Abstract
Aims
TIMI risk score and GRACE risk model are widely available and accepted scores for risk assessment in STEMI patients and include predictors of poor outcomes. CHA2DS2-VASc is a validated score for predicting embolic/stroke risk in patients with non-valvular atrial fibrillation. Its components contribute to the worse prognosis following myocardial infarction. The advantage of the CHA2DS2-VASc score in comparison with other risk scores is that it provides a comprehensive, fast, and simple method for physicians in risk evaluation that requires no calculators or computers. Therefore, we have set out to examine the prognostic significance of CHA2DS2-VASc score following STEMI in diabetic patients without AF.
Methods
A total of 472 patients with diabetes mellitus and STEMI undergoing primary PCI were enrolled. Based on the estimated CHA2DS2-VASc score, the study population was divided into three groups: group 1 (N = 111) with a moderate CHA2DS2-VASc score of 2 or 3; group 2 (N = 257) with a high CHA2DS2-VASc score of 4 or 5; and group 3 (N = 104) with a very high CHA2DS2-VASc score of 6 or higher.
Results
In diabetic patients with STEMI, the median of CHA2DS2-VASc score was 4 (interquartile range 3–5). In-hospital mortality rate was similar across three groups. CHA2DS2-VASc score was not a risk factor of in-hospital mortality. ROC analysis revealed good diagnostic value of CHA2DS2-VASc score in predicting long-term mortality (AUC 0.62 95 % CI 0.57–0.66 P = 0.0003) and stroke (AUC 0.75 95 % CI 0.71–0.79 P = 0.0003), but no value in predicting long-term myocardial infarction. CHA2DS2-VASc score was an independent predictor of 12-month mortality and stroke. One-point increment in CHA2DS2-VASc score was associated with an increase in the risk of 12-month death by 24 % and for 12-month stroke by 101 %.
Conclusions
In diabetic patients with STEMI and no previous AF, median CHA2DS2-VASc score was high (4 points) and predicted 12-month death and stroke. However, it failed to predict in-hospital death and 12-month MI. CHA2DS2-VASc score had a similar discrimination performance in predicting 12-month mortality as TIMI risk score and a better discrimination performance in predicting 12-month stroke than TIMI risk score. Thus, it can serve as an additive tool in identifying high-risk patients that require aggressive management.
Keywords: STEMI, Diabetes mellitus, Risk score, CHA2DS2-VASc, Prognosis
Introduction
Outcomes following ST-segment elevation myocardial infarction (STEMI) have improved over the past two decades. Nevertheless, survivors of STEMI have an elevated risk of subsequent vascular events, including stroke and 12-month mortality approaches 10 % [1]. Risk stratification following STEMI plays an essential role in managing patients and is recommended prior to discharge by the current guidelines. TIMI risk score and GRACE risk model are widely available and accepted scores for risk assessment and include predictors of poor outcomes which were established in large databases of MI patients [2–4]. The algorithms aid clinicians in assessing prognosis and may therefore be useful in guiding management. These two models are both fitted for predicting short- and long-term prognosis and assess the risk in a two-step process which includes: (1) stratifying patients at admission based on demographics, physical examination, presenting signs, and initial laboratory and angiographic data, and (2) identifying long-term risk based on the development of post-event complications. These scores were designed to be simple and practical, although they comprise numerous variables.
Evidence suggests that diabetes mellitus (DM) is among the most important risk factors of poor outcome following STEMI. DM is one of the components of the CHA2DS2-VASc score. Such a high incidence of thromboembolic events observed in these clinical subsets may be attributable to the DM-related prothrombotic state [5]. Generally, the diagnosis of DM is pivotal to assess cardiovascular risk and to guide therapy and lifestyle modification. Particularly, this topic is especially relevant for those patients who have already experienced a major cardiovascular event [6]. In addition, the presence of coronary artery disease is a prognostic factor in DM complications [7, 8]. Accurate risk assessment together with regular follow-up may result in a lower glycemic burden and a lower rate of vascular complications [9].
CHADS2 and the more recent CHA2DS2-VASc are two validated scores for predicting embolic/stroke risk in patients with non-valvular atrial fibrillation (AF) [10, 11]. They aid us in guiding antithrombotic therapy in AF patients. The individual score components not only predict AF-associated stroke risk but also are linked to the development of AF [12–14]. The CHADS2 and CHA2DS2-VASc scores have been reported to identify post-STEMI patients at high risk of AF and ischemic stroke [14].
In addition to predicting the risk of stroke/embolic events in AF patients, CHA2DS2-VASc score components (i.e., increasing age, hypertension, diabetes mellitus, prior cardiovascular events) are traditional risk factors that are associated with atherosclerosis, coronary artery disease, and contribute to the worse prognosis following myocardial infarction (MI). However, only a few studies have examined the prognostic value of CHA2DS2-VASc following MI [15, 16], and no studies have been found to evaluate CHA2DS2-VASc among STEMI or diabetic populations exclusively.
