Abstract
The analgesic mechanism of opioids is known to decrease the excitability of substantia gelatinosa (SG) neurons receiving the synaptic inputs from primary nociceptive afferent fiber by increasing inwardly rectifying K+ current. In this study, we examined whether a µ-opioid agonist, [D-Ala2,N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO), affects the two-pore domain K+ channel (K2P) current in rat SG neurons using a slice whole-cell patch clamp technique. Also we confirmed which subtypes of K2P channels were associated with DAMGO-induced currents, measuring the expression of K2P channel in whole spinal cord and SG region. DAMGO caused a robust hyperpolarization and outward current in the SG neurons, which developed almost instantaneously and did not show any time-dependent inactivation. Half of the SG neurons exhibited a linear I~V relationship of the DAMGO-induced current, whereas rest of the neurons displayed inward rectification. In SG neurons with a linear I~V relationship of DAMGO-induced current, the reversal potential was close to the K+ equilibrium potentials. The mRNA expression of TWIK (tandem of pore domains in a weak inwardly rectifying K+ channel) related acid-sensitive K+ channel (TASK) 1 and 3 was found in the SG region and a low pH (6.4) significantly blocked the DAMGO-induced K+ current. Taken together, the DAMGO-induced hyperpolarization at resting membrane potential and subsequent decrease in excitability of SG neurons can be carried by the two-pore domain K+ channel (TASK1 and 3) in addition to inwardly rectifying K+ channel.
Keywords: DAMGO, K+ current, Opioid, SG neuron, TASK
INTRODUCTION
The substantia gelatinosa (SG, Rexed lamina II) of the spinal cord, is involved in the transmission of nociceptive information from periphery to the central nervous system [1]. SG neurons receive pain signal through glutamatergic synaptic inputs of primary Aδ and C-afferent fibers [2] and are modulated by the descending inhibitory system. In the endogenous descending analgesic system of the spinal SG region, opioids act as an important neurotransmitter and their receptors, particularly the µ-opioid receptors, are located abundantly in the superficial layers of the spinal cord, particularly in SG in rats [3,4,5] and humans [6].
The mechanisms mediated by opioid analgesics also include the inhibition of voltage-gated Ca2+ channels of primary afferent terminals, the decrease of excitability in spinal dorsal horn neuron, descending inhibitory pathway, and perception of pain sensation in the somatosensory cortex. In the spinal cord, opioids have been known that the activation of µ-opioid receptors enhance the opening of K+ channels and then inhibit the nociceptive transmission in rat superficial dorsal horn neurons [7,8]. A previous electrophysiological study using rat spinal cord slices demonstrated that [D-Ala2,N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO), a selective µ-opioid receptor agonist, induced outward currents via activating µ-opioid receptors in SG neurons [9], which suggests that the µ-opioid receptors are associated with the opioid analgesic properties in spinal cord. In addition, the activation of presynaptic µ-opioid receptors inhibited the voltage-gated Ca2+ channel of the dorsal root ganglion (DRG) neurons to reduce primary afferent synaptic transmission [10,11]. These findings demonstrated that the activation of presynaptic and postsynaptic µ-opioid receptor were involved in modulation of synaptic transmission in the spinal dorsal horn.
The excitability of SG neurons is determined by leak K+ channels as well as Gi/o-protein coupled inwardly rectifying K+ (GIRK) channels and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which can contribute to the antinociceptive action of DAMGO in the spinal cord. Previous studies demonstrated that the activation of postsynaptic µ-opioid receptors led to the crossing of the nociceptive threshold by the activation of GIRK channels [9] and the inhibition of HCN channels [12]. However, the effect of DAMGO on leak K+ current, which plays an important role in controlling resting membrane potential (RMP), has not been reported. Leak K+ channels (K2P channels) have four transmembrane sparing region and two-pore forming loops and can be classified into the following six subfamilies; Tandem of pore domains in a weak inwardly rectifying K+ channel (TWIK), TWIK related acid-sensitive K+ channel (TASK), TWIK-related K+ channel (TREK), TWIK-related alkaline pH-activated K+ channel (TLAK), Tandem pore domain halothane-inhibited K+ channel (THIK), and TWIK-related spinal cord K+ channel (TRESK) [13,14]. These also can be modulated by various chemicals, such as neurotransmitters, secondary messengers, lipids, pH, anesthetics, and oxygen pressure [13]. Although the expression of K2P channels has been observed in the SG region [15], there is no evidence about how it is regulated.
