Abstract
Introduction
The objective of this study was to document the initiation of insulin therapy in patients with type 2 diabetes mellitus (T2DM) and its maintenance as a function of time after initiation in a French nationwide representative cohort.
Methods
A retrospective cohort study was conducted on a random sample of ~600,000 beneficiaries registered in the French national health insurance database. Newly insulin treated T2DM patients were selected. Persistence was defined as remaining on insulin without discontinuation (defined over a 6 or a 12-month period).
Results
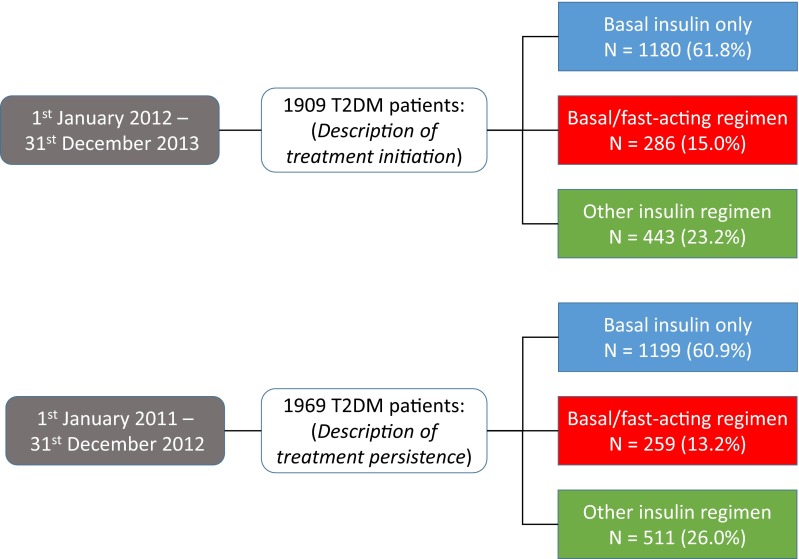
Among 1909 initiations identified in 2012/2013 (basal scheme: 61.8%, basal/rapid: 15%, other schemes: 23.2%) the average age (standard deviation) at initiation was, respectively, 67.5 (14.2), 61.8 (18.1) and 63.2 (18.4) years. Insulin was initiated by general practitioners in 39.3% and prescribed without other antidiabetic drugs in 32.0%. Persistence was studied in 1969 patients initiating insulin in 2011/2012. Among survivors, nearly 25% stopped insulin during the first year (18.4% for basal scheme). Patients discontinuing insulin were younger [64.7 years (18.5) vs 67.3 years (14.3) p = 0.0003] and less often male (45.8% vs 55.7%, p < 0.0001). A proportion of 20.2% did not receive any antidiabetic drug over 12 months after discontinuation. These high percentages were only partly explained by transient intensive insulin regimens in acutely ill patients identifiable in the database.
Conclusion
We observed a high rate of early discontinuation of insulin in T2DM patients (but lower with basal insulin scheme). Further real world studies are warranted to identify factors associated with this poor persistence.
Funding
This study was supported by Sanofi-France.
Keywords: Diabetes mellitus, Drug utilization review, Drug therapy, Insulin
Introduction
Insulin treatment has historically been the mainstay of the management of type 1 diabetes mellitus (T1DM), but has only more recently come to occupy a central role in the management of type 2 diabetes mellitus (T2DM). This change has partly relied on the introduction of modified long-acting insulins (basal insulins) as well as blood glucose control devices that make insulin therapy simpler for the patient. In particular, insulin therapy is recommended in patients who fail to achieve glycemic control with combinations of oral antidiabetic drugs (OADs) [1–3]. In USA, about 33.5% of patients aged 18 years or older with prescribed treatment for diabetes are receiving insulin therapy [4]. In France, it has been estimated that only 25% of the three million patients with prescribed treatment for diabetes are treated with insulin [5].
Both T2DM and T1DM patients have difficulties managing their antidiabetic medications including insulin [6]. Among T2DM patients, this often results in low treatment persistence, which has been defined as the proportion of patients who remained on treatment for a specific time or the duration of time from initiation to discontinuation of therapy.
Treatment adherence and persistence are important factors that influence the effectiveness of antidiabetic medication [7–11] and there is considerable evidence that adherence to and persistence with OADs remains suboptimal [11, 12].
A number of studies have been performed to evaluate persistence in patients prescribed OADs, but less in patients receiving insulin therapy, although renouncement to insulin leaves the patients with limited choice of anti-diabetes therapy [13–17]. Most of these studies were conducted in the US. The objective of this study was to document the initiation of insulin therapy in patients with T2DM and its maintenance as a function of time after initiation in a European nation-wide representative cohort.
Methods
This study was a retrospective analysis of a sample of patients with T2DM documented in a French national prescription claims database [Échantillon Généraliste de Bénéficiaires, (EGB)] performed in 2014. The study included a cross-sectional phase to document initiation of insulin therapy, and a longitudinal phase to document treatment persistence.
