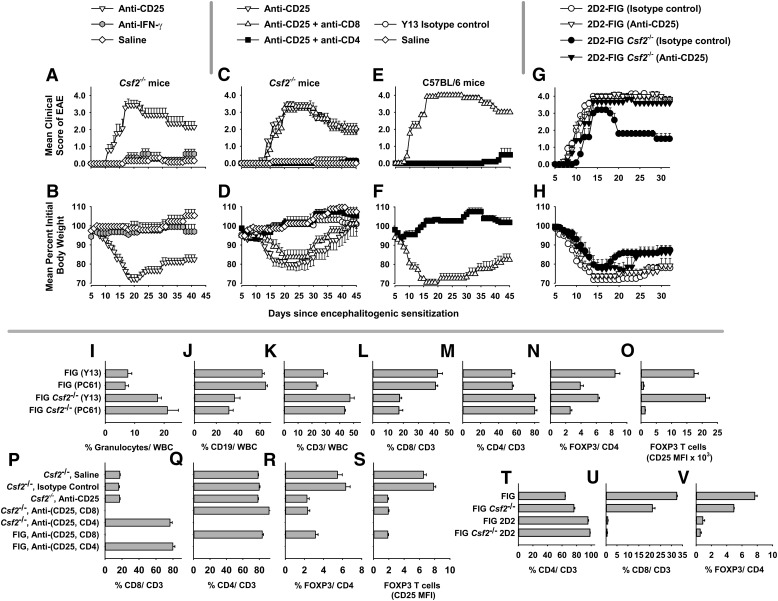
The PC61 mAb was tested side by side with the anti-IFN-γ mAb R4.6A2 as a specificity control in Csf2−/− mice (Fig. 3A and B and Table 2). Conceptually, both PC61 and anti-IFN-γ mAb should augment disease [23–27], although the R4.6A2 mAb should have a minimal impact on EAE based on the paradigm used in Fig. 3, as R4.6A2 would need continuous application throughout the time course to have an effect. Both R4.6A2 and PC61 mAb were rat IgG1 mAb. These mAb were injected on d −4 and −2 (250 µg/dose), followed by active immunization on d 0 with MOG35–55/CFA with injections of Ptx on d 0 and 2. Mice were assessed for EAE and body weight for 41 continuous days. PC61-pretreated mice differed significantly from R4.6A2 mAb or saline-pretreated mice in the mean scores for cumulative disease (77.3 ± 10.0 vs. 8.6 ± 3.0 or 4.3 ± 4.3, P < 0.001), maximal disease (3.6 ± 0.3 vs. 1.0 ± 0.3 or 0.3 ± 3, P ≤ 0.001), and maximal weight loss (29.3 ± 2.0% vs. 7.8 ± 2.9% or 4.0 ± 3.0%, P < 0.001), respectively. The time courses of EAE and weight loss are shown in Fig. 3A and B. The daily mean clinical scores for PC61-pretreated mice differed significantly from the scores for R4.6A2 mAb-pretreated mice on d 11–41 (P ≤ 0.02) or saline-pretreated mice on d 13–37 (P ≤ 0.002). The daily weight loss scores for PC61-pretreated mice significantly differed from those for R4.6A2 mAb-pretreated mice on d 14 and 15 and d 30–37 (P ≤ 0.004), d 16–29 (P ≤ 0.0001), and d 38–41 (P = 0.024). The daily weight loss scores for PC61-pretreated mice also differed significantly from those for saline-pretreated mice on d 14–29 and d 38–41 (P ≤ 0.0001) and d 30–37 (P ≤ 0.006). As the administration of the mAb did not span the time course of disease, no conclusions were rendered regarding the role of IFN-γ in the EAE susceptibility of Csf2−/− mice. Rather, these data indicated that the anti-CD25 specificity of the PC61 mAb was needed to enable EAE susceptibility.
Figure 3. Depletion of CD25+ Tregs enables CD4+ T cell-mediated EAE in the resistant Csf2−/− strain.
(A–H) Shown are EAE and weight-loss time courses for data portrayed in Table 2 (A–F) and Table 3 (G and H). Designated mouse strains were injected i.p. with purified R4.6A2 anti-IFN-γ rat IgG1, PC61 anti-CD25 rat IgG1, Y13 isotype control rat IgG1, GK1.5 anti-CD4 rat IgG2b, and/or 53.6-7 anti-CD8a rat IgG2a on d −5 and −3 (or d −4 and −2). All mAb were injected at a dose of 250 µg/mAb/injection. Mice were immunized on d 0 with 200 µg MOG35–55 in CFA with i.p. injections of Ptx on d 0 and 2. Mice were scored and weighed daily. (I–O) FIG and FIG Csf2−/− mice (n = 4) were injected with PC61 or Y13 mAb on d −4 and −2, and then PBMCs were analyzed for expression of CD45, Ly6G, CD19, CD3, CD4, CD8, FOXP3 (GFP), and CD25 on d 0. FIG vs. FIG Csf2−/− mice (analysis of Y13 groups or PC61 groups separately). (I–M) P < 0.001, both comparisons; (O) P < 0.02, both comparisons; (N) all comparisons, P < 0.005. (O) PC61 vs. Y13, P < 0.001, both comparisons. (P–S) These data represent PBMC analyses (d 0) of mice used in C–F. In groups treated with anti-CD8 and anti-CD4 mAb, percentages were <0.2% for each CD8+ and CD4+ subset, respectively (P < 0.001 vs. all other groups). (R and S) Anti-CD25 (±anti-CD8) groups vs. saline or Y13 groups, P < 0.001. (T–V) Naïve, unmanipulated 2D2-FIG or 2D2-FIG Csf2−/− vs. non-2D2 groups, P < 0.001.
TABLE 2.
EAE in Csf2−/− mice was enabled by an anti-CD25 mAb and reversed by an anti-CD4 mAb
| Figure | Strain | mAb pretreatment | Incidence | Means (±se) cumulative scores | P | Means (±se) maximal scores | P | % Maximal weight loss | P |
|---|---|---|---|---|---|---|---|---|---|
| 3A and B | Csf2−/− | No mAb | 1 of 6 | 4.3 ± 4.3 | * | 0.3 ± 0.3 | * | 4.0 ± 3.0% | * |
| 3A and B | Csf2−/− | Anti-IFN-γ | 7 of 8 | 8.6 ± 3.0 | ns | 1.0 ± 0.3 | ns | 7.8 ± 2.9% | ns |
| 3A and B | Csf2−/− | Anti-CD25 | 7 of 7 | 77.3 ± 10.0 | <0.001 | 3.6 ± 0.3 | <0.001 | 29.3 ± 2.0% | <0.001 |
| 3C and D | Csf2−/− | No mAb | 1 of 5 | 1.5 ± 1.5 | * | 0.1 ± 0.1 | * | 4.4 ± 2.2% | * |
| 3C and D | Csf2−/− | Y13 isotype control | 2 of 7 | 3.7 ± 2.4 | ns | 0.2 ± 0.1 | ns | 6.1 ± 1.7% | ns |
| 3C and D | Csf2−/− | Anti-CD25 | 9 of 9 | 82.3 ± 6.6 | <0.001 | 3.3 ± 0.2 | <0.001 | 21.8 ± 3.5% | 0.008 |
| 3C and D | Csf2−/− | Anti-CD25 + anti-CD8 | 9 of 9 | 80.1 ± 7.1 | <0.001 | 3.6 ± 0.2 | <0.001 | 17.6 ± 3.9% | ns |
| 3C and D | Csf2−/− | Anti-CD25 + anti-CD4 | 1 of 8 | 0.6 ± 0.6 | ns | 0.1 ± 0.1 | ns | 7.9 ± 2.5% | ns |
| 3E and F | C57BL/6 | Anti-CD25 + anti-CD8 | 6 of 6 | 124.8 ± 1.9 | <0.001 | 4.0 ± 0.0 | <0.001 | 32.0 ± 2.0% | <0.001 |
| 3E and F | C57BL/6 | Anti-CD25 + anti-CD4 | 2 of 5 | 2.7 ± 1.7 | ns | 0.5 ± 0.4 | ns | 5.7 ± 1.9% | ns |
These data are portrayed in Fig. 3A–F. (Fig. 3A and B) Designated mouse strains were injected i.p. with purified R4.6A2 anti-IFN-γ rat IgG1 or PC61 anti-CD25 rat IgG1 mAb on d −4 and −2. (Fig. 3C–F) Alternatively, mice were injected with PC61, Y13 (isotype control rat IgG1), GK1.5 (anti-CD4 rat IgG2b), and/or 53.6-7 (anti-CD8a rat IgG2a) mAb on d −5 and −3. All mAb were injected at a dose of 250 µg/injection. Mice were immunized on d 0 with 200 µg MOG35–55 in CFA with i.p. injections of Ptx on d 0 and 2. Mice were scored and weighed daily. Shown are the mean cumulative and maximal scores (±sem). ns, not significant; asterisk indicates control group.
