Abstract
Angiogenesis plays an essential role in embryo development, tissue repair, inflammatory diseases, and tumor growth. In the present study, we showed that endothelial nitric oxide synthase (eNOS) regulates retinal angiogenesis. Mice that lack eNOS showed growth retardation, and retinal vessel development was significantly delayed. In addition, the number of tip cells and filopodia length were significantly reduced in mice lacking eNOS. Retinal endothelial cell proliferation was significantly blocked in mice lacking eNOS, and EMG-2-induced endothelial cell sprouting was significantly reduced in aortic vessels isolated from eNOS-deficient mice. Finally, pericyte recruitment to endothelial cells and vascular smooth muscle cell coverage to blood vessels were attenuated in mice lacking eNOS. Taken together, we suggest that the endothelial cell function and blood vessel maturation are regulated by eNOS during retinal angiogenesis.
Keywords: Angiogenesis, eNOS, Proliferation, Retina, Signal transduction
INTRODUCTION
Angiogenesis is the physiological process that includes migration and proliferation of endothelial cells, formation of new vessel lumen and branches, and maturation of new vessels through the recruitment of perivascular cells, such as pericytes and vascular smooth muscle cells (VSMCs). Angiogenesis is essential for wound healing, tissue regeneration, inflammatory disease and embryonic development [1]. In the development of mouse retinal vessels, the tip cells sense a gradient of vascular endothelial growth factor (VEGF), leading to filopodia formation, and migrate toward the VEGF gradient [2]. The mouse retinal vasculature starts sprouting after birth from the optic nerve into the periphery region. Thereafter, the angiogenic process forms three layers of vasculature, including superficial layer, intermediate layer, and deep layer. From postnatal day 7 (P7), the superficial capillaries start sprouting vertically to form deep layers, which finally form a three-layered vascular system [3,4]. Therefore, retinal angiogenesis is the best model system to study angiogenesis in vivo.
Nitric oxide (NO) plays a central role in vasomotor tone, angiogenesis, and vascular permeability. It is synthesized by NO synthase (NOS). NO is generated by three isoforms of NOS, including neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) [5]. Neuronal NOS and eNOS are regulated in a Ca2+/calmodulin dependent manner and constitutively expressed in neurons or endothelial cells, respectively. However, iNOS activity is not regulated by the intracellular Ca2+ concentration and is not usually expressed in cells. Inducible NOS is induced by cytokines and bacterial lipopolysaccharide. Neuronal NOS is expressed in neurons of the brain and modulates physiological functions, including learning, memory, and neurogenesis [6].
eNOS activation is regulated not only in a Ca2+-dependent manner, but also in a Ca2+-independent manner, including phosphorylation on serine (Ser), threonine (Thr), and tyrosine (Tyr) residues. It has been reported that a variety of upstream kinases phosphorylate and activate eNOS. For example, insulin activates eNOS through the activation of Akt and AMP-activated protein kinase (AMPK). VEGF, an angiogenic stimuli, activates eNOS via the Akt signaling pathway. Moreover, shear stress induces eNOS phosphorylation by activating protein kinase A (PKA) [6]. Although eNOS is phosphorylated and activated by multiple upstream kinases, Akt-dependent phosphorylation of eNOS seems to play an essential role in angiogenesis, i.e. Akt-eNOS signaling axis controlling angiogenesis therby regulating blood flow and tissue repair [7].
The implication of eNOS in angiogenesis was verified by previous studies using a knockout animal model. It has been reported that impaired retinal vascular development has been shown in mice lacking EC-specific Akt1, which is an upstream kinase of eNOS [8]. In this report, the expression of eNOS was significantly reduced in mice lacking Akt1 in comparison with the wild type. Direct evidence was obtained from the studies using eNOS knockout mice. Mice lacking eNOS resulted in impaired recovery of blood flow after hind limb ischemia and impaired mural cell recruitment [9]. In addition, it has been reported that VEGF-induced permeability and endothelial cell precursor mobilization were inhibited in eNOS-deficient mice [10]. However, the exact role of eNOS in retinal vascular development is still ambiguous.