Data Source
The EGB database [18] represents a 1/97 random sample of individuals selected from all beneficiaries of the three main public health insurance funds and provides data of around 600,000 individuals representative of the French population. The sample of the general population is renewed every 3 months, is anonymous and is representative of healthcare expenditure at the national level. The EGB database is updated every month. All information in the database is anonymous.
The insurance funds contributing to the EGB are the Caisse Nationale d’Assurance Maladie des Travailleurs Salariés (CNAMTS), the Mutualité Sociale Agricole (MSA) and the Régime Social des Indépendants (RSI). The CNAMTS is the French health insurance fund for salaried workers, which includes all salaried workers and their relatives and covers 77% of the French population in 2011 (almost 50 million people). The MSA covers agricultural workers and the RSI self-employed people. Individuals remain covered by the same fund if they stop working for any reason, including retirement, unemployment, invalidity or long-term sick leave. If nearly all the population is covered with insurance funds in France, 87% of the population covered is considered in the EGB, as the information system is different for some population sub-groups mainly government workers and students.
This database contains comprehensive reimbursement records of all items of community and hospital healthcare consumption in the public or private sectors eligible for reimbursement by public health insurance. All eligible medical expenditure reimbursed for a given individual is linked through a unique patient identifier. Documented expenditure includes hospitalisations [identified by diagnosis-related group (DRG)], consultations, paraclinical tests, medical devices, medical procedures, medication [identified by Anatomical Therapeutic Chemical classification (ATC)], auxiliary care (for example, nursing care and physiotherapy) and healthcare transport. For each prescribed or reimbursed service, the date of implementation is specified, together with the date of prescription and the healthcare provider. No explicit information is provided for the reason for which the service was prescribed, for example, the diagnosis. No sociodemographic information is available, with the exception of age and gender. Items which are not eligible for reimbursement, such as over-the-counter drugs, are not documented in the database and cannot be identified. In addition, information on inpatient rehabilitation is not available.
The only types of data in the EGB database associated with an explicit diagnosis are hospitalization and eligibility for full insurance coverage due to a severe chronic disease (Long-standing condition status). In the case of hospitalisations, the diagnosis can be identified since each hospital stay is valued on the basis of a unique DRG which is coded using the international classification of disease (ICD-10) codes [19]. The reasons for hospitalization are coded either as primary diagnoses (PD; the condition for which the patient was hospitalized), related diagnoses (RD; any underlying condition which may have been related to the PD) or as associated diagnoses (AD; comorbidities which may affect the course or cost of hospitalization). In the case of long-standing condition status, eligible diseases are identified on a restrictive list established by the CNAMTS which specifies the equivalent ICD-10 disease code.
Study Periods
For the description of initiation of insulin therapy, all new insulin prescriptions documented in the EGB database between January 2012 and December 2013 were considered. A new insulin prescription was defined as an index insulin prescription during the reporting period with no such prescription in the 12 months preceding the index prescription. For the description of treatment persistence, all new index insulin prescriptions documented in the EGB database between January 2011 and December 2012 were considered and insulin prescription followed longitudinally until December 2013.
Selection of the Study Patients
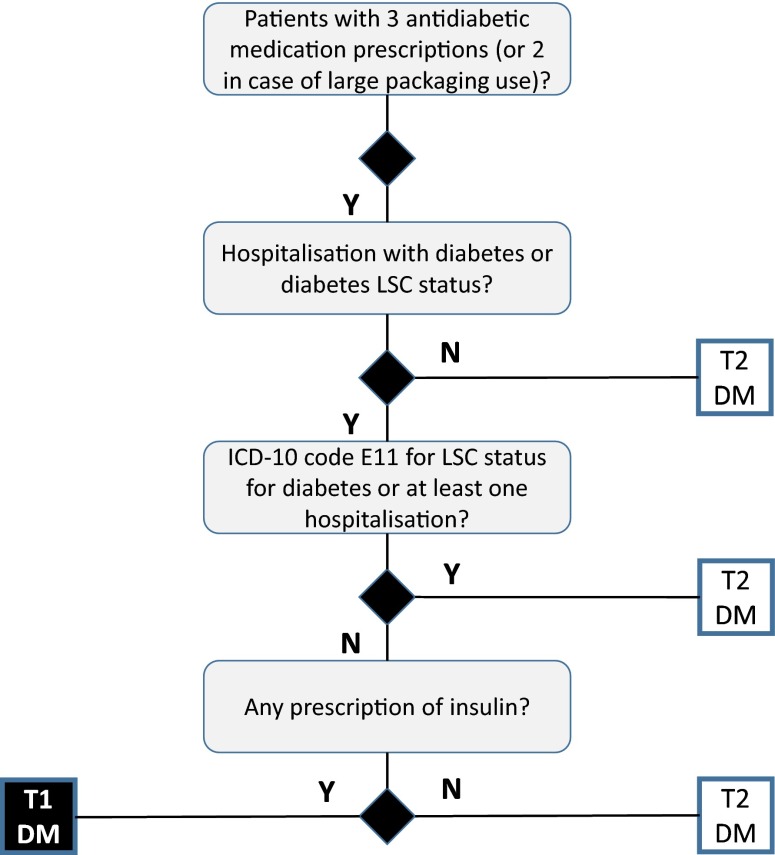
The study aimed to identify all patients with diabetes and treated with insulin documented in the EGB database during the study period. In a first step, these patients were identified by three prescription claims for antidiabetic medication (including insulin products; two when large packaging was used) within two consecutive years. Only adult patients ≥18 years of age were included. A decision tree was used to distinguish patients with T1DM from those with T2DM [20] (Fig. 1). This was based on the identification of hospitalizations with diabetes as an identified diagnosis (PD, RD or AD), the identification of long-standing condition status for diabetes through the associated ICD-10 code (E10 for T1DM and E11 for T2DM), as well as insulin treatment history.
