Abstract
Nontyphoidal Salmonella (NTS; i.e., Salmonella enterica organisms that do not cause typhoid or paratyphoid) are responsible for 94 million infections and 155,000 deaths worldwide annually, 86% of which are estimated to be foodborne. Although more than 50 serogroups and 2,600 serovars have been described, not all Salmonella serovars cause disease in humans and animals. Efforts are being made to develop NTS vaccines, with most approaches eliciting protection against serovars Typhimurium and Enteritidis (serogroups B [O:4] and D [O:9], respectively), as they are widely considered the most prevalent. Here, we show that serogroup C (O:6,7, O:6,8, or O:8 epitopes) is the most common serogroup in the United States, and the prevalence of serovars from this serogroup has been increasing in Europe and the United States over the last decade. They are also the most commonly isolated serovars from healthy cattle and poultry, indicating the underlying importance of surveillance in animals. Four out of the 10 most lethal serovars in the United States are serogroup C, and reports from African countries suggest that strains within this serogroup are highly antibiotic resistant. Serogroup C consists of highly diverse organisms among which 37 serovars account for the majority of human cases, compared to 17 and 11 serovars for serogroups B and D, respectively. Despite these concerning data, no human vaccines targeting serogroup C NTS are available, and animal vaccines are in limited use. Here, we describe the underestimated burden represented by serogroup C NTS, as well as a discussion of vaccines that target these pathogens.
INTRODUCTION
Salmonella enterica is a facultative intracellular pathogen responsible for a high burden of mortality and morbidity worldwide. The species Salmonella enterica contains six subspecies, with 99.5% of all isolated strains belonging to S. enterica subspecies enterica (also known as subspecies I). Further classification into serogroups relies on differences in the surface O antigens, of which individual serovars are distinguished by additional typing of the flagellar H antigen and biochemical tests (1). While almost 2,600 serovars and more than 50 serogroups have been described so far, only a few of these cause disease in humans and animals. Human host-restricted S. enterica serovars Typhi, Paratyphi A, and Paratyphi B cause typhoid and paratyphoid enteric fevers (2). These systemic diseases represent an annual estimated burden of 27 million cases and more than 200,000 deaths worldwide, with sub-Saharan Africa and Asia accounting for 46% and 32% of typhoid fever cases, respectively (3). Other Salmonella serovars have a broader host range and mainly cause gastroenteritis in animals and humans; they are referred to as nontyphoidal Salmonella (NTS). NTS infections cause an estimated 94 million cases and 155,000 deaths worldwide each year (2).
In 2013, diarrheal diseases were the second leading cause of loss of disability-adjusted life-years (DALYs) among communicable diseases (the leading cause was lower respiratory infections) (4). Approximately 80 million (86%) of human NTS infections worldwide are estimated to be foodborne (5). Moreover, multiple outbreaks related to contact with infected domestic or wild animals have been reported (6, 7). In 2010, the World Health Organization (WHO) estimated that nontyphoidal Salmonella was the leading cause of foodborne deaths worldwide (8). NTS thus represents a major public health concern, especially with the increasing number of antibiotic-resistant isolates being reported (9, 10). There are currently three vaccines licensed for use in humans, all targeting typhoidal Salmonella: the live attenuated oral vaccine S. Typhi Ty21a, Vi capsule polysaccharide vaccine, and Vi polysaccharide conjugated with tetanus toxoid (11–14). Despite extensive efforts, no human vaccine targeting NTS has yet been licensed. NTS vaccine developers are mainly targeting serovar Typhimurium (serogroup B, carrying the O:4 antigen) and/or serovar Enteritidis (serogroup D, carrying the O:9 antigen). Although these serovars are some of the most prevalent NTS, other serovars, particularly those belonging to serogroup C, represent underevaluated health and economic burdens for both humans and animals. Serogroup C Salmonella serovars are further subdivided into groups C1 (presence of O:6,7 epitopes, i.e., both O:6 and O:7 epitopes present) and C2 (presence of O:6,8 or O:8 epitopes). One serogroup C1 Salmonella serovar, Paratyphi C, can also express the Vi capsule. This review provides an overview of the burden and clinical syndromes produced by serogroup C NTS, and it describes the existing vaccine strategies against these serovars. In particular, we have analyzed raw serogroup/serovar distribution data published by U.S. and European public health laboratories, with a focus on Salmonella serogroup C infections.
GROUP C NTS DISEASE BURDEN
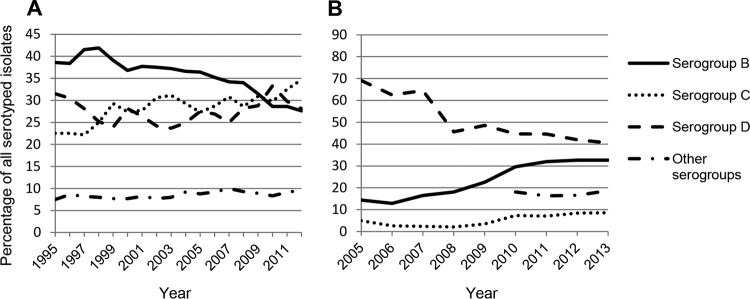
The highest burden of NTS infections (both gastroenteritis and bloodstream infections) has been estimated by the WHO to occur in sub-Saharan Africa (193 to 338 DALYs per 100,000 population), while developed regions such as North America and Europe have a lower prevalence (50 to 67 DALYs per 100,000 population) (8). Invasive NTS are a leading cause of morbidity and mortality in sub-Saharan Africa, with 388 cases per 100,000 children aged 3 to 5 years, 7,500 cases per 100,000 HIV-infected adults, and mortality rates between 10 and 30% (15, 16). Although currently not considered a major concern in Asia, recent reports suggest an increase in the number of NTS infections in that region. The decrease in the S. Typhi prevalence in Vietnam observed between 1994 and 2008 was concomitant with an increase in NTS infections (17). Up to 7% of diarrheal infections in Vietnamese children have been attributed to NTS (18). However, the serovar distribution worldwide varies, leading to a difference in the most common serogroups (Table 1). In Europe in 2012, 42% of cases were serogroup D (almost exclusively attributed to S. Enteritidis), followed by serogroups B (32.7%) and C (8.4%). In the United States in 2012, 25.7% of all reported cases of salmonellosis were caused by serogroup C isolates, followed by serogroup B (20.5% of all cases). Serogroup D accounted for only 16.5% of all reported cases. Global trends over time have also varied between the United States (Fig. 1A) and Europe (Fig. 1B). In Europe, the prevalence of serogroup D has been in decline, from 69.1% in 2005 to 40.6% in 2013, whereas it has remained relatively constant in the United States from 1995 to 2012 (31.5% in 1995 to 28.1% in 2012). In contrast, serogroup B declined in the United States (38.7 to 27.6%) while an increase was observed in Europe (14.4 to 32.7%). A slow but continuous increase in the prevalence of serogroup C has been observed in both regions (22.5 to 34.7% in the United States and 5 to 8.6% in Europe), suggesting that this serogroup may become more relevant in the future.
