Abstract
Maturity-onset diabetes of the young (MODY) is a heterogeneous group of diseases associated with gene mutations leading to dysfunction of pancreatic β-cells. Thirteen identified MODY variants differ from each other by the clinical course and treatment requirement. Currently, MODY subtypes 1–5 are best-studied, descriptions of the other forms are sporadic. This article reports a MODY12 clinical case, caused by a mutation in the gene of the ATP-binding cassette transporter sub-family C member 8 (ABCC8), encoding sulfonylurea receptor 1. Diabetes manifested in a 27-year-old non-obese man with epilepsy in anamnesis. No evidence of ketosis was present, pancreatic antibodies were undetectable, and C-peptide remained within the reference range. During the initial investigation, non-proliferative diabetic retinopathy and elevated albumin excretion rate was revealed. After 4 months, diabetes was complicated by pre-proliferative retinopathy and diabetic macular edema. Recurrent hypoglycemia and an increase in body weight was observed on moderate and even small insulin doses. Taking into account the clinical features and the presence of diabetes in four generations on the maternal side, screening for all MODY subtypes was performed. A mutation in the ABCC8 gene was found in proband and in his mother. After the insulin discontinuation, gliclazide modified release combined with sodium/glucose cotransporter 2 (SGLT2) inhibitors was started. This treatment eliminated hypoglycemia and improved glycemic variability parameters. A decrease in the amplitude of glucose excursions was documented by continuous glucose monitoring. After 3 months of treatment, glycemic control was still optimal, and no hypoglycemic episodes were observed. The case report demonstrates the clinical features of ABCC8-associated MODY and the therapeutic potential of a combination of sulfonylurea with SGLT2 inhibitor in this disease.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-016-0192-9) contains supplementary material, which is available to authorized users.
Keywords: ABCC8, Dapagliflozin, Glucose variability, Maturity-onset diabetes of the young, SGLT2 inhibitor, Sulfonylurea receptor
Introduction
Maturity-onset diabetes of the young (MODY) is a heterogeneous group of disorders caused by the gene mutations leading to the dysfunction of pancreatic β-cells. Diagnostic criteria of MODY include hyperglycemia, usually manifesting before the age of 25 years, autosomal dominant inheritance with transmission across at least three generations, similar phenotypes of the disease among all members of a family, and the C-peptide levels staying within the reference range for years [1–4]. Confirmation of MODY is complicated and expensive, but enables to select the appropriate treatment and conduct genetic consulting [5, 6]. The real prevalence of MODY is unknown. According to CH Lachanse, around 1–2% of diabetes cases can be classified into this type [1].
At present, it is known that a mutation in one of 13 genes can cause a certain subtype of the disease. Subtypes of MODY differ from each other by their prevalence, clinical picture, and treatment requirements [1, 7, 8]. Despite substantial variation in frequency of MODY subtypes in various populations, the most prevalent mutations affect genes of hepatocyte nuclear factor 1α (HNF1A) and glucokinase (GCK), which cause MODY3 and MODY2, respectively [9]. The proportion of these subtypes is up to 90% of all MODY cases [10]. In some European countries, including the United Kingdom, the Netherlands, and Denmark, the most common form of monogenic diabetes is MODY3 (mutation of HNF1A), but in Spain, Italy, France, Germany, and the Czech Republic, the predominant form is MODY2 (mutation of GCK) [11]. The MODY subtypes 1–5 are the best-studied and most thoroughly described forms of the disease. The other subtypes are rare, and the data on their clinical features and treatment options are scarce.
In this report, we describe a case of MODY12, which is caused by a mutation in the ATP-binding cassette transporter sub-family C member 8 gene (ABCC8), encoding the sulfonylurea receptor 1 (SUR1).
Case Presentation
A 28-year-old Caucasian male, at initial examination in our clinic (September 2015) complained of a burning sensation and severe pain in the legs, numbness of toes, headache, and blurred vision.