In the present study, we investigated the effects of DAMGO on the RMP and determined the underlying postsynaptic ionic mechanism of DAMGO in SG neurons. For this purpose we recorded both the membrane potential and the ionic current from SG neurons using whole-cell patch clamp recordings in a spinal cord slice preparation of juvenile rats and showed the mRNA expression of the subtype of K2P channels.
METHODS
All experimental procedures for animal use were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), Medical School, Hanyang University (IACUC approval No. HY-IACUC-15-0057). All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health. The 10~14 days old male Sprague–Dawley rats (OrientBio, Korea) were used. Rats were housed in a conventional facility with a 12:12 h light cycle (lights on 8:00 am) and ad libitum access to water and chow and were acclimatized at least 1 week prior to experiments.
Slice preparation
To prepare the spinal slices, juvenile rats (10~14 days old) were sacrificed by cervical dislocation and a lumbosacral laminectomy was subsequently performed. The lumbosacral segments of the spinal cord were removed and placed in ice-cold artificial cerebrospinal fluid (ACSF). Transverse spinal slices approximately 300 µm thick were cut using a vibratome (752H, Campden Instruments, Loughbrough, UK), incubated at 32℃ for a recovery of 1 h before use, and then were transferred to a recording chamber filled with ACSF equilibrated with 95% O2/5% CO2 at temperature of 22±1℃.
Electrophysiological recordings
SG neurons were visually identified using a fixed-stage microscope (BX50WI, Olympus, Tokyo, Japan) with Nomarski optics and a 40×water-immersion objective. Patch pipettes were fabricated from borosilicate capillaries (Chase Scientific Glass, Inc., Rockwood, TN) by using a pipette puller (PP-83 puller, Narishige, Tokyo, Japan). The resistance of the pipettes was 4~5 MΩ when filled with internal solutions. The currents and voltages of SG neurons were recorded using an EPC-9 amplifier and Pulse software (HEKA, Lambrecht, Germany).
Solutions and drugs
In all experiments, ACSF contained 130 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 1.5 mM MgCl2, 1.25 mM NaH2PO4, 20 mM NaHCO3, 10 mM glucose, and 10 mM sucrose. It was continually aerated with 95% O2/5% CO2, which maintained its pH at approximately 7.4. In some experiments, ACSF with 1 mM CsCl or 1 mM BaCl2 was used to block HCN currents or GIRK currents, respectively. To block the synaptic inputs, 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Sigma), 10 µM bicuculline methiodide (Tocris, Avonmouth, England) and 1 µM strychnine hydrochloride (Tocris) were added to ACSF. Osmolarity and pH of the internal solution in all experiments were adjusted to 295~300 mOsmol and pH 7.4 using KOH. The internal solution for whole-cell recordings consisted of 136 mM K-gluconate, 10 mM NaCl, 1 mM MgCl2, 0.5 mM EGTA, 10 mM HEPES, 2 mM Mg-ATP, and 0.1 mM Na2-GTP. DAMGO and naloxone (Sigma, USA) were first dissolved in DMSO as a stock solution (30 mM) that was then used at the final concentration in ACSF. This final concentration of DMSO had no observable effect on the membrane potential of the SG neurons. All experiments were performed at room temperature (22℃±1℃).