Fig. 1.
Decision tree for identifying patients with type 1 (T1DM) and type 2 (T2DM) diabetes. Y yes, N no, LSC long-standing condition status, ICD international classification of disease
For the purposes of this study, only patients with T2DM (as defined by the decision tree) and starting insulin therapy (index insulin prescription) during the two study periods described above were retained for analysis.
Patients fulfilling the selection criteria were divided into three groups: patients delivered basal insulins only, patients delivered basal and rapid acting insulins, and patients under other insulin treatment regimens.
Data Collection
For each participant, data were retrieved from the EGB database relating to the age and gender of the patient, antidiabetic medication prescribed during the study period and during the year preceding initiation of insulin treatment and the type of physician prescribing insulin.
Longitudinal data on prescription of antidiabetic medication was retrieved covering the period between the initial insulin prescription and the end of the study period (December 31, 2013). Discontinuation of insulin treatment was defined as the absence of reimbursement for insulin over a period of 6 months or 1 year after the initiation index date.
Patients potentially eligible for a short-term transient insulin therapy were identified as patients with an hospital admission for a traumatological (ICD-10 disease classes S00-T98), infectious (ICD-10 disease classes A00-B99) or cardiovascular (ICD-10 disease classes I00-I99) disorder in the 3 months preceding initiation of insulin therapy or patients treated with corticosteroids (ATC class H02AB) or antibiotics (ATC classes A0A, J01, J02, J04 or J05) within 7 days of the initiation of insulin therapy.
Statistical Analysis
Baseline data at initiation of insulin therapy are presented as mean values with standard deviations and median values with range for continuous variables and as frequency counts and percentages for categorical variables. Missing data were not replaced and were excluded from the descriptive analyses. Continuous variables were compared between the three insulin treatment groups using Student’s t test and categorical variables using the χ 2 test. Data on treatment persistence was analyzed using Kaplan–Meier survival analysis. A sensitivity analysis was performed in which patients having potentially received short-term transient insulin therapy were excluded. Patients who left the database, for example, those who died were censored. However, detailed results excluding patients who died during the study period were also provided.
All statistical tests were two-sided and a probability threshold of 0.05 was taken as statistically significant. Data analysis was performed using SAS® V9.3 software (SAS Institute; North Carolina, Unites States).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. Access to the EGB database has been authorized for INSERM Unit U1018-UVSQ.
Results
Participants
Over the 2-year study period (2012 and 2013), 1909 patients in the EGB database were started on insulin therapy. Between January 1, 2011 and December 31, 2012, 1969 patients were started on insulin therapy and these patients were evaluated for persistence of insulin therapy. Patients were divided into three groups on the basis of the insulin treatment regimen prescribed. The distribution of patients between these groups was similar in the 2011–2012 and 2012–2013 cohorts (Fig. 2), with around 60% of patients having been prescribed basal insulin only and around 15.0% a basal-fast acting regimen.
Fig. 2.
Identification of patients with T2DM starting insulin therapy in the EGB database. T2DM type 2 diabetes mellitus, EGB Échantillon Généraliste de Bénéficiaires
The characteristics of both groups are presented in Tables 1, 2.
Table 1.