In February 2014, he noticed bothersome dry mouth, polyuria, and a weight loss (~5 kg for 4 months). A visit to a local clinic revealed high plasma glucose (PG), up to 24 mmol/L, and no signs of ketosis. Besides, non-proliferative diabetic retinopathy and increased urinary albumin excretion rate (microalbuminuria) was detected. Testing for pancreatic islet antibodies yielded negative results. The level of C-peptide was 338 pmol/L (reference range: 298–2350 pmol/L). Type 1 diabetes was diagnosed, and, accordingly, insulin therapy was initiated with glargine 16 U/day and insulin lispro 4–6 U before main meals.
In October 2014, the patient’s vision deteriorated; ophthalmoscopic examination revealed pre-proliferative diabetic retinopathy and diabetic macular edema of both eyes. In the same month, panretinal laser photocoagulation was carried out on the right eye, and in February 2015, the procedure was performed on the left eye.
On insulin therapy, the patient’s body weight increased by 10 kg, with repeated episodes of hypoglycemia, and the daily insulin dose was gradually reduced. In June 2015, the level of glycated hemoglobin A1c (HbA1c) of 5.1% was recorded. Next month, the patient had an episode of severe hypoglycemia. After complete discontinuation of insulin, his PG level increased up to 15 mmol/L, and the injections of insulin were restarted.
The patient was the first child of a 21-year-old woman at 35 week of uneventful pregnancy following spontaneous term delivery; with a birth weight of 2500 g. Hyperkinesis, torsion spasm and cramps were noted in the early neonatal period. Physical and mental development was normal. From the age of 3 years, there were several episodes of seizures lasting 1–2 min with transitional loss of consciousness (grand mal). Since the diagnosis of epilepsy had been established treatment with phenobarbital was administered. The seizures discontinued at the age of 10 years, anticonvulsant therapy was withdrawn 3 years later. No progression of neurological pathology was observed afterwards. The patient graduated from a technical community college. No indication of glucose metabolism disturbances was revealed in medical records until February 2014.
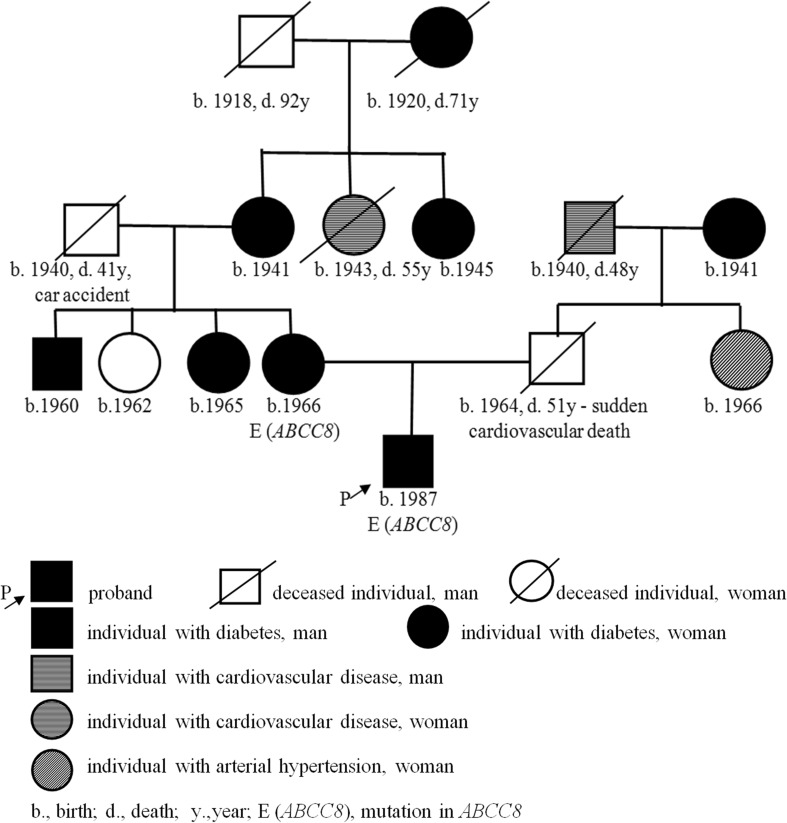
Diabetes was documented within four generations on the maternal side (Fig. 1). Diabetes manifested in all of them in the fourth or fifth decade of life, and all these subjects were treated by oral glucose-lowering agents. On the paternal side, there were cardiovascular diseases for two generations (coronary artery disease and sudden death).