RT-PCR
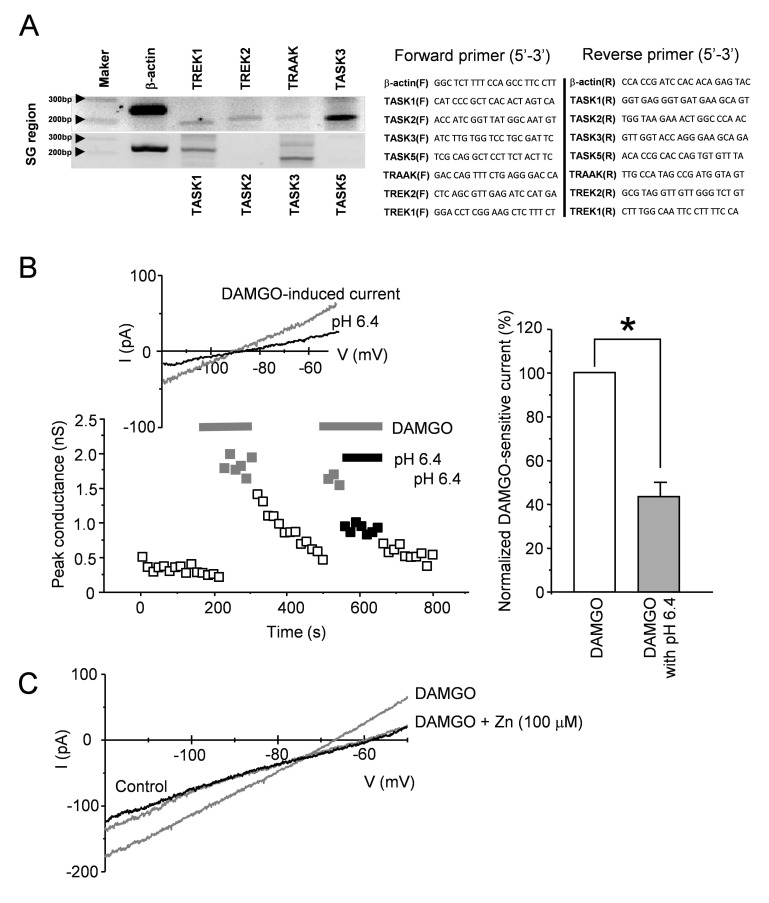
The expression of mRNA encoding two pore domain K+ channels in SG region of spinal cord of 14 days old male rats was confirmed. Total RNA was extracted from each tissue using RNAiso Plus (TaKaRa, Japan). cDNA was generated by the reverse transcription system (Invitrogen Life Technologies), according to the manufacturer's instructions. RT-PCR was performed using cDNA as a template. The primers prepared for this RT-PCR are shown in Fig. 4A. Total RNA was reverse transcribed for 1 h at 42.1℃ using ImProm-II Reverse Transcriptase (Promega, Madison, WI). The PCR products were resolved using 1% agarose gel electrophoresis and were stained using ethidium bromide.
Fig. 4. RT-PCR analysis and electrophysiological property of K2P channels in the SG region.
(A) The mRNA expression of two pore domain K channels (TREK1, TREK2, TRAAK, and TASK3) was observed in SG. Of TASK family, mRNA expression of TASK1 and 3 was only detected in SG, while TASK2 and 5 were not found. Arrow heads indicate 200 and 300 bp, and each size of RT-PCR products is as follows; β-actin 252 bp, TRAAK 198 bp, TREK1 194 bp, TREK2 201 bp, TASK1 244 bp, TASK2 157 bp, TASK3 194 bp, and TASK5 176 bp. (B) The effect of acidic pH on DAMGO-induced current. Low pH (pH 6.4) blocked DAMGO-induced current and increment of slope conductance by DAMGO was reduced in pH 6.4 condition. (C) The effect of Zn2+ on DAMGO-induced current. In some SG neurons (n=4/7), application of ZnCl2 (100 µM) blocked DAMGO-induced current, while others were not altered by ZnCl2 (n=3/7).
Statistical analysis
Statistical analysis of the patch clamp data was performed using Origin 6.1 software (MicroCal, Northampton, MA). A linear regression analysis of reversal potential (Erev) as a function of log [K+]O, Erev=A+slop×[K+]O, was performed against the experimental data. Data were compared using a Student's paired t-test and differences were considered to be significant when the p value was <0.05. All data are presented as mean±SEM; the number of cells tested is indicated in parentheses where applicable.
