Abstract
This trial was conducted to explore the safety of recombinant fusion protein ESAT6-CFP10 as a skin test reagent for the diagnosis of Mycobacterium tuberculosis infection. Twenty-four healthy adult volunteers were recruited and randomized into four groups (groups A to D) to study four increasing doses of ESAT6-CFP10. All subjects in each dose group received an intradermal injection of reagent (0.1 ml) via the Mantoux technique. Then, the vital signs of all subjects were monitored, and skin reactions around injection sites and adverse events were recorded at different detection time points after the skin test. No serious adverse events were observed in this study. A total of 3 subjects had unexpected events. One subject in group A developed subcutaneous hemorrhage 24 h after the skin test, one subject in group B was found with red spots 15 min after the skin test, and another subject in group A showed abnormity during a chest X-ray after the skin test without affecting her health. One of three adverse events (red spots) was probably related to the recombinant ESAT6-CFP10 reagent. A single dose of 1, 5, 10, or 20 μg/ml of recombinant ESAT6-CFP10 as a skin test reagent for M. tuberculosis infection diagnosis is well tolerated and safe in China. (This study has been registered at ClinicalTrials.gov under registration no. NCT01999231.)
INTRODUCTION
Although tuberculosis (TB) has been a curable disease, it still remains a major global problem that seriously threatens human health. According to the WHO Global TB report 2015, there were 9.6 million new cases and nearly 1.5 million TB-related deaths in 2014 (1). Thus, exploring diagnostic techniques with high sensitivity and specificity is crucial for controlling TB development and its drug resistance.
TB is caused by infection with Mycobacterium tuberculosis, an intracellular pathogen transmitted by air (2). Several in vitro assay systems based on the detection of immune reactivity against an M. tuberculosis-specific antigen have been established and widely accepted in recent years. Early secreted antigenic target 6-kDa protein (ESAT6) and culture filtrate protein of 10 kDa (CFP10), two major M. tuberculosis-specific antigens, have been reported to play key roles in the virulence of M. tuberculosis and elicit strong T-cell responses (3, 4). Interferon gamma release assays (IGRAs) based on immune recognition against ESAT6 and CFP10 have been used to help diagnose activated and latent M. tuberculosis infection (5). IGRAs have an increased specificity compared to the conventional tuberculin skin test (TST) because the antigens included in IGRAs, namely, ESAT6 and CFP10, have been selected based on their absence in many environmental nontuberculous mycobacteria and the vaccine strain Mycobacterium bovis Bacillus Calmette-Guérin (BCG), which can only be detected in a number of pathogenic mycobacteria species, including M. tuberculosis, and a minority of nontuberculous mycobacteria (Mycobacteria kansasii, Mycobacteria szulgai, Mycobacteria marinum, and Mycobacteria riyadhense), altogether limiting false-positive reactions (6, 7). However, this diagnostic method is costly and equipment-intensive. It is not always available in many parts of the world, especially in remote areas and areas where TB is endemic and resources are limited. Therefore, developing an inexpensive and simple method with high sensitivity and specificity for the diagnosis of TB and latent M. tuberculosis infection is still necessary.
The conventional TST uses tuberculin or purified protein derivative (PPD) as a skin test reagent and has been used widely to support the diagnosis of TB or the detection of latent M. tuberculosis infection for a long time across the world and even in resource-limited areas (8). This method is inexpensive and easy to operate. PPD contains a large number of antigens that are also present in many other environmental mycobacteria species and BCG vaccine strains (3). Thus, a conventional TST based on immune reactivity against PPD has a limited ability to distinguish the memory T cell responses to M. tuberculosis infections from those of other similar infections. An improved TST based on immune recognition against ESAT6 and CFP10 that is analogous to the IGRAs is speculated to be more specific than the conventional one. A phase II clinical trial showed that the specificity of recombinant ESAT6 and CFP10 (weight ratio of 1:1) as the skin test reagent for the diagnosis of M. tuberculosis infection was 99.3% (95% confidence interval [CI], 96% to 100%). In addition, this combination of recombinant ESAT6 and CFP10 has been demonstrated to be safe in healthy adult volunteers in a first-in-man open clinical trial (9, 10). In the aspect of the preparation process, fusion proteins are more stable than the mixed protein solution (11). Thus, the recombinant fusion protein ESAT6-CFP10 was supposed to be an optimized skin test reagent for the diagnosis of M. tuberculosis infection.