Fig. 1.
Pedigree
On admission to our hospital in September 2015, the patient was taking glargine 13 U at night and insulin lispro 4 U before lunch and dinner (no injections were made before breakfast because of low glycemia). The total daily insulin dose was 0.3 U/kg.
On examination, height 165 cm, weight 68 kg, body-mass index 25 kg/m2, regular pulse 88 per minute, blood pressure (BP) 140/85 mmHg.
Biochemical analysis revealed moderate elevation of alanine aminotransferase (78.6 U/L), aspartate aminotransferase (68.2 U/L), uric acid (422.1 μmol/L), and of LDL-cholesterol (3.41 mmol/L). Other parameters were within reference ranges: creatinine 80.1 μmol/L, urea 6.3 mmol/L, glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration equation, 2009) 115 mL/min/1.73 m2, bilirubin 14.6 μmol/L, alkaline phosphatase 58 U/L, γ-glutamyltranspeptidase 14 U/L, total cholesterol 4.2 mmol/L, and triglycerides 0.7 mmol/L. The HbA1c level was 5.9%, C-peptide 1.35 ng/mL (reference range 0.9–7.1 ng/mL). Testing for hepatitis B and C markers yielded negative results. Urinary albumin excretion was 57.3 mg/L. The reactions for glucose and ketones in the urine were negative.
Twenty-four-hour BP monitoring revealed arterial hypertension with average day-time BP of 157/96 mmHg and nocturnal BP of 155/92 mmHg. Echocardiography showed intact valves, two additional chords of the left ventricle, and the ejection fraction 62%. Ultrasonic Doppler examination indicated atherosclerosis of brachiocephalic arteries at the extracranial level, a hemodynamically insignificant plaque in the left common carotid artery spreading to the internal carotid artery. Ultrasonography of the thyroid gland, abdominal cavity, and kidneys did not reveal any pathology. Electromyography showed pronounced distal sensory neuropathy. The brain MRI uncovered small chronic nonspecific foci in basal ganglia and in the white matter of the hemispheres, atrophic loci in the periventricular white matter (up to 17 mm), moderate internal hydrocephalus, the cyst of transparent partition (43 × 12 × 16 mm), and reduced blood flow through the right vertebral artery. There was no deterioration compared to MRI data from 2008.
Considering case features, such as familial diabetes aggregation, retained C-peptide levels, the absence of islet cell antibodies, and the obvious signs of insulin overdose on relatively small doses, we hypothesized MODY. Whole-exome sequencing was performed on the genes associated with MODY subtypes 1–13. Whole exome libraries were prepared with the Ampliseq Exome Kit (Thermo Fisher Scientific, Waltham, MA, USA). Sequencing was performed using the Ion Proton System (Thermo Fisher Scientific, Waltham, MA, USA). Data processing pipeline included mapping reads to the reference human genome (GRCh37) with the use of Burrows-Wheeler Aligner software. The single nucleotide variant (CNV) calling was conducted with Genome Analysis Toolkit. Ion Reporter™ Software 4.0 (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA) was used for CNV calling. The average exome coverage was 30×.