RESULTS
DAMGO, a µ-opioid agonist, reduced the excitability of SG neurons
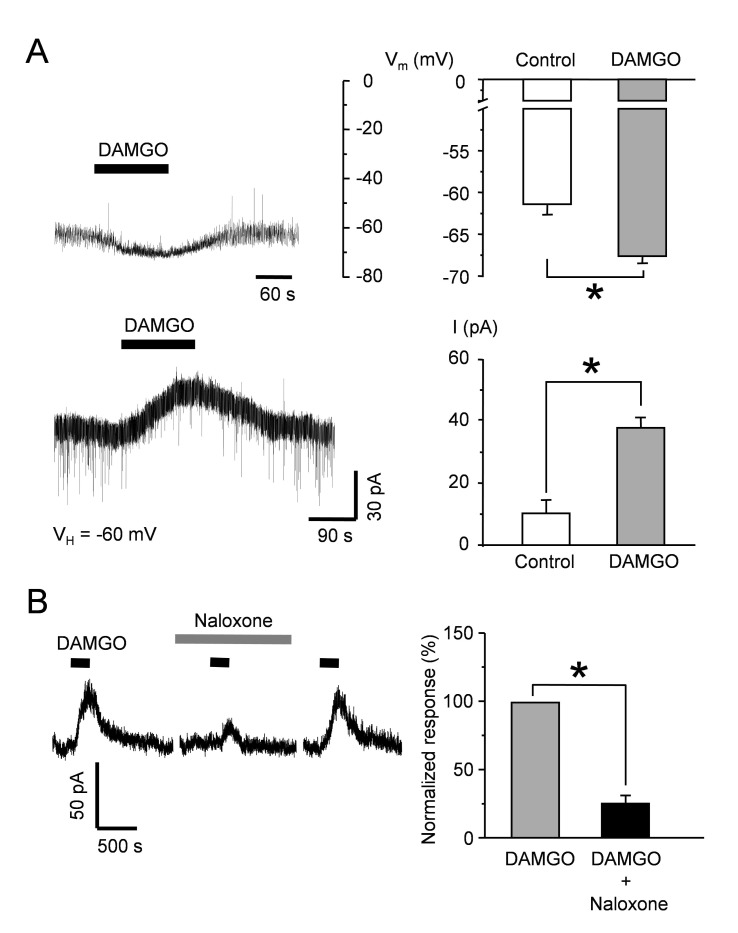
We examined whether DAMGO, a µ-opioid agonist, induces a reversible hyperpolarization of RMP in SG neurons. Application of 1 µM DAMGO (2 min) induced a reproducible and robust hyperpolarization from −61.4±1.2 mV to −67.6±0.8 mV (n=10, p<0.05), with the outward current at a holding potential of −60 mV (control value of 10.1±4.1 pA and DAMGO 37.8±3.2 pA, n=10) (Fig. 1A). Naloxone (1 µM), a broad opioid receptor antagonist, blocked DAMGO-induced outward current (25.3±5.7%; n=8; p <0.05), indicating that the reduced excitability of SG neurons in the presence of DAMGO was mediated through the activation of µ-opioid receptors (Fig. 1B).
Fig. 1. The effect of DAMGO on resting membrane potential (RMP) in SG neuron.
(A) In current clamp condition, the application of DAMGO (1 µM) caused a reversible hyperpolarization of RMP in SGN (−61.4±1.2 mV, control RMP; −67.6±0.8 mV, DAMGO; n=10). In voltage clamp condition at −60 mV, DAMGO induced an outward current in SG neurons with time course similar to the hyperpolarization (control value of 10.1±4.1 pA and DAMGO 37.8±3.2 pA, n=10, respectively). This experiment was conducted in normal ACSF. (B) The inhibitory effect of naloxone (1 µM) on DAMGO-induced outward current. Data are presented as mean±SEM and asterisks (*)indicated a significant difference at p<0.05 (paired t-test).