As EAE in PC61-treated Csf2−/− mice was uncharted, we considered the possibility that alternative effector T cell subsets, including CD8+ effector T cells, may facilitate EAE pathogenesis in this model [28–32]. To test this possibility, PC61 was used to deplete CD25+ Tregs in conjunction with mAb-mediated depletion of either the CD4+ subset (GK1.5 mAb) or the CD8+ subset (53-6.7 mAb; Fig. 3C and D and Table 2). In these experiments, the Y13-259 mAb was used as an isotype control for PC61. The results showed that Csf2−/− mice treated with PC61 alone or with an anti-CD8 mAb exhibited severe, chronic EAE, whereas Csf2−/− mice treated with PC61 and anti-CD4 mAb were resistant to EAE. As expected, the anti-CD4 mAb also inhibited EAE in C57BL/6 mice (Fig. 3E and F and Table 2). Thus, the anti-CD8 mAb had no effect on the disease course, whereas the anti-CD4 mAb abrogated EAE. These data indicated that this model of PC61-dependent EAE in Csf2−/− mice was mediated by CD4+ effector T cells rather than CD8+ T cells. Csf2−/− mice treated with saline or the Y13 isotype control mAb were equally resistant to EAE.
A second experiment focused on 2D2-FIG mice that were or were not bred to include the Csf2−/− genotype (Fig. 3G and H and Table 3). 2D2-FIG mice bear the 2D2 Vβ11/Vα3.2 transgenic TCR specific for I-Ab/MOG35–55 and a GFP reporter of FOXP3 expression. These mice have a deficiency of CD8+ T cells as a result of allelic exclusion and the positive selection of this clonotype on I-Ab in the thymus. Although these mice are Rag1/2 sufficient, these mice nonetheless have a relative deficiency in FOXP3+ T cells compared with WT mice as a result of the clonotypic homogeneity of the 2D2 repertoire. Naïve 2D2-FIG mice typically have a FOXP3+ repertoire ranging from 0.1 to 1.5% of the CD4+ repertoire. 2D2-FIG and 2D2-FIG Csf2−/− mice were pretreated with PC61 or Y13 mAb and were sensitized for EAE induction (Fig. 3G and H). Regardless of mAb pretreatment, all 4 groups had severe acute EAE. However, control (Y13-treated) 2D2-FIG Csf2−/− mice exhibited a partial recovery by d 20, whereas the other 3 groups maintained a severe chronic course of EAE. These data reveal a second model, whereby active immunization of 2D2-FIG Csf2−/− mice drove EAE despite a global deficiency in GM-CSF. The caveat was that the presence of GM-CSF (i.e., 2D2-FIG mice treated with Y13) or depletion of CD25+ Tregs in the absence of GM-CSF (i.e., 2D2-FIG Csf2−/− mice treated with PC61) reversed the spontaneous recovery noted in Y13-pretreated 2D2-FIG Csf2−/− mice. This model reinforces the concept that EAE susceptibility is contingent upon a GM-CSF-dependent balance among conventional and regulatory subsets.
TABLE 3.
Spontaneous recovery in transgenic 2D2 mice was facilitated by endogenous GM-CSF
| Figure | Strain | mAb pretxt | Incidence | Means (±se) maximal scores (overall) | P | Means (±se) maximal scores (d 20–32) | P | % Maximal weight loss (d20–32) | P |
|---|---|---|---|---|---|---|---|---|---|
| 3G and H | 2D2-FIG | Y13 isotype control | 7 of 7 | 4.3 ± 0.2 | ns | 4.3 ± 0.2 | <0.001 | 28.8 ± 3.4% | 0.027 |
| 3G and H | 2D2-FIG | Anti-CD25 | 8 of 8 | 4.3 ± 0.2 | ns | 4.3 ± 0.2 | <0.001 | 25.5 ± 1.8% | ns |
| 3G and H | 2D2-FIG Csf2−/− | Y13 isotype control | 5 of 5 | 3.2 ± 0.4 | * | 1.8 ± 0.2 | * | 14.9 ± 1.9% | * |
| 3G and H | 2D2-FIG Csf2−/− | Anti-CD25 | 6 of 6 | 3.8 ± 0.3 | ns | 3.8 ± 0.3 | =0.008 | 24.4 ± 4.2% | ns |
These data are portrayed in Fig. 3G and H. 2D2-FIG and 2D2-FIG Csf2−/− mice were injected with PC61 or Y13 (isotype control) on d −4 and −2. All mAb were injected at a dose of 250 µg/mAb/injection. Mice were immunized on d 0 with 200 µg MOG35–55 in CFA with i.p. injections of Ptx on d 0 and 2. Mice were scored and weighed daily until the end of the experiment on d 32. Shown are the mean cumulative and maximal scores (±sem). Asterisk indicates the control group.
To assess the efficiency of PC61-mediated depletion of CD25+ FOXP3+ Tregs, FIG Csf2−/− and control FIG mice were treated with 250 µg PC61 or Y13 on d −4 and −2, and PBMCs were analyzed on d 0 (Fig. 3I–O). These analyses revealed that Csf2−/− mice had profound perturbations of leukocyte subsets, including elevated percentages of Ly6G+ granulocytes (Fig. 3I), depressed percentages of CD19+ B cells (Fig. 3J), and increased percentages of CD3+ T cells (Fig. 3K) relative to the total leukocyte/WBC pool. Csf2−/− mice also had depressed CD8+ percentages (Fig. 3L) and elevated CD4+ percentages (Fig. 3M) in the CD3+ T cell compartment. Notably, pretreatment with PC61 did not affect the percentages of these leukocyte and T cell subsets. FIG Csf2−/− mice had decreased percentages of FOXP3+ T cells in the CD4+ compartment compared with FIG mice (Fig. 3N). In that FOXP3+ T cells were comprised of both CD25+ and CD25− subsets, PC61 treatment was found to reduce total percentages of circulating FOXP3+ T cells as a result of the elimination of CD25+ FOXP3+ Tregs but not CD25− FOXP3+ T cells (Fig. 3O). That is, in PC61-treated mice, surviving FOXP3+ Tregs did not express CD25, as measured by MFI. Thus, the ability of PC61 to enable EAE in Csf2−/− mice was correlated with the specific elimination of the CD25+ FOXP3+ subset rather than the CD25− FOXP3+ subset.
In regard to Fig. 3C–F, anti-CD4 or anti-CD8 mAb treatment was shown to eliminate specifically the CD4+ and CD8+ subsets, respectively (Fig. 3P and Q). As an expected consequence of subset-specific deletion, anti-CD4 treatment augmented percentages of CD8+ T cells, and anti-CD8 treatment augmented percentages of CD4+ T cells in the CD3+ pool. Anti-CD4 treatment also completely eliminated Tregs (Fig. 3R), as FOXP3+ T cells were exclusively CD4+ T cells (data not shown). PC61 treatment substantially depleted FOXP3+ Tregs, and surviving Tregs had baseline (negative) expression of CD25 (Fig. 3S). Quality control experiments in reference to Fig. 3G and H showed that CD8+ T cells (0.47% ± 0.21%, 0.77 ± 0.21) and FOXP3+ T cells (0.53% ± 0.15%, 0.87 ± 0.25) each comprised <1% of the “2D2-FIG Csf2−/−” and 2D2-FIG T cell repertoires, respectively (Fig. 3T–V). These data verified that the 2D2-FIG was an EAE model naturally deficient in both CD8+ T cells and FOXP3+ Tregs.