Characteristics of patients (insulin initiation analysis)
| Characteristics of the population (insulin initiation) Total 2012–2013 |
Basal insulin only | Basal-fast-acting | Other insulin regimens | p* | |
|---|---|---|---|---|---|
| Age (years) | N = 1909 | N = 1180 | N = 286 | N = 443 | |
| Mean ± SD | 65.7 ± 16.0 | 67.5 ± 14.2 | 61.8 ± 18.1 | 63.2 ± 18.4 | <0.0001 |
| Median [range] | 67 [18; 101] | 67 [18; 101] | 63.5 [18; 95] | 66 [19; 100] | |
| <40 | 137 (7.2%) | 24 (2.0%) | 44 (15.4%) | 69 (15.6%) | <0.0001 |
| 40–50 | 166 (8.7%) | 100 (8.5%) | 27 (9.4%) | 39 (8.8%) | |
| 50–60 | 329 (17.2%) | 231 (19.6%) | 45 (15.7%) | 53 (12.0%) | |
| 60–70 | 466 (24.4%) | 299 (25.3%) | 67 (23.4%) | 100 (22.6%) | |
| 70–80 | 379 (19.9%) | 241 (20.4%) | 50 (17.5%) | 88 (19.9%) | |
| 80–90 | 361 (18.9%) | 239 (20.3%) | 43 (15.0%) | 79 (17.8%) | |
| ≥90 | 71 (3.7%) | 46 (3.9%) | 10 (3.5%) | 15 (3.4%) | |
| Gender | N = 1818 | N = 1177 | N = 278 | N = 433 | |
| Men | 1000 (53.0%) | 656 (55.7%) | 152 (54.7%) | 192 (44.3%) | 0.0002 |
| Women | 888 (47.0%) | 521 (44.3%) | 126 (45.3%) | 241 (55.7%) | |
| Initial insulin prescription | |||||
| Insulin only | 611 (32.0%) | 223 (18.9%) | 170 (59.4%) | 218 (49.2%) | <0.0001 |
| Insulin + 1 OAD | 437 (22.9%) | 271 (23.0%) | 72 (25.2%) | 94 (21.2%) | |
| Insulin + 2 OAD | 497 (26.0%) | 373 (31.6%) | 32 (11.2%) | 92 (20.8%) | |
| Insulin + 3 OAD | 340 (17.8%) | 294 (24.9%) | 10 (3.5%) | 36 (8.1%) | |
| Insulin + ≥ 4 OAD | 24 (1.3%) | 19 (1.6%) | 2 (0.7%) | 3 (0.7%) | |
| Metformin | 956 (50.1%) | 703 (59.6%) | 91 (31.8%) | 162 (36.6%) | |
| Sulphonylurea | 648 (33.9%) | 526 (44.6%) | 28 (9.8%) | 94 (21.2%) | |
| α-Glucosidase inhibitor | 49 (2.6%) | 42 (3.5%) | 2 (0.7%) | 5 (1.1%) | |
| Glinides | 283 (14.8%) | 211 (17.9%) | 21 (7.3%) | 51 (11.5%) | |
| DPP4i | 467 (24.5%) | 370 (31.4%) | 29 (10.1%) | 68 (15.3%) | |
| GLP-1 receptor agonists | 146 (7.6%) | 125 (10.6%) | 3 (1.1%) | 18 (4.1%) | |
| Physician prescribing insulin | N = 1772 | N = 1105 | N = 257 | N = 410 | |
| Hospital physician | 798 (45.0%) | 381 (34.5%) | 177 (68.9%) | 240 (58.5%) | ND |
| General practitioner | 696 (39.3%) | 527 (47.7%) | 57 (22.2%) | 112 (27.3%) | |
| Community diabetologist | 224 (12.6%) | 164 (14.8%) | 17 (6.6%) | 43 (10.5%) | |
| Other community specialist | 49 (2.8%) | 30 (2.7%) | 4 (1.6%) | 15 (3.7%) | |
| Diabetes treatment before the insulin initiationa | N = 1909 | N = 1180 | N = 286 | N = 443 | |
| None | 415 (21.7%) | 154 (13.1%) | 117 (40.9%) | 144 (32.5%) | <0.0001 |
| 1 OAD | 362 (18.9%) | 337 (16.8%) | 54 (18.9%) | 109 (24.6%) | |
| 2 OAD | 509 (26.7%) | 354 (28.9%) | 66 (23.1%) | 102 (23.1%) | |
| 3 OAD | 542 (28.4%) | 327 (35.9%) | 42 (14.7%) | 76 (17.2%) | |
| ≥4 OAD | 81 (4.2%) | 27 (5.3%) | 7 (2.5%) | 12 (2.7%) | |
Characteristics of patients (insulin initiation analysis)
SD standard deviation, OAD oral antidiabetic drug, DPP4i dipeptidyl peptidase inhibitor, GLP glucagon-like peptide, ND not determined
* Student’s t test for continuous values and χ 2 test for categorical variables
aOver the first or the second quarter before the initial insulin prescription
Table 2.