Mutation Ala1457Thr in the gene ABCC8 was revealed in the proband. The substitution was confirmed by automatic Sanger sequencing. The revealed mutation at position 1457 of the ABCC8 protein is rare and causes a substitution of alanine with threonine (please see Fig. S1 in the supplementary material). The online software PolyPhen-2 (Polymorphism Phenotyping v2, Boston, Harvard, MA, USA), applied for prediction of protein structure [12], showed high probability of an adverse effect of the Ala1457Thr mutation on the product of the ABCC8 gene (please see Fig. S2 in the supplementary material).
In the proband’s mother, we revealed the same mutation in ABCC8 gene. Despite this, some phenotypic differences between proband and his mother were noted. In the mother arterial hypertension and mild fasting hyperglycemia (up to 8 mmol/L) was observed at the age of 30 years. Mild hyperglycemia was corrected successively on diet alone until 38 years of age (2004). Afterwards, glycemic control deteriorated, PG increased up to 14 mmol/L, and the therapy with sulfonylurea was initiated. In September 2015, the level of HbA1c was 7.6% on the treatment with gliclazide modified release (MR) 120 mg/day and vildagliptin 100 mg/day.
Unfortunately, other family members were not available for clinical evaluation and genetic testing.
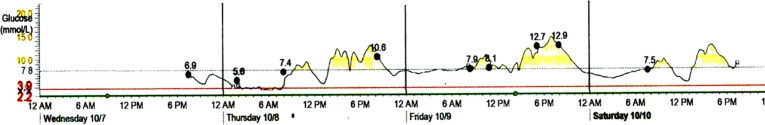
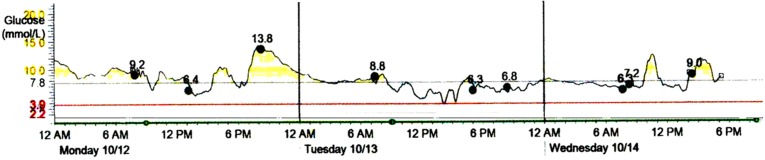
Taking into account the results of genotyping, we attempted to discontinue insulin therapy and started the treatment with gliclazide MR at 60 mg/day. During the first week on gliclazide, glycemic excursions were within the range 3.6–12.9 mmol/L. Real-time continuous glucose monitoring (CGM) with Medtronic Paradigm MMT-722 and Medtronic CareLink ® Pro Software (Medtronic Minimed, Inc, Northridge, CA, USA) was performed. Monitoring data on 4–7 days of gliclazide treatment are shown in Fig. 2. Because of substantial amplitude of glucose excursions and a tendency to fasting hypoglycemia, further increase in gliclazide dose had seemed inappropriate. Thus, SGLT2 inhibitor dapagliflozin at 10 mg/day was added. On combined treatment glycemic excursions diminished, and no hypoglycemic episodes were detected by CGM (Fig. 3).
Fig. 2.
The CGM results on days 4–7 of gliclazide MR treatment. Medtronic CareLink ® Pro Software (Medtronic Minimed, Inc, Northridge, CA, USA). CGM continuous glucose monitoring, MR modified release, Bre breakfast, Lu lunch, Di dinner
Fig. 3.
The CGM results on days 2–4 of treatment with gliclazide MR and dapagliflozin. Medtronic CareLink ® Pro Software (Medtronic Minimed, Inc, Northridge, CA, USA). CGM continuous glucose monitoring, MR modified release, Bre breakfast, Lu lunch, Di dinner
The estimation of glucose variability (GV) at three therapeutic regimens, including insulin, gliclazide and gliclazide plus dapagliflozin, was performed. The GV parameters were derived from three daily six-point measurements of PG for each applied treatment option. The Mean Amplitude of Glucose Excursions (MAGE), Lability Index (LI), Mean Absolute Glucose (MAG), Low Blood Glucose Index (LBGI), and High Blood Glucose Index (HBGI) were computed with EasyGV calculator (EasyGV version 9.0 by Nathan R Hill. © University of Oxford 2010+, UK) [13]. Among these indices, MAGE, LI, and MAG reflect the amplitude and frequency of glucose fluctuations; HBGI indicates the magnitude of hyperglycemic excursions; and LBGI estimates the risk of hypoglycemia [14, 15]. As shown in Table 1, the notable decrease in GV parameters was achieved on gliclazide MR and dapagliflozin combination compared to preceding treatment with insulin and gliclazide MR alone.