DAMGO-induced currents are associated with GIRK channels and K2P channels
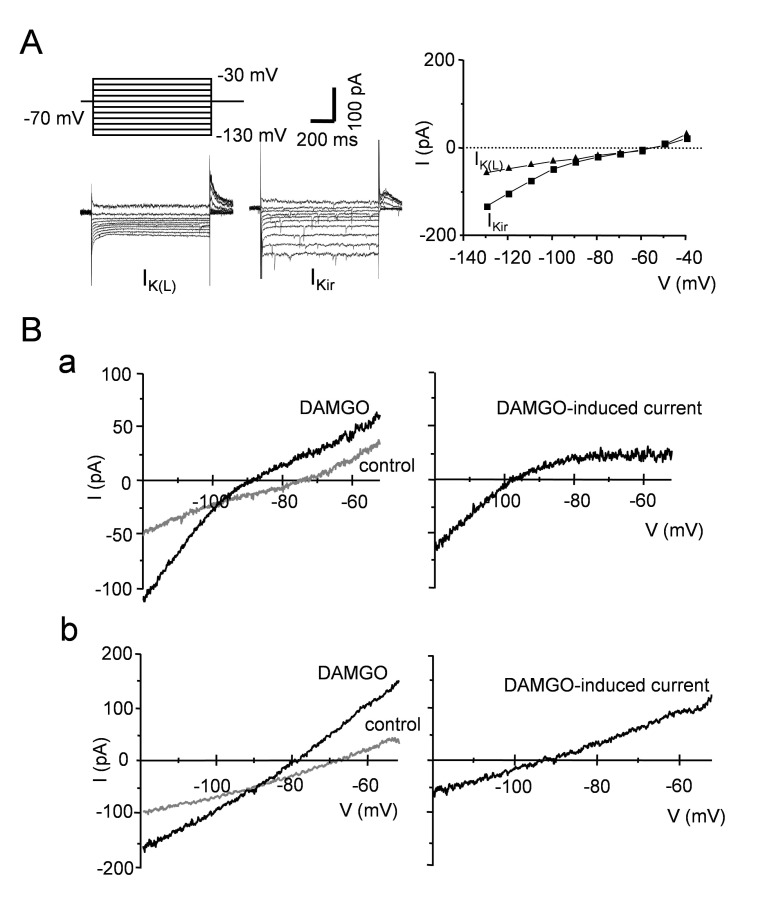
Next, we investigated which channels were involved in the DAMGO- induced current. Using voltage clamp mode, the step pulse developed instantaneously and did not show any time-dependent inactivation. Based on the current-voltage (I~V) relationship, both GIRK channels as well as K2P channels are involved in the maintenance of RMP of SG neurons (Fig. 2A). To obtain the I~V relationship of the DAMGO- induced current, ramp pulses (from −130 to −40 mV, duration of 1 s) were applied to the SG neurons (Fig. 2B). In 3 of 25 neurons, the slope conductance at −50 mV was almost null, exhibiting pure inwardly rectification. This result was consistent with a previous study which reported the activation of GIRK channels by DAMGO. However, half of the neurons (n=13) exhibited a linear I~V relationship with reversal potential of −90 mV, suggesting that DAMGO may open leak K+ channels. In the rest of the neurons (n=9), the DAMGO- induced currents were a mixture of leak K+ channels and GIRK channels (not shown).
Fig. 2. The I-V relationship of DAMGO-induced current.
The I~V relationship of the DAMGO-induced current was diverse. Currents were activated by ramp pulses (from −120 to −40 mV). Three neurons exhibited the pure inward rectification (IKir). Half of neurons (n=13) exhibited a linear relationship suggesting DAMGO opens IK(L).
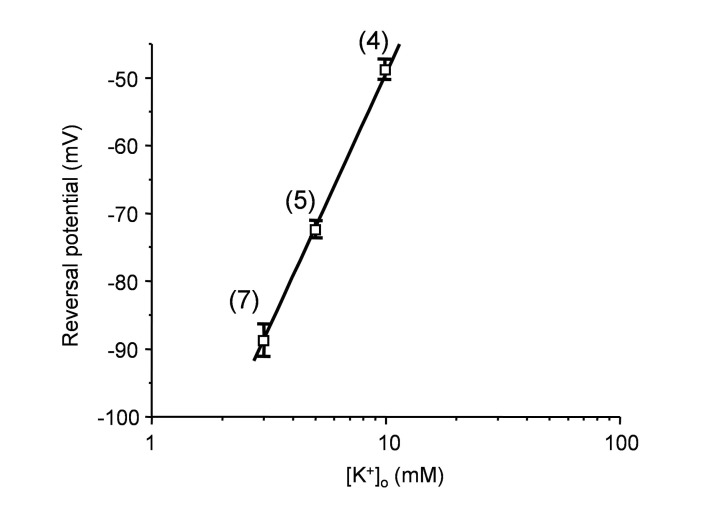
Next, we tested whether the DAMGO- induced current with a linear I~V relationship is related to the change of external K+ concentration ([K+]O). The reversal potential of DAMGO-induced current at different [K+]O was plotted on a semilog scale. The reversal potentials of DAMGO- induced current at [K+]O of 3, 5, and 10 mM were −88.2±2.7 mV, −73.8±1.3 mV, and −48.7±1.8 mV, respectively. The fitting of the reversal potential was linear, and the slope of the regression line was 60.2, which is close to the value predicted by the Nernst equation (58.8 at 23℃), indicating that the DAMGO-induced current is exclusively carried by K+ (Fig. 3).
