PI3Kγ-mediated activation of ERK regulates oxidative burst-dependent netosis, while PI3Kσ and calcium are key signals for netosis independent of oxidative burst induced by Leishmania.
Keywords: parasitic protozoa, netosis, signaling
Abstract
Upon in vitro stimulation, neutrophils undergo a cell death named netosis. This process is characterized by extracellular release of chromatin scaffold associated with granular and cytoplasmic proteins, which together, ensnare and kill microbes. We have previously described that interaction of Leishmania amazonensis with human neutrophils leads to the release of neutrophil extracellular traps, which trap and kill the parasite. However, the signaling leading to Leishmania induced netosis is still unknown. Thus, we sought to evaluate signaling events that drive L. amazonensis induced neutrophil extracellular trap release from human neutrophils. Here, we found that PI3K, independently of protein kinase B, has a role in parasite-induced netosis. We also described that the main isoforms involved are PI3Kγ and PI3Kδ, which work in reactive oxygen species-dependent and -independent ways, respectively. We demonstrated that activation of ERK downstream of PI3Kγ is important to trigger reactive oxygen species-dependent, parasite-induced netosis. Pharmacological inhibition of protein kinase C also significantly decreased parasite-induced neutrophil extracellular trap release. Intracellular calcium, regulated by PI3Kδ, represents an alternative reactive oxygen species-independent pathway of netosis stimulated by L. amazonensis. Finally, intracellular calcium mobilization and reactive oxygen species generation are the major regulators of parasite-induced netosis. Our results contribute to a better understanding of the signaling behind netosis induced by interactions between Leishmania and neutrophils.
Introduction
Neutrophils are the most abundant leukocytes in blood, and their generation represents the primary activity of the hematopoietic cell production in the bone marrow [1]. Generally, neutrophils do not leave blood vessels, except when injury or infection provides attracting signals for them to migrate from the bloodstream to the affected site [2].
Leishmaniasis is an infectious disease caused by the intracellular protozoan parasite Leishmania, which infects >1.6 million people worldwide [3]. Upon transmission to mammals by female phlebotomine sand flies, Leishmania is inoculated into a pool of blood in the dermis from where neutrophils are rapidly recruited to the parasite inoculation site [4]. Neutrophils constitute the early cellular defense against the parasite and modulate subsequent immune responses by cross-talking with other cells through secretion of several immune modulators [5].
Neutrophils are endowed with powerful mechanisms to confront invasive microbes, including phagocytosis and degranulation [6]. Moreover, it has been recently demonstrated that neutrophils kill microbes by a distinct mechanism, which consists of the extracellular release of traps composed of chromatin, decorated with several granular and cytoplasmic proteins, and named “NETs” [7, 8]. NETs are capable of trapping a plethora of microbes, avoiding infection dissemination, and killing ensnared microorganisms through contact with toxic proteins. A long list of NET inducers has been described, including bacteria, fungi, viruses, cytokines, chemokines, and crystal salts [9]. Our group demonstrated that Leishmania induces NET release by human neutrophils and that the parasite is susceptible to histones associated with these fibers [10].
In vitro neutrophils undergo several ultrastructural changes during NET formation. These include nuclear membrane swelling and disintegration, chromatin decondensation with subsequent association with several proteins, and extrusion to the extracellular milieu. Together, these features define a unique cell-death mechanism named netosis [11]. Although some signaling pathways leading to netosis have been determined for certain stimuli, the pathways downstream of most of NET inducers remain poorly understood. A requirement for ROS generation by NOX2 seems to be involved in most of the netosis-induction processes [12–14], and both pharmacological inhibition and genetic mutations on NOX2, such as in chronic granulomatous disease, abrogate NET release by ROS-dependent inducers [11]. In contrast, ROS-independent NET release has also been reported for different stimuli [15–18]. Additionally, it has been demonstrated that the MAPK pathway and PI3K are involved in NET release downstream of some stimuli [19–21]. Therefore, the aim of this work was to identify signaling molecules associated with NET release induced by L. amazonensis promastigotes to provide a better characterization of this process.
MATERIALS AND METHODS
Purification of human neutrophils
Neutrophils were purified from buffy coats of healthy volunteers, provided by the Hemotherapy Service of the HUCFF (Universidade Federal do Rio de Janeiro, Brazil). All procedures were approved by the Institutional Review Board for Human Subjects (HUCFF).
Neutrophils were purified by density gradient and hypotonic lysis of RBCs, as described previously [10]. Purified neutrophils were resuspended in RPMI 1640 medium (LGC Biotecnologia, São Paulo, Brazil) and kept on ice until use.
Parasite culture
L. amazonensis (WHOM/BR/75/Josefa) promastigotes were maintained at 26°C in Schneider’s Insect Medium (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% heat-inactivated FCS and 40 µg/ml gentamicin. Stationary-phase promastigotes were obtained from 5- to 6-d-old cultures.
Reagents/inhibitors
Pharmacological intervention was carried out using the following inhibitors: Wort (PI3K inhibitor; Sigma-Aldrich), AKT inhibitor (Calbiochem, San Diego, CA, USA), AS60 (selective inhibitor of PI3Kγ; Tocris, Bristol, United Kingdom), IC87 (selective inhibitor of PI3Kδ; Cayman Chemical, Ann Arbor, MI, USA), DPI (flavoenzymes inhibitor; Sigma-Aldrich), PD98 (MEK inhibitor; Sigma-Aldrich), RO (PKC inhibitor; Calbiochem), BAPTA ([Ca2+]i chelator; Calbiochem), Fura-2/AM (Thermo Fisher Scientific, Waltham, MA, USA), and PMA (Calbiochem).
NET induction assay
Neutrophils (1 × 106) were incubated in the presence or absence of inhibitors for 30 min and then stimulated with PMA (100 nM) or L. amazonensis promastigotes at a 10:1 neutrophil/parasite ratio for 2 h at 35°C, 5% CO2.
NET quantification and analysis
Culture supernatants from each sample were distributed into 96-well opaque plates, and NET-DNA was quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific). Analysis was done using a spectrofluorometer reader (Molecular Devices, Sunnyvale, CA, USA), using 485/538 nm excitation/emission. NET-DNA concentration (nanograms/milliliter) was calculated based on a standard curve made with herring DNA (Sigma-Aldrich). Data were normalized to NET-DNA (nanograms/milliliter), released by cells stimulated with the parasite or PMA.