The advantage of the CHA2DS2-VASc score in comparison with other risk scores is that it provides a comprehensive, fast, and simple method for physicians in risk evaluation that requires no calculators or computers. Therefore, we have set out to examine the prognostic significance of CHA2DS2-VASc score following STEMI in diabetic patients. In the interest of averting a possible effect of AF on the outcomes, we have performed the analysis in a population of patients without AF.
Materials and methods
The study conforms to the Declaration of Helsinki. Informed consent for data analysis was obtained from the patients according to the Polish law on patients’ rights regarding data registration. Approval for analyzing recorded data was waived by the local bioethics committee on human research given the retrospective nature of the study. Patients admitted with diagnosis of STEMI, within 12 h from symptom onset were enrolled in the study. Patients with a history of or newly diagnosed AF were excluded. This is a single-center, cross-sectional, retrospective study.
A total of 472 patients with diabetes mellitus and STEMI undergoing primary PCI were enrolled. Based on the estimated CHA2DS2-VASc score, the study population was divided into three groups: group 1 (N = 111) with a moderate CHA2DS2-VASc score of 2 or 3; group 2 (N = 257) with a high CHA2DS2-VASc score of 4 or 5; and group 3 (N = 104) with a very high CHA2DS2-VASc score of 6 or higher.
The components of CHA2DS2-VASc score include: congestive heart failure (1 point), hypertension (1 point), age >75 years (2 points), diabetes mellitus (1 point), history of stroke (2 points), history of vascular disease (1 point), age >65 years (1 point), and female sex (1 point) [11]. The history of myocardial infarction was regarded as ‘vascular disease’, and the current STEMI was counted as 1 point. As the study investigated only diabetic patients, all patients have received a minimum score of 2 points.
All patients received loading doses of antiplatelet medications (aspirin, clopidogrel) before admission to our hospital (either in the referring hospital or ambulance) according to the guidelines. Diabetes mellitus was defined as: (a) preexisting condition diagnosed before STEMI (patients on insulin, oral glucose-lowering drugs, or on a diet) and (b) newly diagnosed diabetes mellitus based on fasting plasma glucose (FPG) ≥7.0 mmol/L or 2-h plasma glucose ≥11.1 mmol/L during an oral glucose tolerance test (OGTT) [17]. To avoid acute hyperglycemia, FPG was taken into consideration after the third day of hospital stay. For that reason, OGTT was performed on day four of hospital stay or later. STEMI was defined as: (1) ST-segment elevation consistent with MI of at least 2 mm in contiguous precordial leads and/or ST-segment elevation of at least 1 mm in two or more limb leads or new left bundle branch block, and (2) positive cardiac necrosis markers: CK-MB mass (upper limit of normal: 4.9 mg/mL) and/or troponin T (upper limit of normal: 0.014 ng/mL). Patients received 300 mg of acetylsalicylic acid (ASA) loading dose and 600 mg of clopidogrel loading dose, followed by 75 mg of ASA maintenance dose and 75 mg of clopidogrel maintenance dose [18]. Coronary angiography and percutaneous coronary interventions were performed using standard protocols and guidelines. A culprit lesion was described in the presence of an acute occlusion, intraluminal filling defects (or thrombus), ulcerated plaques, dissection, or intraluminal flaps. Successful PCI was defined as a post-procedural residual-diameter stenosis <30 %, with TIMI 3 flow in the infarct-related artery and no procedural complications.
All patients were scheduled for an elective 12-month clinical follow-up. We clinically monitored the patients for cardiovascular events. The major adverse cardiac and cerebrovascular events (MACCEs) included death, rehospitalization for myocardial infarction, and stroke.
Statistical analysis
Quantitative data are presented as means ± standard deviations (SDs) or medians with interquartile ranges (lower and upper quartiles). Qualitative data are presented as frequencies. The Shapiro–Wilk test was used to determine whether random samples came from a normal distribution. The Chi-square test with Yates’ correction was used to compare categorical variables. One-way analysis of variance (ANOVA) and Kruskal–Wallis ANOVA tests were used to compare continuous variables between groups for variables normally and not normally distributed, respectively. In-hospital and one-year survival was estimated with the Kaplan–Meier method and compared with the log-rank test. The relationship between CHA2DS2-VASc score and clinical variables was evaluated by Spearman’s rank correlation coefficient. Receiver-operating characteristic (ROC) curves were estimated for CHA2DS2-VASc score. ROC analysis was used to determine the cutoff values of CHA2DS2-VASc score to predict in-hospital and 12-month mortality, myocardial infarction, and stroke. The effects of the clinical and angiographic variables on the in-hospital and 12-month mortality were assessed using the multivariate Cox proportional hazard regression models with the results expressed as hazard ratios (HRs) and 95 % confidence intervals (CIs). Variables with a significant influence on mortality in univariate analysis were entered into the multivariate model. These included: history of myocardial infarction, left ventricular ejection fraction (per 1 % increment), success of PCI (final TIMI 3 flow) in the culprit vessel, cardiogenic shock, and time form symptom onset (per 1-h increment). Variables with a significant influence on 12-month stroke in univariate analysis were entered into the multivariate model. These included: history of myocardial infarction, anterior myocardial infarction on admission, and left ventricular ejection fraction (per 1 % increment).