In this study, we investigated the role of eNOS in retinal angiogenesis. We provided direct evidence that eNOS plays an important role in angiogenesis in vivo. Moreover, we showed that eNOS was required for EC proliferation and pericyte recruitment.
METHODS
Animals
Mice lacking eNOS (eNOS–/–, B6.129P2-Nos3tm1Unc/J) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). All animal procedures were done in accordance with our institutional guidelines for animal research and were approved by our institutional animal care and use committee (PNU-2015-0998).
Materials
Anti-eNOS antibody was purchased from BD Biosciences (San Jose, CA, USA). Anti-SM22a antibody was obtained from Abcam (Cambridge, UK). Anti-actin antibody was purchased from MP Biomedicals (Aurora, OH, USA). NG2 antibody was obtained from Millipore Bioscience (Temecula, CA, USA). Anti-pH3 antibody was purchased from Cell Signaling Technology (Boston, MA, USA). GSL I-isolectin B4 was obtained from Vector Laboratories (Burlingame, CA, USA). Alexa Fluor 405- and 488-conjugated streptavidin, and Cy3-conjugated goat anti-rabbit secondary antibodies were purchased from Molecular Probes, Inc. (Carlsbad, CA, USA). IRDye700- and IRDye800-conjugated rabbit and mouse secondary antibodies were obtained from Li-COR Bioscience (Lincoln, NE, USA).
Tissue isolation and western blotting
The heart, aorta, lung, liver, spleen and retina were isolated from eNOS+/+ and eNOS–/– mice. Tissues were homogenized and lysed in 20 mM Tris-HCl, pH 7.4, 1 mM EGTA/EDTA, 1% Triton X-100, 1 mM Na3VO4, 10% glycerol, 1 µg/ml leupeptin and 1 µg/ml aprotinin. After centrifugation at 12,000 rpm for 10 min, 30 µg of total protein was separated by a 10% polyacrylamide gel and transferred onto the nitrocellulose membrane. The membranes were incubated with indicated primary antibodies and IRDye-conjugated secondary antibodies, and the protein bands were visualized by an infrared image analyzer (Li-COR Bioscience).
Whole-mount staining of retina
Eyes were isolated from eNOS+/+ and eNOS–/– mice at P6 and were fixed in 4% paraformaldehyde for 12 h at 4℃. The cornea, sclera, lens, and hyaloids vessels were removed, and the retinas were blocked and permeabilized in a blocking buffer (1% BSA and 0.3% Triton X-100 in PBS) for 12 h at 4℃. For immunostaining, IB4 was diluted in a PBlec solution (1% Triton X-100, 1 mM CaCl2, 1 mM MnCl2 and 1 mM MgCl2 in PBS, pH 6.8), and other primary antibodies were incubated overnight in a blocking buffer at 4℃. Secondary antibodies were diluted in a blocking buffer and incubated at room temperature for 2 h. Retinas were mounted flat with an anti-fading reagent (2% n-propylgalate in 80% glycerol/PBS solution), and images were obtained with a confocal microscope (FV1000-ZDC, Olympus, Japan). The area of the angiogenic region and the distance of the sprouting vessel were analyzed using image J (National Institutes of Health, MD, USA).
Aortic sprouting assay
Growth factor-reduced matrigel was plated into 24-well plates, and incubated for 30 min at 37℃ to allow polymerization. Thoracic aortas were dissected from 6- to 7-week-old eNOS+/+ and eNOS–/– mice, and the surrounding fat and connective tissues were discarded. Aortas were cut into 0.8-mm length, and embedded in matrigel-coated wells. The aortic rings were stimulated with 0.2% EBM or EMG-2 medium every 2 days. Bright field images were obtained with a fluorescence microscope at ×5 magnification (Axiovert200, Carl Zeiss, Jena, Germany).
Statistical analysis
Results are expressed as the means±SEM of multiple experiments. When comparing the two groups, an unpaired Student's t-test was used to assess the differences. p-values of less than 0.05 were considered significant, which were indicated by an asterisk (*).