Characteristics of patients (persistence analysis)
| Characteristics of the population (persistence study) Total 2011–2012 |
|
|---|---|
| Age (years) | N = 1969 |
| Mean ± SD | 66.4 (16.1) |
| Median [Range] | 68.0 [18.0; 101] |
| <40 | 145 (7.4%) |
| 40–50 | 157 (8.0%) |
| 50–60 | 306 (15.5%) |
| 60–70 | 453 (23.0%) |
| 70–80 | 423 (21.5%) |
| 80–90 | 417 (21.2%) |
| ≥90 | 68 (3.5%) |
| Gender | N = 1936 |
| Men | 1004 (51.9%) |
| Women | 932 (48.1%) |
| Initial insulin prescription | |
| Insulin only | 686 (34.8%) |
| Insulin + 1 OAD | 486 (24.7%) |
| Insulin + 2 OAD | 488 (24.8%) |
| Insulin + 3 OAD | 280 (14.2%) |
| Insulin + ≥ 4 OAD | 29 (1.5%) |
| Metformin | 896 (45.5%) |
| Sulphonylurea | 651 (33.1%) |
| α-Glucosidase inhibitor | 68 (3.5%) |
| Glinides | 295 (14.9%) |
| DPP4i | 399 (20.3%) |
| GLP-1 receptor agonists | 109 (5.5%) |
| Physician prescribing insulin | N = 1826 |
| Hospital physician | 728 (39.9%) |
| General practitioner | 796 (43.6%) |
| Community diabetologist | 297 (16.3%) |
| Other community specialist | 5 (0.3%) |
| Diabetes treatment before the insulin initiationa | N = 1969 |
| None | 526 (26.7%) |
| 1 OAD | 407 (20.7%) |
| 2 OAD | 511 (25.9%) |
| 3 OAD | 447 (22.7%) |
| ≥4 OAD | 8 (0.4%) |
SD standard deviation, OAD oral antidiabetic drug, DPP4i dipeptidyl peptidase inhibitor, GLP glucagon-like peptide, ND not determined
aOver the first or the second quarter before the initial insulin prescription
Initiation of Insulin Prescription
Insulin therapy with basal insulin only was mainly initiated by a general practitioner, whereas other insulin regimens were principally initiated by a hospital physician. During the previous 6 months, 18.9% of all patients starting an insulin therapy (all schemes) had been treated with an OAD in monotherapy and 59.3% with an association of two or more OADs. The remaining 21.7% of patients had no previous diabetes treatment documented in the EGB database. This proportion was three times higher in the patients starting basal-fast-acting regimens (40.9%) and other insulin treatment regimens (32.5%) than in patients initiating treatment with basal insulin only (13.1%). To ensure that this absence of previous treatment was not an artifact due to a gap between documentation of the last recorded OAD prescription and the initial insulin prescription, the EGB database was searched for prescription of diabetes medications to these patients in the 12 months preceding insulin prescription. This appeared generally not to be the case, since among the 415 patients who had no previous diabetes treatment during the previous 6 months only ten patients (2.4%) had been prescribed an antidiabetic medication between twelve and 6 months prior to starting insulin therapy.
The reimbursement form of the initial insulin prescription did not include any other antidiabetic drug in 32.0% of the patients, although this proportion was considerably higher in patients starting basal-fast-acting regimens and other insulin treatment regimens than in patients initiating treatment with basal insulin only (Table 1). For the patients who were also prescribed concomitant OADs, they received a single OAD therapy (22.9% of patients) or a dual OAD therapy (26.0%), or a triple therapy (17.8%) in addition to insulin. In patients starting a basal-fast-acting insulin regimen or another insulin treatment regimen, the use of concomitant OAD with insulin was less frequent (Table 1). Overall metformin was associated to insulin in 50.1% of patients (59.6% in patients starting a basal only insulin regimen).
Persistence with Insulin Therapy
This analysis was performed in the 1969 patients who initiated insulin therapy between 1st January 2011 and 31st December 2012 (Table 2). Among them, 205 deaths were observed during the study period (until 31st December 2013). Excluding deaths, insulin therapies were discontinued 1 year later in nearly one third of patients, when discontinuation was defined as a 6 months interruption of insulin treatment. If discontinuation is set as a 12 months interruption of insulin treatment, 24.9% of patients had discontinued insulin therapy within 1 year after its initiation (excluding deaths) (Table 3).
Table 3.