A value of P < 0.05 was considered significant.
Results
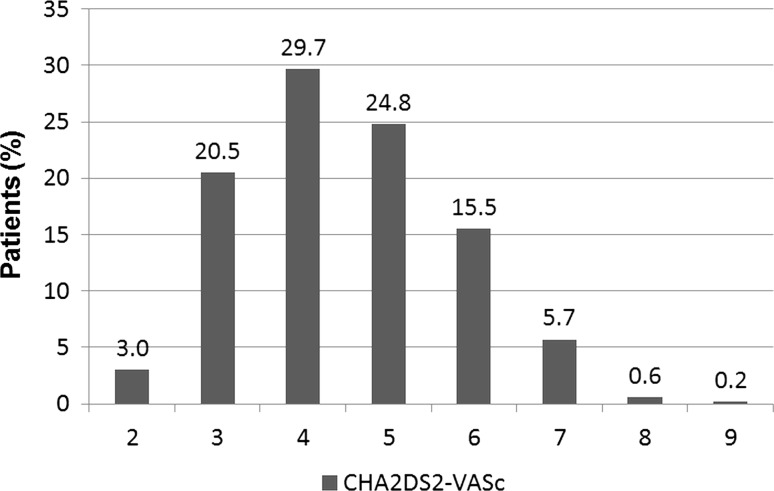
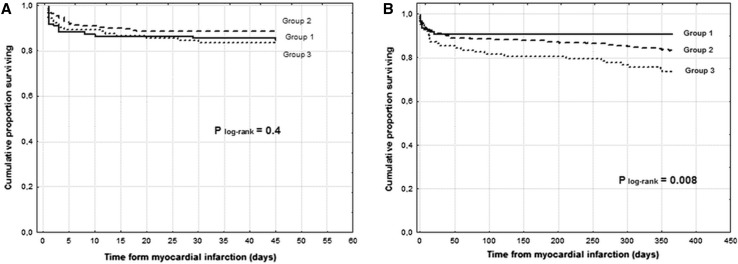
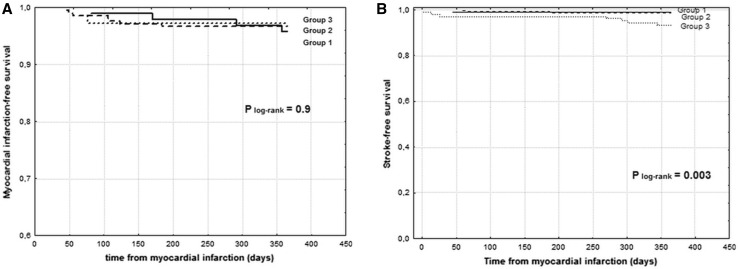
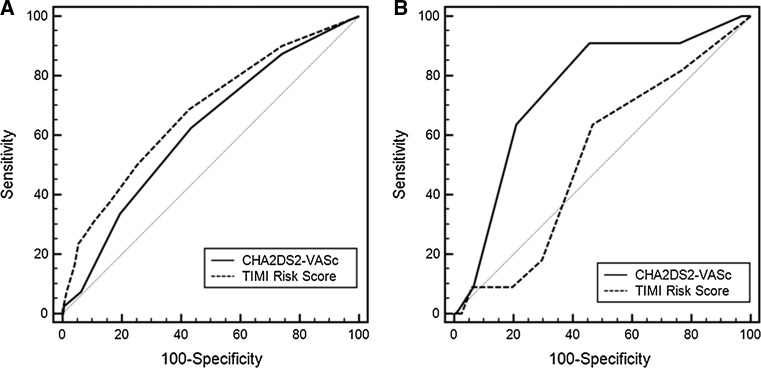
In diabetic patients with STEMI, the median of CHA2DS2-VASc score was 4 (interquartile range 3–5). The number of patients falling in each CHA2DS2-VASc score category is depicted in Fig. 1. Baseline clinical characteristics are featured in Table 1. Patients with moderate CHA2DS2-VASc score (group 1) were younger and more frequently men. Hypertension and a lower ejection fraction were more prevalent among patients with a high and very high CHA2DS2-VASc score (groups 2 and 3). Angiographic data are presented in Table 2. There was a trend toward a worse initial TIMI flow in group 3. Initial TIMI 3 flow was the least prevalent in group 3. Notwithstanding, final TIMI flow was similar in all three groups. ROC analysis demonstrated no value of CHA2DS2-VASc score in predicting in-hospital mortality AUC 0,503 (95 % CI 0.463–0.555) P = 0.83. In-hospital mortality rate was similar across three groups (Fig. 2a). CHA2DS2-VASc score was not a risk factor of in-hospital mortality (Table 3). During 12-month follow-up, there were 10 deaths (9.0 %) in group 1, 43 deaths (16.7 %) in group 2, and 27 deaths (16.2 %) in group 3 (P = 0.003) (Table 3; Fig. 2b). The rate of non-fatal myocardial infarction was similar across all groups, and the rate of stroke was higher in group 3 (Table 4; Fig. 3a, b). ROC analysis revealed good diagnostic value of CHA2DS2-VASc score in predicting 12-month mortality and stroke, but no value in predicting 12-month myocardial infarction (Tables 5, 6; Fig. 4a, b). CHA2DS2-VASc score was an independent predictor of 12-month mortality and stroke. One-point increment in CHA2DS2-VASc score was associated with an increase in the risk of 12-month death by 24 % and for 12-month stroke by 101 %. CHA2DS2-VASc score was positively correlated with age (Spearman R = 0.67 P < 0.0001) and TIMI risk score (Spearman R = 0.40 P < 0.0001), and negatively correlated with ejection fraction (Spearman R = 0.28 P < 0.0001), time to stroke during follow-up (Spearman R = −0.13 P = 0.003), and time to death during follow-up (Spearman R = −0.15 P = 0.001).