TABLE 1.
Worldwide serogroup distribution of NTS isolated from humans
| Region | Country | % of isolates belonging to serogroup (epitope[s]): |
Yr(s) covered | Source or reference | |||
|---|---|---|---|---|---|---|---|
| B (O:4) | C (O:7, O:8) | D (O:9) | Other | ||||
| Africa | 30.9 | 19.5 | 44.6 | 5 | 2014 | WHO GFN | |
| Ethiopia | 16.9 | 28.6 | 47.9a | 3 | 1982–2012 | 19 | |
| Kenya | 57.5 | 9.3 | 33.2 | 0 | 2002–2004 | 20 | |
| Tunisia | 10.8 | 45.7 | 24.1 | 14.4 | 1994–2004 | 21 | |
| Asia | 26.2 | 27.3 | 45.4 | 1.1 | 2009 | WHO GFN | |
| Taiwan | 39 | 23 | 29 | 9 | 2004–2012 | 22 | |
| China | 56.9 | 6.0 | 14.6 | 21.0 | 2007–2012 | 23 | |
| Middle East | Saudi Arabia | 19.6 | 33.5 | 34.2 | 12.6 | 2008–2011 | 24 |
| Europe | 32.7 | 8.4 | 42 | 16.9 | 2012 | 26 | |
| North America | United States | 20.5 | 25.7 | 16.5 | 17.8 | 2012 | 25 |
Reported data include information for S. Typhi as well as NTS isolates.
FIG 1.
Frequency of nontyphoidal Salmonella serogroups associated with human infections, by year. (A) NTS serovars in the United States from 1995 to 2012. (B) NTS serovars in Europe from 2005 to 2013. Data were obtained from the U.S. Centers for Disease Control and Prevention and the European Surveillance System (TESSy).
Access to detailed serogroup data is more complicated in countries or regions without an established surveillance network, such as in certain parts of Africa. The WHO, with the aid of its member institutions within the Global Foodborne Infections Network (GFN; http://www.who.int/gfn/en/), has established a surveillance system for tracking Salmonella. As of January 2016, only 85 countries worldwide were participants in this system. Although the NTS burden on the African continent is poorly characterized, the limited data suggest that serogroup C is the third leading serogroup (19.5% of all cases reported by African countries), after serogroup D (44.6%) and serogroup B (30.9%). Data from independent studies performed in several countries suggest a high variability in NTS serogroup distributions between countries (19–26) (Table 1). In Ethiopia, serogroup C represents 28.6% of all serotyped isolates, compared to only 9.3% in Kenya (20). The majority of studies with African isolates did not determine serovars other than S. Typhimurium and S. Enteritidis. For example, a study of isolates from The Gambia showed that although 40% of typed bacteria were S. Typhimurium and 10% were S. Enteritidis, 47% of all isolates belonged to other serovars that were not further serotyped (27). The presence of several country-specific isolates (such as S. Concord [serogroup C1] in Ethiopia, accounting for 34% of all identified strains) emphasizes the need for broader surveillance and more complete identification of Salmonella isolates in this region (19). In Asia, a similar lack of surveillance makes it difficult to gather information on serovar distributions. According to WHO GFN 2009 data for the entire Asian continent, serogroup C was the second leading cause of Salmonella infections, with 27.3% of reported cases, after serogroup D (45.4% of reported cases). In Taiwan, however, a very large study analyzed 18,280 human Salmonella isolates collected between 2004 and 2012 and found that 39% of isolates belonged to serogroup B, 29% to serogroup D, and 23% to serogroup C (Table 1) (22). The leading serovars in each serogroup were S. Typhimurium, S. Enteritidis, and S. Newport (serogroup C2), respectively. Two consecutive studies conducted on NTS isolated in Taiwan between 1993 and 2007 showed that while serogroup E was predominant in 1993 (32.4% of all cases), its incidence decreased to 17% in 2007 (28, 29). In the same period, the proportion of NTS infections caused by group C serovars increased from 15.4 to 26%. In Saudi Arabia, serogroups D (34.2% of reported cases), C (33.5%), and B (19.6%) accounted for the majority of Salmonella strains isolated from patients with gastroenteritis or diarrhea between 2008 and 2011 (24). Moreover, 37% of group C isolates were found to be resistant to at least one antibiotic, compared to only 12.6% of group D isolates.
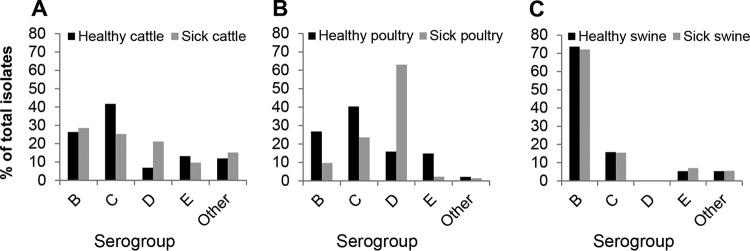
In the United States, 74% of Salmonella outbreaks in 2012 were foodborne (25). Many of these were linked to contaminated animal products (30–32). Comparison of Salmonella isolates obtained from both healthy and sick farm animals indicates that isolates found associated with livestock are not necessarily the same as those that cause disease (Fig. 2). In cattle and poultry, serogroup C is the serogroup most commonly associated with healthy animals but only the second most common serogroup in diseased animals. It is worth noting that the serovar most commonly isolated from broiler meat is S. Kentucky (O:8,20), accounting for 49% of typed isolates between 1998 and 2010 (33). These findings suggest that serovars carried by farm animals, which can potentially cause outbreaks in humans via the food chain, are different from the serovars that cause disease in those animals. These silent infections in commercial livestock present difficulties in surveillance and disruption of transmission. These findings bear relevance to continued efforts in preventing transmission from animals to humans.
FIG 2.
Serogroups of Salmonella isolates from animals in the United States in 2012. Data depict the 20 most common serovars for cattle (A), poultry (B), and swine (C), identified in healthy animals or sick animals and reported to the U.S. Centers for Disease Control and Prevention.