Discontinuation rates with initial insulin therapy (% patients still treated with insulin)
| All subjects (N = 1969) (%) | Basal insulin only (N = 1199) (%) | Basal-fast-acting insulin (N = 259) (%) | Other regimens (N = 511) (%) | |
|---|---|---|---|---|
| Insulin discontinuation defined by a 6 months interruption | ||||
| Including deaths | ||||
| After 6 months | 24.0 | 19.0 | 23.4 | 37.2 |
| After 12 months | 33.4 | 27.5 | 35.5 | 46.9 |
| Excluding deaths | ||||
| After 6 months | 24.6 | 19.1 | 24.6 | 39.3 |
| After 12 months | 33.8 | 28.7 | 35.4 | 48.5 |
| Insulin discontinuation defined by a 12 months interruption | ||||
| Including deaths | ||||
| After 6 months | 18.3 | 13.1 | 19.0 | 30.9 |
| After 12 months | 24.9 | 18.4 | 17.7 | 39.6 |
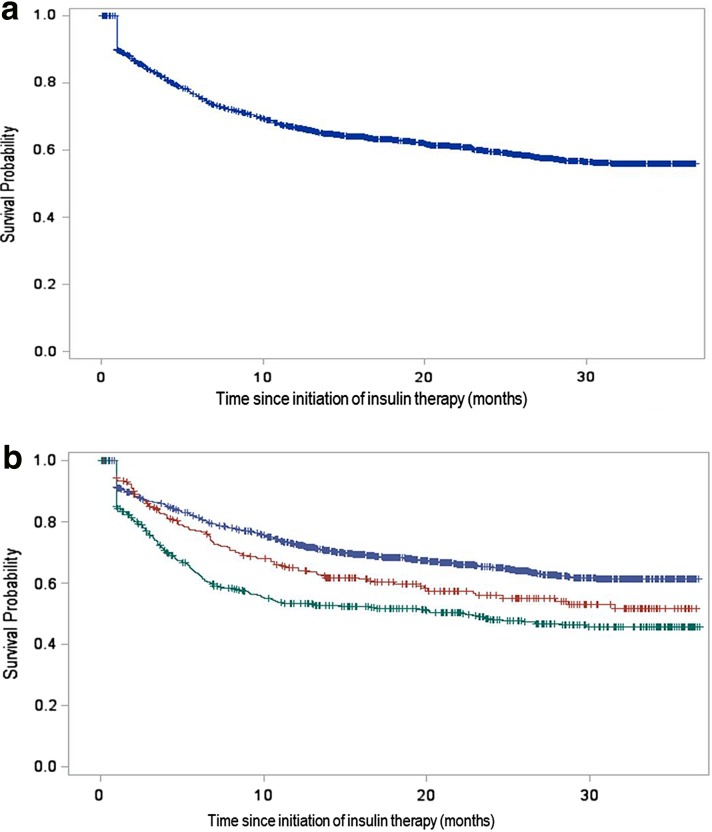
Persistence was better in the patients starting a treatment with a basal insulin only and worse in those starting other insulin treatment regimens (Table 3). The median treatment duration was longest in patients’ prescribed basal insulin only (17 months) and lowest in those prescribed another insulin regimen (9 months). Kaplan–Meier survival curves for all patients and by insulin treatment group are provided in Fig. 3. There was limited variation in the rates of persistence with insulin therapy according to the specialty of the initial prescriber (General practitioner vs Diabetes specialist, data not shown).
Fig. 3.
a, b Kaplan–Meier survival curves for the persistence with insulin therapy (discontinuation of insulin defined by a 6 months period without insulin) in the newly treated patients. Left all patients, right by treatment group: blue curve basal insulin only, red curve basal-fast-acting insulin regimen, green curve other insulin treatment regimens
Patients discontinuing insulin were younger (64.7 ± 18.5 years) than those who did not (67.3 ± 14.3 years; p = 0.0003) and were more frequently women (54.2% vs 44.3%, p < 0.0001). Interestingly, among patients who interrupted their insulin treatment during the first 6 months and were still alive 1 year later, insulin resumption was observed in 22.3% of patients, 57.4% were treated with antidiabetic treatment, and 20.2% did not received any antidiabetic drug.
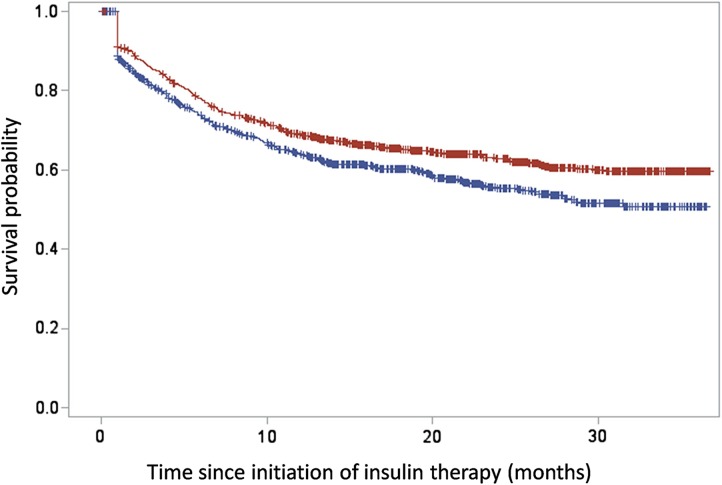
A sensitivity analysis was conducted, in which the patients who had potentially received short-term transient insulin therapy were excluded and analyzed separately. This concerned 901 patients (45.8% of all patients) who were identified as being hospitalized for a traumatological, infectious or cardiovascular disorder in the 3 months preceding initiation of insulin therapy, or being treated with corticosteroids or antibiotics within 7 days prior to the initiation of insulin. Persistence with insulin therapy was quite similar in patients who had a reason to receive short-term transient insulin therapy and those who did not (Fig. 4).
Fig. 4.
Kaplan–Meier survival curves for the persistence with insulin therapy in the newly treated patients (discontinuation of insulin defined by a 6 months period without insulin). Red curve exclusion of patients with a reason for short-term transient insulin therapy, blue curve patients with a reason for short-term transient insulin therapy
Discussion
This study was designed to document the maintenance of insulin therapy in patients with T2DM after initiation in a representative sample of the French national insurance claims database. The main finding was that excluding deaths and defining discontinuation in a rather conservative way (at least 12 months without any reimbursement of insulin therapy) around 25% of patients starting insulin therapy had discontinued treatment within a year. This high rate is of concern as the mean age of the patients is over 65, and the decision of insulin initiation suggests a need. This observation is somewhat counter-intuitive because insulin therapy is usually a late, often delayed, option in patients with T2DM; indeed, the mean age of the participants to this study is over 65.