In this study, we evaluate the safety and tolerability of the recombinant fusion protein ESAT6-CFP10 by performing a single-center, randomized, and open-label phase I clinical trial. These results provide a basis for approval and wide use of the recombinant fusion protein ESAT6-CFP10 for diagnosis of M. tuberculosis infection.
MATERIALS AND METHODS
Study design.
The study was designed as an open-label, single-center, randomized phase I clinical trial to assess the safety and tolerability of the recombinant protein ESAT6-CFP10 as a skin test reagent for TB. This study was performed at the Department of Tuberculosis of the Shanghai Public Health Clinical Center affiliated with Fudan University in China in accordance with the principles of the Declaration of Helsinki and the good clinical practice guidelines of the Chinese Food and Drug Administration (CFDA; no. 2013L01038). Written informed consent was obtained from each subject for blood sampling, tuberculin skin testing, electrocardiography, and hepatic and renal functions tests after an adequate explanation of the objective, methodology, and possible risks of the study. The study protocol, the investigator's brochure, and other trial-related information were approved by the Medical Ethics Committee of Shanghai Public Health Clinical Center (no. 201 [3]; Approved 7 August 2013). The study protocol was reviewed, approved, and registered at ClinicalTrials.gov under registration no. NCT01999231 (12).
Skin test reagent.
ESAT6-CFP10 is a recombinant fusion protein of ESAT6 and CFP10, which was manufactured in Escherichia coli at Anhui Longcom Biologic Pharmacy Co. Ltd. under good manufacturing practice (GMP) requirements and Chinese Pharmacopoeia (2010) requirements. ESAT6-CFP10 manufacturing was overseen by regulators. The expression and purification of the recombinant protein were conducted by standard protocols (12, 13). The purity of this recombinant fusion protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and high-performance liquid chromatography (HPLC) and was ≥95% purity in this study. This reagent was supplied as a liquid formulation (500 μg/ml, 0.3 ml per bottle), consisting of ESAT6-CFP10 antigen, phosphate-buffered saline (1.0 mmol/liter), 3% phenol, and 0.005% Tween 80. The final product was verified by the National Institutes for Food and Drug Control. The stock solution was diluted with 1.0 mmol/liter phosphate-buffered saline containing 3% phenol and 0.005% Tween 80 to prepare the different concentrations of the proteins.
Study subjects.
The trial population consisted of 24 volunteers aged 18 to 40 years with body mass indexes (BMIs) between 20 and 27, who were mainly recruited by advertising in newspapers. To enroll, participants had to be healthy, which was confirmed by physical examination, laboratory tests, and review of their recorded medical history. Subjects were excluded from the study if they met one or more of the following criteria: (i) had a history of TB or known contact with tuberculosis patients, especially with patients who were sputum smear-positive within the last 3 weeks; (ii) had congenital and/or acquired immune dysfunction or received immunosuppressive therapy or other immune-modulating drugs; (iii) had been vaccinated with live vaccines within the preceding 3 months by a standard interview; (iv) suffered from any serious illness, such as cancer, autoimmune disease, progressive atherosclerosis, diabetes, chronic obstructive pulmonary disease, acute or progressive liver disease, or kidney disease; (v) had a history of epilepsy, encephalopathy, or neurological diseases; (vi) had an acute febrile illness, infectious disease, or were hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV seropositive; (vii) had attended other clinical trials within the last 3 months or any ongoing test; (viii) were pregnant or breastfeeding within the trial period; or (ix) suffered from mental or physical disability.
Allocation in study groups.
Twenty-four subjects were included in our study and were randomized into four groups (groups A to D) with 6 volunteers per group, to study 4 increasing doses of the reagent (1 μg/ml, 5 μg/ml, 10 μg/ml, and 20 μg/ml). The selected doses were based on results from a previous clinical trial performed with an 11-kDa recombinant ESAT6 (14). Randomization was performed using the Rv.Uniform function in SPSS 19.0 analysis software with a random number of 24. In the actual study, included subjects were given a specific random number and then assigned into a specific group (groups A to D). In addition, subjects were stratified according to sex at randomization. Each subject received a single dose of ESAT6-CFP10, with a total volume of 0.1 ml. This dose-escalation trial was initiated when subjects in group A received intradermal injections of ESAT6-CFP10 of 1 μg/ml. If any adverse event was observed in more than half of the subjects or was considered to be serious (“serious” was defined as an occurrence of life-threatening events associated with this test), the trial would be terminated at any dose.