RESULTS
eNOS is required for retinal angiogenesis
As shown in Fig. 1A, eNOS-deficient mice showed growth retardation compared with its wild type counterpart. To elucidate the expression of eNOS in various organs, we isolated the lung, liver, spleen, aorta, heart, and retina from mice lacking eNOS. The expression of eNOS was selectively ablated in tissues from eNOS-deficient mice (Fig. 1B). To verify the role of eNOS in retinal angiogenesis, we isolated retinas from the eNOS knockout mice at P6, and analyzed the retinal vascular development by staining the endothelial cells (ECs) with IB4. As shown in Fig. 1C, retinal vascular outgrowth was delayed in eNOS knockout mice. In addition, the angiogenic area and sprouting distance from optic nerve (ON) were significantly impaired in mice lacking eNOS (Fig. 1D). These results indicate that eNOS regulates the development of retinal vessels.
Fig. 1. eNOS plays an essential role in retinal angiogenesis.
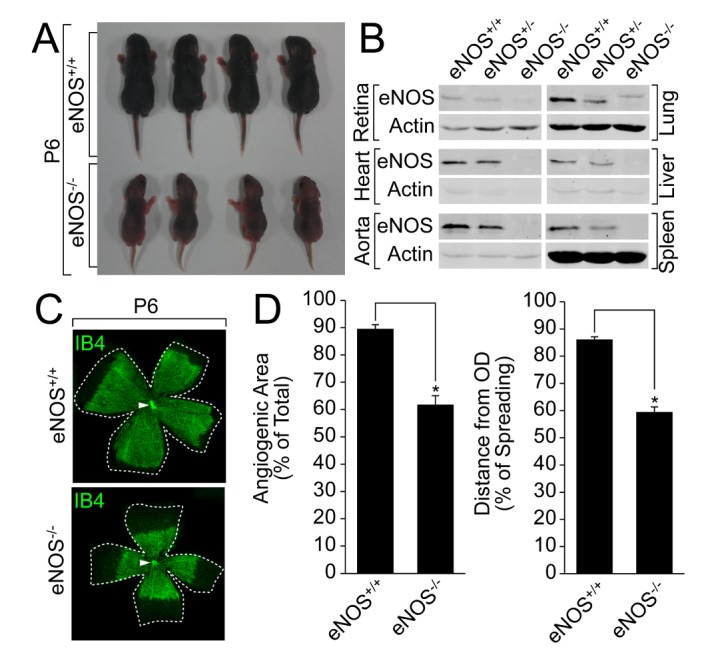
(A)A comparison of the body size between the wild type and eNOS knockout littermates was visualized by a digital camera at P6. (B) Lung, liver, spleen, heart, aorta, and retina were isolated from the wild type or eNOS-deficient mice, and the expression of eNOS was verified by a Western blot analysis with the indicated antibodies. (C and D) Retinas were isolated from the wild type and eNOS knockout mice at P6 and stained with IB4 (green). Angiogenesis was analyzed by measuring the angiogenic area and distance. Images were captured on confocal microscope at ×2.5 (zoom ×0.5) magnification. Angiogenic area and sprouting distance were quantified using Image J (National Institutes of Health, MD, USA) software. White arrowheads indicate optic nerve (ON). Data are presented as the means±SEM. Asterisks indicate statistical significance (p<0.05). Scale bars, 800 µm.
eNOS is required for endothelial cell activation
Tip cells lead to sprouting angiogenesis, and filopodia on the tip cells sense the angiogenic factors, leading to the proliferation and migration of endothelial cells. As shown in Fig. 2A and 2B, the number of tip cells and filopodia length were significantly reduced in the retinas isolated from mice lacking eNOS. These results indicate that eNOS plays an essential role in the activation of tip cells during retinal angiogenesis.
Fig. 2. eNOS regulates the activation of tip cells during retinal angiogenesis.