Fluorescence microscopy and decondensed chromatin quantification
Neutrophils (1 × 105), plated in 0.001% poly-l-lysine-coated glass coverslips, were incubated with L. amazonensis promastigotes for 90 min and fixed with 4% paraformaldehyde. Cells were stained with anti-neutrophil elastase (1:250; Calbiochem), anti-DNA/Histone 1 (8.8 µg/ml; EMD Millipore, Billerica, MA, USA), and Hoechst dye (10 nM; Thermo Fisher Scientific) for 10 min and gently washed twice with PBS between steps. Images were captured using a Zeiss Axioplan 2 microscope (Carl Zeiss, Oberkochen, Germany), equipped with a Color View XS digital video camera. For the video microscopy, alive neutrophils were incubated with promastigotes (1:1 ratio) in the presence of 10 μM Sytox Green and interaction recorded during 90 min. For chromatin decondensation, the nuclear area of 150–400 cells from at least 3 different donors was quantified using ImageJ 1.46r (U.S. National Institutes of Health, Bethesda, MD, USA), and the mean nuclear area of a given field was plotted using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). The chromatin area of each cell was measured using ImageJ, and at least 100 cells were analyzed in each of 3 different fields.
Intracellular ROS measurement
Neutrophils (1 × 106) were preincubated with the inhibitors described above at the same concentrations used for the NET inhibition assays for 30 min at 35°C, 5% CO2. Then, DHR 123 (1.2 µM; Sigma-Aldrich) was added, and neutrophils were stimulated with PMA (100 nM; Merck, Darmstadt, Germany) or promastigotes of L. amazonensis (1 × 105) for 15 min at 35°C, 5% CO2. Gated cells (10,000) were analyzed by flow cytometry with a FACSCalibur flow cytometer, and further analyses were performed using Summit v4.3 software.
Detection of intracellular phosphorylated proteins by flow cytometry
For the assessment of AKT and ERK phosphorylation, freshly isolated neutrophils were treated as previously indicated and fixed with 2% formaldehyde for 20 min at room temperature. Cells were then incubated with 5% PBS-BSA (Sigma-Aldrich) and 5% PBS-AB human serum (Sigma-Aldrich). After 20 min, neutrophils were treated with permeabilization buffer (eBioscience, San Diego, CA, USA) for 30 min at room temperature, then incubated with rabbit anti-human phosphorylated p44/42 MAPK (1:150; Cell Signaling Technology, Danvers, MA, USA) or rabbit anti-human phosphorylated AKT (1:400; Cell Signaling Technology), and stained with mouse anti-rabbit Alexa Fluor 488 (1:500; BioLegend, San Diego, CA, USA). Gated cells (50,000) were analyzed by flow cytometry with a FACSCalibur flow cytometer, and further analyses were performed using Summit v4.3 software.
[Ca2+]i measurement
Neutrophils were plated on 0.001% poly-l-lysine-coated glass coverslips and allowed to adhere for 15 min at 37°C, 5% CO2. Next, cells were loaded with 5 µM Fura-2/AM, and/or 10 µM BAPTA in RPMI 1640 medium containing 2.5 mM probenecid (Sigma-Aldrich) under the same conditions [22, 23]. Cells were then washed twice, seeded in a chamber whose base was formed by a coverslip, and maintained at 37°C in fresh RPMI 1640 medium. Cytoplasmic [Ca2+] of groups of 15–25 cells were monitored continuously with the use of a fluorescence imaging spectrofluorimeter (EasyRatioPro, equipped with a DeltaRAM X illuminator, Olympus IX71 microscope, QuantEM:5125C camera, and Image-Pro Plus V 6.3 software; Photon Technology International, Princeton, NJ, USA). Fura-2/AM, was alternatively excited at 340 and 380 nm, and the emission ratio at 510 nm was recorded as described [22, 23]. The ratio measurement, which is proportional to the cytoplasmic [Ca2+], was determined every 100 ms. L. amazonensis was gently added to the system after the fluorescence baseline was stabilized (∼1 min) and maintained for 10 min. When indicated, cytoplasmic [Ca2+] detected by Fura-2/AM, was also quantified in a spectrofluorometer reader (Molecular Devices).
Inhibitor cytotoxicity to neutrophils
Human neutrophils (1 × 106) were incubated with the different inhibitors for 2 h under the same conditions used for the previous assays, and cell viability was then evaluated from culture supernatants by the presence of LDH using the CytoTox kit (Promega, Madison, WI, USA). Tween-lysed neutrophils and purified LDH were used as positive controls. Reaction readings were carried out at 490 nm. Additionally, to assess apoptosis, cells were labeled with annexin V (Thermo Fisher Scientific), and apoptotic neutrophils were obtained by exposing cells to UV light for 2 h.
Inhibitor toxicity to parasites
To mimic better the inhibitor concentration faced by the parasites, neutrophils (1 × 106) were incubated with the different inhibitors for 30 min and centrifuged, and supernatants were collected. Leishmania promastigotes (1 × 106) were then exposed to these supernatants for 1 h, and parasite viability was checked by propidium iodide (100 µg/ml; Sigma-Aldrich), added immediately before analysis. Cells (50,000) were analyzed by flow cytometry with a FACSCalibur flow cytometer, and further analyses were performed using Summit v4.3 software.
Statistical analysis
Data were analyzed by unpaired Student's t test with GraphPad Prism 5.0. Each experiment was performed at least 3 times on independent occasions. P < 0.05 was considered significant.
RESULTS
Netosis induced by L. amazonensis requires a PI3K cascade independent of AKT
Leishmania promastigotes induce NET release in human neutrophils (Supplemental Video). PI3K signaling regulates several immune functions of neutrophils [24]. PI3K inhibition abolishes PMA-induced netosis [25]. To address whether PI3Ks could also have a role in NET formation induced by Leishmania as well, neutrophils were treated with Wort, a pharmacological irreversible pan inhibitor of PI3K, before stimulation with L. amazonensis promastigotes. Neutrophils were also stimulated with PMA as a positive control for PI3K inhibition. Our results are shown as the following: 1) normalized data relative to stimulated cells to address the variability of NET-DNA generated by each donor in response to stimuli and 2) the correlation between NET-DNA concentration (nanograms/milliliter) of untreated and treated cells to evaluate each donor’s response to inhibitor treatment. We observed that Wort significantly inhibited parasite-induced NET-DNA (∼40%; Fig. 1A and Supplemental Fig. 1A). As expected, Wort inhibited PMA-induced netosis (Fig. 1B and Supplemental Fig. 1B).