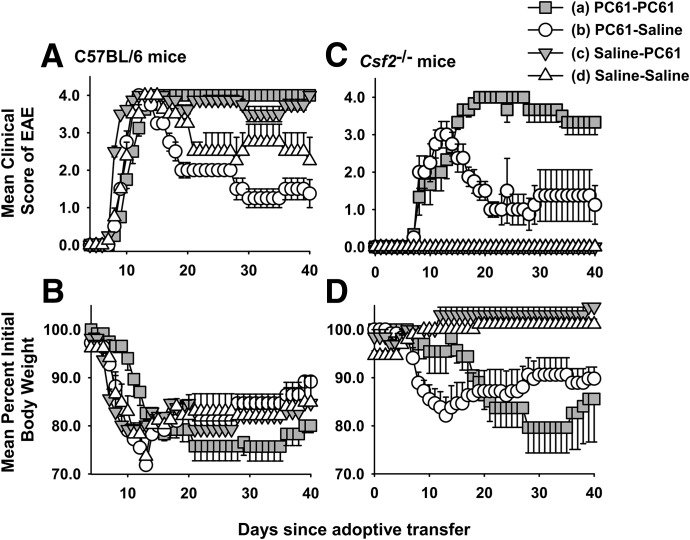
A passive model of EAE was also used to assess whether PC61 enabled the induction of GM-CSF-deficient encephalitogenic T cells in Csf2−/− mice (Table 4 and Fig. 4). Donor Csf2−/− mice and donor C57BL/6 mice were or were not treated with 250 µg PC61 on d −5 and −3 and then were immunized with MOG35–55 in CFA on d 0. Draining lymph nodes and splenocytes from each of the 4 donor groups were pooled on d 11 and were cultured for 3 d with 1 µM MOG35–55 and 10 ng/ml IL-23. Activated, nonfractionated donor T cells (107/recipient) were then adoptively transferred into recipients. Csf2−/− T cells were injected into Csf2−/− recipients, and C57BL/6 T cells were injected into C57BL/6 recipients. On d 7 after adoptive transfer, each recipient group was or was not injected with 250 µg PC61 (8 groups, n = 4/group). Two conclusions were evident. First, PC61 treatment of Csf2−/− donors enabled the differentiation of encephalitogenic T cells and the adoptive transfer of paralytic EAE into Csf2−/− recipients. PC61 pretreatment of Csf2−/− donors resulted in cumulative and maximal disease scores of 53.8 and 3.0, respectively, in recipients, whereas control Csf2−/− donor T cells did not transfer EAE to recipients [(b) vs. (d); Table 4]. The main conclusion was that PC61 enabled encephalitogenic sensitization of Csf2−/− donor T cells. Second, PC61 treatment of both donors and recipients enabled chronic EAE in recipient Csf2−/− mice, whereas pretreatment of donors alone resulted in an initial bout of severe EAE in recipients, followed by a partial recovery [(b) vs. (a); Table 4 and Fig. 4C]. These data provided evidence that CD4+ CD25+ Tregs prevented the differentiation of encephalitogenic T cells in Csf2−/− donor mice and blunted the action of established pathogenic T cells in Csf2−/− recipient mice. Differences in the time courses of EAE were paralleled by differences in maximal and average weight loss (Fig. 4B and D). All 4 groups of C57BL/6 recipients exhibited severe paralytic EAE (Fig. 4A). Nonetheless, PC61 treatment of recipient C57BL/6 mice prevented partial recovery and maintained EAE scores near a maximum of 4.0 (full hindlimb paralysis of both legs) during the entire 40 d observation period. These data provided evidence that CD4+ CD25+ Tregs may also modify passively induced EAE in wt recipients.
TABLE 4.
The anti-CD25 PC61 mAb enables the adoptive transfer of EAE in Csf2−/− mice
| Strain | Donors | Recipients | Incidence | Mean cumulative score | Median cumulative score | Mean maximal score | Median maximal score | Mean maximal weight loss | Mean average weight loss |
|---|---|---|---|---|---|---|---|---|---|
| (a) Csf2−/− | PC61 | PC61 | 4 of 4 | 109.7 ± 7.4 | 108.5 | 4.0 ± 0.0 | 4.0 | 20.4% | 13.7% |
| (b) Csf2−/− | PC61 | Saline | 4 of 4 | 53.8 ± 19.1 | 60.3 | 3.0 ± 0.7 | 3.5 | 17.9% | 12.4% |
| (c) Csf2−/− | Saline | PC61 | 0 of 4 | 0.0 ± 0.0 | 0.0 | 0.0 ± 0.0 | 0.0 | 0.0% | −3.0% |
| (d) Csf2−/− | Saline | Saline | 0 of 4 | 0.0 ± 0.0 | 0.0 | 0.0 ± 0.0 | 0.0 | 0.0% | −0.8% |
| (e) C57BL/6 | PC61 | PC61 | 4 of 4 | 119.8 ± 1.8 | 120.3 | 4.0 ± 0.0 | 4.0 | 26.9% | 21.2% |
| (f) C57BL/6 | PC61 | Saline | 4 of 4 | 67.6 ± 4.6 | 71.0 | 4.0 ± 0.0 | 4.0 | 28.1% | 17.7% |
| (g) C57BL/6 | Saline | PC61 | 4 of 4 | 123.5 ± 2.1 | 123.0 | 4.0 ± 0.0 | 4.0 | 22.5% | 18.4% |
| (h) C57BL/6 | Saline | Saline | 4 of 4 | 91.9 ± 12.8 | 81.0 | 4.0 ± 0.0 | 4.0 | 27.3% | 18.0% |
| (c) and (d) vs. (a), (e), (g), and (h) | P = 0.0286 | P ≤ 0.002 | P ≤ 0.001 | P ≤ 0.001 | P ≤ 0.017 | ||||
These data are portrayed in Fig. 4. Donor Csf2−/− and control C57BL/6 mice were or were not treated with 250 µg PC61 on d −5 and −3 and were immunized with 200 µg MOG35–55 in CFA on d 0. On d 11, draining lymph nodes and spleen were pooled within each of the 4 donor groups. These cells were cultured for 3 d with 1 µM MOG35–55 and 10 ng/ml IL-23. Activated donor T cells were then adoptively transferred into recipients (107 cells/recipient). Csf2−/− T cells were injected into Csf2−/− recipients, and C57BL/6 T cells were injected into C57BL/6 recipients. On d 7, each recipient group was or was not injected with 250 µg PC61. Shown are the means and the sem for cumulative and maximal EAE scores. Differences in EAE and weight-loss values were assessed by nonparametric and parametric ANOVA, respectively. Differences in disease incidence were assessed pairwise by a Fisher’s exact test.
Figure 4. The PC61 anti-CD25 mAb enable the passive induction of EAE in Csf2−/− mice.
Shown are time courses of data portrayed in Table 4. PC61-PC61, both donors and recipients were treated with PC61; PC61-Saline, donors but not recipients were treated with PC61; Saline-PC61, recipients but not donors were treated with PC61; Saline-Saline, neither donors nor recipients were treated with PC61. Shown are the means and the sem for the EAE score (A and C) and weight loss (B and D) time courses of C57BL/6 (A and B) and Csf2−/− (C and D) mice. (C) Nonparametric ANOVA; (a) vs. (c) and (d), P < 0.004 from d 8 to 40; (b) vs. (d), P < 0.001 from d 8 to 17 and P < 0.05 from d 18 to 40; and (b) vs. (a), P < 0.05 from d 17 to 29. (D) Parametric ANOVA; (a) vs. (c) and (d), P < 0.020 from d 27 to 38; (b) vs. (d), P < 0.010 from days 9 to 18; (b) vs. (c), P < 0.02 from d 9 to 20 and P < 0.05 from d 21 to 26; and (b) vs. (a), P < 0.32 from d 13 to 15.