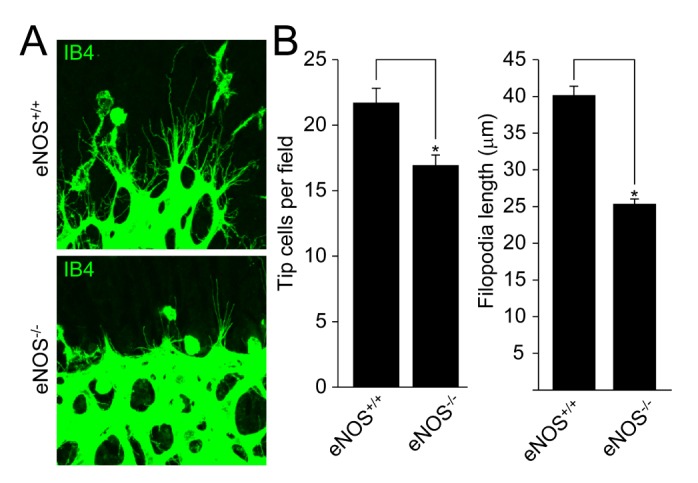
(A) Retinas from the wild type and eNOS-deficient mice at P6 were stained with IB4 (green). (B) Tip cell number and filopodia length were measured using Image J (National Institutes of Health, MD, USA) software. Images were visualized on confocal microscope at ×80 magnification. Data are presented as the means±SEM. Asterisks indicate statistical significance (p<0.05). Scale bars, 100 µm.
eNOS is required for endothelial cell proliferation
Since eNOS is involved in the activation of EC, we examined the effect of eNOS on EC proliferation. Retinas were isolated from mice lacking eNOS at P6 stage and were stained with antibody against phospho-H3. As shown in Figs. 3A and 3B, EC proliferation was significantly reduced in retinas isolated from mice lacking eNOS. To verify the requirement of eNOS in EC proliferation, we isolated aortas from either eNOS+/+ or eNOS–/– mice, and examined for EGM-2-induced EC sprouting. EC sprouting was markedly reduced in aortic rings isolated from mice lacking eNOS (Fig. 3C and Supplemental Fig. S1). These results suggest that eNOS is required for endothelial cell proliferation.
Fig. 3. eNOS is necessary for endothelial cell proliferation and angiogenic sprouting.
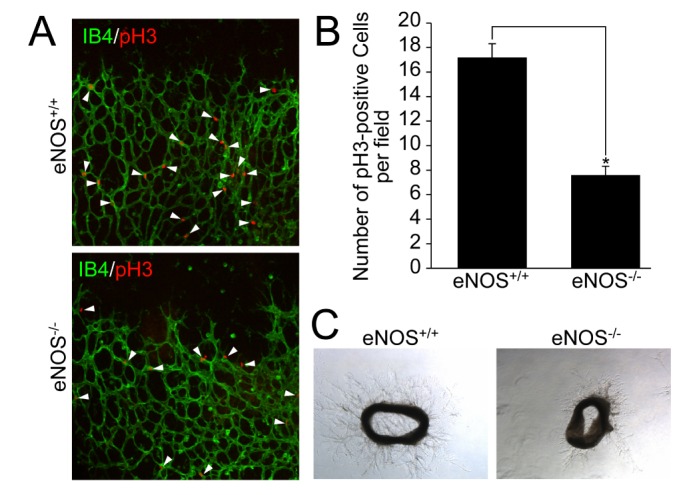
(A and B) Retinas isolated from the wild type and eNOS knockout mice at P6 were stained with IB4 (green) and pH3 (red). Images were visualized on confocal microscope at ×20 magnification. White arrowheads indicate pH3-positive cells. The number of pH3-positive cells was quantified using Image J (National Institutes of Health, MD, USA) software. Data are presented as the means±SEM. Asterisks indicate statistical significance (p<0.05). Scale bars, 100 µm. (C) Aortas were isolated from the wild type and eNOS knockout mice, and embedded in growth factor-reduced matrigel-coated plates in the presence of EGM-2. After 6 days, bright field images were captured under a microscope at ×5 magnification. Scale bars, 500 µm.
eNOS is required for pericyte recruitment and vessel stabilization
We next explored the role of eNOS in the stabilization and maturation of blood vessels. As shown in Fig. 4A, retinas isolated from mice lacking eNOS showed a significant reduction in pericyte recruitment of endothelial cells, especially in the periphery rather than the ON site. In addition, VSMC coverage to blood vessels was significantly impaired in mice lacking eNOS (Fig. 4B). These results indicate that eNOS regulates blood vessel stabilization through pericyte recruitment and VSMC coverage.