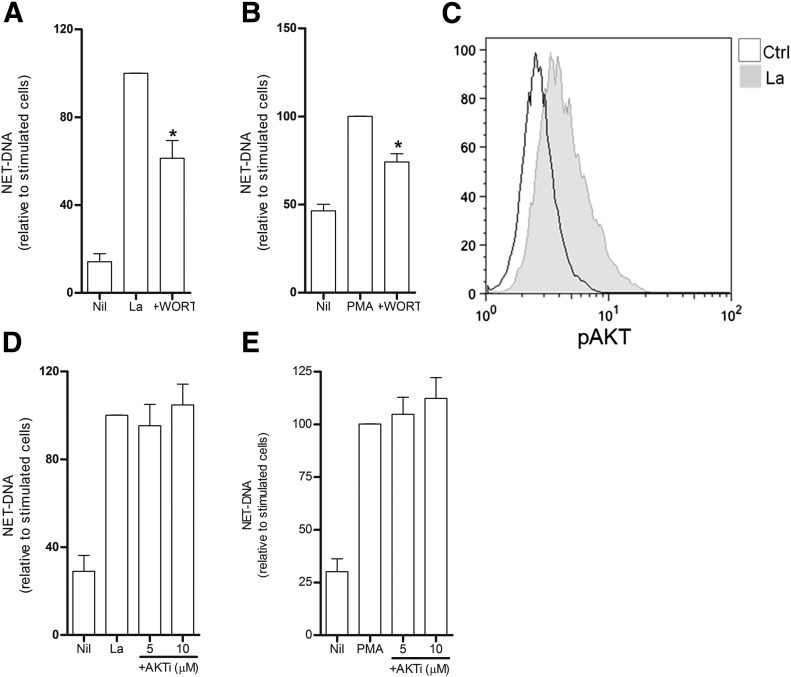
Figure 1. Netosis induced by L. amazonensis requires a PI3K cascade independent of AKT.
Neutrophils (1 × 106) were left untreated or preincubated with (A and B) Wort (200 nm) or (D and E) AKT inhibitor (AKTi; 5 and 10 µM) at 5% CO2, 35°C. Next, cells were stimulated with promastigotes [L. amazonensis (La); 10:1 neutrophils/parasite] or PMA (100 nM). NET-DNA was then quantified in the supernatant as described. (C) Neutrophils were stimulated for 5 min with Leishmania and stained for intracellular phosphorylated AKT [control (Ctrl)]. Data are shown as a representative histogram. Data represent means ± sem of normalized data relative to stimulated cells; n ≥ 4 donors. NET-DNA raw numbers: PMA-stimulated cells, 811.2 ± 94.26 (Wort), 2225.0 ± 536.4 (AKTi) ng/ml; La-stimulated cells, 7229.0 ± 1898.0 (Wort), 4210.0 ± 573.0 (AKTi) ng/ml. *P < 0.05 compared with stimulated cells.
One of the conventional targets of PI3K is AKT [24]; therefore, we evaluated whether L. amazonensis could activate this protein. We observed increased fluorescence intensity of phosphorylated AKT on neutrophils incubated for 5 min with promastigotes (Fig. 1C). We then evaluated whether AKT would also be involved in Leishmania induced netosis by using an AKT inhibitor. Our results revealed that AKT exerted no effect over NET release triggered by the parasite or PMA (Fig. 1D and E and Supplemental Fig. 1C and D). Taken together, these data indicate a role for the PI3K family in regulating induction of NET release by L. amazonensis independently of AKT.
PI3Kγ and -δ control NET release dependently and independently of ROS generation, respectively
PI3Kγ and PI3Kδ are isoforms highly enriched within leukocytes [26]. With the use of the PI3Kγ-specific inhibitor AS60, we detected ∼43% reduction of NET-DNA release induced by the parasite at the higher concentrations used (Fig. 2A). Additionally, we observed a dose-dependent response of PMA-induced netosis to AS60 (Fig. 2B). Accordingly, most donors displayed an inhibition profile with the higher AS60 dose with either Leishmania or PMA (Supplemental Fig. 1E and F). Furthermore, our results demonstrate that IC87, a selective inhibitor of PI3Kδ, reduced parasite-induced NET-DNA release by 30% (Fig. 2C and Supplemental Fig. 1G) but did not affect PMA-induced netosis (Supplemental Fig. 1H).
Figure 2. PI3Kγ and -δ control NETs release dependent and independent of ROS generation.
Neutrophils were left untreated (Nil) or preincubated with (A and B) AS (1, 5, 10 µM) or (C–E) IC87 (1 µM) and incubated under 5% CO2, 35°C. Association of AS60 plus IC87 (D) or AS60 plus IC87 and DPI (2.5 µg/ml; E) was also tested under the same conditions as above. Next, cells were stimulated with L. amazonensis or PMA, and then NET-DNA in the supernatants was quantified. Data represent means ± sem of normalized data relative to stimulated cells. N, nil. (D) ROS generation by DHR 123 probing was measured from untreated or AS60- or IC87-pretreated neutrophils and stimulated with the parasite. PMA-stimulated neutrophils reaching the “R2” marker were set as actively ROS-generating cells (inset) and our positive control. Data represents means ± sem of the percentage of neutrophils in the R2 zone, as described in Supplemental Fig. 1. NET-DNA raw numbers: PMA-stimulated cells (A) 1682 ± 409 ng/ml; La-stimulated cells (B and C) 5043 ± 1405 ng/ml; n ≥ 6 donors. *P < 0.05 compared with stimulated cells; #P < 0.05 compared with IC87 pretreatment.