Table 1.
Dynamics of GV parameters
| Glucose-lowering therapy | MAGE, mmol/L | LI, (mmol/L)2/h | MAG, (mmol/L)/h | LBGI, a.u. | HBGI, a.u. |
|---|---|---|---|---|---|
| Insulin | 5.7 | 3.0 | 1.1 | 5.3 | 5.4 |
| Gliclazide MR | 4.6 | 2.8 | 0.8 | 2.8 | 4.8 |
| Gliclazide MR + dapagliflozin | 2.7 | 1.0 | 0.6 | 0.3 | 3.4 |
GV glucose variability, HBGI High Blood Glucose Index, LBGI Low Blood Glucose Index, LI Lability Index, MAG Mean Absolute Glucose, MAGE Mean Amplitude of Glucose Excursions, MR modified release, a.u. arbitrary units
At 3-month follow-up, on treatment with gliclazide MR 60 mg/day and dapagliflozin 10 mg/day, the level of HbA1c was 6.0%, and 24-h urinary glucose excretion was 719 mmol/day (~40 g/day). No presence of ketones in the urine was detected. Self-monitored fasting glucose remained in the range of 4.3–6.2 mmol/L, no episode of hypoglycemia was recorded. The body weight has decreased by 4 kg.
Written informed consent was obtained from the patient prior to any medical procedures. Additional informed consent was obtained for identifying information included in this article.
Discussion
The presented case demonstrates the clinical features of diabetes associated with a rare mutation in the ABCC8 gene encoding SUR1 (MODY subtype 12). Initially, disease was misclassified with type 1 diabetes, despite the absence of ketonuria and antipancreatic antibodies. The possibility of considering the case as MODY was supported by the presence of diabetes within four generations, by stable levels of C-peptide, and by obvious signs of overdose on relatively small doses of insulin in the second year of the clinical course of the disease.
Genetic testing revealed a mutation in the ABCC8 gene leading to a substitution of alanine with threonine in the protein encoded. This protein is the second subunit of ATP-sensitive K+ channels in pancreatic β-cells, or SUR1. It is known that closure of ATP-sensitive K+ channels is necessary for glucose-stimulated secretion of insulin by β-cells, whereas the opening of these channels inhibits the insulin secretion. Gene ABCC8 in humans is located on chromosome 11. Mutations in ABCC8 are associated with MODY, type 2 diabetes and gestational diabetes. Some mutations in ABCC8 cause hyperinsulinemia in newborns [16, 17]. Besides, some ABCC8 mutations were revealed in subjects with neonatal diabetes [18–20]. Recently a mutation in the ABCC8 gene within three generations of one family was reported; the phenotype resembles a deficiency of GCK (MODY2), with “mild” hyperglycemia that does not require pharmacotherapy [21]. It was demonstrated that ABCC8 mutations cause variable clinical phenotypes with glucose intolerance, overt diabetes, or insulin-requiring diabetes from a young age to adulthood. Several factors may explain this variability, also seen within families, such as the type and location of the mutation itself or other modifier genetic and superimposed environmental factors [22]. In our patient, we observed the relatively late clinical manifestation of diabetes (at age of 27 years) with a dramatic increase in glycemia to high levels. Surely, the latent course of the disease prior to clinical manifestation cannot be ruled out. In proband’s mother, mild hyperglycemia was corrected successfully for a long time by diet alone. These observations indicate heterogeneity of genotype–phenotype correlations in ABCC8-associated diabetes.