In Europe, serovars isolated from broiler-associated human salmonellosis cases were shown to belong to serogroups C (42.6%), D (42.2%), and B (14.45%) (12). In 2006, member states of the European Union (EU) implemented breeding flock control programs aiming for 1% or fewer positive poultry flocks for five target serovars: S. Enteritidis, S. Typhimurium, S. Infantis, S. Virchow, and S. Hadar. The latter three of these five target serovars belong to serogroup C, thus emphasizing the importance of this serogroup in animals destined for human consumption.
INVASIVENESS AND LETHALITY OF SALMONELLA SEROGROUP C
Although they generally produce gastroenteritis, salmonellae can become invasive and cause septicemia, as well as focal infections such as meningitis, endocarditis, or osteomyelitis (34, 35). A recent meta-analysis found that 18 to 21% of bloodstream infections in infants and adults in Africa were due to Salmonella enterica serovars, for which 87 to 97% were due to nontyphoidal Salmonella organisms (16). NTS is known to become invasive in about 5% of cases worldwide (this proportion increases to 12% in people 65 years or older), and the case-fatality rate can reach up to 47% in some regions (34, 36, 37). Certain NTS serovars have been associated with a higher mortality rate than others (Table 2). When examining case-fatality rates, 4 out of the 10 most lethal serovars belong to serogroup C and 2 to serogroup E (S. Muenster and S. Anatum), whereas there is only one serogroup D serovar (S. Dublin).
TABLE 2.
Serogroup and associated mortality rates of the 10 deadliest serovars isolated in the United States between 1996 and 2006a
| Rank | Serovar | Serogroup | Mortality rate (%) |
|---|---|---|---|
| 1 | Dublin | D | 3 |
| 2 | Muenster | E | 2 |
| 3 | Choleraesuis | C | 1.8 |
| Cerro | K | 1.8 | |
| 5 | Johannesburg | R | 1.5 |
| 6 | Tennessee | C | 1.3 |
| 7 | Manhattan | C | 1 |
| Anatum | E | 1 | |
| 9 | Bovismorbificans | C | 0.9 |
| Adelaide | O | 0.9 |
Data obtained from reference 37.
Overall, there is no significant difference in invasiveness at the serogroup level between serogroups B, C, and D or other groups isolated in the United States between 1996 and 2006 (37). However, some serovars are more likely to become invasive than others, and several of these belong to serogroup C (2). In Ethiopia, 30% of isolates of the group C1 serovar S. Concord (responsible for 34% of all NTS infections in that country) have been isolated from blood, compared to 14% of S. Typhimurium isolates (38). One of the leading serovars in Asia, S. Choleraesuis (group C1) has been found to be invasive in up to 56% of cases (37, 39). In Taiwan, S. Choleraesuis has a much higher odds ratio (44:1) of being recovered from blood rather than feces, compared to other NTS serovars (40). S. Dublin (serogroup D) is also one of the most invasive NTS serovars, with 64% of strains isolated from sterile sites (37). There is also a positive correlation (Spearman coefficient of 0.42; P < 0.002) between the invasiveness of a serovar and the hospitalization rate due to infection by the serovar (Fig. 3). Targeting these invasive serovars would therefore be important to reduce health care costs as well as indirect economic burdens (e.g., due to lost work days). In the United States alone, the annual costs of all Salmonella infections have recently been estimated to be $3.3 billion (41). The economic burden of Salmonella infection in humans has been estimated by the European Food Safety Authority to be 3 billion euros in the EU (42).
FIG 3.
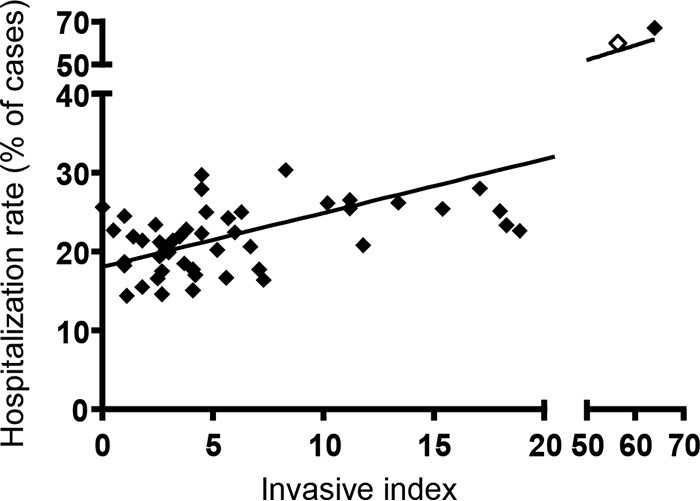
Correlation between invasiveness of a serovar and hospitalization rate. Hospitalization rates were defined as the percentages of salmonellosis patients who were hospitalized within 7 days following specimen collection. The invasive index was defined as the ratio of the number of specimens isolated from normally sterile sites and the total number of isolates of a serovar. Data for S. Choleraesuis (empty symbol) and S. Dublin (filled symbol) are shown on a separate scale, as they are much more invasive than other NTS. Data obtained from reference 37.
MULTIDRUG RESISTANCE OF SALMONELLA SEROGROUP C
In addition to invasiveness, emerging antibiotic resistance is a major concern for the control of nontyphoidal Salmonella. Many studies have reported an increase in multidrug-resistant Salmonella isolates (20, 22, 24, 43–45).
Analysis of antibiotic resistance patterns of the four most frequently isolated serovars in Greece between 2011 and 2012 (S. Enteritidis, S. Typhimurium, S. Newport, and S. Hadar) showed that 17% of serogroup C isolates were resistant to more than four antibiotics, compared to only 9% of the serogroup B isolates and none of the serogroup D strains (46). In Ethiopia, 81% of isolates of the most common serovar, S. Concord (serogroup C1), were found to be multidrug resistant (19).
The intensive use of antibiotics on animal farms has been linked to the appearance and spread of antibiotic resistance genes among several bacterial genera and species, including Salmonella (47). Even in countries that had reduced their use of antibiotics in animals destined for the food industry, persistence of previously acquired antimicrobial resistance was observed, suggesting that consequences of antimicrobial misuse may not be easily reversed (47). A study of NTS isolates from animals in Senegal and The Gambia showed that 83% of group C2 isolates were resistant to more than four antibiotics, compared to 50% of group B isolates (27). Only one group D isolate was tested, and it was found to be sensitive to all antibiotics used in this study. Strikingly, isolates of S. Hadar (serogroup C2) were found to be resistant to up to nine different antibiotics. A recent report of two S. Newport isolates obtained from the stools of pilgrims attending Hajj in Saudi Arabia being resistant to the “last-resort” antibiotic colistin only reinforces the need for another strategy to control Salmonella infections and prevent transmission to humans (48).