PI3Kγ has been shown to regulate ROS generation, whereas PI3Kδ is dispensable for ROS generation [27]. It has been demonstrated previously that ROS production is a key step for netosis [11], and we also observed that L. amazonensis promastigotes trigger classic netosis dependently of ROS generation [28]. Thus, we evaluated the effect of pharmacological PI3Kγ and PI3Kδ inhibition on ROS induction by the parasite. Whereas Nil generated low levels of ROS under our conditions, neutrophils activated with PMA generated larger amounts of ROS, shifting toward the R2 marker (Fig. 2D, inset). Therefore, we used R2 as the marker of cells actively producing ROS. Our results revealed that AS60 completely abolished Leishmania induced ROS generation, showing that PI3Kγ modulate parasite-induced netosis through ROS generation. In contrast, we could not detect any effect of IC87 over ROS induction by Leishmania (Fig. 2D). The combination of IC87 and AS60 showed an additive effect compared with the association of IC87 with DPI, a flavoprotein inhibitor that inhibits NOX, both combinations increasing over 2-fold the inhibition of parasite-induced NET-DNA (Fig. 2E). These results indicate that PI3Kδ signals a ROS-independent pathway and that the NET inhibitory effect is potentiated by shutting down PI3Kδ together with DPI or PI3Kγ.
ERK activation downstream of PI3Kγ and upstream of ROS generation is required for netosis triggered by L. amazonensis
The MAPK pathway is one of the most evolutionary conserved signaling pathways in eukaryotic cells. To address whether L. amazonensis interaction with neutrophils would activate ERK, we stained cells for intracellular phosphorylated ERK. We observed a significant increase in ERK phosphorylation following stimulation of cells, as indicated by both histogram and MFI representations (Fig. 3A and B). Hence, we performed a pharmacological inhibition with PD98, a selective inhibitor of ERK phosphorylation, and observed a 40% inhibition of NET-DNA release induced by the parasite (Fig. 3C). Accordingly, the amount of NET-DNA released from PD98-treated neutrophils was reduced in all donors compared with Nil (Supplemental Fig. 1I). As previously shown, inhibition of ERK phosphorylation significantly affects PMA-induced NET release (Fig. 3D and Supplemental Fig. 1J). Furthermore, ROS generation induced by L. amazonensis was reduced to basal levels when neutrophils were treated with PD98 (Fig. 3E). To address the interplay between PI3Kγ and ERK activation, we evaluated Leishmania induced ERK phosphorylation on AS60-treated neutrophils. This experiment revealed that PI3Kγ inhibition reduced ERK phosphorylation to almost basal levels, similar to PD98-treated cells (Fig. 3F). Finally, neutrophils pretreated with PD98 and AS60 showed no effect over parasite induction of netosis greater than that observed with each inhibitor alone (Fig. 3G). Taken together, our data indicate that both proteins are part of the same pathway and that ERK activation is involved in L. amazonensis induced netosis downstream of PI3Kγ and upstream of ROS generation.
Figure 3. ERK activation upstream of ROS generation is required for netosis triggered by L. amazonensis.
Neutrophils were left untreated (Nil) or preincubated with PD98 (30 or 60 µM) or AS60 (10 µM) at 5% CO2, 35°C. Cells were then incubated with promastigotes or PMA for 5 or 15 min and stained for intracellular phosphorylated ERK (pERK). (A) Histogram of representative data and (B and F) normalized MFI. Additionally, neutrophils preincubated with PD98 and/or AS60 were stimulated with (C, D, and G) La (10 neutrophils:1 parasite) or PMA (100 nM), and then NET-DNA in the supernatant was quantified as described earlier. Data represent means ± sem of normalized data relative to stimulated cells. Sec Ab, Secondary antibody. ROS generation by DHR 123 probing (E) was measured from untreated or PD98-pretreated neutrophils and stimulated with the parasite or PMA, which was used as positive control. Data represent mean percentages ± sem of neutrophils in the R2 zone. NET-DNA raw numbers: PMA-stimulated cells, 1529 ± 155 ng/ml; La-stimulated cells, 4175 ± 923 ng/ml. *P < 0.05 compared with stimulated cells; #P < 0.05 compared with Nil.
Blockage of PKC partially inhibits NET formation
PMA is a 1,2-diacylglycerol analog and consequently, directly activates PKC; therefore, we tested whether PKC would also have a role in NET stimulation by Leishmania. To do this, we used the pharmacological PKC inhibitor RO and observed a 36% reduction in NET extrusion by parasite-stimulated neutrophils (Fig. 4A). NET release by stimulated neutrophils from all donors was negatively affected by the pharmacological treatment (Supplemental Fig. 1K). Accordingly, PMA-induced netosis was diminished to control levels upon RO treatment (Fig. 4B and Supplemental Fig. 1L).
Figure 4. Blockage of PKC by pharmacological treatment partially inhibits netosis.
Neutrophils were left untreated (Nil) or preincubated with RO (3 µM) under 5% CO2, 35°C. Next, cells were stimulated with L. amazonensis (A) or PMA (B), and NET-DNA in the supernatant was quantified. Data represent means ± sem of data normalized to stimulated cells. NET-DNA raw numbers: PMA-stimulated cells, 1435 ± 107 ng/ml; La-stimulated cells, 4664 ± 609 ng/ml. *P < 0.05 compared with stimulated cells.