The prognosis of complications in this subtype of MODY is unknown. In the observed subject, the appearance of microvascular complications and, especially, rapid progression of retinopathy within 2 years of clinical course of the disease, should be noted. The combination of hyperglycemia, dyslipidemia, arterial hypertension and, possibly, genetic features, could be responsible for the early manifestation of complications. Since the early signs of retinopathy and nephropathy were prior to insulin treatment, we hardly believe that the emergence of microvascular complications could be attributed to hypoglycemic events. Nonetheless, the role of recurrent hypoglycemia in progression of retinopathy and diabetic macular edema development could not be excluded. The presence of arterial hypertension, dyslipidemia, hyperuricemia, and subclinical carotid atherosclerosis may indicate a higher cardiovascular risk.
Neurological disorders, including epilepsy and atrophic loci in the periventricular white matter revealed by MRI, should also be mentioned. It is a matter of debate whether these changes resulted from a vascular or neurodegenerative mechanism, or if they are residual signs of prenatal brain impairment. At the same time, a mechanism specific to the underlying genetic defect could also be suspected. The SUR1-regulated ATP-sensitive K+ channels are not constitutively expressed in the brain, but could be transcriptionally upregulated in many forms of central nervous system pathology, including cerebral ischemia, traumatic brain injury, and subarachnoid hemorrhage. The channel is linked to microvascular dysfunction, edema formation, and accidental necrotic cell death in the brain [23]. Developmental delay, epilepsy and neonatal diabetes syndrome represents the most severe clinical form of permanent neonatal diabetes caused by ABCC8 mutations [18]. A milder clinical picture, without generalized epilepsy and with less severe developmental delay, was also described [20]. Mild atrophy of frontal–temporal regions of the brain revealed by MRI was reported in a 5-month-old boy with monoallelic missense mutation (c.142A>T) in ABCC8 gene [18]. In our patient the sings of brain impairment emerged in the early neonatal period, while diabetes manifested in adulthood. Further observations are needed to assess the dynamics of neurological disorders.
Identification of a MODY subtype is crucial for the choice of adequate treatment. In the present case verification of the diagnosis by genetic testing enabled the discontinuation of insulin therapy, which had produced obvious adverse effects, such as hypoglycemia and an increase in body weight. It was demonstrated that impaired insulin secretion in response to glucose is a major metabolic feature in adult ABCC8 mutation carriers [22]. Diabetic patients with mutations in ABCC8 are usually responsive to treatment with sulfonylurea [18, 22]. In these patients, switching from insulin to sulfonylurea leads to the improvement of metabolic control [19]. Interestingly, that sulfonylurea in neonatal diabetes secondary to mutations in potassium-channel subunits produces measurable improvements in neuropsychomotor impairments, which are greater in younger patients [19, 24]. Furthermore, it was speculated that rather than a drug that targets both SUR1 and SUR2 isoforms, such as glibenclamide, a SUR1-specific drug, such as gliclazide, might influence neurological symptoms more effectively as only SUR1 isoforms are present in neuronal K ATP channels [18]. In our case, pilot treatment with gliclazide MR at an average therapeutic dose turned out to be effective, but was characterized by large glucose fluctuations and increased GV. A growing body of evidence suggests that high GV is a risk factor of diabetic vascular complications [15, 25]. Taking into account the rapid development of vascular damage in this patient, it seemed essential to prevent the episodes of both hypoglycemia and hyperglycemia and, thereby, to reduce GV. It was demonstrated recently that SGLT2 inhibitors decrease GV in patients with type 2 diabetes [26] and in type 1 diabetes [27]. We observed attenuation of glucose excursions and reduction of GV parameters on combined treatment with gliclazide MR and dapagliflozin in our patient with MODY.
