Abstract
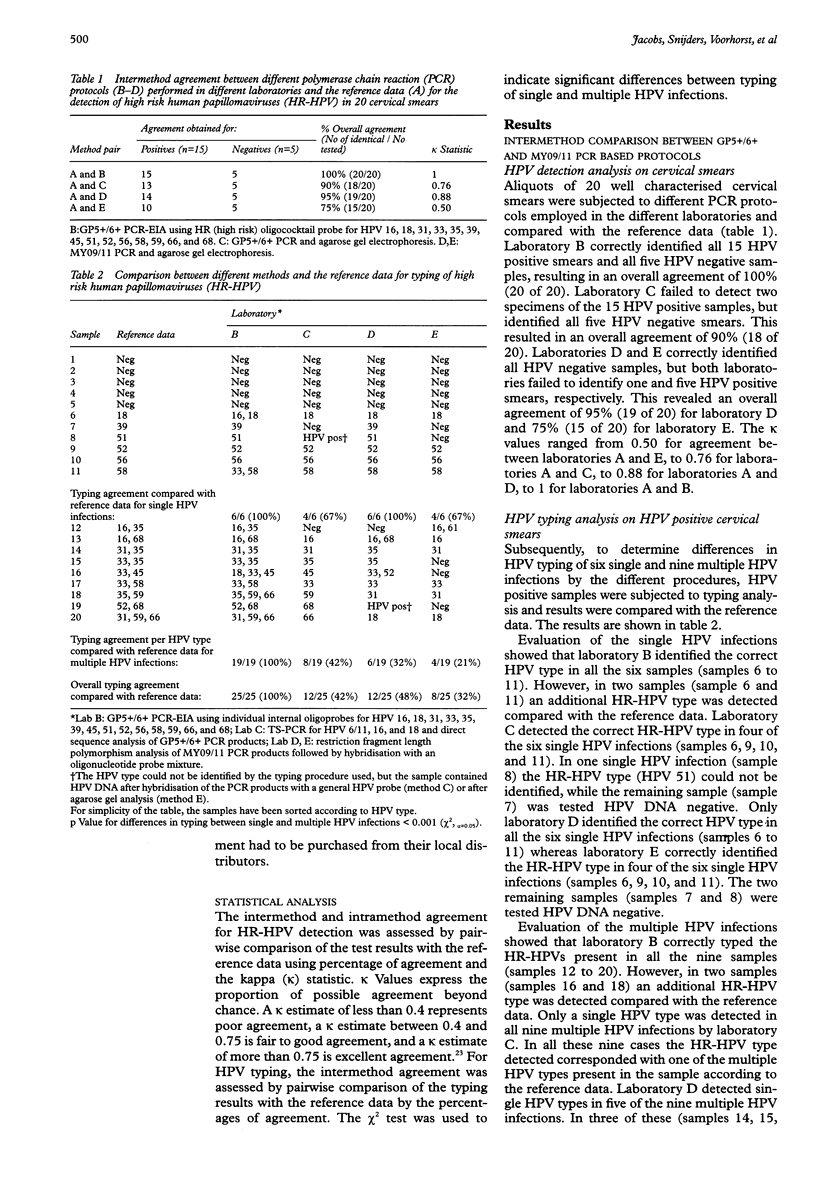
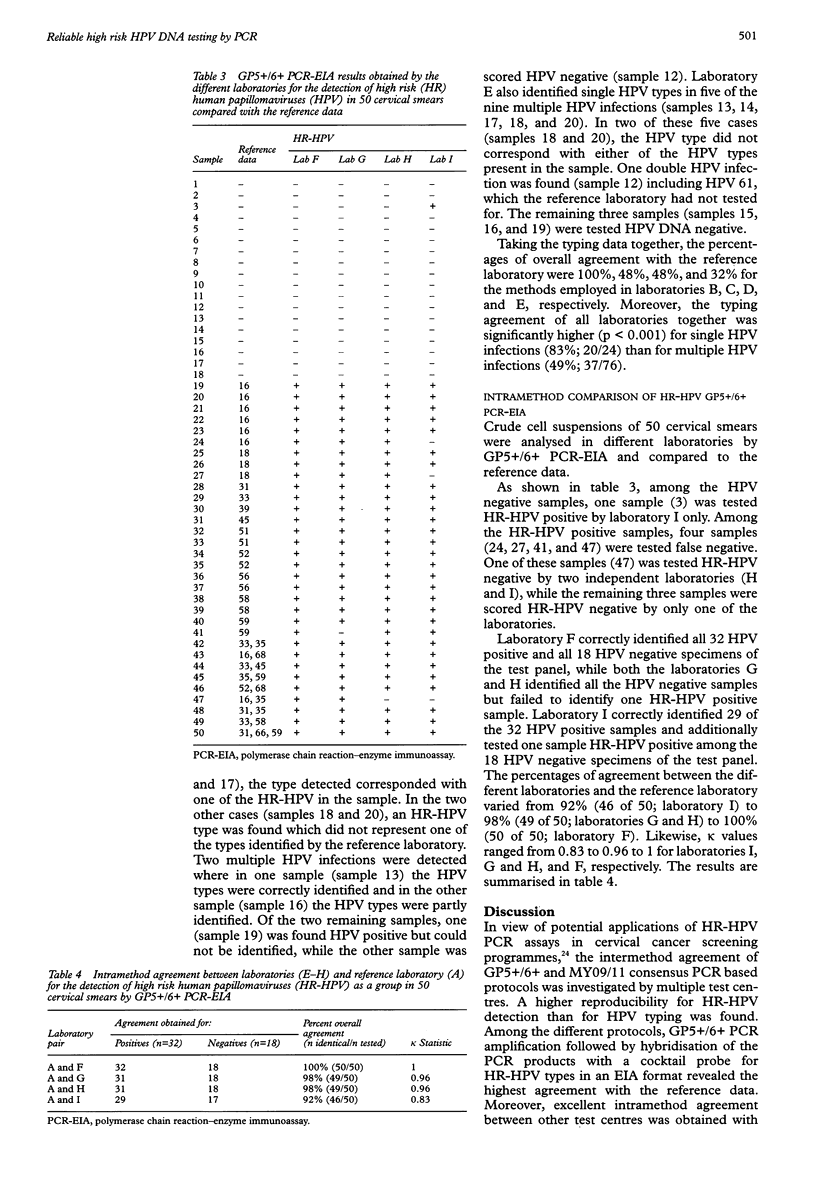
BACKGROUND: The development of a reproducible, sensitive, and standardised human papillomavirus (HPV) polymerase chain reaction (PCR) test is required to implement HPV testing in cervical cancer screening programmes and for triaging women with mild to moderate dysplasia. AIMS: To determine the intermethod agreement between different GP5+/6+ and MY09/11 PCR based protocols for the detection and typing of high risk (HR) HPV DNA in cervical smears and to assess the intramethod reproducibility of the GP5+/6+ PCR enzyme immunoassay (EIA) for HR-HPV detection. METHODS: For the intermethod comparison, crude aliquots of 20 well characterised cervical smears comprising five HPV negative samples, and six and nine samples containing single and multiple HPV infections, respectively, were coded and sent from reference laboratory (A) to three other laboratories. One of these (laboratory B) used the GP5+/6+ PCR-EIA and was provided with standard protocols. Another laboratory (C) used GP5+/6+ PCR combined with sequence analysis and type specific PCR, whereas two laboratories (D and E) used MY09/11 PCR followed by restriction fragment length polymorphism (RFLP) analysis for the detection and typing of HR-HPV. The intramethod agreement of GP5+/6+ PCR-EIA was analysed in a subsequent study with four other laboratories (F to I) on crude aliquots of 50 well characterised cervical smears, consisting of 32 HR-HPV positive and 18 HPV negative samples. Standardised protocols, primers, and probes were also provided by the reference laboratory for HR-HPV detection. RESULTS: In the intermethod comparison, pairwise agreement of the different laboratories with reference laboratory A for the detection of HR-HPV varied between 75% and 100% (kappa values: 0.5 to 1). Typing data revealed a broader range in pairwise agreement rates between 32% and 100%. The highest agreement was found between laboratories A and B using standardised protocols and validated reagents. In the intramethod evaluation, pairwise comparison of the laboratories F to I with reference laboratory A revealed excellent agreement rates from 92% to 100% (kappa values: 0.88 to 1.0) with an overall sensitivity of 97.5% (195/200) and specificity of 99.5% (199/200). CONCLUSIONS: The detection of HR-HPV as a group is highly reproducible with GP5+/6+ PCR-EIA provided that standardised protocols and validated reagents are used.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chichareon S., Herrero R., Muñoz N., Bosch F. X., Jacobs M. V., Deacon J., Santamaria M., Chongsuvivatwong V., Meijer C. J., Walboomers J. M. Risk factors for cervical cancer in Thailand: a case-control study. J Natl Cancer Inst. 1998 Jan 7;90(1):50–57. doi: 10.1093/jnci/90.1.50. [DOI] [PubMed] [Google Scholar]