Calcium works as an alternative ROS-independent pathway for Leishmania induction of NET
The rise of [Ca2+]i in neutrophils is intimately associated with the proinflammatory functions of these cells [29]. Therefore, we first tested the [Ca2+] response of Fura-2/AM-loaded neutrophil preparations. Promastigotes and PMA significantly augmented [Ca2+] mobilization, and ionomycin, used as a control, induced a typical rise in the fluorescence ratio, which then rapidly decreased after addition of EGTA (Fig. 5A, inset). Mobilization of [Ca2+] induced Leishmania, increased [Ca2+]i within the first minutes that persisted for 8–10 min (Fig. 5B). Furthermore, treatment with the [Ca2+]i chelator BAPTA before parasite stimulation prevented [Ca2+]i from rising (Fig. 5B). With the evaluation of whether BAPTA pretreatment would affect Leishmania induction of NET, we observed a decrease of ∼32% in the parasite-induced netosis (Fig. 5C and Supplemental Fig. 1M) and ∼50% of the NET-DNA induced by PMA (Fig. 5D and Supplemental Fig. 1N). To address whether [Ca2+]i would signal through a ROS-dependent or -independent pathway, we pretreated neutrophils with BAPTA before assessing parasite-induced ROS generation. It is noteworthy that [Ca2+]i sequestration did not induce any effect on the parasite-induced ROS production (Fig. 5E). Additionally, to correlate better the [Ca2+] signal with PI3K isoforms, we pretreated the neutrophil with IC87 or AS60 before assessing [Ca2+] mobilization induced by the parasite. We observed that IC87 prevented [Ca2+] rising, whereas AS60 increased parasite-induced [Ca2+] mobilization (Fig 5F). Accordingly, we combined BAPTA with IC87 or AS60 and evaluated parasite induction of NET-DNA, and whereas the former had no further impact on NET-DNA liberation, AS60 showed a significant 1.8-fold decrease on NET-DNA release (Supplemental Fig. 2A). Finally, to confirm further that [Ca2+]i triggered by Leishmania regulates netosis in a ROS-independent pathway, neutrophils were incubated in the presence of BAPTA alone or in combination with DPI before parasite stimuli. Our results showed a reduction of NET-DNA to basal levels when both pharmacological interventions were combined (Fig. 5G and Supplemental Fig. 1O). Accordingly, fluorescence microscopy confirmed reduction of parasite-induced NET generation by BAPTA treatment, and furthermore, the combination of BAPTA and PD98 substantially prevented NET extrusion (Supplemental Fig. 3). Thus, in conclusion, PI3Kδ-regulated [Ca2+] formation and PI3Kγ-ERK-ROS seem to be independent pathway regulators for Leishmania induction of NET.
Figure 5. Role of calcium in the Leishmania induction of NETs.
(A) Neutrophils plated on poly-l-lysine-precoated coverslips were loaded with 5 µM Fura-2/AM. (Inset) EGTA was added (dashed line) as a negative control of calcium mobilization induced by ionomycin (IONO; 5 µM; arrow). (B) Additionally, cells were left untreated or treated with BAPTA (10 µM) and kept under 5% CO2 and 35°C. Then, Fura-2/AM-loaded cells were maintained in a chamber with the same conditions, and cytoplasmic [Ca2+] were monitored continuously with the use of a fluorescence imaging spectrofluorimeter after gentle addition of L. amazonensis (1 neutrophil:1 parasite) or PMA (100 nM) for 8–10 min. (A and B) Means ± sem of normalized data relative to time 0. (C, D, and G) Neutrophils were incubated with BAPTA or BAPTA combined with DPI (2.5 µg/ml) and stimulated or not with promastigotes or PMA. NET-DNA in the supernatant was quantified as described above. Data represent means ± sem of normalized data relative to stimulated cells. ROS generation was measured by DHR 123 probing from (E) neutrophils that were either untreated or BAPTA pretreated and then stimulated with the parasite. PMA-stimulated neutrophils were set as a positive control. Data represent mean percentages ± sem of neutrophils in the R2 zone. (F) Calcium mobilization was assessed in Fura-2/AM-loaded cells pretreated or not with indicated inhibitors and quantified in a spectrofluorimeter. NET-DNA raw numbers: PMA-stimulated cells, 1950 ± 588.0 ng/ml; La-stimulated cells, 4624 ± 998.7. *P < 0.05 compared with stimulated cells; ≠P < 0.05 compared with Nil (unstimulated cells); #P < 0.05 compared with BAPTA pretreatment; n ≥ 4.
Chromatin decondensation and apoptosis
One of the remarkable neutrophil morphology changes upon netosis is the loss of compact and polymorphic nuclear shape as a result of chromatin decondensation, which allows DNA to associate with some proteins from granules and cytoplasm [10]. Thus, we evaluated whether our pharmacological treatments could affect chromatin decondensation induced by L. amazonensis by quantifying the chromatin area of each cell nucleus. In agreement with our NET-DNA release data, the chromatin area was significantly reduced upon the pharmacological treatments previously mentioned (Supplemental Fig. 2B). Additionally, none of the inhibitors used in this work showed any toxicity to neutrophils or parasites (data not shown). We also performed annexin V labeling to address whether AKT and ERK inhibition, as previously demonstrated, would favor apoptosis over netosis cell death [19, 30]. We observed a 37% increase in neutrophil apoptosis after PD98 treatment (Supplemental Fig. 2C), whereas AKT inhibition showed no effect on cell apoptosis. Therefore, ERK activation might trigger netosis by modulating the apoptotic cell fate, at least partially, whereas AKT inhibition does not seem to have an impact on apoptosis under our experimental conditions. Likewise, none of the neutrophil stimuli induced necrosis in our experimental conditions (Supplemental Fig. 2D).
DISCUSSION
Neutrophils are the major leukocytes circulating in the bloodstream and key innate cells involved in the launch of inflammation as a result of their rapid recruitment to infected tissues and ability to kill pathogens efficiently by mechanisms that include the release of extracellular traps. NETs can ensnare a plethora of pathogens, restricting microbial spread, and also killing microbes by exposing them to an environment with highly concentrated microbicidal peptides attached to the chromatin scaffold. Leishmania parasites are among the pathogens susceptible to NETs. Neutrophils are rapidly recruited to the parasite inoculation site and thus, represent one the first cell types to interact with the parasite and initiate an immune response [31]. Nevertheless, work to understand the neutrophil–Leishmania interaction is still in progress. The same is also true for the molecular mechanisms that drive neutrophils and parasite interaction. Here, we elucidated the molecular mechanisms associated with L. amazonensis induction of NETs by human neutrophils. We demonstrated the role of PI3K, specifically the PI3Kγ and PI3Kδ isoforms, ERK activation, PKC, and [Ca2+]i mobilization.