Data on the use of SGLT2 inhibitors for the treatment of MODY are scarce. It was reported recently that dapagliflozin seems to be less efficient in MODY3 (HNF1A mutations) than in MODY2 (GCK mutations), that can be attributed to impaired SGLT2 function [28]. To the best of our knowledge, we provide here the first description of treatment with dapagliflozin in diabetes caused by mutation in the ABCC8 gene. The insulin-independent mechanism of action of SGLT2 inhibitors makes these agents a rather promising option for the treatment of patients with genetically mediated dysfunction of β-cells, who are not prone to ketoacidosis, especially when sulfonylurea effect is inappropriate.
Conclusion
The combination of MODY with prenatal brain impairment and epilepsy could be a phenotypic expression of a mutation in the ABCC8 gene, encoding SUR1. The combined therapy of sulfonylurea and SGLT2 inhibitors could be considered as a possible treatment option for patients with ABCC8-associated diabetes manifested at adulthood. Personalized diagnostics and treatment is essential for young subjects with non-classical diabetic forms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Genetic testing and publication costs were covered by an RSF grant (14-15-00496). All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
A. K. Ovsyannikova, O. D. Rymar, E. V. Shakhtshneider, V. V. Klimontov, E. A. Koroleva, N. E. Myakina and M. I. Voevoda have nothing to disclose.
Compliance with Ethics Guidelines
Written informed consent was obtained from the patient prior to any medical procedures. Additional informed consent was obtained for identifying information included in this article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6DE4F0606D766C11.
References
- 1.Lachanse CH. Practical aspects of monogenic diabetes: a clinical point of view. Can J Diabetes. 2016 doi: 10.1016/j.jcjd.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Cabezas O, Hattersley AT, Njolstad PR, Mlynarski W, Ellard S, White N, et al. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2014;15(20):47–64. doi: 10.1111/pedi.12192. [DOI] [PubMed] [Google Scholar]
- 3.Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34(8):1878–1884. doi: 10.2337/dc11-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanik J, Dusatkova P, Cinek O, Valentinova L, Huckova M, Skopkova M, et al. De novo mutations of GCK, HNF1a and HNF 4a may be more frequent in MODY than previously assumed. Diabetologia. 2014;57:480–484. doi: 10.1007/s00125-013-3119-2. [DOI] [PubMed] [Google Scholar]
- 5.Voevoda MI, Ivanova AA, Shakhtshneider EV, et al. Molecular genetics of maturity-onset diabetes of the young. Ther Arch. 2016;88(4):117–124. doi: 10.17116/terarkh2016884117-124. [DOI] [PubMed] [Google Scholar]
- 6.Pihoker C, Gilliam MK, Ellard S, Dabelea D, Davis C, Dolan LM, et al. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1a, HNF4a, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab. 2013;98:4055–4062. doi: 10.1210/jc.2013-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald TJ, Ellard S. Maturity onset diabetes of the young: identification and diagnosis. Ann Clin Biochem. 2013;50(Pt 5):403–415. doi: 10.1177/0004563213483458. [DOI] [PubMed] [Google Scholar]
- 8.Bonnefond A, Philippe J, Durand E, Dechaume A, Huyvaert M, Montagne L, et al. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. PLoS One. 2012;7(6):e37423. doi: 10.1371/journal.pone.0037423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delvecchio M, Ludovico O, Menzaghi C, Di Paola R, Zelante L, Marucci A, et al. Low prevalence of HNF1A mutations after molecular screening of multiple MODY genes in 58 Italian families recruited in the pediatric or adult diabetes clinic from a single italian hospital. Diabetes Care. 2014;37:e258–e260. doi: 10.2337/dc14-1788. [DOI] [PubMed] [Google Scholar]
- 10.Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY) BMJ. 2011;343:d6044. doi: 10.1136/bmj.d6044. [DOI] [PubMed] [Google Scholar]