- Cox J. T., Lorincz A. T., Schiffman M. H., Sherman M. E., Cullen A., Kurman R. J. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995 Mar;172(3):946–954. doi: 10.1016/0002-9378(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Cuzick J., Szarewski A., Terry G., Ho L., Hanby A., Maddox P., Anderson M., Kocjan G., Steele S. T., Guillebaud J. Human papillomavirus testing in primary cervical screening. Lancet. 1995 Jun 17;345(8964):1533–1536. doi: 10.1016/s0140-6736(95)91086-7. [DOI] [PubMed] [Google Scholar]
- Devesa S. S., Young J. L., Jr, Brinton L. A., Fraumeni J. F., Jr Recent trends in cervix uteri cancer. Cancer. 1989 Nov 15;64(10):2184–2190. doi: 10.1002/1097-0142(19891115)64:10<2184::aid-cncr2820641034>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Giard R. W., Bosman F. T. Een normaal uitstrijkje en toch baarmoederhalskanker. Ned Tijdschr Geneeskd. 1992 Nov 21;136(47):2311–2314. [PubMed] [Google Scholar]
- Hatch K. D., Schneider A., Abdel-Nour M. W. An evaluation of human papillomavirus testing for intermediate- and high-risk types as triage before colposcopy. Am J Obstet Gynecol. 1995 Apr;172(4 Pt 1):1150–1157. doi: 10.1016/0002-9378(95)91473-0. [DOI] [PubMed] [Google Scholar]
- Hsing A. W., Burk R. D., Liaw K. L., Chen C. J., Zhang T., Schiffman M., Greer C. E., You S. L., Hsieh C. Y., Huang T. W. Interlaboratory agreement in a polymerase chain reaction-based human papillomavirus DNA assay. Cancer Epidemiol Biomarkers Prev. 1996 Jun;5(6):483–484. [PubMed] [Google Scholar]
- Jacobs M. V., Snijders P. J., van den Brule A. J., Helmerhorst T. J., Meijer C. J., Walboomers J. M. A general primer GP5+/GP6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol. 1997 Mar;35(3):791–795. doi: 10.1128/jcm.35.3.791-795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. V., van den Brule A. J., Snijders P. J., Helmerhorst T. J., Meijer C. J., Walboomers J. M. A non-radioactive PCR enzyme-immunoassay enables a rapid identification of HPV 16 and 18 in cervical scrapes after GP5+/6+ PCR. J Med Virol. 1996 Jul;49(3):223–229. doi: 10.1002/(SICI)1096-9071(199607)49:3<223::AID-JMV11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Koss L. G. The Papanicolaou test for cervical cancer detection. A triumph and a tragedy. JAMA. 1989 Feb 3;261(5):737–743. [PubMed] [Google Scholar]