Csf2−/− mice exhibited alterations in the relative distribution of lymphocyte subsets
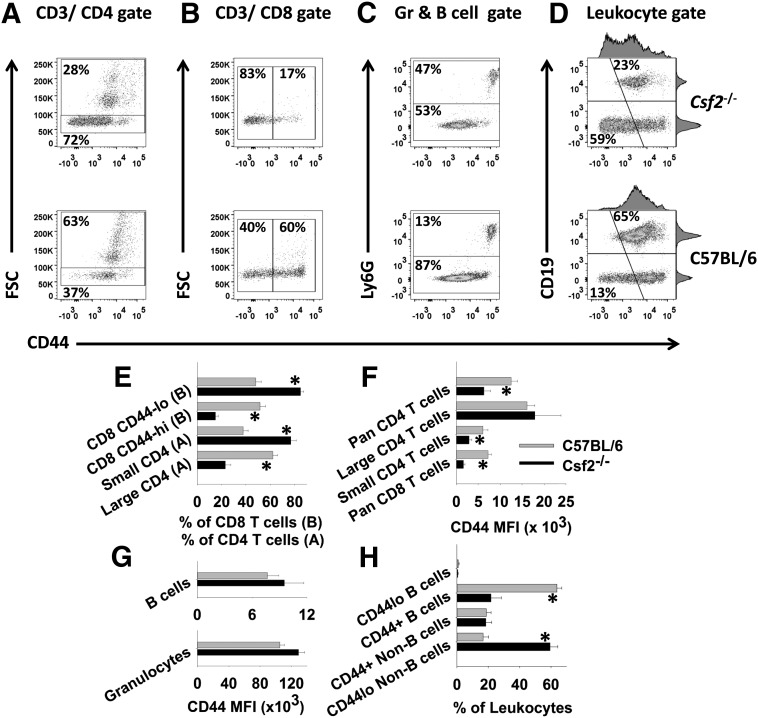
As noted previously in Figs. 1 and 3, Csf2−/− mice had profoundly altered percentages of leukocyte and lymphoid subsets. In CD4+ and CD8+ subsets, quiescent FSClow T cells from Csf2−/− peripheral blood had low levels of CD44 surface expression compared with WT controls (Fig. 5A and B). Experiments comparing Csf2−/− with WT splenocytes showed essentially equivalent differences among leukocyte subset percentages and CD44 expression as those noted in PBMCs (data not shown). FSClow CD4+ T cells constituted the majority of CD4+ T cells in Csf2−/− blood (72%) and a relative minority of CD4+ T cells (37%) in C57BL/6 blood (Fig. 5A). These representative percentages are portrayed as bar graphs (n = 6) in Fig. 5E. Not only were FSClow CD4+ T cells predominant in Csf2−/− blood, but also, these Csf2−/− CD4+ T cells expressed substantially less CD44 than the equivalent FSClow CD4+ subset in C57BL/6 blood (Fig. 5A and F). In contrast, enlarged FSChigh Csf2−/− CD4+ T cells had CD44 levels that were commensurate with WT controls (Fig. 5A and F). Overall, these findings revealed a significant difference in quiescent memory between the 2 mouse strains. Similar findings were noted for the CD8+ T cell subset. In both Csf2−/− and C57BL/6 blood, most CD8+ T cells had a small FSClow phenotype, and FSClow CD8+ T cells from Csf2−/− blood expressed substantially lower levels of CD44 than FSClow CD8+ T cells from C57BL/6 blood (Fig. 5B, E, and F).
Figure 5. Csf2−/− mice show altered T cell expression of CD44.
PBMCs from 6 Csf2−/− and 6 C57BL/6 were stained with fluorochrome-conjugated antibodies against CD45, CD3, CD4, CD8, CD44, Ly-6G, or CD19. Dot plots (upper) show data from a representative mouse from each group, and the associated bar graphs (lower) show the means and sd of each group (n = 6 mice). Gated populations of CD3+CD4+ T cells (A, E, and F) and CD3+CD8+ T cells (B, E, and F) were analyzed for FSC and CD44 expression. (C and G) A granulocyte (Gr)–B cell gate was used to analyze CD44 levels on the respective subsets. (D and H) A leukocyte gate (excluding granulocytes) was used to analyze CD44 expression on B cells and non-B cells (mostly T cells). The bar graphs show the mean percentage of the designated subsets (E and H) or the MFI for CD44 expression (F and G) together with the sd (*P < 0.001). These data are representative of 4 independent experiments.
In contrast to FSClow T cells, Csf2−/− granulocytes had CD44 levels similar to that of WT controls (Fig. 5C and G). Csf2−/− PBMCs had significantly higher percentages of Ly-6G+ granulocytes compared with C57BL/6 PBMCs, although the frequencies of CD11b+ blood monocytes were similar between the 2 strains (data not shown). Indeed, the average percentage of Ly-6G+ granulocytes (n = 6) was 3.8-fold greater than in C57BL/6 mice. As noted in splenocytes (not shown), CD19+ B cells were substantially less frequent in Csf2−/− PBMCs (excluding granulocytes; 23%) compared with WT PBMCs (65%; Fig. 5D). All B cells expressed CD44, and Csf2−/− B cells had CD44 levels similar to that of C57BL/6 B cells (Fig. 5D and G). As shown in Fig. 5D, both Csf2−/− and C57BL/6 CD19+ B cells had similar levels of CD44, whereas the non-B cell fraction (mostly T cells) exhibited profound differences in the percentages of CD44low vs. CD44high subsets (Fig. 5G and H).
Absolute leukocyte counts assessed by use of reference beads revealed no significant differences in the absolute numbers of viable PBMCs and CD45+ leukocytes in the blood of Csf2−/− and C57BL/6 mice. Absolute leukocyte counts in PBMCs revealed that the Csf2−/− mutation was associated with profound alterations in major leukocyte subsets, including CD4+CD3+ T cells, CD8+CD3+ T cells, B cells, and granulocytes. Csf2−/− PBMCs contained a 3.4-fold increase in numbers of CD11b+Ly-6G+ granulocytes, a 1.9-fold increase in numbers of CD3+ T cells, a 1.5-fold increase in CD3+ CD8+ T cells, and a 2.4-fold increase in CD3+ CD4+ T cells. Conversely, C57BL/6 PBMCs contained a 2.8-fold increase in CD19+ B cells (n = 6). Overall, the altered frequencies of lymphoid cell types were consistent between the blood and the spleen, such that the blood was a reliable indicator of the cell-type percentages in the spleen. The increased numbers of CD4+ and CD8+ T cells in Csf2−/− mice were largely attributed to increased cellularity of the naïve CD44low T cell compartments. Increased absolute numbers but decreased percentages of CD8+ T cells (e.g., Figs. 1 and 3) simply reflected the predominant accumulation of naïve CD4+ T cells.