DIVERSITY OF SEROGROUP C SEROVARS CAUSING HUMAN DISEASE
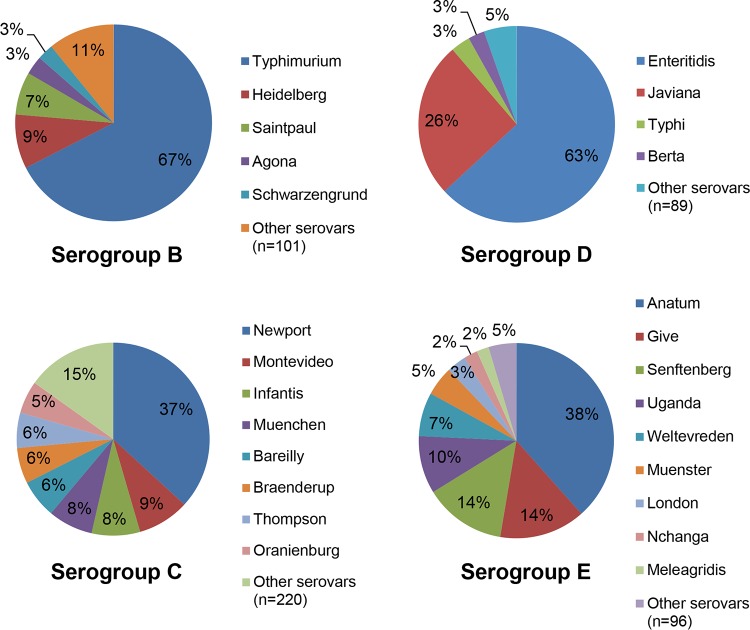
The overall number of serovars reported to have caused disease is highest for serogroup C; 228 different serogroup C serovars were isolated from human patients in the United States in 2012, compared to 106, 93, and 105 serovars of serogroups B, D, and E, respectively. When excluding serovars that cause less than 10 cases per year (accounting altogether for less than 1% of all cases), the remaining Salmonella infections due to serogroups B, D, and E were attributed to 17, 11, and 17 serovars, respectively (Fig. 4). However, infections due to serogroup C were caused by 37 serovars. The contributions of individual serovars within each serogroup is also striking (Fig. 5). While two (S. Enteritidis and S. Javiana) and three (S. Typhimurium, S. Heidelberg, and S. Saintpaul) serovars account for the majority of cases (80% or more) within serogroups D and B, respectively, seven serovars (S. Newport, S. Montevideo, S. Infantis, S. Muenchen, S. Bareilly, S. Braenderup, and S. Thompson) account for the same proportion within serogroup C cases. Serogroup E exhibits an intermediate distribution, with five serovars accounting for 80% or more of cases. These data suggest that successful vaccines against Salmonella serogroup C would need to elicit cross-protection against the entire serogroup rather than protection against a few individual serovars.
FIG 4.
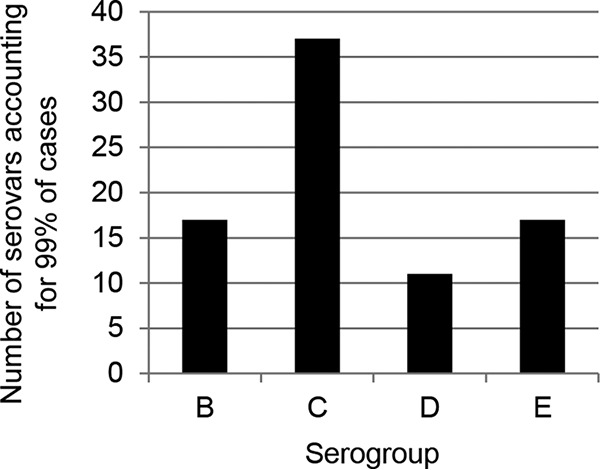
Number of serovars within each serogroup accounting for 99% of NTS cases in 2012. Data are from the U.S. Centers for Disease Control and Prevention.
FIG 5.
Contribution of individual serovars within serogroups isolated in the United States in 2012. Only serovars responsible for 2% or more of cases within each serogroup are represented individually. In 2012 in the United States, serovar I 4,[5],12:i:− accounted for 15.8% of all cases associated with serogroup B NTS. These data are included within the S. Typhimurium slice of the pie chart. Data are from the U.S. Centers for Disease Control and Prevention.
VACCINES AGAINST SEROGROUP C NTS
Although there has been very little work on development of vaccines that can protect humans against Salmonella serogroup C infections, there are a variety of vaccines licensed for use in animals. Lessons learned from these vaccines can help to guide development of human vaccines.
Animal vaccines.
Several vaccines have been developed to protect swine against S. Choleraesuis (serogroup C1) (49–53). They all rely on a single approach, wherein live attenuated strains of S. Choleraesuis have been obtained by chemical mutagenesis, targeted gene deletions (such as Δcya and Δcrp), or removal of the virulence plasmid. Several of these S. Choleraesuis vaccines have been licensed worldwide for use in animals (Table 3). The reason for targeting S. Choleraesuis in swine is due to its high carriage rate; it constituted 57.3% of isolates from swine in the United States in 2003 (54, 55). Moreover, this serovar is documented as among the most invasive Salmonella serovars. Protecting swine, along with poultry and other animals of agronomical importance, is an imperative under the recently introduced One Health Initiative (an initiative aimed at collaboration between physicians, veterinarians, and other scientific health and environmental professionals), another measure to reduce human infectious disease.
TABLE 3.