Fig. 1.
Number of patients falling in each CHA2DS2-VASc score category
Table 1.
Patients’ baseline and clinical characteristics
| Group 1 N = 111 | Group 2 N = 257 | Group 3 N = 104 | P | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 57 ± 8† | 64 ± 8† | 73 ± 6† | 0.0001 |
| Sex, men, N (%) | 106 (95.5 %) | 150 (58.4 %) | 15 (14.4 %) | 0.0001 |
| Systemic hypertension, N (%) | 41 (36.9 %) | 198 (77.0 %) | 100 (96.1 %) | 0.0001 |
| Anterior myocardial infarction, N (%) | 34 (30.6 %) | 89 (34.6 %) | 36 (34.6 %) | 0.7 |
| Prior myocardial infarction, N (%) | 34 (30.6 %) | 78 (30.5 %) | 32 (30.8 %) | 0.9 |
| Time from symptom onset, hours [median (interquartile range)] | 5.0 (3.0–7.0) | 4.5 (3.0–7.0) | 4.5 (3.0–7.5) | 0.3 |
| Cardiogenic shock, N (%) | 15 (13.5 %) | 33 (12.8 %) | 16 (15.4 %) | 0.8 |
| LVEF, (%) [median (interquartile range)] | 50 (41–55)* | 40 (35–46)* | 40 (35–45)* | 0.0001 |
| Hospital stay, days [median (interquartile range)] | 8 (6–11) | 9 (6–12) | 8 (6–12) | 0.09 |
| TIMI risk score | 3 (2–4)§ | 3 (2–5)§ | 4 (3–6)§ | <0.0001 |
| HbA1c (%) [median (interquartile range)] | 7.6 (7.0–8.0) | 7.6 (6.9–8.0) | 7.5 (6.9–8.2) | 0.9 |
| Admission glycemia (mmol/l) [median (interquartile range)] | 8.1 (7.0–11.4) | 7.8 (6.8–10.2) | 8.0 (6.2–10.3) | 0.2 |
| Fasting plasma glucose (mmol/L) [median (interquartile range)] | 6.7 (5.3–7.8) | 6.8 (5.8–7.9) | 6.6 (5.2–8.3) | 0.6 |
| In-hospital death, N (%) | 17 (15.3 %) | 30 (11.7 %) | 17 (16.3 %) | 0.4 |
SD standard deviation, LVEF left ventricular ejection fraction
* Group 1 versus group 2 P < 0.001, group 1 versus group 3 P < 0.001, and group 2 versus group 3 P = 0.7
†Group 1 versus group 2 P < 0.0001, group 1 versus group 3 P < 0.0001, and group 2 versus group 3 P < 0.0001
§Group 1 versus group 2 P = 0.01, group 1 versus group 3 P < 0.0001, and group 2 versus group 3 P < 0.0001
Table 2.