PI3Ks are intracellular signaling enzymes, classified as lipid and protein kinases, that signal by phosphorylating phosphoinositides in the cell membrane [24]. Neutrophils have an extensive number of surface receptors that signal through the PI3K pathway. Therefore, as expected, PI3K regulates several aspects of neutrophil microbicidal systems [32]. In light of the central role of PI3K for neutrophil immune functions, we sought to evaluate whether PI3K would also regulate the release of NETs. Indeed, we observed that pan inhibition of PI3K by Wort had a significant impact on NET-DNA release induced by the parasite or PMA. Moreover, previous work showing the role of PI3K in netosis induction by different stimuli, such as the Pseudomonas virulence factor pyocyanin, pseudogout-associated microcrystals, and immune complexes, indicates a conserved pathway for PI3Ks in that mechanism [12, 20, 21]. Furthermore, inhibition of autophagy by Wort on PMA-stimulated neutrophils prevented chromatin decondensation and cell vacuolization, demonstrating the role of PI3K in NET release through autophagy regulation [25]. Here, we report that Wort also diminished chromatin decondensation in neutrophils stimulated with Leishmania promastigotes and also reduced NET-DNA levels induced by PMA. Moreover, L. amazonensis has been reported to induce autophagy on macrophages, a process that modulates the outcome of parasite infection on host cells [31, 33, 34]. Therefore, it is quite reasonable to consider that autophagy could participate in netosis induced by the parasite, although further investigation must be done to address that. One of the most conventional targets of PI3K lipid products is the serine/threonine kinase AKT [24]. Interestingly, although we observed phosphorylation of AKT after 5 min stimulation of human neutrophils, treatment with an AKT inhibitor did not show any effect over NET-DNA release induced by the parasite. AKT is a well-known regulator of the survival/death cell-fate decision, primarily through regulation of apoptosis [35]. Accordingly, Leishmania species have been shown to induce resistance of macrophages to apoptosis by engaging PI3K/AKT signaling in infected cells [36]. Additionally, it has been demonstrated that PMA-induced NET formation is dependent on AKT, which diverts neutrophil fate from apoptosis to netosis [30]. Contrary to these studies, however, we did not observe any effect of AKT inhibition on PMA-induced NET-DNA release under our conditions. These differences could be because Douda et al. [30] used 4-fold less PMA and pretreated neutrophils for longer times with 2 distinct AKT inhibitors (XI and MK2206). Additionally, another AKT inhibitor (VIII) has been shown to partially affect NET release induced by immobilized immune complexes [12]. However, the aforementioned work also differs from Douda et al. [30] concerning the requirement of ROS to trigger AKT phosphorylation. Thus, inconsistency in data regarding the role of AKT in netosis could be explained by the use of distinct conditions and AKT inhibitors.
PI3Ks can be categorized as class I, II, and III, based mainly on their distinct substrate preferences. Class I enzymes can be subdivided further into IA (PI3Kα, PI3Kβ, and PI3Kδ) and IB (PI3Kγ), which express variants of the same catalytic subunits (p110). PI3Kγ and PI3Kδ are preferentially expressed on leukocytes and together, regulate many functions of the immune system [26]. However, those isoforms play distinct roles in neutrophil ROS generation. Whereas PI3Kγ is essential for TNF-α-primed neutrophils to produce ROS after stimulation with fMLP, PI3Kδ works only as an amplifier of a second wave of ROS production dependent on the first wave initiated by PI3Kγ [27]. Here, we showed that blocking PI3Kγ with a selective isoform inhibitor, AS60, led to significant inhibition of NET extrusion and chromatin decondensation induced by Leishmania and NET-DNA release induced by PMA. In contrast, selective inhibition of PI3Kδ only affected parasite-induced netosis, which is in agreement with previous data showing that PMA induction of NET is fully dependent on ROS generation [11]. Indeed, we showed that ROS generation induced by the parasite was abolished by AS60 but unaffected by IC87, a PI3Kδ inhibitor. Accordingly, IC87 prevented [Ca2+] mobilization induced by the parasite, whereas AS60 enhanced the [Ca2+] signal, indicating a compensation mechanism. Moreover, association of DPI, which inhibits NOX ROS generation with IC87, increased the effect of the latter by 2-fold, similarly to the IC87–AS60 association, corroborating our hypothesis that PI3Kδ comprises an alternative netosis pathway independent of ROS generation. Thus, our data indicate that Leishmania can trigger netosis in a ROS-dependent manner through PI3Kγ or independent through PI3Kδ.
MEK signaling is one of the most evolutionary conserved pathways used by eukaryotes and modulates vital cell functions, such as proliferation, differentiation, survival, and death. The MAPK pathway consists of sequential phosphorylation and activation of 3 kinases: a MEK kinase that phosphorylates and activates a MEK, which then activates MAPK, which in turn, might activate cytoplasmic substrates or modulate transcription factors. MEK/ERK represents, respectively, MEK and MAPK, the latter being associated with chemoattractant and cytokine receptor transduction in neutrophils. PMA-induced netosis is dramatically affected by inhibition of ERK activation, preventing ROS generation and the up-regulation of the antiapoptotic protein myeloid leukemia cell differentiation protein 1 [19]. Additionally, Leishmania major has been shown to induce phosphorylation of ERK in human neutrophils, which regulates antiapoptotic effects exerted by the parasite [37]. In the present work, we demonstrated that L. amazonensis can also trigger phosphorylation of ERK in a time-dependent manner and that inhibition of ERK activation by PD98 significantly decreased parasite induction of NET-DNA, chromatin decondensation, and ROS generation. Accordingly, PMA treatment was significantly affected by PD98. Furthermore, we described here that parasite-induced ERK activation is dependent on PI3Kγ, as AS60 pretreatment prevented ERK phosphorylation. Indeed, it was demonstrated that neutrophils from PI3Kγ−/− mice have impaired phosphorylation activity of ERK [38]. Moreover, as previously demonstrated, inhibition of ERK activation leads to a significant increase of neutrophil apoptosis [19], suggesting that ERK modulates apoptosis to favor NET release. Hence, we showed here that Leishmania induction of NET works, in part, by activating PI3Kγ, which in turn, modulates ERK phosphorylation, leading to ROS generation, apoptosis inhibition, and chromatin decondensation.