Vaccines against group C Salmonella currently licensed or in development
| Vaccine name | Type | Principle | Serovar(s) (serogroup) included | Usage | Company and/or reference |
|---|---|---|---|---|---|
| Licensed vaccinesa | |||||
| Nitro-Sal | Live attenuated | Chemical mutagenesis | Choleraesuis (C1) | Swine | Arko Laboratories (Jewel, IA, USA) |
| Nobl | Live attenuated | Loss of virulence plasmid | Choleraesuis (C1) | Swine | 51 |
| Argus-SC | Live attenuated | Gene deletions (Δcya and Δcrp) | Choleraesuis (C1) | Swine | 50 |
| Enterisol Salmonella T/C | Live attenuated | Public information not available | Choleraesuis (C1), Typhimurium (B) | Swine | Boehringer Ingelheim GmbH (Ingelheim, Germany) |
| Enterisol SC-54 | Live attenuated | Loss of virulence plasmid | Choleraesuis (C1) | Swine | Boehringer Ingelheim GmbH |
| Suisaloral | Live attenuated | Attenuating gene mutation | Choleraesuis (C1) | Swine | IDT Biologika GmbH (Desau-Roslau, Germany); 53 |
| Newport SRP | Component vaccine | Purified siderophore receptor and porin | Newport (C2) | Cattle | Zoetis Inc. (Florham Park, NJ, USA) |
| Vaccines in developmentb | |||||
| Salenvac | Killed vaccine | Inactivated whole-cell vaccine | Typhimurium (B), Mbandaka (C1), Orion (E) | Poultry | Intervet Schering-Plough Australia; 59 |
| Unknown | Killed vaccine (with adjuvant) | Inactivated whole-cell vaccine | Typhimurium (B), Infantis (C1), Enteritidis (D) | Poultry | 58 |
| TBD | Live attenuated | Attenuating gene deletions | Paratyphi C (C1), Newport (C2) | Human | F. J. Fuche and S. M. Tennant, Center for Vaccine Development (unpublished data) |
| TBD | Conjugate vaccine | Core O polysaccharide conjugated to flagellin | Paratyphi C (C1), Newport (C2) | Human | G. Ramachandran and R. Simon, Center for Vaccine Development (unpublished data) |
Data include vaccines with conditional licenses.
TBD, vaccine name to be determined.
Only one vaccine targets serogroup C2: the SRP vaccine (siderophore receptor and porin), developed by Zoetis Inc. This is a subunit component vaccine under conditional license, and it contains purified outer membrane proteins from S. Newport. This vaccine formulation has been shown to induce antibody production in cattle; however, no significant difference in fecal shedding of Salmonella or symptoms of disease were observed between vaccinated and unvaccinated groups (56, 57). Surprisingly, it was observed that vaccinated cattle produced more milk than unvaccinated cattle, although the mechanism for this remains unclear (56).
Vaccines currently in development include two trivalent whole-cell killed vaccines. The first vaccine targets serogroups B, C1, and E (serovars Typhimurium, Mbandaka, and Orion, respectively), and the second vaccine targets serogroups B, C1, and D (serovars Typhimurium, Infantis, and Enteritidis, respectively). Both were shown to confer protection against colonization and shedding of Salmonella in animals. Colonization after vaccination with the Typhimurium-Mbandaka-Orion-based vaccine was reduced from 50% of nonvaccinated animals to 9% of vaccinated hens challenged with S. Typhimurium, from 58% to 8% when challenged with S. Mbandaka (group C1), and from 17% to no detectable colonization when challenged with S. Orion (group E) (58, 59). Cross-protection was found to be complete against other serogroup C (S. Infantis) and E (S. Zanzibar) serovars but was only partial against the serogroup B serovar Agona. One study found incomplete protection against rechallenge in chickens that had previously been infected with wild-type serogroup C1 serovars (60). Here, despite eliciting both humoral (specific IgA, IgM, and IgG) and cellular immune responses (especially CD4- and CD8-positive T cells), infection with S. Virchow failed to protect against rechallenge with the same strain.
Human vaccines.
Most NTS vaccine efforts have been directed against S. Typhimurium and S. Enteritidis, as they are recognized as the main causes of gastroenteritis in developed countries such as the United States and of invasive disease in sub-Saharan Africa (2, 16). However, according to our reanalysis of existing data, in the United States serogroup C is one of the most prevalent serogroups and was the most common serogroup in 2012 (Fig. 1). Therefore, in an effort to prevent Salmonella-induced gastroenteritis, as well as invasive Salmonella infections, a vaccine that targets serogroup C Salmonella along with S. Typhimurium and S. Enteritidis is desirable. However, due to the diversity within serogroup C, cross-protection against seven serovars from that serogroup would be required to prevent >80% of Salmonella serogroup C infections. There are several vaccines in development against invasive NTS, such as S. Typhimurium and S. Enteritidis. A discussion of the S. Typhimurium and S. Enteritidis candidate vaccines is beyond the scope of this review and has been discussed elsewhere (14, 35). No licensed human vaccine against serogroup C NTS currently exists. Here at the Center for Vaccine Development of the University of Maryland School of Medicine, efforts to develop a live attenuated vaccine are under way, based on well-characterized attenuating gene deletions (14). Another strategy that has proven successful in generating functional immunity and protection against S. Typhimurium and S. Enteritidis relies on conjugate vaccines in which flagellin serves as a carrier for core O polysaccharide (61). We expect that, based on differences in their O polysaccharides, we would need vaccines against both serogroups C1 and C2 to elicit broad protection against all serogroup C serovars. Ultimately, one could combine these live attenuated or conjugate vaccines to protect against serogroups B, D, C1, and C2, therefore preventing the majority of salmonellosis cases (up to 88% of cases in the United States).
Vaccines in development should ideally generate both antibody- and cell-mediated immunity, as T cells are required for ultimate resolution of Salmonella infections (62–64). It was recently reported that cell-mediated immunity might be crucial for protection against NTS in individuals coinfected with malaria, a common comorbidity in parts of Africa and Asia where these pathogens are endemic (65). In that study, the protection elicited by an S. Typhimurium live attenuated vaccine was transiently lost as CD4 and CD8 T cells levels decreased during acute malarial episodes, despite elevated antibody titers. This loss of protection was shown to be reversible upon resolution of malaria, as CD4 and CD8 T cell levels recovered to premalaria levels, suggesting that antibodies alone were not sufficient to protect against S. Typhimurium. It was also shown in mice that despite generating high antibody titers, intraperitoneal immunization with three doses of formalin-killed S. Bovismorbificans (serogroup C2) did not protect against homologous challenge (66). Protection against challenge with a 100× 50% lethal dose (LD50) of virulent S. Bovismorbificans was, however, achieved by immunizing the mice twice with a live attenuated derivative strain (carrying an aroA deletion) via either the intraperitoneal route or the oral route: 75% of mice survived in either case. Attempts to generate a vaccine against S. Choleraesuis in the late 1980s demonstrated that an S. Typhimurium-based vaccine carrying the attenuating mutation ΔgalE conferred protection to BALB/c mice, whereas the corresponding S. Choleraesuis vaccine did not (67). The lack of protection was later suggested to be due to the inability of the vaccine strain to colonize the livers and spleens of vaccinated mice (68). The type of immunity generated is therefore an important factor for consideration when developing vaccines against serogroup C Salmonella.