Fig. 3. The reversal potential of DAMGO-induced current with linear relationship.
The reversal potential of DAMGO-induced current at different [K+]O was plotted on a semilog scale. The slope of a regression line (60.2) is close to the value predicted by the Nernst equation (58.8 at 23℃), indicating that the current is through K+-selective channels.
Two-pore domain K+ ion channel in spinal SG was activated by DAMGO
Next, we examined whether K2P channels in the SG region are related to the DAMGO-induced leak K+ channel. Of the K2P channels, members of the TREK, TRAAK and TASK families, which have previously documented expression in the superficial dorsal horn (SDH) [15], were selected. As shown in Fig. 4A, mRNAs of TREK1, TREK2, TRAAK, and TASK3 were detected in the SG region and TASK3 was more highly expressed compared with others (TREK1, TREK2 and TRAAK). In the next experiment, we confirmed the expression of the TASK subfamily (TASK1, 2, 3, and 5). We detected mRNA expression of TASK1 and 3, but not TASK2 and 5. This result is consistent with the finding reported by a previous study [15], which demonstrated that there was transcriptional expression of TASK1 and 3, TREK1 and 2, and TRAAK in SDH and that TASK3 was expressed at the highest levels in SDH.
Because TASK1 and 3 channels show sensitivity to extracellular acidic pH [16], we tested whether extracellular pH 6.4 inhibited DAMGO-induced current. In Fig. 4B, DAMGO-induced currents and the increment of slope conductance by DAMGO were blocked by pH 6.4, an acidic extracellular condition (46.2%±7.4%; n=8; p<0.05), indicating that DAMGO-induced current has the acid-sensitive property of TASK1 and 3 channels. To distinguish between TASK1 and TASK 3, we examined whether 100 µM Zn2+ exclusively inhibits DAMGO-induced current. In the presence of Zn2+, some DAMGO-induced currents (4 of 7 SG neurons) were reduced, but others (n=3) were not reduced (data not shown). This result indicates that DAMGO-induced currents may be mediated by a TASK1 homodimer, TASK3 homodimer and TASK1/TASK3 heterodimer.
DISCUSSION
The main question addressed in this study is whether DAMGO modulates two pore domain K+ channels of SG neurons and, if so, which subtypes of two pore domain K+ channels are associated with DAMGO-induced currents. The results can be summarized as follows: (1) DAMGO activates two pore domain K+ channels as well as GIRK channels in juvenile rat SG neurons. (2) TASK1 and TASK3 of two pore domain K+ channels subfamilies are involved in DAMGO-induced hyperpolarization. From these results it was suggested that facilitatory effect of DAMGO on TASK1 and TASK3 contribute to the reduction excitability in SG neurons and the inhibition of nociceptive transmission in the spinal cord.
Opioid-containing neurons and axon terminals as well as opiate binding sites highly distributed in the superficial layers of the spinal cord and remained in charge of endogenous opioidergic antinociception [17,18,19]. It has been shown that opioids reduce the excitability of SG neurons via the activation of a µ-opioid receptor in presynaptic and postsynaptic sites. The µ-opioid receptor is localized presynaptically on small-diameter nociceptive primary afferents and postsynaptically on excitatory or inhibitory interneurons in SG region [18,19,20,21], which may play an important role in the spinal antinociception mechanisms. Presynaptically, µ-opioid receptor agonists inhibit synaptic transmission by blocking voltage-gated Ca2+ channels of primary afferent neurons [10,11], thereby reducing neurotransmitter release from primary afferent nociceptors [22]. Postsynaptically, activation of µ-opioid receptors in SG neurons increase the conductance of pertussis toxin-sensitive GIRK channels [9], and inhibit HCN channels of SG neurons [12]. The postsynaptic action of opioid agonists on SG neurons leads to robust membrane hyperpolarization and reducing the excitability of excitatory SG neurons.