Skin test procedure.
Subjects were tested for the recombinant protein ESAT6-CFP10 reagent via the Mantoux technique. The ESAT6-CFP10 reagent (0.1 ml) was injected with a 1-ml syringe that was fitted with a short bevel needle (21 gauge/0.51 mm) in the volar aspect of the lower one-third of the left forearm. Each subject received one dose. The needles were pierced into the dermal surface, with the bevel of the needle upward on a 5° to 10° angle. The correct injection technique was performed when a small papule was observed at the injection site in patients. The volunteers were monitored closely for local skin reactions and adverse events at 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, 24 h, 48 h, 72 h, and 96 h after the skin test by the team of investigators. Digital photographs of all injection sites from all subjects were taken 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, 24 h, 48 h, 72 h, and 96 h after the skin test. Calipers were used to measure the longitudinal and transverse diameters of the skin induration, redness, and/or swelling around the injection site. Redness, swelling, induration, and blister reactions were graded according to the criteria listed in Table 1, which are based on the “principle of quantitative criterion and grading system for adverse events from vaccine for clinical trials” released by the China Food and Drug Administration in 2005 (http://www.sda.gov.cn/WS01/CL1616/83435.html).
TABLE 1.
Classification of local reaction after skin testa
| Local reaction | Mild (grade 1) | Moderate (grade 2) | Severe (grade 3) | Potentially life-threatening (grade 4) |
|---|---|---|---|---|
| Pain | Without prejudice to activities | Impacts activities or more often use of a nonnarcotic pain medication | Interferes with daily activities or repeated use of narcotic pain medication | Emergency room or hospital |
| Indurationb | <15 mm | 15 to ∼30 mm | >30 mm | Gangrene or exfoliative dermatitis |
| Rednessb | <15 mm | 15 to ∼30 mm | >30 mm | Gangrene or exfoliative dermatitis |
| Swellingc | <15 mm and without prejudice to activities | 15 to ∼30 mm or impacts activities | >30 mm or restrictions on daily activities | Gangrene |
| Skin rash (injection site) | <15 mm | 15 to ∼30 mm | >30 mm | |
| Itching | Injection site micro-itch | Injection body itch | Whole body itches | |
| Mucocutaneous lesion | Red, itchy | Diffusion, maculopapular rash, desquamation | Bubbly wet desquamation or ulcers | Skin dermatitis, trojan and mucosal erythema or polymorphism, or suspected Stevens-Johnson syndrome |
These guidelines are from the preventive vaccine clinical trial adverse reaction classification.
Apart from most directly by measuring the diameter of grading evaluation of local reactions, also records changes in measurement.
The evaluation and classification of swelling should be based on the grading system and actual measurement results.
Safety.
Safety was assessed using the following clinical assessments: (i) regular physical examination for vital signs, including respiratory rate, heart rate, blood pressure, and body temperature at 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, 24 h, 48 h, 72 h, and 96 h after the skin test; (ii) recordings of the longitudinal and transverse diameters of induration, redness, and/or swelling around the injection site, local skin reactions, including rash, pain, and itch, as well as adverse events at 24 h, 48 h, 72 h, and 96 h after the skin test; and (iii) routine blood and urine tests, liver and kidney function tests, electrocardiography, chest X-ray, and monitoring of adverse events 1 day before and 7 days after the skin test. If any abnormality was observed during examination, the volunteer would receive another examination the next week.
All systemic and local adverse events, including anaphylactic shock, systemic allergic rash, generalized urticaria, lymphangitis, allergic purpura, and fever, generated during this clinical trial were recorded, regardless of their causal relationship with the recombinant ESAT6-CFP10 antigen. Unexpected reactions or damage occurring with the received dose and during the skin test period were considered adverse reactions. Serious adverse events were defined as life-threatening events associated with this test. Emergency measures were prepared in the event of a volunteer developing a significant clinical event or disease after injection of the recombinant ESAT6-CFP10 antigen. The relationships between the study reagent and adverse events were determined by investigators as “irrelevant,” “unrelated,” “probable,” “certain,” or “related” according to a predefined algorithm based on the modified World Health Organization Uppsala Monitoring Centre (WHO-UMC) causality assessment method by the National Center for Adverse Drug Reaction (ADR) Monitoring (China) as described previously (15).