Cytoplasmic changes in neutrophil [Ca2+] levels have been consistently related to several cell functions, including motility, vesicle trafficking, and transduction of signals coming from chemoattractants and FcRs [39, 40]. We reported here that intracellular levels of [Ca2+] rise within the first minutes after contact with PMA or L. amazonensis promastigotes. Furthermore, upon BAPTA pretreatment, neutrophils reduced NET formation induced by both Leishmania and PMA. However, we could not detect any alterations in the levels of parasite-induced ROS in neutrophils pretreated with BAPTA. Furthermore, association of BAPTA and DPI completely abolished parasite induction of NET and shows that BAPTA-PD98 association prevented parasite-induced NET, as shown by immunofluorescence. Together, we may conclude that [Ca2+] mobilization and ROS generation constitute the 2 primary independent signals that drive Leishmania induction of NET release. Accordingly, NET formation by ionomycin has been described to be independent of respiratory burst [18]. More recently, it has been demonstrated that NOX-independent netosis induced by [Ca2+] influx is dependent on the activation of the small conductance calcium-activated potassium channel 3 and mitochondrial ROS. In contrast, ERK activation was shown to be dispensable for [Ca2+]-induced netosis [41]. Additionally, we combined BAPTA treatment with IC87 or AS60, and whereas the former had no further impact on NET-DNA reduction, AS60 showed an additive effect, which corroborates our data that [Ca2+] influx and PI3Kγ-ERK-ROS comprise distinct pathways for parasite-induced netosis. However, AS60 association did not show the same impact of DPI association, as probably DPI is affecting other flavoprotein-dependent ROS sources, which are not regulated by PI3Kγ or detected by DHR probing. Gupta et al. [42] showed that intracellular and extracellular sources of [Ca2+] are required for netosis induction by IL-8. However, we could not investigate the role of extracellular sources of [Ca2+], as a result of high cytotoxicity of EGTA treatment under our experimental conditions (data not shown), which differed from Gupta and colleagues’ [42] conditions, in terms of neutrophil isolation and supplementation of their cultures with 2% heat-inactivated human serum. Activation of many PKC isoforms is modulated by [Ca2+]i levels [43], and we observed a significant inhibition of NET-DNA release when neutrophils were treated with a specific PKC pharmacological inhibitor. However, as a result of fluorescence interference from this inhibitor, we could not further characterize the role of PKC in ROS generation. However, we hypothesize that PKC isoforms might participate in ROS-dependent and -independent pathways, as PKC isoforms can be modulated by [Ca2+]i levels and directly activate NOX [43].
In conclusion, we provide here new insights into cell signaling that dictates induction of netosis on human neutrophils. Although pharmacological inhibition assays have specificity limitations, it is still the most used method for cell signaling studies. Our work also contributes to a better understanding of the molecular mechanisms of Leishmania interaction with human neutrophils, which could be used for further modulation of netosis during treatment of leishmaniasis.
AUTHORSHIP
T.D-V., A.G-C., M.T.N., P.M.P., and E.M.S. conceived of and designed the experiments, analyzed the data, and wrote the paper. T.D-V., A.G-C., N.C.R., M.T.N., M.N.L., P.S.L.G., and R.M.M. performed the experiments. R.M.M. and P.M.P. contributed reagents/materials/analysis tools.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors thank Dr. Alessandra P. Granato (Instituto de Microbiologia Paulo de Góes, Universidade Federal do Rio de Janeiro, Brazil) for assistance on intracellular detection of phosphorylated protein assays and Dr. Flávio Lara (Lab. de Microbiologia Celular, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil) for the video microscopy analysis. The authors are also grateful to the Hemotherapy Service Hospital Clementino Fraga Filho (Universidade Federal do Rio de Janeiro, Brazil) for the buffy coats.
Glossary
- AKT
protein kinase B
- AS60
AS605240
- [Ca2+]
calcium ion concentration
- DHR
dihydrorhodamine
- DPI
diphenyleneiodonium
- HUCFF
Clementino Fraga Filho Universitario Hospital
- IC87
IC-87114
- [Ca2+]i
intracellular calcium concentration
- LDH
lactate dehydrogenase
- MFI
mean fluorescence intensity
- NET
neutrophil extracellular trap
- Nil
unstimulated neutrophils
- NOX2
NADPH oxidase 2
- PD98
PD98059
- PKC
protein kinase C
- RO
RO-31-7549
- ROS
reactive oxygen species
- Wort
wortmannin
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Borregaard N. (2010) Neutrophils, from marrow to microbes. Immunity 33, 657–670. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182. [DOI] [PubMed] [Google Scholar]
- 3.Alvar, J., Vélez, I. D., Bern, C., Herrero, M., Desjeux, P., Cano, J., Jannin, J., den Boer, M.; WHO Leishmaniasis Control Team (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno I., Domínguez M., Cabañes D., Aizpurua C., Toraño A. (2010) Kinetic analysis of ex vivo human blood infection by Leishmania. PLoS Negl. Trop. Dis. 4, e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro-Gomes F. L., Sacks D. (2012) The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front. Cell. Infect. Microbiol. 2, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- 8.Urban C. F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., Brinkmann V., Jungblut P. R., Zychlinsky A. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5, e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauseef W. M., Borregaard N. (2014) Neutrophils at work. Nat. Immunol. 15, 602–611. [DOI] [PubMed] [Google Scholar]
- 10.Guimarães-Costa A. B., Nascimento M. T., Froment G. S., Soares R. P., Morgado F. N., Conceição-Silva F., Saraiva E. M. (2009) Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA 106, 6748–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. (2007) Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behnen M., Leschczyk C., Möller S., Batel T., Klinger M., Solbach W., Laskay T. (2014) Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcγRIIIB and Mac-1. J. Immunol. 193, 1954–1965. [DOI] [PubMed] [Google Scholar]
- 13.Röhm M., Grimm M. J., D’Auria A. C., Almyroudis N. G., Segal B. H., Urban C. F. (2014) NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect. Immun. 82, 1766–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azevedo E. P., Guimarães-Costa A. B., Torezani G. S., Braga C. A., Palhano F. L., Kelly J. W., Saraiva E. M., Foguel D. (2012) Amyloid fibrils trigger the release of neutrophil extracellular traps (NETs), causing fibril fragmentation by NET-associated elastase. J. Biol. Chem. 287, 37206–37218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabriel C., McMaster W. R., Girard D., Descoteaux A. (2010) Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 185, 4319–4327. [DOI] [PubMed] [Google Scholar]