Our results show that the modulation of excitability of the SG neurons by DMAGO is determined by K2P channels and GIRK channels. DAMGO-induced currents showed an inwardly rectifying and a linear I~V relationship with a reversal potential of approximately −90 mV (theoretical EK), indicating that DAMGO-induced currents are mediated by K+ as charge carrier. In the present study, we focused on the DAMGO-induced current with the linear I~V relationship. The reversal potential of the DAMGO-induced current obtained in different extracellular concentrations of K+ closely matched the theoretical reversal potential for a pure K+ conductance, supporting the possibility that DAMGO acted on a K+-selective conductance. Based on the presence of a linear I~V relationship and the time-independent manner [14], we propose that the increment of conductance by DAMGO is due to an activation of leak K+ channels.
Leak K+ currents have an important role in the regulation of the RMP and excitability in CNS. As major sources of this leak current, K2P channels classified into six subfamilies have been recognized. K2P channels can be regulated in a distinct manner by diverse modulatory factors such as pH, neurotransmitters, anesthetics, oxygen levels, and polyunsaturated fatty acids [14]. We have examined selected members of the TREK and TASK families, which have previously documented expression in the spinal cord [15]. Our data show that mRNA expressions of TASK1, TASK3, TREK1, TREK2, and TRAAK are detected in the SG region, and TASK3 is expressed at the highest levels in juvenile rats, which is consistent with the previous studies [15,23,24]. Because TASK, but not TREK current, was highly sensitive to changes in extracellular pH, a hallmark of the TASK family [16,25,26], we tested whether DAMGO-induced current was inhibited by extracellular acidic pH. Our results revealed the inhibitory effect of acidic pH on DAMGO-induced current, which may be mediated via TASK channels rather than TREK. In a recent study, however, Devilliers et al. reported the direct activation of the TREK1 channel downstream from the m-opioid receptor of presynaptic or postsynaptic sites [27]. This discrepancy may be caused by differences between the diverse recording systems. We confirmed the inhibition of TASK1 and TASK3 by µ-opioid receptor activation in SG neurons, whereas they demonstrated the action of morphine in COS cells with TREK1 and µ-opioid receptors [27]. Taken together, it is suggested that opioids can induce the enhancement of TASK1 and TASK3 channels of SG neurons as well as TREK1. To address whether DAMGO-induced current is mediated by TASK1 or TASK3, we tested its sensitivity to Zn2+, since TASK3 homodimer is sensitive to 100 µM Zn2+, whereas TASK1/TASK3 heterodimers and TASK1 homodimers are not affected by Zn2+ [28]. We found that 57% (n=4/7) of SG neurons with DAMGO-induced currents were blocked by Zn2+ and the rest were not sensitive to Zn2+. Thus, we suggest that DAMGO-induced current in SG neurons is mediated by a TASK1 homodimer, a TASK3 homodimer, or TASK1/TASK3 heterodimers. In native tissues, the TASK1/TASK3 heterodimers are assembled as efficiently as TASK1 or TASK3 homodimer and are detected in cerebellar granule neurons, motoneurons, carotid body glomus cells, and hippocampal CA1 neurons [24,25,29,30].
In conclusion, our study suggest that activation of K2P channels (TASK1 and 3) via µ-opioid receptors reduces the excitability of the juvenile rat SG neurons and this postsynaptic action of µ-opioid receptor contributes to a least part of an analgesic mechanism of opioid in spinal cord.
ACKNOWLEDGEMENTS
This work was supported by the research fund of Hanyang University (HY-2012-N).
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflict of interest.
Author contributions: P.Y.C. and J.Z.I. performed the cell-based assay experiments. H.K.L. performed patch clamp measurement. S.H.L performed PCR measurement. J.S.J. and S.H.L. supervised and coordinated the study. J.S.J. and P.Y.C. wrote the manuscript.