Statistical analysis.
All data in our study were verified by a third-party that was blinded to treatment allocation and grouping. The process for data verification was as follows. Data entry was completed using EpiData 3.0 software (EpiData Association, Odense, Denmark) by data editors. Data information recorded by editors included the number of included subjects, information for exclusion and dropping out of our study, basic demographic features of subjects, and results from the study. They also investigated the balance of data in each group and the comparability among groups. If they had any questions about the data, they directed inquiries to the supervisor who passed them on to our team. Then, researchers from our team offered answers in writing to the data editors after a careful examination of the whole study process. Statistical analysis was performed with SAS 9.3 software (SAS Institute, Cary, NC, USA). The differences in demographic characteristics among groups were calculated with one-way analysis of variance (ANOVA) or Fisher's exact test. All adverse events were evaluated descriptively.
RESULTS
Subjects.
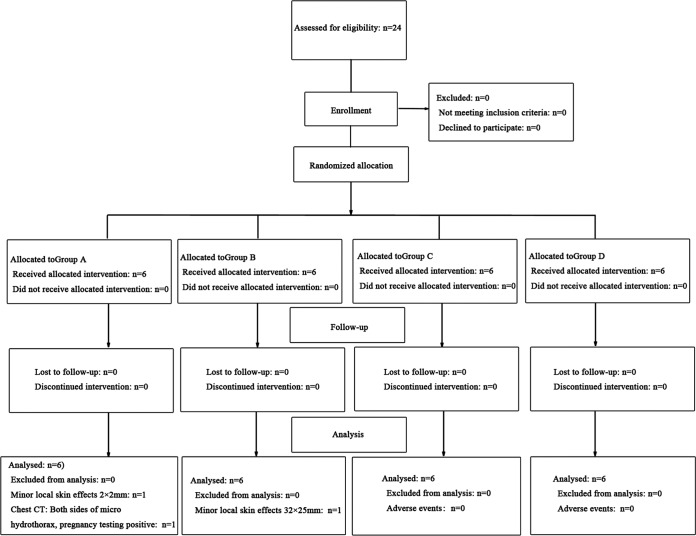
A total of 24 healthy volunteers, 12 male and 12 female, meeting the inclusion criteria were enrolled in this trial. All of them completed the study trial, and no one dropped out during the test. None of the participants included in our study had any history of prior TST after a standard interview. The study design is shown in Fig. 1. After grouping, there were no significant differences among the four groups in terms of age, gender, ethnic group, height, weight, or body mass index (Table 2). Additionally, laboratory tests for HIV, HBV, HCV, and syphilis and sputum smear microscopy for acid-fast bacilli were all negative in the four groups. The volunteers among the four groups had no history of allergies.
FIG 1.
Flow diagram of study design. All subjects in this trial received an intradermal injection with a single dose of ESAT6-CFP10, with a total volume of 0.1 ml. The increasing doses of the reagent were 1 μg/ml (group A), 5 μg/ml (group B), 10 μg/ml (group C), and 20 μg/ml (group D).
TABLE 2.
Demographic characteristics
| Demographic data | Study cohort |
P value | |||
|---|---|---|---|---|---|
| Group A (1 μg/ml) (n = 6) | Group B (5 μg/ml) (n = 6) | Group C (10 μg/ml) (n = 6) | Group D (20 μg/ml) (n = 6) | ||
| Age (mean ± SD) (yr) | 25 ± 4 | 26 ± 4 | 30 ± 4 | 28 ± 4 | 0.1466 |
| Sex (male/female) | 3/3 | 3/3 | 3/3 | 3/3 | 1.0000 |
| Nationality (Han/others) | 5/1 | 6/0 | 6/0 | 6/0 | 1.0000 |
| Height (mean ± SD [range]) (cm) | 169.2 ± 12.0 (158.0–189.0) | 169.0 ± 9.7 (160.0–181.0) | 163 ± 5.1 (156.0–168.0) | 164.8 ± 11.1 (150.0–178.0) | 0.6296 |
| Weight (mean ± SD [range]) (kg) | 64.5 ± 13.1 (50.0–83.0) | 66.0 ± 8.9 (54.0–75.0) | 58.8 ± 6.6 (51.0–67.0) | 62.3 ± 9.5 (48.0–76.0) | 0.6183 |
| BMI (mean ± SD [range]) (kg/m2) | 22.3 ± 2.0 (20.0–25.4) | 23.1 ± 2.1 (20.8–26.6) | 22.1 ± 1.1 (20.8–23.7) | 22.9 ± 2.1 (21.3–26.7) | 0.7636 |
Group A (subjects received 1 μg/ml of ESAT6-CFP10).