- 11.Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504–2508. doi: 10.1007/s00125-010-1799-4. [DOI] [PubMed] [Google Scholar]
- 12.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol The. 2011;13(9):921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill NR, Nick SO, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Service FJ. Glucose variability. Diabetes. 2013;62(5):1398–1404. doi: 10.2337/db12-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klimontov VV, Yakima NE. Glycaemic variability in diabetes: a tool for assessing the quality of glycaemic control and the risk of complications. Diabetes Mellitus. 2014;17(2):76–82. doi: 10.14341/DM2014276-82. [DOI] [Google Scholar]
- 16.Haghverdizadeh P, Sadat Haerian M, Haghverdizadeh P, Sadat Haerian B. ABCC8 genetic variants and risk of diabetes mellitus. Gene. 2014;545(2):198–204. doi: 10.1016/j.gene.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Baier LJ, Muller YL, Remedi MS, Traurig M, Piaggi P, Wiessner G, et al. ABCC8 R1420H loss-of-function variant in a Southwest American Indian community: association with increased birth weight and doubled risk of type 2 diabetes. Diabetes. 2015;64(12):4322–4332. doi: 10.2337/db15-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwaveling-Soonawala N, Hagebeuk EE, Slingerland AS, Ris-Stalpers C, Vulsma T, van Trotsenburg AS. Successful transfer to sulfonylurea therapy in an infant with developmental delay, epilepsy and neonatal diabetes (DEND) syndrome and a novel ABCC8 gene mutation. Diabetologia. 2011;54(2):469–471. doi: 10.1007/s00125-010-1981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanciullo L, Iovane B, Gkliati D, Monti G, Sponzilli I, Cangelosi AM, et al. Sulfonylurea-responsive neonatal diabetes mellitus diagnosed through molecular genetics in two children and in one adult after a long period of insulin treatment. Acta Biomed. 2012;83(1):56–61. [PubMed] [Google Scholar]
- 20.Busiah K, Drunat S, Vaivre-Douret L, Bonnefond A, Simon A, Flechtner I, et al. Neuropsychological dysfunction and developmental defects associated with genetic changes in infants with neonatal diabetes mellitus: a prospective cohort study. Lancet Diabetes Endocrinol. 2013;1(3):199–207. doi: 10.1016/S2213-8587(13)70059-7. [DOI] [PubMed] [Google Scholar]
- 21.Gonsorcikova L, Vaxillaire M, Pruhova S, Dechaume A, Dusatkova P, Cinek O, et al. Familial mild hyperglycemia associated with a novel ABCC8-V84I mutation within three generations. Pediatr Diabetes. 2011;12(3 Pt 2):266–269. doi: 10.1111/j.1399-5448.2010.00719.x. [DOI] [PubMed] [Google Scholar]
- 22.Riveline JP, Rousseau E, Reznik Y, Fetita S, Philippe J, Dechaume A, et al. Clinical and metabolic features of adult-onset diabetes caused by ABCC8 mutations. Diabetes Care. 2012;35(2):248–251. doi: 10.2337/dc11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32(9):1699–1717. doi: 10.1038/jcbfm.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltrand J, Elie C, Busiah K, Fournier E, Boddaert N, Bahi-Buisson N, et al. Sulfonylurea therapy benefits neurological and psychomotor functions in patients with neonatal diabetes owing to potassium channel mutations. Diabetes Care. 2015;38(11):2033–2041. doi: 10.2337/dc15-0837. [DOI] [PubMed] [Google Scholar]
- 25.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–2369. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura R, Osonoi T, Kanada S, Jinnouchi H, Sugio K, Omiya H, et al. Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, crossover study. Diabetes Obes Metab. 2015;17(8):800–804. doi: 10.1111/dom.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38(3):412–419. doi: 10.2337/dc13-2955. [DOI] [PubMed] [Google Scholar]
- 28.Hohendorff J, Szopa M, Skupien J, Klupa T, Malecki MT. Response to SGLT2 inhibitor may be altered in HNF1A-MODY. Diabetes. 2016;65(S.1):A308–A308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.