However, the 1-year persistence rates in our study are consistent with the results from the claims studies conducted among North-American patients with diabetes starting injectable therapy, despite differences in the health insurance systems. In a study of the General Electric Centricity electronic medical record database between November 2004 and August 2010, Wu et al. [21] found that about a third of patients (32.9%) initiating an insulin therapy discontinued (defined as a 90-day gap) the index insulin within 12 months from initiation. Wei et al. [15] performed a pooled analysis of three prescription claims studies performed in the USA and reported a persistence rate of 65% at 1 year for patients starting basal insulin therapy. In another study from Wang et al. [17] during the 1-year follow-up, only 54.5% of patients were persistent to insulin glargine medication. Other researchers reported a similar rate of discontinuation in the USA [22, 23] or even worse: in the study from Cooke et al. [13] the percentage of patients treated with insulin who were persistent at 1 year was only 28.7%. In Japan, 22% of patients discontinued basal insulin in the year after initiation according to a reimbursement claim base [24]. In Germany, the discontinuation rate was also around 20% and 30% after 1 year with glargine and NPH, respectively [16]. In this work, in France, a persistence rate of 75.0% after initiation of insulin (and 82% when the insulin scheme was limited to basal insulin use) is somewhat higher, although it should be noted that the definition of persistence was not exactly identical in the aforementioned studies.
The type of physician initiating the insulin therapy differed according to the treatment regimens; the general practitioners initiated a significant number (39%) of the basal insulin therapies, whereas the majority of the other regimens were initiated in a hospital setting. This distribution is likely related to a combination of reasons: either the severity of diabetes in hospitalized patients with comorbidities requires a more intensive regimen, or the basal-fast-acting insulin regimen was chosen in an outpatient setting but its complexity was found to require initiation during hospitalization. Unfortunately, it is not possible to assess the reasons for prescription or the detailed clinical features of the patient from the data available in the EGB database. Community-based endocrinologists or diabetologists appeared to play a relatively minor role in initiation of insulin therapy, whatever the treatment regimen prescribed. With regard to concomitant therapies, the most frequent treatment combinations were insulin with metformin and insulin with metformin and a sulphonylurea, which are the recommended combinations in the French guidelines [2]. Nonetheless, around 30% of patients were apparently prescribed insulin without concomitant OADs and around 10% insulin together with three or more OADs. These combinations are deviations from the latest French guidelines.
Our study, like every analysis of insurance claim databases, has several inherent limitations. First, diagnoses are only directly documented in the EGB database in case of hospitalizations and long-standing condition status, and even in these cases, it is not possible to ascertain the diagnosis independently. However, the decision tree used to ascertain the diagnosis of diabetes on the basis of the use of drugs has been externally validated [20]. Second, no information is available on the clinical status of the patient, except fatality cases, or on the reasons for which a given medication was prescribed. Nevertheless, insurance claims databases have many advantages for documenting prescription trends. The EGB database is representative of insured patients reimbursed for health care in France and contains exhaustive and reliable information on all reimbursed prescription medication. The almost universal coverage of the French insurance system limits dramatically missing data and losses of follow-up. Insulin therapy is only available as a prescription medication and is not prescribed to a significant extent to patients without diabetes.
The study does not provide any information about the reason of early discontinuation of insulin therapy, i.e., it was not possible to identify hypoglycemia events, a strong factor for the patients to stop any of antidiabetic treatment. Drugs and visits are fully reimbursed for people with diabetes in France and this economic aspect has no influence as compare to other countries. Further work is needed to develop answers on this question.
Conclusion
Information from the large EGB claim database is useful to analyze the condition of use of insulin therapy in patients with diabetes in France. As in previous studies worldwide, we found that a significant proportion of patients with T2DM starting insulin therapy discontinue treatment within a year. Because very few options are available for patients failing insulin therapy, it is important to conduct further real world studies aiming at understanding why patients do not pursue their therapy and identify how to optimize persistence.
Acknowledgments
Sponsorship for this study was funded by Sanofi, France. Medical writing assistance for this study was provided by Mr. Adam Dobble of Foxymed Inc. and funded by Sanofi, France. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for the authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
R. Roussel reports receiving lecturing and consulting fees from AbbVie, AstraZeneca, Boehringer Ingelheim, Sanofi, Janssen, Eli Lilly, MSD, Novo Nordisk. B. Charbonnel has received honoraria for consultations/advisory board from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck-Sharpe & Dohme, Novartis, Novo Nordisk, Sanofi, Takeda. M. Behar is employed by Sanofi, Paris, France. C. Emery is employed by CEMKA-EVAL, a consultancy team working for numerous private companies and public national and international institutions in health care. B. Detournay is employed by CEMKA-EVAL, a consultancy team working for numerous private companies and public national and international institutions in health care. J. Gourmelen has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. Access to the EGB database has been authorized for INSERM Unit U1018-UVSQ.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/8FD4F0607194F080.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 2.Haute Autorité de la Santé—Agence Nationale de Sécurité du Médicament, Diabète de type 2. Stratégie médicamenteuse du contrôle glycémique du diabète de type 2. Published: January 2013.