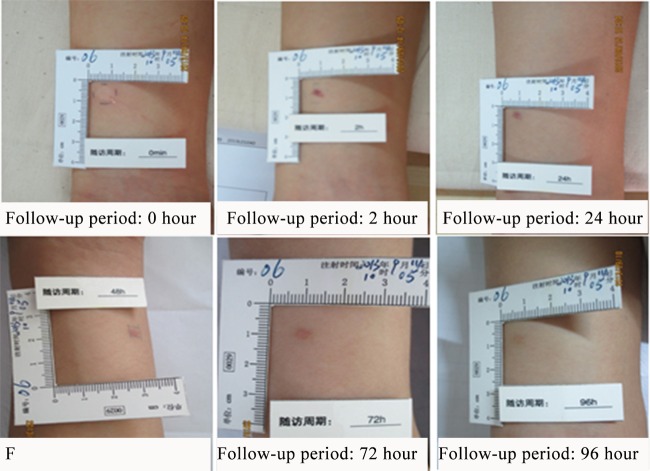
Group A consisted of 3 men and 3 women aged 25.00 ± 3.93 years (21.3 to 31.5 years old). One subject (no. 6) developed a 2- by 2-mm subcutaneous hemorrhage at the injection site 24 h after the skin test, which flattened at 48 and 72 h and subsided 96 h after the skin test. Following the classification criteria of local reactions (Table 1), it was a mild local skin reaction (Fig. 2). Another subject (no. 12) had a positive pregnancy test after the skin test, although a urine pregnancy test was negative before the trial. Additionally, a chest X-ray examination revealed trace effusions before her enrollment and mild bilateral hydrothorax on the 7th day after the skin test in the same subject. On the 9th day after the skin test, her pregnancy was confirmed by ultrasound. She had no other symptoms and refused to go to the hospital for a review. She told us that she terminated the pregnancy in hospital 13 days after the skin test during a follow-up by telephone. No other volunteers in group A developed any local skin reaction or adverse events.
FIG 2.
Mild skin reactions in one subject (no. 6) in group A (subjects received 1 μg/ml of ESAT6-CFP10), who developed a 2- by 2-mm subcutaneous hemorrhage at the injection site 24 h after the skin test, which flattened at 48 and 72 h and subsided at 96 h after the skin test.
Group B (subjects received 5 μg/ml of ESAT6-CFP10).
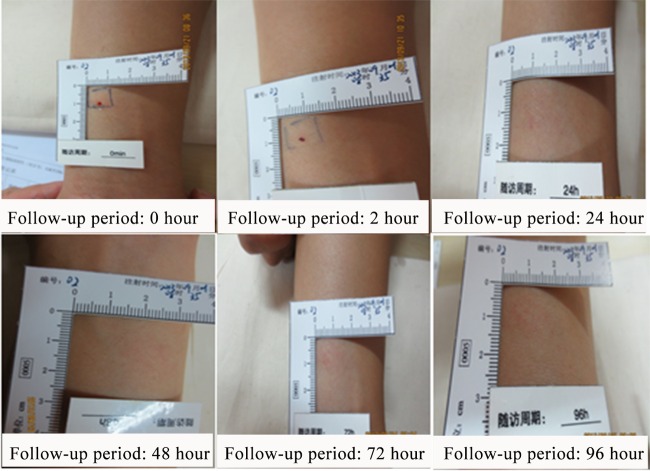
Group B included 3 men and 3 women aged 25.63 ± 4.13 years (19.3 to 30.5 years old). One subject (no. 2) developed a mild local reaction of red spots scattered within an area of 32 by 25 mm at the injection site 15 min after the skin test. The size of the scattered red spots remained unchanged 30 min after the skin test. These red spots disappeared on the next day after the skin test (Fig. 3). No other skin reactions or adverse events were found in group B.