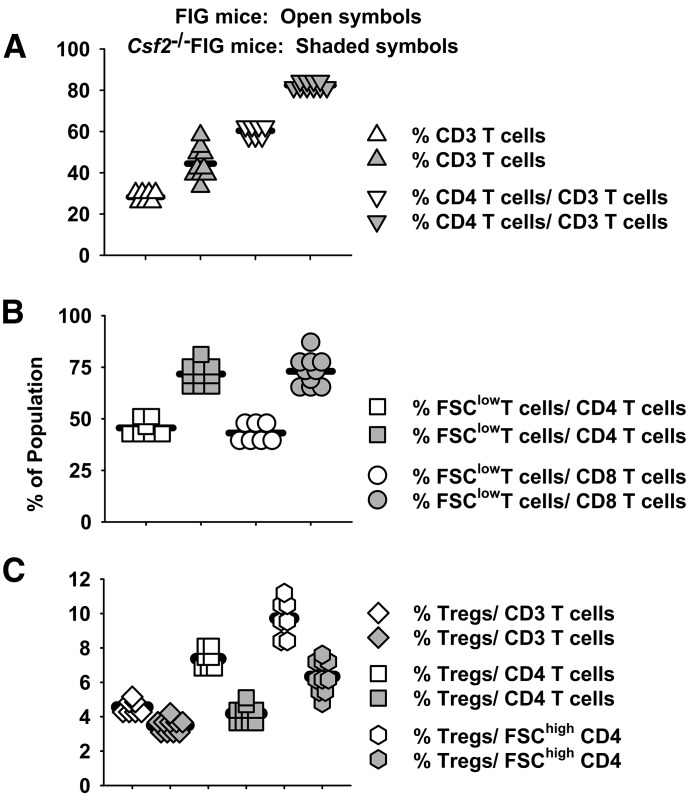
Given that GM-CSF deficiency caused profound perturbations of major T cell subsets, an important question was how the GM-CSF deficiency-affected percentages of CD4+ CD25+ FOXP3+ Tregs. FIG Csf2−/− and FIG mice were used in these experiments. Like Csf2−/− PBMC and splenocytes, FIG Csf2−/− PBMCs had elevated frequencies of total CD3+ T cells and a predominance of CD4+ T cells (Fig. 6A). Shown are the percentages of CD3+ T cells in reference to total leukocytes (excluding granulocytes) and the percentages of CD4+ T cells in the CD3+ T cell pool (Fig. 6A). As shown for Csf2−/− mice (Fig. 5), elevated percentages of CD3+ T cells in FIG Csf2−/− PBMC were associated with increased frequencies of small, quiescent CD4+ and CD8+ subsets (Fig. 6B). The percentages of FSClow CD4+ and FSClow CD8+ T cells are shown in reference to the total pools of CD4+ and CD8+ T cells, respectively. Essentially, all GFP+ (FOXP3+) T cells were CD4+ (data not shown). Frequencies of CD4+ CD25+ FOXP3+ T cells were reduced in FIG Csf2−/− PBMCs when total CD3+ T cells were used as the baseline (Fig. 6C). These reductions were modest but statistically significant (P < 0.001). Larger reductions were noted when total CD4+ T cells or FSChigh CD4+ T cells were used as the denominator of the ratio. Thus, Treg percentages in FIG Csf2−/− mice were modestly decreased when normalized to CD3+ T cells and more substantially decreased when normalized to CD4+ T cells or FSChigh CD4+ T cells. These data indicate that the GM-CSF deficiency is not associated with elevated Treg percentages (Fig. 6C) or Treg numbers (data not shown).
Figure 6. Percentages of CD4+ CD25+ FOXP3+ Tregs in Csf2−/− exhibit modest reductions in frequency relative to the pool of total CD3+ T cell.
PBMCs were obtained from 7 Csf2−/− FIG mice and 10 FIG mice. (A) Shown are the percentages of CD3+ T cells among total leukocytes (excluding granulocytes) and the percentage of CD4+ T cells among the total CD3+ T cell pool. (B) Shown are the percentages of FSClow CD4+ T cells among the total CD4+ T cell pool and the percentages of FSClow CD8+ T cells among the total CD8+ T cell pool. (C) Shown are the percentages of CD4+ CD25+ FOXP3+ Tregs among the CD3+ T cell pool, the CD3+ CD4+ T cell pool, and the FSChigh CD3+ CD4+ T cell pool. t Tests revealed significant differences for all 7 pairwise comparisons (A–C; P < 0.001). These data are representative of 3 independent experiments.
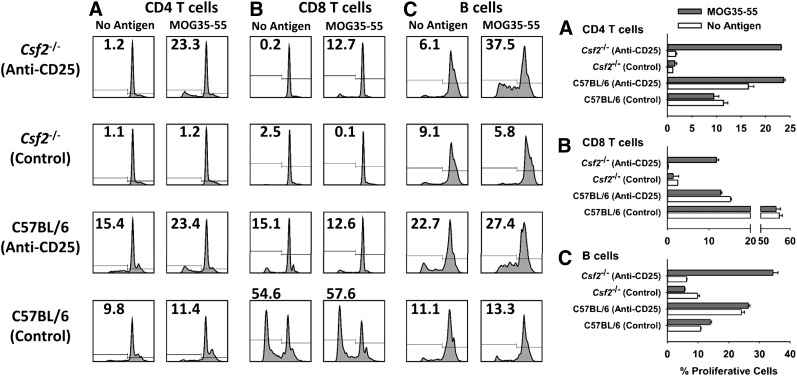
The large accumulation of naïve CD44low T cells in Csf2−/− mice suggested that GM-CSF deficiency conferred deficient immunogenic sensitization to environmental antigens. Likewise, GM-CSF deficiency, at least in part, may confer EAE resistance by undermining encephalitogenic sensitization in MOG/CFA-challenged mice, as previously noted in Fig. 4. Deficient Tconv responses balanced by a functionally intact Treg repertoire would accommodate the main observation of this study—that PC61 liberates encephalitogenic responses in Csf2−/− mice. To test this hypothesis, Csf2−/− and C57BL/6 mice were pretreated with PC61 or Y13 mAb on d −4 and −2, were challenged on d 0 with MOG35–55 in CFA, and were given Ptx on d 0 and 2. On d 12, these sensitized C57BL/6 mice and PC61-pretreated Csf2−/− mice began exhibiting clinical signs of EAE (score of 3.0 and 2.0, respectively), whereas Y13-pretreated Csf2−/− mice remained resistant to EAE. Lymphoid cells were isolated from draining nodes, labeled with CellTrace Violet (Thermo Fisher Scientific), and cultured with 1 µM MOG35–55 for 4 d. The results showed that CD4+ T cells from the draining lymph nodes of PC61-pretreated Csf2−/− mice, but not those from Y13-pretreated Csf2−/− mice, exhibited robust MOG-specific proliferation (Fig. 7A). Similar MOG-specific proliferative responses were noted in the CD8+ T and B cell compartments (Fig. 7B and C). The latter observation presumably reflected bystander proliferation as a result of cytokine production by MOG-stimulated CD4+ T cells. Parallel cultures of C57BL/6 showed high levels of background proliferation. This observation may reflect more efficient transport of MOG from the MOG/CFA emulsion into the draining lymphatics, as in vitro proliferation was not dependent on exogenously added MOG. We also noted that CD8+ T cell proliferation from Y13-pretreated C57BL/6 mice was substantially higher than that from PC61-treated C57BL/6 mice. This result may reflect PC61-mediated depletion of CD25+ CD8+ precursors in vivo. Based on the analysis of PC61 vs. Y-13-treated Csf2−/− mice, these data indicate that GM-CSF is needed for MOG-specific CD4+ T cells to overcome Treg barriers during encephalitogenic sensitization in the lymphatics draining a MOG/CFA inoculation.
Figure 7. MOG-specific sensitization is deficient in Csf2−/− mice.
Csf2−/− and C57BL/6 mice were pretreated with 250 µg PC61 or Y13 mAb on d −4 and −2 and were challenged on d 0 with MOG35–55 in CFA. Ptx was given on d 0 and 2. On d 12, lymphoid cells were isolated from draining nodes; labeled with CellTrace Violet; cultured in triplicate, with or without 1 µM MOG35–55 for 4 d; and analyzed for expression of CD45, CD3, CD4, CD19, and MHC class II. B Cells were gated as the CD19+, MHC class II+ population. CellTrace Violet dye dilution was used as a measure of proliferation. (A–C) PC61-pretreated Csf2−/− cultures ± 1 µM MOG35–55 (P < 0.001). Y13-pretreated Csf2−/− cultures ± 1 µM MOG35–55 (not significant). These data are representative of 3 replicate experiments.