- Kuypers J. M., Critchlow C. W., Gravitt P. E., Vernon D. A., Sayer J. B., Manos M. M., Kiviat N. B. Comparison of dot filter hybridization, Southern transfer hybridization, and polymerase chain reaction amplification for diagnosis of anal human papillomavirus infection. J Clin Microbiol. 1993 Apr;31(4):1003–1006. doi: 10.1128/jcm.31.4.1003-1006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nindl I., Jacobs M., Walboomers J. M., Meijer C. J., Pfister H., Wieland U., Meyer T., Stockfleth E., Klaes R., von Knebel Doeberitz M. Interlaboratory agreement of different human papillomavirus DNA detection and typing assays in cervical scrapes. Int J Cancer. 1999 May 17;81(4):666–668. doi: 10.1002/(sici)1097-0215(19990517)81:4<666::aid-ijc25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Qu W., Jiang G., Cruz Y., Chang C. J., Ho G. Y., Klein R. S., Burk R. D. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997 Jun;35(6):1304–1310. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M. H., Kiviat N. B., Burk R. D., Shah K. V., Daniel R. W., Lewis R., Kuypers J., Manos M. M., Scott D. R., Sherman M. E. Accuracy and interlaboratory reliability of human papillomavirus DNA testing by hybrid capture. J Clin Microbiol. 1995 Mar;33(3):545–550. doi: 10.1128/jcm.33.3.545-550.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H. L., Bollen L. J., Tjong-A-Hung S. P., Vonk J., Van Der Velden J., Ten Kate F. J., Kaan J. A., Mol B. W., Ter Schegget J. Intermethod variation in detection of human papillomavirus DNA in cervical smears. J Clin Microbiol. 1995 Oct;33(10):2631–2636. doi: 10.1128/jcm.33.10.2631-2636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H. L., Tieben L. M., Tjong-A-Hung S. P., Jebbink M. F., Minnaar R. P., Jansen C. L., ter Schegget J. Detection and typing of human papillomaviruses present in fixed and stained archival cervical smears by a consensus polymerase chain reaction and direct sequence analysis allow the identification of a broad spectrum of human papillomavirus types. J Gen Virol. 1992 Dec;73(Pt 12):3263–3268. doi: 10.1099/0022-1317-73-12-3263. [DOI] [PubMed] [Google Scholar]
- Snijders P. J., van den Brule A. J., Schrijnemakers H. F., Snow G., Meijer C. J., Walboomers J. M. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990 Jan;71(Pt 1):173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- de Roda Husman A. M., Snijders P. J., Stel H. V., van den Brule A. J., Meijer C. J., Walboomers J. M. Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction. Br J Cancer. 1995 Aug;72(2):412–417. doi: 10.1038/bjc.1995.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roda Husman A. M., Walboomers J. M., van den Brule A. J., Meijer C. J., Snijders P. J. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995 Apr;76(Pt 4):1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]