FIG 3.
Mild skin reactions in one subject (no. 2) in group B (subjects received 5 μg/ml of ESAT6-CFP10), who was observed with red spots scattered within an area of 32 by 25 mm at the injection site 15 min after the skin test. These red spots disappeared on the next day after the skin test.
Group C (subjects received 10 μg/ml of ESAT6-CFP10).
There were also three women and three men included in group C. Their ages ranged from 25.0 years old to 34.3 years old, with a mean age of 30.07 ± 4.00 years (19.3 to 30.5 years). None of the volunteers in this group developed a local skin reaction or an adverse event.
Group D (subjects received 20 μg/ml of ESAT6-CFP10).
As with the other groups, 3 women and 3 men were enrolled in group D with a mean age of 27.68 ± 3.79 years, ranging from 23 years old to 33.1 years old. None of the volunteers in this group showed a local skin reaction or an adverse event.
Safety outcomes.
For vital signs, such as blood pressure, respiration, heart rate, body temperature, and nighttime axillary temperature, no significant changes were observed for any of the volunteers at different detection time points after the test. Additionally, there were no significant changes in white and red blood cell counts, erythrocyte and platelet counts, hemoglobin level, urine protein level, or liver and kidney function among the volunteers before and after the skin test.
Outcomes for adverse events are shown in Table 3. No serious adverse events were found in any subject during the whole experiment. Three of the 24 volunteers developed episodes of adverse events. One subject in group A and one subject in group B had mild local reactions. The abnormality during the chest X-ray examination in one subject in group A did not affect the health of the volunteer and was supposed to be not related to this skin test or the recombinant fusion protein as judged by clinicians.
TABLE 3.
Summary of adverse events and local skin reactions around injection site
| Adverse event | Study cohort |
|||
|---|---|---|---|---|
| Group A (1 μg/ml) (n = 6) | Group B (5 μg/ml) (n = 6) | Group C (10 μg/ml) (n = 6) | Group D (20 μg/ml) (n = 6) | |
| Systemic adverse event | Mild bilateral hydrothorax during chest X-ray examination | None | None | None |
| Local adverse events and skin reactions around injection site | ||||
| Total (n [%]) | 1 (16.7) | 1 (16.7) | 0 (0) | 0 (0) |
| Injection site pain | None | None | None | None |
| Injection site induration | None | None | None | None |
| Injection site redness | None | Red spots scattered within an area 32 by 25 mm | None | None |
| Injection site swelling | None | None | None | None |
| Injection site skin rash | None | None | None | None |
| Injection site itching | None | None | None | None |
| Injection site mucocutaneous lesion | None | None | None | None |
| Other local adverse events | 2- by 2-mm subcutaneous hemorrhage | None | None | None |
DISCUSSION
TB remains a public health problem that seriously affects human health (1). It is essential to develop a diagnostic method with high sensitivity and specificity for the detection of M. tuberculosis infection. Several diagnostic methods, including bacteriological tests, imaging test, as well as immunological methods, can be used for the diagnosis of TB, while only indirect immunological means are able to detect the latent infection of M. tuberculosis (16, 17). TSTs using tuberculin or PPD as the skin test reagent are a major means of immunological diagnosis and have been applied for more than 100 years owing to their low cost and ease of operation (18, 19). The main disadvantage for this diagnosis method is low specificity (20). Recently, M. tuberculosis-secreted antigens serving as TB skin test reagents have attracted a lot of interest in the diagnosis of TB (21).These antigens, including ESAT6, CFP10, PE13, PE5, MPB70, TB10.4, and TB27.4, have been tested. ESAT6 and CFP10 are powerful antigens that elicit humoral and cellular immunity, so they are considered the most promising antigens as skin test reagents (22). Additionally, when used in combination, they showed higher diagnostic value than when used alone (23). Bergstedt et al. indicated that the combination of ESAT6 with CFP10 in a novel skin test reagent appeared safe by performing a small-scale first-in-man phase I clinical trial (9). Nevertheless, considering the preparation process, the mixed protein solution of these two recombinant proteins is less stable than the recombinant fusion proteins (11). Thus, using recombinant fusion protein ESAT6-CFP10 as a skin test reagent should be effective and more stable for the diagnosis of M. tuberculosis infection. Excitingly, this fusion protein has been demonstrated to induce a significantly higher cellular immunity response than the BCG vaccine as well as a protection efficacy similar to that of BCG in a preclinical study in mice (24). Therefore, the recombinant ESAT6-CFP10 protein may have potential for clinical application.