DISCUSSION
Severe chronic EAE can be elicited in Csf2−/− mice
This study was based on the observation that pretreatment with the PC61 anti-CD25 mAb enabled the expression of severe EAE in the otherwise resistant Csf2−/− strain. Although Csf2−/− mice are profoundly resistant to EAE, previous studies provided evidence that resistance could be bypassed by intracranial inoculation of LPS or TLR9 agonists simultaneously with the adoptive transfer of MBP-specific Csf2−/− T cells into WT recipients [19]. Although recipients were GM-CSF replete, EAE effector T cells represented the key source of GM-CSF, as MBP–TCR transgenic T cells transferred EAE into WT and Csf2−/− recipients, whereas MBP-TCR Csf2−/− T cells lacked the ability to transfer EAE into WT recipients. Presumably, direct stimulation of TLRs in the CNS compensated for the lack of GM-CSF production by MBP-specific Csf2−/− T cells in the CNS, thereby resulting in the activation of microglia, CNS infiltration of peripheral myeloid cells, enhanced effector T cell infiltration, and the reconstitution of EAE. MBP–TCR transgenic mice were used to bypass any role that GM-CSF may play during clonal expansion and in vivo sensitization. However, as recipients were GM-CSF replete, and many cell types produce GM-CSF, these studies could not exclude the possibility that TLR-stimulated GM-CSF in the host contributed to EAE in this model [19].
Data shown in Figs. 1–4 complement and extend these studies by showing that severe chronic EAE can be readily induced in an unambiguous GM-CSF-deficient model, including active induction of EAE in Csf2−/− mice and adoptive transfer of Csf2−/− T cells into Csf2−/− recipients. The mechanism of EAE resistance in Csf2−/− mice was abrogated by treatment of Csf2−/− mice with the PC61 anti-CD25 mAb that eliminated CD4+ CD25+ FOXP3+ Tregs. PC61-treated Csf2−/− mice exhibited disease time courses and severities that were essentially the same as those in WT mice. As PC61 did not affect CD25− FOXP3+ Tregs, the implication was that the normal effector CD25+ FOXP3+ Treg subset represented a major mechanism of disease resistance in Csf2−/− mice. We considered 2 overlapping possibilities to explain the role of CD25+ Tregs in GM-CSF-deficient mice. First, Tconv responses may be impaired, whereas Treg barriers may be essentially normal and functionally intact. Second, Tconv responses may be essentially normal, whereas Treg responses may be expanded or hyperfunctional.
The first hypothesis (i.e., impaired Tconv repertoire) was directly supported by the observation that both CD4 and CD8 T cell repertoires were dominated by quiescent, naïve CD44low T cells (Fig. 5). The large accumulation of CD44low naïve T cells in Csf2−/− mice most likely reflected a requirement for GM-CSF in normal sensitization to environmental antigens. This hypothesis was also directly supported by the observation that Csf2−/− mice had a profound defect in encephalitogenic sensitization (Fig. 4). Initial studies showing that Csf2−/− mice were profoundly resistant to EAE also revealed that MOG35–55/CFA-sensitized mice exhibited depressed MOG-specific splenocyte proliferation and reduced production of IL-2, IL-6, and IFN-γ [6]. GM-CSF was also needed for induction of CD103+ DCs in peripheral lymphoid tissues and the consequent differentiation of naïve, myelin-reactive T cells into clonally expanded IFN-γ effector T cells [33]. These observations showed that GM-CSF has an important role in sensitization of encephalitogenic responses. This finding was confirmed by data in Fig. 7. MOG/CFA-immunized Csf2−/− mice lacked ex vivo reactivity to MOG, whereas PC61 pretreatment of Csf2−/− mice enabled vigorous anti-MOG CD4+ T cell responses ex vivo, together with recruitment of CD8+ and B cell responses. We also noted that the draining lymph nodes of MOG35–55/CFA-sensitized Csf2−/− mice were at least 2 times smaller, on average, than the nodes of WT mice (data not shown). Overall, these observations indicate that Csf2−/− mice had a profound defect in antigenic priming.
The second hypothesis (i.e., Treg hyper-reactivity) was less consistent with our experimental observations, particularly in that the activated/effector subset of CD25+ FOXP3+ Tregs in Csf2−/− mice did not exhibit evidence of expansion in blood or secondary lymphoid organs (Figs. 1, 3, and 6). If the Treg repertoire were expanded or hyperfunctional, one would expect exaggerated tolerogenic responses. However, immunization of Csf2−/− mice with MOG did not increase numbers, percentages, or activation phenotypes of Tregs compared with WT mice, and tolerogenic vaccination of Csf2−/− mice did not elicit more Tregs than observed in WT mice (data not shown). Rather, Csf2−/− mice exhibited deficient immunogenic responsiveness, not hyper-reactive tolerance, as the unresponsive state was readily reversed by administration of exogenous GM-CSF [6]. Likewise, EAE in WT mice was blocked by anti-GM-CSF therapy, but withdrawal of the antibody resulted in the rapid return of severe clinical symptoms [6, 14]. If GM-CSF deficiency conferred the hyperinduction of tolerance, then the unresponsive state would persist beyond the simple reconstitution of GM-CSF. In the absence of GM-CSF, weakened Tconv responses apparently lacked the activation energy to overcome the normal barriers imposed by CD25+ Tregs. However, upon mAb-mediated depletion of CD25+ Tregs, encephalitogenic sensitization was unimpeded by Tregs and culminated in severe chronic EAE. Overall, this study supports the concept that EAE resistance of Csf2−/− mice was a result of a functional imbalance of functionally deficient effector responses and functional CD25+ Tregs. These observations favor the view that the PC61 sensitivity of Csf2−/− mice is not a result of gross alteration of the Treg repertoire but rather, reflected subjugation of a compromised Tconv repertoire.
An anti-CD25 mAb represented the tool used to deplete FOXP3+ Tregs in this study. FOXP3+ Tregs exist as CD25+ and CD25– subsets, and the PC61 mAb specifically depleted CD25+ FOXP3+ Tregs but not CD25− FOXP3+ T cells (Fig. 3). CD25+ FOXP3+ Tregs represent activated, functional Tregs, whereas CD25− FOXP3+ T cells are quasi-stable Tregs that represent transitional phases between Tconv and functional Tregs [34–36]. Although PC61 is typically thought to work by depletion of FOXP3+ Tregs, this study cannot exclude the possibility that PC61 enabled EAE in Csf2−/− mice by depletion of alternative CD25+ regulatory cells aside from the FOXP3 lineage. Additional research focusing on more direct manipulation of the FOXP3+ T cell subset will be needed to address this possibility.
GM-CSF is required for pathogenic invasion of the CNS
Several lines of evidence implicated T cell-mediated production of GM-CSF as a critical proinflammatory mediator in the effector phase of EAE. For example, adoptive transfer of IFN-γ−/− T cells into IL-17RA−/− recipients was completely inhibited by treatment with an anti-GM-CSF antibody [15]. Additional adoptive transfer studies also implicated T cell-mediated production of GM-CSF as a key event in the effector phase of CNS pathogenesis [16]. IL-17−/−, IFN-γ−/−, or dual-deficient polyclonal T cells or IL-17−/− 2D2 T cells had potent EAE transfer activity, but Csf2−/− polyclonal T cells or polarized Th1/Th17 Csf2−/− 2D2 T cells lacked EAE-inductive activity. GM-CSF-deficient and -replete Th1 and Th17 T cells from the transgenic MBP(Ac1–11) clonotype exhibited similar dependency of GM-CSF production for adoptive EAE [14]. Csf2−/− effector cells exhibited early CNS infiltration but did not sustain CNS inflammation as a result of defects in the recruitment of peripheral myeloid CD45high, CD11b myeloid cells [6, 14]. Conditional gene targeting revealed that GM-CSF responsiveness of peripheral CCR2+ Ly6Chigh monocytes was required for EAE [37]. Whether GM-CSF is important for surmounting Treg barriers at the effector phase remains an important question. PC61 treatment of recipients in the Csf2−/− adoptive transfer system prevented partial recovery from EAE (Fig. 4). Likewise, PC61 pretreatment of actively immunized 2D2-FIG Csf2−/− mice also prevented spontaneous recovery (Fig. 3). These data indicate that GM-CSF has a continuing role to counter Treg responses during the effector/recovery phase of chronic EAE.