Fig. 4. eNOS regulates pericyte recruitment and VSMC coverage.
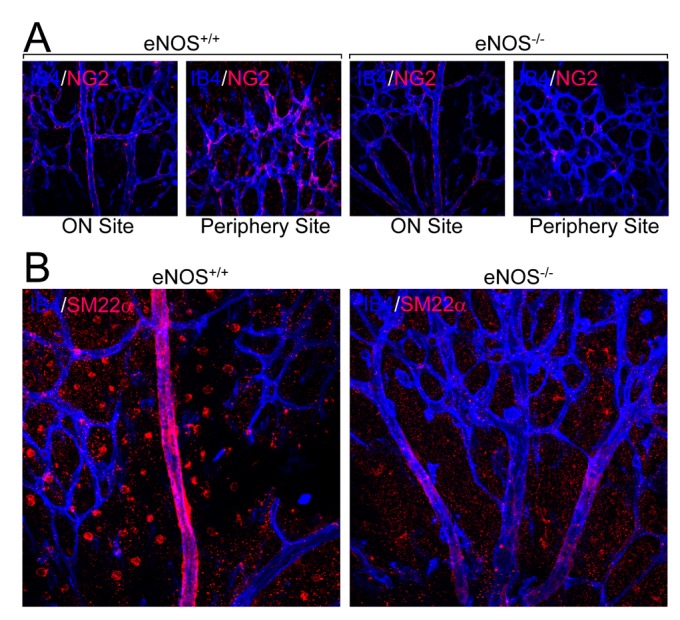
(A and B) Retinas from the wild type and eNOS knockout mice at P6 were stained with IB4 (blue) and either pericyte marker protein (NG2, red, panel A) or VSMC marker protein (SM22α, red, panel B). Images were visualized under a confocal microscope at ×40 magnification. Scale bars, 100 µm.
DISCUSSION
In the present study, we demonstrated that eNOS regulates endothelial cell functions and retinal angiogenesis. We observed a smaller body size in eNOS knockout littermates. The loss of eNOS resulted in reduced endothelial cell proliferation—both ex vivo and in vivo. In addition, retinal vessel development was delayed concomitantly with insufficient vascular maturation in mice lacking eNOS.
NO, produced by eNOS in endothelial cells, affects a variety of functions in EC itself, as well as in nearby VSMCs. Consequently, the production of NO by eNOS affects the blood vessel itself, as well as organism development. For example, it has been reported that eNOS knockout mice showed lower body weights compared with the wild type or eNOS hetero mice [11]. Consistent with this, our results showed that eNOS knockout littermates have smaller body size than the wild type mice at P6 (Fig. 1A). It is noteworthy that the size of retarded organisms is not due to systemic effect of eNOS knockout, since the expression of eNOS is restricted to ECs.
It has been reported that the production of NO is an important regulator of endothelial cell function and angiogenesis. Regulation of NO production by eNOS acts as a molecular switch in the proangiogenic and antiangiogenic effect. For example, VEGF or angiopoietin-2 facilitates angiogenesis by phosphorylation of eNOS and subsequent production of NO, whereas endostatin or somatostatin suppresses angiogenesis via the opposite effect on eNOS [12,13,14,15]. Our results also illustrated that the ablation of eNOS suppressed a variety of angiogenic parameters, including tip cell activation, EC proliferation, EC sprouting, and vascular stabilization (Figs. 1,2,3,4). Therefore, it is likely that the eNOS pathway plays a vital role in the regulation of endothelial cell function.
Among the extracellular stimuli, VEGF plays a crucial role in the proliferation and directional migration of EC during angiogenesis. For instance, the secretion of VEGF from astrocytes and macrophages, which reside in a hypoxic condition, forms a concentration gradient, in which the tip cells use its sensory faculty of lead to filopodia formation and directional migration [2]. In particular, eNOS plays a central role in VEGF-induced angiogenesis. For example, ablation of eNOS suppresses VEGF-induced angiogenesis, as well as vascular leakage [16,17,18]. Currently it is well known that NO plays an essential role in VSMC relaxation [19]; however, the role of NO in EC is relatively unclear. Recently, it has been shown that NO promotes VEGF production. For example, the expression of VEGF is significantly reduced in ischemic limbs from mice lacking eNOS [9]. Therefore, angiogenesis seems to be regulated by a positive-forward mechanism of VEGF and eNOS.