Angiographic findings
| Group 1 N = 111 | Group 2 N = 257 | Group 3 N = 104 | P | |
|---|---|---|---|---|
| Multivessel CAD, N (%) | 49 (44.1 %) | 117 (45.5 %) | 47 (45.2 %) | 0.6 |
| Initial TIMI flow, N (%) | ||||
| 0 | 63 (56.8 %) | 150 (58.6 %) | 56 (53.8 %) | 0.08 |
| 1 | 18 (16.2 %) | 46 (17.9 %) | 18 (17.3 %) | |
| 2 | 14 (12.6 %) | 35 (13.7 %) | 25 (24.0 %) | |
| 3 | 16 (14.4 %) | 26 (10.1 %) | 5 (4.8 %) | |
| Initial TIMI 3 flow, N (%) | 16 (14.4 %) | 26 (10.1 %) | 5 (4.8 %) | 0.05 |
| Final TIMI flow, N (%) | ||||
| 0 | 5 (4.5 %) | 17 (6.6 %) | 8 (7.1 %) | 0.8 |
| 1 | 1 (0.9 %) | 3 (1.2 %) | 2 (1.9 %) | |
| 2 | 11 (10.0 %) | 19 (7.4 %) | 9 (8.7 %) | |
| 3 | 94 (84.6 %) | 218 (85.1 %) | 85 (81.3 %) | |
| Final TIMI 3 flow, N (%) | 94 (84.6 %) | 218 (85.1 %) | 85 (81.3 %) | 0.7 |
Fig. 2.
a Kaplan–Meier in-hospital survival curves. b Kaplan–Meier 12-month survival curves. Group 1 versus 2 P = 0.06; group 1 versus 3 P = 0.001; group 2 versus 3 P = 0.04
Table 3.
Predictors of in-hospital and 12-month mortality
| In-hospital mortality | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| HR | 95 % CI | P | HR | 95 % CI | P | |
| CHA2DS2-VASc score (per one-point increment) | 0.99 | 0.81–1.21 | 0.9 | – | – | – |
| Twelve-month mortality | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | |||||
| HR | 95 % CI | P | HR | 95 % CI | P | |
| CHA2DS2-VASc score (per one-point increment) | 1.31 | 1.11–1.54 | 0.001 | 1.24 | 1.06–1.45 | 0.008 |
| Twelve-month myocardial infarction | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| HR | 95 % CI | P | HR | 95 % CI | P | |
| CHA2DS2-VASc score (per one-point increment) | 0.78 | 0.52–1.16 | 0.22 | – | – | – |
| Twelve-month stroke | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted** | |||||
| HR | 95 % CI | P | HR | 95 % CI | P | |
| CHA2DS2-VASc score (per one-point increment) | 1.81 | 1.18–2.79 | 0.006 | 2.01 | 1.25–3.23 | 0.004 |
HR hazard ratio, CI confidence interval
* Adjusted for: history of myocardial infarction, left ventricular ejection fraction (per 1 % increment), successful percutaneous coronary intervention (final TIMI 3 flow) in the culprit vessel, cardiogenic shock, time form symptom onset (per 1-h increment), platelet distribution width (per 1fL increment), and platelet count (per 104/mm3 increment)
** Adjusted for: history of myocardial infarction, left ventricular ejection fraction (per 1 % increment), anterior myocardial infarction during index hospitalization
Table 4.
12-month follow-up
| Group 1 N = 111 | Group 2 N = 257 | Group 3 N = 104 | P | |
|---|---|---|---|---|
| All-cause mortality, N (%) | 10 (9.0 %) | 43 (16.7 %) | 27 (16.2 %) | 0.003 |
| Non-fatal myocardial infarction, N (%) | 5 (4.5 %) | 9 (3.5 %) | 3 (2.9 %) | 0.7 |
| Stroke, N (%) | 0 (0 %) | 3 (1.1 %) | 7 (6.7 %) | 0.004 |
Fig. 3.
a Kaplan–Meier 12-month myocardial infarction-free survival curves. b Kaplan–Meier 12-month stroke-free survival curves. Group 1 versus 2 P = 0.8; group 1 versus 3 P = 0.02; group 2 versus 3 P = 0.03
Table 5.
Receiver-operating characteristics curves identifying the discrimination thresholds of CHA2DS2-VASc score for predicting 12-month mortality, non-fatal myocardial infarction, and stroke
| Cut off | AUC | 95 % CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | P | |
|---|---|---|---|---|---|---|---|---|
| All-cause mortality | >4 | 0.62 | 0.57–0.66 | 62 | 56 | 23 | 88 | 0.0003 |
| Myocardial infarction | 0.58 | 0.45–0.62 | 0.3 | |||||
| Stroke | >4 | 0.75 | 0.71–0.79 | 91 | 54 | 5 | 99 | 0.0003 |
Table 6.