Here, we reported the first in vivo use of ESAT6-CFP10, a recombinant fusion protein of ESAT6 and CFP10, as a skin test reagent for M. tuberculosis, with four doses of 1 μg/ml, 5 μg/ml, 10 μg/ml, and 20 μg/ml in this phase I clinical study in China. This fusion protein is obtained using genetic engineering technology. The main aim of this study was to explore the safety and tolerability of ESAT6-CFP10 in a small-scale trial. Different from previous studies with mixed protein solutions of recombinant ESAT6 and CFP10, our study was performed with the fusion protein of ESAT6 and CFP10. Following our results, no serious adverse events or recombinant ESAT6-CFP10 protein-related adverse events were found during the whole study. Among 24 healthy volunteers, three subjects developed three cases of adverse events. Two of the adverse events were mild local reactions. The reaction of one subject in group A, who was found with subcutaneous hemorrhage around the injection site after the skin test, was speculated to be caused by a needle stick injury during the skin test. One subject in group B developed red spots, which might be caused by an allergy to alcohol or the study reagent. The relationship between the study reagent and the adverse events was considered “probable.” Abnormity in the chest X-ray of the remaining subject was not related to the skin test or the recombinant ESAT6-CFP10 protein. Except for the above adverse events, no other significant clinical changes were identified in volunteers in terms of blood pressure, respiration, heart rate, body temperature, night axillary temperature, blood, urine, liver and kidney function, electrocardiogram, and chest computed tomography (CT) as a result of the recombinant ESAT6-CFP10 skin test. Vital signs were all normal for each volunteer throughout this trial. Furthermore, clinical follow-up exhibited no signs of sequelae from this skin test.
Our results indicated that the recombinant ESAT6-CFP10 antigen as a skin test reagent for the diagnosis of M. tuberculosis infection in the healthy, adult Chinese population was safe and well tolerated. However, larger studies are still required to give a true impression of the adverse reaction profile of this skin test reagent. Additionally, since the main aim of this study was to explore the safety and tolerability of ESAT6-CFP10, the diagnostic performance for this fusion protein as a skin test reagent was not evaluated.
In conclusion, recombinant ESAT6-CFP10 as a skin test reagent is safe and well tolerated in healthy adults in China. Our results provide a basis for further phase II and phase III clinical trials for the recombinant ESAT6-CFP10 protein-based skin test for the detection of TB as well as the latency infection of M. tuberculosis in China.
ACKNOWLEDGMENT
We declare that we have no conflict of interests.