- 16.Pilsczek F. H., Salina D., Poon K. K., Fahey C., Yipp B. G., Sibley C. D., Robbins S. M., Green F. H., Surette M. G., Sugai M., Bowden M. G., Hussain M., Zhang K., Kubes P. (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185, 7413–7425. [DOI] [PubMed] [Google Scholar]
- 17.Byrd A. S., O’Brien X. M., Johnson C. M., Lavigne L. M., Reichner J. S. (2013) An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 190, 4136–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker H., Dragunow M., Hampton M. B., Kettle A. J., Winterbourn C. C. (2012) Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 92, 841–849. [DOI] [PubMed] [Google Scholar]
- 19.Hakkim A., Fuchs T. A., Martinez N. E., Hess S., Prinz H., Zychlinsky A., Waldmann H. (2011) Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 7, 75–77. [DOI] [PubMed] [Google Scholar]
- 20.Rada B., Jendrysik M. A., Pang L., Hayes C. P., Yoo D. G., Park J. J., Moskowitz S. M., Malech H. L., Leto T. L. (2013) Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS One 8, e54205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang L., Hayes C. P., Buac K., Yoo D. G., Rada B. (2013) Pseudogout-associated inflammatory calcium pyrophosphate dihydrate microcrystals induce formation of neutrophil extracellular traps. J. Immunol. 190, 6488–6500. [DOI] [PubMed] [Google Scholar]
- 22.Malgaroli A., Milani D., Meldolesi J., Pozzan T. (1987) Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal layers. J. Cell Biol. 105, 2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Da Cruz C. M., Ventura A. L., Schachter J., Costa-Junior H. M., da Silva Souza H. A., Gomes F. R., Coutinho-Silva R., Ojcius D. M., Persechini P. M. (2006) Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X7-associated pore or channel formation. Br. J. Pharmacol. 147, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins P. T., Stephens L. R., Suire S., Wilson M. (2010) PI3K signaling in neutrophils. Curr. Top. Microbiol. Immunol. 346, 183–202. [DOI] [PubMed] [Google Scholar]
- 25.Remijsen Q., Vanden Berghe T., Wirawan E., Asselbergh B., Parthoens E., De Rycke R., Noppen S., Delforge M., Willems J., Vandenabeele P. (2011) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 21, 290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341. [DOI] [PubMed] [Google Scholar]
- 27.Condliffe A. M., Davidson K., Anderson K. E., Ellson C. D., Crabbe T., Okkenhaug K., Vanhaesebroeck B., Turner M., Webb L., Wymann M. P., Hirsch E., Ruckle T., Camps M., Rommel C., Jackson S. P., Chilvers E. R., Stephens L. R., Hawkins P. T. (2005) Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106, 1432–1440. [DOI] [PubMed] [Google Scholar]
- 28.Rochael N. C., Guimarăes-Costa A. B., Nascimento M. T., DeSouza-Vieira T. S., Oliveira M. P., Garcia de Souza L. F., Oliveira M. F., Saraiva E. M. (2015) Classical ROS-dependent and early/rapid ROS-independent release of neutrophil extracellular traps triggered by Leishmania parasites. Sci. Rep. 5, 18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tintinger G., Steel H. C., Anderson R. (2005) Taming the neutrophil: calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin. Exp. Immunol. 141, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douda D. N., Yip L., Khan M. A., Grasemann H., Palaniyar N. (2014) Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 123, 597–600. [DOI] [PubMed] [Google Scholar]
- 31.Guimarães-Costa A. B., Nascimento M. T., Wardini A. B., Pinto-da-Silva L. H., Saraiva E. M. (2012) ETosis: a microbicidal mechanism beyond cell death. J. Parasitol. Res. 2012, 929743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Futosi K., Fodor S., Mócsai A. (2013) Reprint of neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 17, 1185–1197. [DOI] [PubMed] [Google Scholar]
- 33.Cyrino L. T., Araújo A. P., Joazeiro P. P., Vicente C. P., Giorgio S. (2012) In vivo and in vitro Leishmania amazonensis infection induces autophagy in macrophages. Tissue Cell 44, 401–408. [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro R. O., Nunes M. P., Pinheiro C. S., D’Avila H., Bozza P. T., Takiya C. M., Côrte-Real S., Freire-de-Lima C. G., DosReis G. A. (2009) Induction of autophagy correlates with increased parasite load of Leishmania amazonensis in BALB/c but not C57BL/6 macrophages. Microbes Infect. 11, 181–190. [DOI] [PubMed] [Google Scholar]
- 35.Zhu D., Hattori H., Jo H., Jia Y., Subramanian K. K., Loison F., You J., Le Y., Honczarenko M., Silberstein L., Luo H. R. (2006) Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc. Natl. Acad. Sci. USA 103, 14836–14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruhland A., Leal N., Kima P. E. (2007) Leishmania promastigotes activate PI3K/Akt signalling to confer host cell resistance to apoptosis. Cell. Microbiol. 9, 84–96. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar A., Aga E., Bussmeyer U., Bhattacharyya A., Möller S., Hellberg L., Behnen M., Solbach W., Laskay T. (2013) Infection of neutrophil granulocytes with Leishmania major activates ERK 1/2 and modulates multiple apoptotic pathways to inhibit apoptosis. Med. Microbiol. Immunol. (Berl.) 202, 25–35. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki T., Irie-Sasaki J., Jones R. G., Oliveira-dos-Santos A. J., Stanford W. L., Bolon B., Wakeham A., Itie A., Bouchard D., Kozieradzki I., Joza N., Mak T. W., Ohashi P. S., Suzuki A., Penninger J. M. (2000) Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287, 1040–1046. [DOI] [PubMed] [Google Scholar]
- 39.Hübner K., Surovtsova I., Yserentant K., Hänsch M., Kummer U. (2013) Ca2+ dynamics correlates with phenotype and function in primary human neutrophils. Biophys. Chem. 184, 116–125. [DOI] [PubMed] [Google Scholar]
- 40.Jaconi M. E., Lew D. P., Carpentier J. L., Magnusson K. E., Sjögren M., Stendahl O. (1990) Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J. Cell Biol. 110, 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douda D. N., Khan M. A., Grasemann H., Palaniyar N. (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 112, 2817–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A. K., Giaglis S., Hasler P., Hahn S. (2014) Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One 9, e97088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosentino-Gomes D., Rocco-Machado N., Meyer-Fernandes J. R. (2012) Cell signaling through protein kinase C oxidation and activation. Int. J. Mol. Sci. 13, 10697–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.