Comparison of the receiver-operating characteristics curves identifying the discrimination thresholds of CHA2DS2-VASc score and TIMI risk score for long-term hospital mortality and stroke
| Cut off | AUC | 95 % CI | |
|---|---|---|---|
| Twelve-month mortality | |||
| CHA2DS2-VASc score | >4 | 0.67 | 0.63–0.71 |
| TIMI risk score | >4 | 0.60 | 0.56–0.65 |
| Difference between areas CHA2DS2-VASc score and TIMI risk score | 0.06 | ||
| 95 % CI | −0.03–0.15 | ||
| P | 0.18 | ||
| Twelve-month stroke | |||
| CHA2DS2-VASc score | >4 | 0.75 | 0.71–0.79 |
| TIMI risk score | >4 | 0.52 | 0.48–0.57 |
| Difference between areas CHA2DS2-VASc score and TIMI risk score | 0.22 | ||
| 95 % CI | 0.06–0.38 | ||
| P | 0.006 | ||
Fig. 4.
Receiver-operating characteristic (ROC) curves for the discrimination performance of CHA2DS2-VASc score and TIMI risk score for prediction of 12-month mortality (a) and 12-month stroke (b)
Discussion
In the current study, we have set out to investigate the utility of CHA2DS2-VASc score in predicting poor outcomes in patients with diabetes mellitus and STEMI. In particular, we have sought to determine whether CHA2DS2-VASc score commonly used for prediction of thromboembolic events in AF patients can be used as a risk assessment tool in STEMI patients without AF.
There are several major findings of the study. First and foremost, CHA2DS2-VASc score was high across the entire study population with a median of 4 points. Second, it had no influence on the in-hospital mortality. The third major finding was that CHA2DS2-VASc score, both unadjusted and adjusted for potential cofounders, correlated with 12-month stroke and all-cause mortality, but it had no influence on 12-month myocardial infarction. CHA2DS2-VASc score showed similar predictive value with respect to 12-month mortality as TIMI risk score. More importantly, it demonstrated a better predictive value with respect to 12-month stroke than TIMI risk score. And finally, CHA2DS2-VASc score showed a negative correlation with the time to subsequent stroke or death during follow-up.
CHADS2 score was developed to identify AF patients at risk for stroke or thromboembolic event and thus to guide anticoagulation therapy [10]. However, patients with an intermediate risk (CHADS2 score 1) presented a challenge in everyday practice because some of them were at low–intermediate risk and others were at high–intermediate risk. Consequently, CHA2DS2-VASc score was introduced to improve predictive value for thromboembolic events in patients at low and intermediate risk [11]. The utility of the score goes beyond the benefits of risk stratification for thromboembolic events. Apiyasawat et al. [19] reported it to be an independent prognostic marker of mortality following hospitalization for AF. All elements of the CHA2DS2-VASc score are essential risk factors in cardiovascular disease. Hypertension, diabetes mellitus, and congestive heart failure were all found to be predictors of poor outcomes following acute MI [20].
As pointed out in the introduction to this paper, there is little published reports on the utility of CHA2DS2-VASc score in risk stratification following acute MI. Poci et al. [21] analyzed 2335 patients with acute coronary syndromes and reported that CHADS2 score was associated with long-term mortality (HR 1.38 95 % CI 1.28–1.48 P < 0.0001) and stroke (HR 1.46 95 % CI 1.27–1.68 P < 0.0001). Kim et al. [15] performed a retrospective analysis of 15,681 patients with acute MI who were enrolled in the Korean Working Group in Acute Myocardial Infarction (KORMI) registry. They reported that CHA2DS2-VASc score was associated with long-term cardiac events (MI, all-cause death) (HR 1.384 P < 0.0001). Interestingly, they found that CHA2DS2-VASc score was a more important predictor in STEMI patients (HR 1.455 P < 0.0001) than in NSTEMI patients (HR 1.298 P = 0.048). Piccini et al. [22] proposed a modified R-CHA2DS2-VASc score by adding renal function parameters (glomerular filtration rate and urea), performance of a revascularization procedure, and history of atrial fibrillation. Based on that modified R-CHA2DS2-VASc score, a study showed a good calibration and high discriminative performance of that score in the prediction of post-discharge ischemic stroke and all-cause mortality [23]. Recently, Podolecki et al. [16] studied 2980 patients with acute coronary syndromes and no AF and reported that an increment of one point in the CHA2DS2-VASc score was independently associated with a 41 % increase in stroke risk and a 23 % increase in mortality rate (P < 0.001 for both). Our results agree with the aforementioned studies. We found that one-point increment in CHA2DS2-VASc score was associated with an increase in the risk of 12-month death by 24 % and for 12-month stroke by 101 %. The present study validates prior findings and refines their estimates among patients with diabetes mellitus. In addition, we demonstrated that the score was negatively correlated with the time to stroke and the time to death during follow-up. Furthermore, we demonstrated that CHA2DS2-VASc score yielded a moderately high negative predictive value (NPV) of 88 % for identifying patients at low risk of 12-month death and a high NPV of 99 % for identifying patients at low risk for 12-month stroke. Similar observations were made by Melgaard et al. [24] in a population of patients with heart failure and no AF.