Paradoxically, GM-CSF can be used to induce tolerance in EAE
One might surmise that a major action of GM-CSF is to enable Tconv to overcome Treg barriers during sensitization and effector phases of EAE. However, the regulatory action of GM-CSF is complex. A substantial literature linked the presence (not the absence) of GM-CSF to the induction of "regulatory" DCs and Tregs in several models of autoimmune disease [38–48]. Likewise, GM-CSF–neuroantigen fusion proteins had potent tolerogenic and therapeutic action in rat and mouse models of EAE [49–51]. GM-CSF–neuroantigen fusion proteins mediated a dominant tolerogenic action even when introduced into the strongly proinflammatory environment of the CFA lymphatic drainage [52]. Thus, GM-CSF apparently facilitates dominance of Tconv or Treg responses based on adaptive cues in the local environment.
GM-CSF is required for the normal balance of leukocyte subsets, including granulocytes, B cells, and naïve vs. effector T cells
Given that GM-CSF has direct action on myeloid rather than lymphoid cells as a result of the presence of the respective receptor on myeloid APCs but not on T and B cells, it was notable that GM-CSF deficiency had profound effects on numbers and percentages of lymphoid subsets. Most importantly, Csf2−/− mice had elevated percentages of CD3+ T cells and increased percentages of CD44null-low naïve T cells in CD4+ and CD8+ T cell compartments. The likelihood is that Csf2−/− DCs and T cells exhibit an altered antigen-driven development as a result of intertwined mechanisms, in which the lack of GM-CSF production by antigen-stimulated T cells causes deficient DC maturation. The lack of myeloid DC differentiation may, in turn, engender deficient presentation of ubiquitous environmental antigens that, in turn, impair the differentiation of naïve T cells into memory cells. This bottleneck in T cell development may account for the accumulation of naïve, quiescent CD44null/low T cells and the deficiency in CD44high memory T cells in Csf2−/− mice.
The main myeloid perturbation noted in this study was an ∼3-fold increase in the percentages of granulocytes in Csf2−/− PBMCs. The impact of GM-CSF upon the granulocytic lineage may be multifold. Granulocytes express the GM-CSFR heterodimer and directly respond to GM-CSF by induction of effector activities. However, GM-CSF-stimulated production of reactive oxygen intermediates may shorten the granulocyte lifespan. GM-CSF production by tissue-resident myeloid APCs and effector-memory T cells may induce chemokines that promote migration of granulocytes from the blood into peripheral tissues. Thus, the absence of GM-CSF in Csf2−/− mice may thereby lengthen the granulocyte lifespan and/or persistence of granulocytes in the circulation and thereby, increase detection of granulocytes in blood. Another possibility is that the balance of GM-CSF and G-CSF may influence the proportional differentiation of monocytic and granulocytic lineages in the bone marrow. The absence of GM-CSF in Csf2−/− mice may result in unbalanced G-CSF-induced differentiation of myeloid precursors into the granulocytic lineage. However, percentages of CD11b+ monocytes were not significantly different in Csf2−/− and WT PBMCs (data not shown). Many other nonexclusive possibilities also may account for the accentuated frequencies of granulocytes in Csf2−/− mice.
Conclusion
The presence of maximal EAE in the complete absence of GM-CSF revealed that GM-CSF is not an obligate effector molecule in all forms of EAE. This study instead supports the alternative explanation that the EAE resistance of Csf2−/− mice, at least in part, was a result of a functional imbalance of Tregs and effector T cells. These observations reveal a link between deficient T cell differentiation, a functional Treg/Tconv imbalance, and EAE resistance in Csf2−/− mice.
AUTHORSHIP
D.G. performed the majority of the experiments. A.D.C. and D.S.W. facilitated the experimentation, including the histological analysis and mAb purification/quality control. M.D.M. played a major role in the conception and design of the study, generation of antibody supernatants, compilation of the data, and writing of the manuscript. All authors were important intellectual contributors to this study.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Neurological Disorders and Stroke, U.S. National Institutes of Health (Grants R15-NS075830 and R01-NS072150, to M.D.M.) and the Harriet and John Wooten Laboratory for Alzheimer’s and Neurodegenerative Disease Research (to M.D.M) and by a research grant from AlzNC, Alzheimer’s North Carolina.
Glossary
- CD25
IL-2Rα
- Csf2−/−
GM-CSF deficient
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- FIG
forkhead box p3-internal ribosome entry site-GFP knock-in
- FOXP3+
forkhead box P3+
- FSC
forward-scatter
- i.p.
intraperitoneal
- MBP
myelin basic protein
- MFI
mean fluorescence intensity
- MOG
myelin oligodendrocyte glycoprotein
- Ptx
pertussis toxin
- Tconv
conventional T cell
- Treg
regulatory T cell
- WT
wild-type
DISCLOSURES
The authors have no financial or commercial conflicts of interest in regard to this work.
REFERENCES
- 1.Robinson A. P., Harp C. T., Noronha A., Miller S. D. (2014) The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 122, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Nun A., Kaushansky N., Kawakami N., Krishnamoorthy G., Berer K., Liblau R., Hohlfeld R., Wekerle H. (2014) From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J. Autoimmun. 54, 33–50. [DOI] [PubMed] [Google Scholar]
- 3.Simmons S. B., Pierson E. R., Lee S. Y., Goverman J. M. (2013) Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 34, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangachari M., Kuchroo V. K. (2013) Using EAE to better understand principles of immune function and autoimmune pathology. J. Autoimmun. 45, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuerten S., Lehmann P. V. (2011) The immune pathogenesis of experimental autoimmune encephalomyelitis: lessons learned for multiple sclerosis? J. Interferon Cytokine Res. 31, 907–916. [DOI] [PubMed] [Google Scholar]
- 6.McQualter J. L., Darwiche R., Ewing C., Onuki M., Kay T. W., Hamilton J. A., Reid H. H., Bernard C. C. (2001) Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J. Exp. Med. 194, 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendel I., Katz A., Kozak N., Ben-Nun A., Revel M. (1998) Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur. J. Immunol. 28, 1727–1737. [DOI] [PubMed] [Google Scholar]
- 8.Samoilova E. B., Horton J. L., Hilliard B., Liu T. S., Chen Y. (1998) IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 161, 6480–6486. [PubMed] [Google Scholar]
- 9.Becher B., Segal B. M. (2011) T(H)17 cytokines in autoimmune neuro-inflammation. Curr. Opin. Immunol. 23, 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Lee Y. S., Yu C. R., Egwuagu C. E. (2008) Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 180, 6070–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitnis T., Najafian N., Benou C., Salama A. D., Grusby M. J., Sayegh M. H., Khoury S. J. (2001) Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J. Clin. Invest. 108, 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens F., Tak P. P., Ostergaard M., Stoilov R., Wiland P., Huizinga T. W., Berenfus V. Y., Vladeva S., Rech J., Rubbert-Roth A., Korkosz M., Rekalov D., Zupanets I. A., Ejbjerg B. J., Geiseler J., Fresenius J., Korolkiewicz R. P., Schottelius A. J., Burkhardt H. (2015) MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 74, 1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deiß A., Brecht I., Haarmann A., Buttmann M. (2013) Treating multiple sclerosis with monoclonal antibodies: a 2013 update. Expert Rev. Neurother. 13, 313–335. [DOI] [PubMed] [Google Scholar]
- 14.El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G. X., Dittel B. N., Rostami A. (2011) The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroenke M. A., Chensue S. W., Segal B. M. (2010) EAE mediated by a non-IFN-γ/non-IL-17 pathway. Eur. J. Immunol. 40, 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Codarri L., Gyülvészi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., Becher B. (2011) RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567. [DOI] [PubMed] [Google Scholar]