- 3.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Nice Guideline. NG28 Published: December 2015. [PubMed]
- 4.Centers for Disease Control and Prevention . National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. Atlanta: US Department of Health and Human Services; 2014. [Google Scholar]
- 5.Mandereau-Bruno L, Denis P, Fagot-Campagna A, Fosse-Edorh S. Prevalence of people pharmacologically treated for diabetes and territorial variations in France in 2012. Bull Epidemiol Hebd. 2014;30–31:493–499. [Google Scholar]
- 6.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Perez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence DB, Ragucci KR, Long LB, Parris BS, Helfer LA. Relationship of oral antihyperglycemic (sulfonylurea or metformin) medication adherence and hemoglobin A1c goal attainment for HMO patients enrolled in a diabetes disease management program. J Manag Care Pharm. 2006;12(6):466–471. doi: 10.18553/jmcp.2006.12.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 10.Pladevall M, Williams LK, Potts LA, Divine G, Xi H. Lafata JE Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31–41. [PubMed] [Google Scholar]
- 12.Blackburn DF, Swidrovich J, Lemstra M. Non-adherence in type 2 diabetes: practical considerations for interpreting the literature. Patient Prefer Adherence. 2013;7:183–189. doi: 10.2147/PPA.S30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke CE, Lee HY, Tong YP, Haines ST. Persistence with injectable antidiabetic agents in members with type 2 diabetes in a commercial managed care organization. Curr Med Res Opin. 2010;26(1):231–238. doi: 10.1185/03007990903421994. [DOI] [PubMed] [Google Scholar]
- 14.Davies MJ, Gagliardino JJ, Gray LJ, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabet Med. 2013;30(5):512–524. doi: 10.1111/dme.12128. [DOI] [PubMed] [Google Scholar]
- 15.Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes. Endocr Pract. 2014;20(1):52–61. doi: 10.4158/EP13159.OR. [DOI] [PubMed] [Google Scholar]
- 16.Pscherer S, Chou E, Dippel FW, Rathmann W, Kostev K. Treatment persistence after initiating basal insulin in type 2 diabetes patients: a primary care database analysis. Primary Car Diabetes. 2015;9:377–384. doi: 10.1016/j.pcd.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wei W, Miao R, Xie L, Baser O. Real-world outcomes of US employees with type 2 diabetes mellitus treated with insulin glargine or neutral protamine Hagedorn insulin: a comparative retrospective database study. BMJ Open. 2013;3(4):e002348. doi: 10.1136/bmjopen-2012-002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merlière Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58(4):286–290. doi: 10.1016/j.respe.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . International Classification of Diseases 10th Revision. 4. Geneva: WHO; 2010. [Google Scholar]
- 20.Quantin C. Caisse nationale d’assurance maladie des travailleurs salariés. Etude des algorithmes de définition de pathologies dans le système national d’information inter-régimes de l’assurance maladie (SNIIRAM). Août 2015. http://www.ameli.fr/fileadmin/user_upload/documents/Rapport_Etude_algorithmes_partie1.pdf. Accessed 12 July 2016.
- 21.Wu N, Aagren M, Boulanger L, Friedman M, Wilkey K. Assessing achievement and maintenance of glycemic control by patients initiating basal insulin. Curr Med Res Opin. 2012;28(10):1647–1656. doi: 10.1185/03007995.2012.722989. [DOI] [PubMed] [Google Scholar]
- 22.Xie L, Zhou S, Pinsky BW, Buysman EK, Baser O. Impact of initiating insulin glargine disposable pen versus vial/syringe on real-world glycemic outcomes and persistence among patients with type 2 diabetes mellitus in a large managed care plan: a claims database analysis. Diabetes Technol Ther. 2014;16(9):567–575. doi: 10.1089/dia.2013.0312. [DOI] [PubMed] [Google Scholar]
- 23.Grabner M, Chu J, Raparla S, Quimbo R, Zhou S, Conoshenti J. Clinical and economic outcomes among patients with diabetes mellitus initiating insulin glargine pen versus vial. Postgrad Med. 2013;125(3):204–213. doi: 10.3810/pgm.2013.05.2656. [DOI] [PubMed] [Google Scholar]
- 24.Hadjiyianni I, Desai U, Ivanova JI, Kirson NY, Enloe CJ, Cummings AG, Birnbaum HG, Suzuki SJ, Duan R, Raibouaa A, Cao D, Perez-Nieves M. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in Japan. Value Health. 2015;18(7):A611–A612. doi: 10.1016/j.jval.2015.09.2121. [DOI] [Google Scholar]