CHA2DS2-VASc score comprises a cluster of common cardiovascular risk factors associated with thromboembolism. It is possible it may identify underlying conditions that may lead to AF, stroke, or death during follow-up. It is reported to predict incident AF in patients without preexisting AF [25–27]. More importantly, CHA2DS2-VASc score is recognized to be related to mortality and ischemic stroke in patients without AF and stroke [28, 29], heart failure [24], acute coronary syndromes [25], undergoing CABG [30]. Interestingly, Podolecki et al. analyzing patients with acute coronary syndromes failed to show any discrimination performance of CHA2DS2-VASc score in predicting incident MI during follow-up [16]. This finding is in agreement with our observations. Moreover, cerebral atherosclerosis is reported to be more common in patients with higher CHADS2 score. Kim et al. [31] demonstrated that patients with higher CHADS2 score had more carotid (both extra- and intracranial) stenosis. This finding may, in turn, result in an elevated risk of atherothrombotic stroke even in the absence of AF.
Overall, CHA2DS2-VASc score seems to be a well-known, simple tool that (1) comprises clusters of conditions that are associated with poor outcomes, (2) may increase the risk of incident AF in patients without preexisting AF leading to thromboembolic stroke, and (3) may be associated with a more extensive atherosclerosis leading to atherothrombotic stroke. All of the aforementioned mechanisms indicate the role of CHA2DS2-VASc score in predicting an increased morbidity and mortality. Taken together, these findings further support the potential role of CHA2DS2-VASc score in identifying high-risk patients that may require more aggressive management strategies.
Strengths and limitations
We investigated a real-life population of diabetic patients with STEMI in which we did not exclude patients with severe comorbidities, including cardiogenic shock. These comorbidities may have predisposed some of the patients to a greater risk of stroke. Single-center design has also its shortcomings. We cannot exclude that some of the patients have had undiagnosed AF, as vascular disease (acute MI in this instance) is associated with a higher AF prevalence. Moreover, we lack data on the development of AF during follow-up, which may have resulted in the increased risk of stroke. In any way, we were able to demonstrate the role of CHA2DS2-VASc score in identifying patients at risk of increased 12-month mortality and stroke regardless the underlying mechanisms. Given the relatively low study size, however, these promising results should be verified on much larger cohorts.
Conclusions
In diabetic patients with STEMI and no previous AF, median CHA2DS2-VASc score was high (4 points) and predicted 12-month death and stroke. However, it failed to predict in-hospital death and 12-month MI. CHA2DS2-VASc score had a similar discrimination performance in predicting 12-month mortality as TIMI risk score and a better discrimination performance in predicting 12-month stroke than TIMI risk score. Thus, it can serve as an additive tool in identifying high-risk patients that require aggressive management.
Compliance with ethical standards
Funding
None.
Conflict of interest
The authors have no commercial associations or sources of support that might pose a conflict of interests.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study. Informed consent for data analysis was obtained from the patients according to the Polish law on patients’ rights regarding data registration. Approval for analyzing recorded data was waived by the local bioethics committee on human research given the retrospective nature of the study.
References
- 1.McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrow DA, Antman EM, Parsons L, et al. Application of the TIMI risk score for ST-elevation MI in the National Registry of Myocardial Infarction 3. JAMA. 2001;286:1356–1359. doi: 10.1001/jama.286.11.1356. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 4.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 5.Pomero F, Di Minno MN, Fenoglio L, Gianni M, Ageno W, Dentali F. Is diabetes a hypercoagulable state? A critical appraisal. Acta Diabetol. 2015;52:1007–1016. doi: 10.1007/s00592-015-0746-8. [DOI] [PubMed] [Google Scholar]
- 6.Luzi A, Rizza S, Cardellini M, et al. A1c value for diabetes diagnosis in subjects with established cardiovascular disease. Acta Diabetol. 2015;52:999–1001. doi: 10.1007/s00592-015-0729-9. [DOI] [PubMed] [Google Scholar]
- 7.Bergis D, Bergis PM, Hermanns N, Zink K, Haak T. Coronary artery disease as an independent predictor of survival in patients with type 2 diabetes and charcot neuro-osteoarthropathy. Acta Diabetol. 2014;51:1041–1048. doi: 10.1007/s00592-014-0669-9. [DOI] [PubMed] [Google Scholar]