REFERENCES
- 1.World Health Organization. 2015. Global tuberculosis report 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Gomez JE, McKinney JD. 2004. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Guo S, Xue R, Li Y, Wang SM, Ren L, Xu JJ. 2012. The CFP10/ESAT6 complex of Mycobacterium tuberculosis may function as a regulator of macrophage cell death at different stages of tuberculosis infection. Med Hypotheses 78:389–392. doi: 10.1016/j.mehy.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Uvarova EA, Belavin PA, Permyakova NV, Zagorskaya AA, Nosareva OV, Kakimzhanova AA, Deineko EV. 2013. Oral immunogenicity of plant-made Mycobacterium tuberculosis ESAT6 and CFP10. Biomed Res Int 2013:316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pai M, Zwerling A, Menzies D. 2008. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen P, Munk M, Pollock J, Doherty T. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099–1104. doi: 10.1016/S0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 7.Brock I, Weldingh K, Leyten EM, Arend SM, Ravn P, Andersen P. 2004. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J Clin Microbiol 42:2379–2387. doi: 10.1128/JCM.42.6.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arend SM, Franken WPJ, Aggerbeck H, Prins C, van Dissel JT, Thierry-Carstensen B, Tingskov PN, Weldingh K, Andersen P. 2008. Double-blind randomized phase I study comparing rdESAT-6 to tuberculin as skin test reagent in the diagnosis of tuberculosis infection. Tuberculosis (Edinb) 88:249–261. doi: 10.1016/j.tube.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Bergstedt W, Tingskov PN, Thierry-Carstensen B, Hoff ST, Aggerbeck H, Thomsen VO, Andersen P, Andersen AB. 2010. First-in-man open clinical trial of a combined rdESAT-6 and rCFP-10 tuberculosis specific skin test reagent. PLoS One 5:e11277. doi: 10.1371/journal.pone.0011277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggerbeck H, Giemza R, Joshi P, Tingskov PN, Hoff ST, Boyle J, Andersen P, Lewis DJ. 2013. Randomised clinical trial investigating the specificity of a novel skin test (C-Tb) for diagnosis of M. tuberculosis infection. PLoS One 8:e64215. doi: 10.1371/journal.pone.0064215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark EDB. 2001. Protein refolding for industrial processes. Curr Opin Biotechnol 12:202–207. doi: 10.1016/S0958-1669(00)00200-7. [DOI] [PubMed] [Google Scholar]
- 12.Ganguly N, Giang PH, Gupta C, Basu SK, Siddiqui I, Salunke DM, Sharma P. 2008. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10:ESAT6 complex inhibit lipopolysaccharide-induced NF-κB transactivation by downregulation of reactive oxidative species (ROS) production. Immunol Cell Biol 86:98–106. doi: 10.1038/sj.icb.7100117. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Xue Y, Gao H, Wang L, Ding T, Bai W, Fan A, Zhang J, An Q, Xu Z. 2008. Expression and purification of Mycobacterium tuberculosis ESAT-6 and MPT64 fusion protein and its immunoprophylactic potential in mouse model. Protein Expr Purif 59:189–196. doi: 10.1016/j.pep.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Wen JS, Du WX, Zhao WD, He WS, Xie SH, Yang XM, Wu J. 2009. Safety and dose standardization of a 11 kDa recombinant antigen from Mycobacterium tuberculosis in clinical trial. Jiang Xi Yi Yao 44:1180–1182. [Google Scholar]
- 15.Wei X, Xie YM. 2012. Principle of adverse drug reaction causality judgement and interpretation of causality assessment method both in China and abroad. Zhongguo Zhong Yao Za Zhi 37:2744–2747. (In Chinese.) [PubMed] [Google Scholar]
- 16.Grode L, Ganoza CA, Brohm C, Weiner J III, Eisele B, Kaufmann SHE. 2013. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 31:1340–1348. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 17.Katoch V. 2004. Newer diagnostic techniques for tuberculosis. Indian J Med Res 120:418–428. [PubMed] [Google Scholar]
- 18.Mendel F. 1908. Die von Pirquet'sche Hautreaktion und die intravenöse tuberkulinbehandlung. Medizinische Klinik, München 4:402–404. [Google Scholar]
- 19.Lordi GM, Reichman LB. 1994. Tuberculin skin testing, p 33–38. In Schlossberg D. (ed), Tuberculosis. Springer, New York, NY. [Google Scholar]
- 20.Press C. 2000. Tuberculosis: a comprehensive international approach. CRC Press, Boca Raton, FL. [Google Scholar]
- 21.Bekmurzayeva A, Sypabekova M, Kanayeva D. 2013. Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberculosis (Edinb) 93:381–388. doi: 10.1016/j.tube.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol 51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aagaard C, Govaerts M, Meikle V, Vallecillo A, Gutierrez-Pabello J, Suarez-Güemes F, McNair J, Cataldi A, Espitia C, Andersen P, Pollock JM. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J Clin Microbiol 44:4326–4335. doi: 10.1128/JCM.01184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Peng P, Miao S, Zhao Y, Mao F, Wang L, Bai Y, Xu Z, Wei S, Shi C. 2010. Recombinant Mycobacterium smegmatis expressing an ESAT6-CFP10 fusion protein induces anti-mycobacterial immune responses and protects against Mycobacterium tuberculosis challenge in mice. Scand J Immunol 72:349–357. doi: 10.1111/j.1365-3083.2010.02448.x. [DOI] [PubMed] [Google Scholar]