CONCLUSIONS
NTS infections are the leading cause of DALYs in the United States among major foodborne pathogens (Campylobacter spp., Escherichia coli O157, Listeria monocytogenes, Clostridium perfringens, Toxoplasma gondii, and norovirus) (69). Among pathogens that cause gastroenteritis in developed countries, NTS is also the leading cause of hospitalization (0.6% to 3.9% of all NTS cases) and death (37 per 100,000 cases). Moreover, NTS infections are responsible for an underestimated burden of sequelae: 1 to 2.8% of cases can lead to reactive arthritis, and 5.7 to 16.6% of infections are thought to induce postinfectious irritable bowel syndrome (69, 70). A multivalent NTS vaccine would not only protect against acute gastroenteritis but also protect against these secondary diseases, therefore reducing the costs associated with long-term health care.
Vaccination of animals is an important way to protect both humans and animals, since most human disease sources are linked to animal-related food products. However, vaccinating only farm animals might not be sufficient to prevent disease in humans. A major outbreak of S. Thompson in the Netherlands in 2012 was attributed to smoked salmon (71). Multiple outbreaks have also been linked to fruits and vegetables. Cases of contamination after handling pet food have also been reported (6). Moreover, following introduction of vaccination and improved sanitation in swine in Taiwan, a decrease in human cases due to S. Choleraesuis was observed between 2004 (4.3% of all serogroup C infections) and 2007 (0.84%) (72). However, that decrease was associated with an increase in infections due to other serogroup C serovars (from 16.5% of all serogroup C cases in 2004 to 33.7% in 2007). This suggests that targeting only one serovar may lead to emergence of other serovars, indicating that a multivalent vaccine that can protect against all serogroup C Salmonella (and possibly all NTS) is needed for complete protection.
Funding Statement
This work was funded by Project 4, Vaccine Strategy for Broad-Spectrum Protection against Non-Typhoidal Salmonella (project leaders, Sharon M. Tennant and Raphael Simon), NIH/NIAID Centers for Excellence in Translation Research grant U19 AI109776-01, awarded to Myron M. Levine.
REFERENCES
- 1.Grimont PAD, Weill F-X. 2007. Antigenic formulae of the Salmonella serovars, 9th ed WHO Collaborating Centre for Reference and Research on Salmonella. Institut Pasteur, Paris, France. [Google Scholar]
- 2.Gal-Mor O, Boyle EC, Grassl GA. 2014. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckle GC, Walker CLF, Black RE. 2012. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2:010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease 2013 DALYs and HALE Collaborators. 2015. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet 386:2145–2191. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 6.Cavallo SJ, Daly ER, Seiferth J, Nadeau AM, Mahoney J, Finnigan J, Wikoff P, Kiebler CA, Simmons L. 2015. Human outbreak of Salmonella Typhimurium associated with exposure to locally made chicken jerky pet treats, New Hampshire, 2013. Foodborne Pathog Dis 12:441–446. doi: 10.1089/fpd.2014.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behravesh CB, Brinson D, Hopkins BA, Gomez TM. 2014. Backyard poultry flocks and salmonellosis: a recurring, yet preventable public health challenge. Clin Infect Dis 58:1432–1438. doi: 10.1093/cid/ciu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C, Stein C, Angulo FJ, Devleesschauwer B. 2015. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 12:e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerra B, Fischer J, Helmuth R. 2014. An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet Microbiol 171:290–297. doi: 10.1016/j.vetmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Taneja N, Appannanavar SB, Kumar A, Varma G, Kumar Y, Mohan B, Sharma M. 2014. Serotype profile and molecular characterization of antimicrobial resistance in non-typhoidal Salmonella isolated from gastroenteritis cases over nine years. J Med Microbiol 63:66–73. doi: 10.1099/jmm.0.061416-0. [DOI] [PubMed] [Google Scholar]
- 11.Germanier R, Füer E. 1975. Isolation and characterization of galE mutant Ty21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis 131:553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 12.Messens W, Vivas-Alegre L, Bashir S, Amore G, Romero-Barrios P, Hugas M. 2013. Estimating the public health impact of setting targets at the European level for the reduction of zoonotic Salmonella in certain poultry populations. Int J Environ Res Public Health 10:4836–4850. doi: 10.3390/ijerph10104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacket CO, Ferreccio C, Robbins JB, Tsai CM, Schulz D, Cadoz M, Goudeau A, Levine MM. 1986. Safety and immunogenicity of two Salmonella typhi Vi capsular polysaccharide vaccines. J Infect Dis 154:342–345. doi: 10.1093/infdis/154.2.342. [DOI] [PubMed] [Google Scholar]
- 14.Tennant SM, Levine MM. 2015. Live attenuated vaccines for invasive Salmonella infections. Vaccine 33(Suppl 3):C36–C41. doi: 10.1016/j.vaccine.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy EA, Shaw AV, Crump JA. 2010. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nga TVT, Parry CM, Le T, Lan NPH, Diep TS, Campbell JI, Hoang NVM, Dung LT, Wain J, Dolecek C, Farrar JJ, Chau NVV, Hien TT, Day JN, Baker S. 2012. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern Vietnam. Trans R Soc Trop Med Hyg 106:26–34. doi: 10.1016/j.trstmh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Carrique-Mas JJ, Bryant JE. 2013. A review of foodborne bacterial and parasitic zoonoses in Vietnam. Ecohealth 10:465–489. doi: 10.1007/s10393-013-0884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadesse G. 2014. Prevalence of human salmonellosis in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis 14:88. doi: 10.1186/1471-2334-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J, Githinji JW, Kagendo D, Munyalo A, Hart CA. 2006. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol 55:585–591. doi: 10.1099/jmm.0.46375-0. [DOI] [PubMed] [Google Scholar]