References
- 1.Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978;177:417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura M, Jessell TM. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Merchenthaler I, Maderdrut JL, Altschuler RA, Petrusz P. Immunocytochemical localization of proenkephalin-derived peptides in the central nervous system of the rat. Neuroscience. 1986;17:325–348. doi: 10.1016/0306-4522(86)90250-2. [DOI] [PubMed] [Google Scholar]
- 4.Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- 5.Gouardères C, Beaudet A, Zajac JM, Cros J, Quirion R. High resolution radioautographic localization of [125I]FK-33-824-labelled mu opioid receptors in the spinal cord of normal and deafferented rats. Neuroscience. 1991;43:197–209. doi: 10.1016/0306-4522(91)90427-p. [DOI] [PubMed] [Google Scholar]
- 6.Faull RL, Villiger JW. Opiate receptors in the human spinal cord: a detailed anatomical study comparing the autoradiographic localization of [3H]diprenorphine binding sites with the laminar pattern of substance P, myelin and nissl staining. Neuroscience. 1987;20:395–407. doi: 10.1016/0306-4522(87)90100-x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura M, North RA. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983;305:529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]
- 8.Grudt TJ, Williams JT. mu-Opioid agonists inhibit spinal trigeminal substantia gelatinosa neurons in guinea pig and rat. J Neurosci. 1994;14:1646–1654. doi: 10.1523/JNEUROSCI.14-03-01646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider SP, Eckert WA, 3rd, Light AR. Opioid-activated postsynaptic, inward rectifying potassium currents in whole cell recordings in substantia gelatinosa neurons. J Neurophysiol. 1998;80:2954–2962. doi: 10.1152/jn.1998.80.6.2954. [DOI] [PubMed] [Google Scholar]
- 10.Rusin KI, Moises HC. mu-Opioid receptor activation reduces multiple components of high-threshold calcium current in rat sensory neurons. J Neurosci. 1995;15:4315–4327. doi: 10.1523/JNEUROSCI.15-06-04315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taddese A, Nah SY, McCleskey EW. Selective opioid inhibition of small nociceptive neurons. Science. 1995;270:1366–1369. doi: 10.1126/science.270.5240.1366. [DOI] [PubMed] [Google Scholar]
- 12.Seol GH, Kim J, Cho SH, Kim WK, Kim JW, Kim SJ. The inhibitory effect of opioid on the hyperpolarization-activated cation currents in rat substantia gelatinosa neurons. Korean J Physiol Pharmacol. 2001;5:373–380. [Google Scholar]
- 13.Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 15.Blankenship ML, Coyle DE, Baccei ML. Transcriptional expression of voltage-gated Na+ and voltage-independent K+ channels in the developing rat superficial dorsal horn. Neuroscience. 2013;231:305–314. doi: 10.1016/j.neuroscience.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro-da-Silva A, Pioro EP, Cuello AC. Substance P- and enkephalin-like immunoreactivities are colocalized in certain neurons of the substantia gelatinosa of the rat spinal cord: an ultrastructural double-labeling study. J Neurosci. 1991;11:1068–1080. doi: 10.1523/JNEUROSCI.11-04-01068.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW. delta-Opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci. 1995;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp T, Spike RC, Watt C, Todd AJ. The mu-opioid receptor (MOR1) is mainly restricted to neurons that do not contain GABA or glycine in the superficial dorsal horn of the rat spinal cord. Neuroscience. 1996;75:1231–1238. doi: 10.1016/0306-4522(96)00333-8. [DOI] [PubMed] [Google Scholar]
- 21.Kerchner GA, Zhuo M. Presynaptic suppression of dorsal horn inhibitory transmission by mu-opioid receptors. J Neurophysiol. 2002;88:520–522. doi: 10.1152/jn.2002.88.1.520. [DOI] [PubMed] [Google Scholar]
- 22.Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J Neurosci. 1994;14:4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 24.Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- 26.Morton MJ, O'Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and -2. Pflugers Arch. 2003;445:577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- 27.Devilliers M, Busserolles J, Lolignier S, Deval E, Pereira V, Alloui A, Christin M, Mazet B, Delmas P, Noel J, Lazdunski M, Eschalier A. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat Commun. 2013;4:2941. doi: 10.1038/ncomms3941. [DOI] [PubMed] [Google Scholar]
- 28.Clarke CE, Veale EL, Green PJ, Meadows HJ, Mathie A. Selective block of the human 2-P domain potassium channel, TASK-3, and the native leak potassium current, IKSO, by zinc. J Physiol. 2004;560:51–62. doi: 10.1113/jphysiol.2004.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torborg CL, Berg AP, Jeffries BW, Bayliss DA, McBain CJ. TASK-like conductances are present within hippocampal CA1 stratum oriens interneuron subpopulations. J Neurosci. 2006;26:7362–7367. doi: 10.1523/JNEUROSCI.1257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]