- 8.Bjornstad P, Maahs DM, Rivard CJ, et al. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta Diabetol. 2014;51:783–791. doi: 10.1007/s00592-014-0611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anjana RM, Shanthirani CS, Unnikrishnan R, et al. Regularity of follow-up, glycemic burden, and risk of microvascular complications in patients with type 2 diabetes: a 9-year follow-up study. Acta Diabetol. 2015;52:601–609. doi: 10.1007/s00592-014-0701-0. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 11.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 12.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau KK, Chan PH, Yiu KH, et al. Roles of the CHADS2 and CHA2DS2-VASc scores in post-myocardial infarction patients: risk of new occurrence of atrial fibrillation and ischemic stroke. Cardiol J. 2014;21:474–483. doi: 10.5603/CJ.a2014.0034. [DOI] [PubMed] [Google Scholar]
- 15.Kim KH, Kim W, Hwang SH, Kang WY, Cho SC, Jeong MH. The CHA2DS2VASc score can be used to stratify the prognosis of acute myocardial infarction patients irrespective of presence of atrial fibrillation. J Cardiol. 2015;65:121–127. doi: 10.1016/j.jjcc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Podolecki T, Lenarczyk R, Kowalczyk J, et al. Stroke and death prediction with CHA2DS2-vasc score after myocardial infarction in patients without atrial fibrillation. J Cardiovasc Med (Hagerstown). 2015;16:497–502. doi: 10.2459/JCM.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 17.Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The task force on diabetes and cardiovascular diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehm124. [DOI] [PubMed] [Google Scholar]
- 18.Silber S, Albertsson P, Aviles FF, et al. Guidelines for percutaneous coronary interventions. The task force for percutaneous coronary interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804–847. doi: 10.1093/eurheartj/ehi564. [DOI] [PubMed] [Google Scholar]
- 19.Apiyasawat S, Tangcharoen T, Wisaratapong T, Yamwong S, Wiboonpolprasert S, Sritara P. CHA(2)DS(2)-VASc scores predict mortality after hospitalization for atrial fibrillation. Int J Cardiol. 2015;185:293–296. doi: 10.1016/j.ijcard.2015.03.180. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson F, Kober L, Torp-Pedersen C, Hildebrandt P, Ottesen MM, Sonne B, Carlsen J. Long-term prognosis after acute myocardial infarction in patients with a history of arterial hypertension. TRACE study group. Eur Heart J. 1998;19:588–594. doi: 10.1053/euhj.1997.0822. [DOI] [PubMed] [Google Scholar]
- 21.Poci D, Hartford M, Karlsson T, Herlitz J, Edvardsson N, Caidahl K. Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest. 2012;141:1431–1440. doi: 10.1378/chest.11-0435. [DOI] [PubMed] [Google Scholar]
- 22.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 23.Barra S, Almeida I, Caetano F, et al. Stroke prediction with an adjusted R-CHA2DS2VASc score in a cohort of patients with a Myocardial Infarction. Thromb Res. 2013;132:293–299. doi: 10.1016/j.thromres.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314:1030–1038. doi: 10.1001/jama.2015.10725. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell LB, Southern DA, Galbraith D, Ghali WA, Knudtson M, Wilton SB. Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart. 2014;100:1524–1530. doi: 10.1136/heartjnl-2013-305303. [DOI] [PubMed] [Google Scholar]
- 26.Seo WK, Kang SH, Jung JM, Choi JY, Oh K. Novel composite score to predict atrial fibrillation in acute stroke patients: AF predicting score in acute stroke. Int J Cardiol. 2016;209:184–189. doi: 10.1016/j.ijcard.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Saliba W, Gronich N, Barnett-Griness O, Rennert G. Usefulness of CHADS and CHADS-VASc scores in the prediction of new-onset atrial fibrillation: a population-based study. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Ntaios G, Lip GY, Makaritsis K, et al. CHADS(2), CHA(2)S(2)DS(2)-VASc, and long-term stroke outcome in patients without atrial fibrillation. Neurology. 2013;80:1009–1017. doi: 10.1212/WNL.0b013e318287281b. [DOI] [PubMed] [Google Scholar]
- 29.Saliba W, Gronich N, Barnett-Griness O, Rennert G. The role of CHADS and CHA DS -VASc scores in the prediction of stroke in individuals without atrial fibrillation: a population-based study. J Thromb Haemost. 2016;14:1155–1162. doi: 10.1111/jth.13324. [DOI] [PubMed] [Google Scholar]
- 30.Verdejo HBP, Zalaquett R, Heuete I, Corbalan R. CHA2DS2-VASc score predicts the occurrence of perioperative stroke in CABG patients without post-operative atrial fibrillation. Circulation. 2015;132:A18978. [Google Scholar]
- 31.Kim YD, Cha MJ, Kim J, et al. Increases in cerebral atherosclerosis according to CHADS2 scores in patients with stroke with nonvalvular atrial fibrillation. Stroke. 2011;42:930–934. doi: 10.1161/STROKEAHA.110.602987. [DOI] [PubMed] [Google Scholar]