- 21.Ben Aissa R, Al-Gallas N, Troudi H, Belhadj N, Belhadj A. 2007. Trends in Salmonella enterica serotypes isolated from human, food, animal, and environment in Tunisia, 1994-2004. J Infect 55:324–339. doi: 10.1016/j.jinf.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Kuo H-C, Lauderdale T-L, Lo D-Y, Chen C-L, Chen P-C, Liang S-Y, Kuo J-C, Liao Y-S, Liao C-H, Tsao C-S, Chiou C-S. 2014. An association of genotypes and antimicrobial resistance patterns among Salmonella isolates from pigs and humans in Taiwan. PLoS One 9:e95772. doi: 10.1371/journal.pone.0095772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ke B, Sun J, He D, Li X, Liang Z, Ke C. 2014. Serovar distribution, antimicrobial resistance profiles, and PFGE typing of Salmonella enterica strains isolated from 2007-2012 in Guangdong, China. BMC Infect Dis 14:338. doi: 10.1186/1471-2334-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elhadi N, Aljindan R, Aljeldah M. 2013. Prevalence of nontyphoidal Salmonella serogroups and their antimicrobial resistance patterns in a university teaching hospital in Eastern Province of Saudi Arabia. Infect Drug Resist 6:199–205. doi: 10.2147/IDR.S51184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. 2014. FoodNet surveillance report for 2012 (final report). CDC, Atlanta, GA. [Google Scholar]
- 26.Eurosurveillance Editorial Team. 2012. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. Euro Surveill 17(10):pii=20113 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20113. [PubMed] [Google Scholar]
- 27.Kwambana-Adams B, Darboe S, Nabwera H, Foster-Nyarko E, Ikumapayi UN, Secka O, Betts M, Bradbury R, Wegmüller R, Lawal B, Saha D, Hossain MJ, Prentice AM, Kampmann B, Anderson S, Dalessandro U, Antonio M. 2015. Salmonella infections in The Gambia, 2005-2015. Clin Infect Dis 61:S354–S362. doi: 10.1093/cid/civ781. [DOI] [PubMed] [Google Scholar]
- 28.Bangtrakulnonth A, Pornreongwong S, Pulsrikarn C, Sawanpanyalert P, Hendriksen RS, Wong DMALF, Aarestrup FM. 2004. Salmonella serovars from humans and other sources in Thailand, 1993-2002. Emerg Infect Dis 10:131–136. doi: 10.3201/eid1001.02-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domingues AR, Vieira AR, Hendriksen RS, Pulsrikarn C, Aarestrup FM. 2014. Spatio-temporal analysis of Salmonella surveillance data in Thailand. Epidemiol Infect 142:1614–1624. doi: 10.1017/S095026881300215X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aseffa A, Mengistu G, Tiruneh M. 1994. Salmonella newport: outbreak of food poisoning among college students due to contaminated undercooked eggs. Ethiop Med J 32:1–6. [PubMed] [Google Scholar]
- 31.CDC. 2002. Outbreak of multidrug-resistant Salmonella newport—United States, January-April 2002. MMWR Morb Mortal Wkly Rep 51:545–548. [PubMed] [Google Scholar]
- 32.Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. 2013. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998-2008. Emerg Infect Dis 19:1239–1244. doi: 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.USDA Food Inspection Service. 2010. Serotypes profile of Salmonella isolates from meat and poultry products: January 1998 through December 2010. USDA, Washington, DC. [Google Scholar]
- 34.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. 2015. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis 21:941–949. doi: 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLennan CA, Martin LB, Micoli F. 2014. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccin Immunother 10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbour EK, Ayyash DB, Alturkistni W, Alyahiby A, Yaghmoor S, Iyer A, Yousef J, Kumosani T, Harakeh S. 2015. Impact of sporadic reporting of poultry Salmonella serovars from selected developing countries. J Infect Dev Ctries 9:1–7. doi: 10.3855/jidc.5065. [DOI] [PubMed] [Google Scholar]
- 37.Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, Hurd S, Medus C, Cronquist A, Angulo FJ. 2008. Salmonellosis outcomes differ substantially by serotype. J Infect Dis 198:109–114. doi: 10.1086/588823. [DOI] [PubMed] [Google Scholar]
- 38.Beyene G, Nair S, Asrat D, Mengistu Y, Engers H, Wain J. 2011. Multidrug resistant Salmonella Concord is a major cause of salmonellosis in children in Ethiopia. J Infect Dev Ctries 5:23–33. doi: 10.3855/jidc.906. [DOI] [PubMed] [Google Scholar]
- 39.Vugia DJ, Samuel M, Farley MM, Marcus R, Shiferaw B, Shallow S, Smith K, Angulo FJ, Emerging Infections Program FoodNet Working Group. 2004. Invasive Salmonella infections in the United States, FoodNet, 1996-1999: incidence, serotype distribution, and outcome. Clin Infect Dis 38:S149–S156. doi: 10.1086/381581. [DOI] [PubMed] [Google Scholar]
- 40.Hendriksen RS, Bangtrakulnonth A, Pulsrikarn C, Pornruangwong S, Noppornphan G, Emborg H-D, Aarestrup FM. 2009. Risk factors and epidemiology of the ten most common Salmonella serovars from patients in Thailand: 2002-2007. Foodborne Pathog Dis 6:1009–1019. doi: 10.1089/fpd.2008.0245. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann S, Batz MB, Morris JG Jr. 2012. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- 42.European Food Safety Authority. 2014. EFSA fact sheet on Salmonella. European Food Safety Authority, Parma, Italy. [Google Scholar]
- 43.Bae D, Cheng C-M, Khan AA. 2015. Characterization of extended-spectrum β-lactamase (ESBL) producing non-typhoidal Salmonella (NTS) from imported food products. Int J Food Microbiol 214:12–17. doi: 10.1016/j.ijfoodmicro.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Dione MM, Geerts S, Antonio M. 2012. Characterisation of novel strains of multiply antibiotic-resistant Salmonella recovered from poultry in Southern Senegal. J Infect Dev Ctries 6:436–442. doi: 10.3855/jidc.1530. [DOI] [PubMed] [Google Scholar]