- 17.Flügel A., Berkowicz T., Ritter T., Labeur M., Jenne D. E., Li Z., Ellwart J. W., Willem M., Lassmann H., Wekerle H. (2001) Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity 14, 547–560. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami N., Lassmann S., Li Z., Odoardi F., Ritter T., Ziemssen T., Klinkert W. E., Ellwart J. W., Bradl M., Krivacic K., Lassmann H., Ransohoff R. M., Volk H. D., Wekerle H., Linington C., Flügel A. (2004) The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J. Exp. Med. 199, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponomarev E. D., Shriver L. P., Maresz K., Pedras-Vasconcelos J., Verthelyi D., Dittel B. N. (2007) GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 178, 39–48. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke M. A., Carlson T. J., Andjelkovic A. V., Segal B. M. (2008) IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 205, 1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeachy M. J. (2011) GM-CSF: the secret weapon in the T(H)17 arsenal. Nat. Immunol. 12, 521–522. [DOI] [PubMed] [Google Scholar]
- 22.Dranoff G., Crawford A. D., Sadelain M., Ream B., Rashid A., Bronson R. T., Dickersin G. R., Bachurski C. J., Mark E. L., Whitsett J. A., Mulligan R. C. (1994) Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264, 713–716. [DOI] [PubMed] [Google Scholar]
- 23.Lublin F. D., Knobler R. L., Kalman B., Goldhaber M., Marini J., Perrault M., D’Imperio C., Joseph J., Alkan S. S., Korngold R. (1993) Monoclonal anti-gamma interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity 16, 267–274. [DOI] [PubMed] [Google Scholar]
- 24.Duong T. T., Finkelman F. D., Singh B., Strejan G. H. (1994) Effect of anti-interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. J. Neuroimmunol. 53, 101–107. [DOI] [PubMed] [Google Scholar]
- 25.Krakowski M., Owens T. (1996) Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 26, 1641–1646. [DOI] [PubMed] [Google Scholar]
- 26.Willenborg D. O., Fordham S., Bernard C. C., Cowden W. B., Ramshaw I. A. (1996) IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 157, 3223–3227. [PubMed] [Google Scholar]
- 27.Chu C. Q., Wittmer S., Dalton D. K. (2000) Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki K., Bean A., Shah S., Schutten E., Huseby P. G., Peters B., Shen Z. T., Vanguri V., Liggitt D., Huseby E. S. (2014) Relapsing-remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J. Immunol. 192, 3029–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Q., Goverman J. (2007) Experimental autoimmune encephalomyelitis mediated by CD8+ T cells. Ann. N. Y. Acad. Sci. 1103, 157–166. [DOI] [PubMed] [Google Scholar]
- 30.Ford M. L., Evavold B. D. (2005) Specificity, magnitude, and kinetics of MOG-specific CD8+ T cell responses during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 35, 76–85. [DOI] [PubMed] [Google Scholar]
- 31.Sun D., Whitaker J. N., Huang Z., Liu D., Coleclough C., Wekerle H., Raine C. S. (2001) Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J. Immunol. 166, 7579–7587. [DOI] [PubMed] [Google Scholar]
- 32.Huseby E. S., Liggitt D., Brabb T., Schnabel B., Ohlén C., Goverman J. (2001) A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J. Exp. Med. 194, 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King I. L., Kroenke M. A., Segal B. M. (2010) GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J. Exp. Med. 207, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huynh A., DuPage M., Priyadharshini B., Sage P. T., Quiros J., Borges C. M., Townamchai N., Gerriets V. A., Rathmell J. C., Sharpe A. H., Bluestone J. A., Turka L. A. (2015) Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 16, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komatsu N., Okamoto K., Sawa S., Nakashima T., Oh-hora M., Kodama T., Tanaka S., Bluestone J. A., Takayanagi H. (2014) Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68. [DOI] [PubMed] [Google Scholar]
- 36.Ohkura N., Hamaguchi M., Morikawa H., Sugimura K., Tanaka A., Ito Y., Osaki M., Tanaka Y., Yamashita R., Nakano N., Huehn J., Fehling H. J., Sparwasser T., Nakai K., Sakaguchi S. (2012) T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799. [DOI] [PubMed] [Google Scholar]
- 37.Croxford A. L., Lanzinger M., Hartmann F. J., Schreiner B., Mair F., Pelczar P., Clausen B. E., Jung S., Greter M., Becher B. (2015) The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43, 502–514. [DOI] [PubMed] [Google Scholar]
- 38.Gopisetty A., Bhattacharya P., Haddad C., Bruno J. C. Jr., Vasu C., Miele L., Prabhakar B. S. (2013) OX40L/Jagged1 cosignaling by GM-CSF-induced bone marrow-derived dendritic cells is required for the expansion of functional regulatory T cells. J. Immunol. 190, 5516–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng J. R., Muthusamy T., Prabhakar B. S., Meriggioli M. N. (2011) GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J. Neuroimmunol. 240-241, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattacharya P., Gopisetty A., Ganesh B. B., Sheng J. R., Prabhakar B. S. (2011) GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J. Leukoc. Biol. 89, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganesh B. B., Cheatem D. M., Sheng J. R., Vasu C., Prabhakar B. S. (2009) GM-CSF-induced CD11c+CD8a—dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int. Immunol. 21, 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheatem D., Ganesh B. B., Gangi E., Vasu C., Prabhakar B. S. (2009) Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin. Immunol. 131, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng J. R., Li L. C., Ganesh B. B., Prabhakar B. S., Meriggioli M. N. (2008) Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin. Immunol. 128, 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaudreau S., Guindi C., Ménard M., Besin G., Dupuis G., Amrani A. (2007) Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J. Immunol. 179, 3638–3647. [DOI] [PubMed] [Google Scholar]
- 45.Sheng J. R., Li L., Ganesh B. B., Vasu C., Prabhakar B. S., Meriggioli M. N. (2006) Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J. Immunol. 177, 5296–5306. [DOI] [PubMed] [Google Scholar]
- 46.Gangi E., Vasu C., Cheatem D., Prabhakar B. S. (2005) IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J. Immunol. 174, 7006–7013. [DOI] [PubMed] [Google Scholar]
- 47.Vasu C., Dogan R. N., Holterman M. J., Prabhakar B. S. (2003) Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J. Immunol. 170, 5511–5522. [DOI] [PubMed] [Google Scholar]
- 48.Gaudreau S., Guindi C., Ménard M., Benabdallah A., Dupuis G., Amrani A. (2010) GM-CSF induces bone marrow precursors of NOD mice to skew into tolerogenic dendritic cells that protect against diabetes. Cell. Immunol. 265, 31–36. [DOI] [PubMed] [Google Scholar]
- 49.Mannie M. D., Blanchfield J. L., Islam S. M., Abbott D. J. (2012) Cytokine-neuroantigen fusion proteins as a new class of tolerogenic, therapeutic vaccines for treatment of inflammatory demyelinating disease in rodent models of multiple sclerosis. Front. Immunol. 3, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott D. J., Blanchfield J. L., Martinson D. A., Russell S. C., Taslim N., Curtis A. D., Mannie M. D. (2011) Neuroantigen-specific, tolerogenic vaccines: GM-CSF is a fusion partner that facilitates tolerance rather than immunity to dominant self-epitopes of myelin in murine models of experimental autoimmune encephalomyelitis (EAE). BMC Immunol. 12, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanchfield J. L., Mannie M. D. (2010) A GMCSF-neuroantigen fusion protein is a potent tolerogen in experimental autoimmune encephalomyelitis (EAE) that is associated with efficient targeting of neuroantigen to APC. J. Leukoc. Biol. 87, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Islam S. M., Curtis A. D. II, Taslim N., Wilkinson D. S., Mannie M. D. (2014) GM-CSF-neuroantigen fusion proteins reverse experimental autoimmune encephalomyelitis and mediate tolerogenic activity in adjuvant-primed environments: association with inflammation-dependent, inhibitory antigen presentation. J. Immunol. 193, 2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]