Our results highlight some important issues of blood vessel maturation. Maturation of vasculature requires mural cell recruitment, and platelet-derived growth factor (PDGF) and angiopoietin signaling pathways regulate mural cell coverage to new blood vessels. Our results showed that pericyte recruitment and VSMC coverage was significantly reduced in mice lacking eNOS (Fig. 4). Likewise, it has been reported that the expression of VEGF and PDGF was reduced in ischemia-induced vessels isolated from eNOS knockout mice, leading to the impairment of mural cell coverage [9]. In addition, VSMC coverage in B16 tumor vessels was significantly impaired by the inhibition of eNOS with L-NMMA and in mice lacking eNOS [20]. Therefore, it is likely that the presence of eNOS in EC is required for pericyte recruitment, as well as VSMC coverage. Indeed, it has been reported that the production of PDGF from EC is necessary for pericyte recruitment to EC, and expression of Jag1 is required for Notch3 activation in VSMC, which in turn regulates VSMC coverage [21,22]. Hence, further studies on the eNOS-dependent expression of PDGF and Jag1 would provide better understanding of the underlying mechanism of eNOS-dependent blood vessel maturation.
In conclusion, the present study shows that eNOS is essential for endothelial activation, thereby important in regulating the proliferation, migration and vascular stabilization. Coordinated regulation of these processes by eNOS is required for angiogenesis. Further studies regarding the downstream signaling pathway of eNOS should provide more relevant mechanism in retinal angiogenesis.
Acknowledgements
This work was supported by the Financial Supporting Project of Long-term Overseas Dispatch of PNU's Tenure-track Faculty, 2013.
Footnotes
Author contributions: J.M.H. performed experiments. S.Y.J. and H.S.L. assisted data analysis. H.K.S., D.H.L., S.H.S. and C.D.K. provided critical comments on the manuscript. S.S.B. generated main idea and wrote manuscript. Y.C.K., S.Y.K., H.S., E.H.J., H.S.K., S.W.L., S.J.L. and I.W.J. performed clinical and pharmacological assistance and wrote paper.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
SUPPLEMENTARY MATERIALS
Supplementary data including three figures can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp020-05-11-s001.pdf.
eNOS regulates angiogenic sprouting. Aortas were isolated from the wild type and eNOS knockout mice, and embedded in growth factor-reduced matrigel-coated plates in the presence of EGM-2. After 6 days, images were captured on a bright field microscope at ×5 magnification.
References
- 1.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 2.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uemura A, Kusuhara S, Katsuta H, Nishikawa S. Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res. 2006;312:676–683. doi: 10.1016/j.yexcr.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 6.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang PL, Sessa WC. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal. 2009;2:ra41. doi: 10.1126/scisignal.2000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci U S A. 2014;111:12865–12870. doi: 10.1073/pnas.1408472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papapetropoulos A, García-Cardeña G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- 14.Urbich C, Reissner A, Chavakis E, Dernbach E, Haendeler J, Fleming I, Zeiher AM, Kaszkin M, Dimmeler S. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 2002;16:706–708. doi: 10.1096/fj.01-0637fje. [DOI] [PubMed] [Google Scholar]
- 15.Le Romancer M, Reyl-Desmars F, Cherifi Y, Pigeon C, Bottari S, Meyer O, Lewin MJ. The 86-kDa subunit of autoantigen Ku is a somatostatin receptor regulating protein phosphatase-2A activity. J Biol Chem. 1994;269:17464–17468. [PubMed] [Google Scholar]
- 16.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 18.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Zoghbi JF, Bai Y, Sanderson MJ. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J Gen Physiol. 2010;135:247–259. doi: 10.1085/jgp.200910365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Kennard S, Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eNOS regulates angiogenic sprouting. Aortas were isolated from the wild type and eNOS knockout mice, and embedded in growth factor-reduced matrigel-coated plates in the presence of EGM-2. After 6 days, images were captured on a bright field microscope at ×5 magnification.