- 45.Miriagou V, Tassios PT, Legakis NJ, Tzouvelekis LS. 2004. Expanded-spectrum cephalosporin resistance in non-typhoid Salmonella. Int J Antimicrob Agents 23:547–555. doi: 10.1016/j.ijantimicag.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Maraki S, Papadakis IS. 2014. Serotypes and antimicrobial resistance of human nontyphoidal isolates of Salmonella enterica from Crete, Greece. Interdiscip Perspect Infect Dis 2014:256181. doi: 10.1155/2014/256181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Migura L, Hendriksen RS, Fraile L, Aarestrup FM. 2014. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: the missing link between consumption and resistance in veterinary medicine. Vet Microbiol 170:1–9. doi: 10.1016/j.vetmic.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Olaitan AO, Dia NM, Gautret P, Benkouiten S, Belhouchat K, Drali T, Parola P, Brouqui P, Memish Z, Raoult D, Rolain J-M. 2015. Acquisition of extended-spectrum cephalosporin- and colistin-resistant Salmonella enterica subsp. enterica serotype Newport by pilgrims during Hajj. Int J Antimicrob Agents 45:600–604. doi: 10.1016/j.ijantimicag.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Kelly SM, Bosecker BA, Curtiss R. 1992. Characterization and protective properties of attenuated mutants of Salmonella choleraesuis. Infect Immun 60:4881–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy MJ, Yancey RJ, Sanchez MS, Rzepkowski RA, Kelly SM, Curtiss R. 1999. Attenuation and immunogenicity of Δcya Δcrp derivatives of Salmonella choleraesuis in pigs. Infect Immun 67:4628–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roof MB, Doitchinoff DD. 1995. Safety, efficacy, and duration of immunity induced in swine by use of an avirulent live Salmonella choleraesuis-containing vaccine. Am J Vet Res 56:39-44. [PubMed] [Google Scholar]
- 52.Ku Y-W, McDonough SP, Palaniappan RUM, Chang C-F, Chang Y-F. 2005. Novel attenuated Salmonella enterica serovar Choleraesuis strains as live vaccine candidates generated by signature-tagged mutagenesis. Infect Immun 73:8194–8203. doi: 10.1128/IAI.73.12.8194-8203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schöll W, Grünert G. 1980. Suisaloral “Dessau”: a Salmonella cholerae suis live vaccine for oral, parenteral and combined applications. Arch Exp Veterinärmed 34:91–97. [PubMed] [Google Scholar]
- 54.Chiu C-H, Su L-H, Chu C. 2004. Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clin Microbiol Rev 17:311–322. doi: 10.1128/CMR.17.2.311-322.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clothier KA, Kinyon JM, Frana TS. 2010. Comparison of Salmonella serovar isolation and antimicrobial resistance patterns from porcine samples between 2003 and 2008. J Vet Diagn Invest 22:578–582. doi: 10.1177/104063871002200412. [DOI] [PubMed] [Google Scholar]
- 56.Hermesch DR, Thomson DU, Loneragan GH, Renter DR, White BJ. 2008. Effects of a commercially available vaccine against Salmonella enterica serotype Newport on milk production, somatic cell count, and shedding of Salmonella organisms in female dairy cattle with no clinical signs of salmonellosis. Am J Vet Res 69:1229–1234. doi: 10.2460/ajvr.69.9.1229. [DOI] [PubMed] [Google Scholar]
- 57.Smith GW, Alley ML, Foster DM, Smith F, Wileman BW. 2014. Passive immunity stimulated by vaccination of dry cows with a Salmonella bacterial extract. J Vet Intern Med 28:1602–1605. doi: 10.1111/jvim.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deguchi K, Yokoyama E, Honda T, Mizuno K. 2009. Efficacy of a novel trivalent inactivated vaccine against the shedding of Salmonella in a chicken challenge model. Avian Dis 53:281–286. doi: 10.1637/8516-110908-Reg.1. [DOI] [PubMed] [Google Scholar]
- 59.Pavic A, Groves PJ, Cox JM. 2010. Utilization of a novel autologous killed tri-vaccine (serogroups B [Typhimurium], C [Mbandaka] and E [Orion]) for Salmonella control in commercial poultry breeders Avian Pathol 39:31–39. doi: 10.1080/03079450903454277. [DOI] [PubMed] [Google Scholar]
- 60.Salisbury A-M, Leeming G, Nikolaou G, Kipar A, Wigley P. 2014. Salmonella Virchow infection of the chicken elicits cellular and humoral systemic and mucosal responses, but limited protection to homologous or heterologous re-challenge. Front Vet Sci 1:6. doi: 10.3389/fvets.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon R, Levine MM. 2012. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Hum Vaccin Immunother 8:494–498. doi: 10.4161/hv.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. 1993. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun 61:3981–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moon JJ, McSorley SJ. 2009. Tracking the dynamics of Salmonella specific T cell responses. Curr Top Microbiol Immunol 334:179–198. doi: 10.1007/978-3-540-93864-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McSorley SJ, Jenkins MK. 2000. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun 68:3344–3348. doi: 10.1128/IAI.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mooney JP, Lee S-J, Lokken KL, Nanton MR, Nuccio S-P, McSorley SJ, Tsolis RM. 2015. Transient loss of protection afforded by a live attenuated non-typhoidal Salmonella vaccine in mice co-infected with malaria. PLoS Negl Trop Dis 9:e0004027. doi: 10.1371/journal.pntd.0004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukkur TK, Stocker BA, Walker KH. 1991. Genetic manipulation of Salmonella serotype Bovismorbificans to aromatic-dependence and evaluation of its vaccine potential in mice. J Med Microbiol 34:57–62. doi: 10.1099/00222615-34-1-57. [DOI] [PubMed] [Google Scholar]
- 67.Nnalue NA, Stocker BA. 1987. Test of the virulence and live-vaccine efficacy of auxotrophic and galE derivatives of Salmonella choleraesuis. Infect Immun 55:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nnalue NA, Stocker BAD. 1989. Vaccination route, infectivity and thioglycollate broth administration: effects on live vaccine efficacy of auxotrophic derivatives of Salmonella choleraesuis. Microb Pathog 7:299–310. doi: 10.1016/0882-4010(89)90048-X. [DOI] [PubMed] [Google Scholar]
- 69.Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. 2015. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect 143:2795–2804. doi: 10.1017/S0950268814003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haagsma JA, Siersema PD, De Wit NJ, Havelaar AH. 2010. Disease burden of post-infectious irritable bowel syndrome in The Netherlands. Epidemiol Infect 138:1650–1656. doi: 10.1017/S0950268810000531. [DOI] [PubMed] [Google Scholar]
- 71.Friesema I, de Jong A, Hofhuis A, Heck M, van den Kerkhof H, de Jonge R, Hameryck D, Nagel K, van Vilsteren G, van Beek P, Notermans D, van Pelt W. 2014. Large outbreak of Salmonella Thompson related to smoked salmon in the Netherlands, August to December 2012. Euro Surveill 19(39):pii=20918. doi: 10.2807/1560-7917.ES2014.19.39.20918. [DOI] [PubMed] [Google Scholar]
- 72.Chiou C-S, Lin J-M, Chiu C-H, Chu C-H, Chen S-W, Chang Y-F, Weng B-C, Tsay J-G, Chen C-L, Liu C-H, Chu C. 2009. Clonal dissemination of the multidrug resistant Salmonella enterica serovar Braenderup, but not the serovar Bareilly, of prevalent serogroup C1 Salmonella from Taiwan. BMC Microbiol 9:264. doi: 10.1186/1471-2180-9-264. [DOI] [PMC free article] [PubMed] [Google Scholar]