Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease affecting motor neurons of the brain, brainstem and spinal cord. To date, mutations in more than 30 genes have been linked to the pathogenesis of ALS. Among them, SOD1, FUS and TARDBP are ranked as the three most common genes associated with ALS. However, no mutation analysis has been reported in central-southern China. In this study, we sequenced SOD1, FUS and TARDBP in a central-southern Chinese cohort of 173 patients with ALS (15 familial ALS and 158 sporadic ALS) to detect mutations. As a result, five missense mutations in SOD1, namely, p.D101N, p.D101G, p.C111Y, p.N86S and p.V87A, were identified in three unrelated familial probands and three sporadic cases; two mutations in FUS were found in two unrelated familial probands, including an insertion mutation (p.P525_Y526insY) and a missense mutation (p.R521H); no variants of TARDBP were observed in patients. Therefore, SOD1 mutations were present in 20.0% of familial ALS patients and 1.9% of sporadic ALS patients, while FUS mutations were responsible for 13.3% of familial ALS cases, and TARDBP mutations were rare in either familial or sporadic ALS cases. This study broadens the known mutational spectrum in patients with ALS and further demonstrates the necessity for genetic screening in ALS patients from central-southern China.
Amyotrophic lateral sclerosis (ALS) is a progressive adult-onset disorder that affects upper and lower motor neurons, consequently resulting in muscular weakness and atrophy. Patients eventually die of respiratory failure within 3 to 5 years1. It is a clinically heterogeneous disease characterized by different ages of onset, sites of onset, lengths of disease and neurological signs2. Further, it has overlapping clinical, genetic and pathological characteristics with frontotemporal dementia (FTD). It is reported that approximately 5–15% patients with ALS meet the criteria for FTD diagnosis. In contrast, clinical features of ALS exist in more than 15% of FTD patients3,4. The pathogenesis of ALS is complicated, and genetic factors play a relatively important role in patients with ALS. The pattern of inheritance can be autosomal dominant, autosomal recessive, or X-linked5. Approximately 5–10% of patients with ALS have a positive family history of the disease.
To date, mutations in more than 30 genes have been linked to the pathogenesis of ALS, but mutations in only a few of them, including SOD1, FUS, TARDBP and C9orf72, are present in a significant number of ALS cases6,7. SOD1 was the first gene associated with ALS to be identified, and more than 166 mutations have been successively reported2, which could account for the occurrence of 20% of familial ALS (fALS) cases and 1–4% of sporadic ALS (sALS) cases8. FUS mutations have been detected in 4–6% of fALS cases and 0.7–1.8% of sALS cases9. Most of these mutations are located in exons 5–6 and exons 14–15 of FUS10. With regards to TARDBP, more than 42 mutations have been found in 5% of fALS cases and 2% of sALS cases5. However, there are still no reports of these mutations in central-southern China. Therefore, we performed a comprehensive and systematic screening of SOD1, FUS and TARDBP genes to further analyze the types of mutations and their frequency in patients with ALS from central-southern China. In addition, we further analyzed the clinical characteristics of ALS patients carrying genetic mutations.
Results
Genetic Results
Three reported mutations of SOD1 (p.D101N, p.D101G and p.C111Y) were identified in three unrelated fALS patients, and three reported mutations of SOD1 (p.N86S, p.V87A and p.C111Y) were identified in three unrelated sALS patients. With regards to FUS, one novel mutation (p.P525_Y526insY) and one previously reported mutation (p.R521H) were detected in two unrelated fALS probands. However, no mutations in TARDBP were identified. None of these mutations were found in the 500 healthy individuals testd. Genetic results and pathogenic predictions are presented in Table 1. In addition, the mean age of onset for mutation carriers and non-carriers was 44.0 ± 15.2 and 49.0 ± 12.2 years, respectively. The Mann-Whitney U test revealed that there was no significant difference between the groups (p = 0.31). No significant differences was found between groups when comparing in male to female ratio (p = 0.44).
Table 1. The mutations on SOD1 and FUS gene found in this study.
| N0. | Gene | Inheritance | Location | Position | cDNA | Protein | Mutation type | Value | Reference/Novel |
|---|---|---|---|---|---|---|---|---|---|
| M13948 | SOD1 | F | Exon4 | 33039635 | c.304G>A | p.D101N | Missense | 0.001 damaging | Jones (1994) |
| M23969 | SOD1 | F | Exon4 | 33039666 | c.335G>A | p.C111Y | Missense | 0.000 damaging | Eisen34 |
| M31584 | SOD1 | F | Exon4 | 33039667 | c.305A>G | p.D101G | Missense | 0.000 damaging | Yulug31 |
| M23225 | SOD1 | S | Exon4 | 33039591 | c.260A>G | p.N86S | Missense | 0.000 damaging | Hayward28 |
| M23362 | SOD1 | S | Exon4 | 33039594 | c.263T>C | p.V87A | Missense | 0.000 damaging | Andersen29 |
| M18301 | SOD1 | S | Exon 4 | 33039666 | c.335G>A | p.C111Y | Missense | 0.000 damaging | Eisen34 |
| M17748 | FUS | F | Exon 15 | 31202740 | c.1562G>A | p.R521H | Missense | 0.000 damaging | Kwiatkowski39,41 |
| M17299 | FUS | F | Exon15 | 31202753 | c.1575_1576insTAT | p.P525_Y526insY | Insertion | NA | In this study |
SOD1 mutation in fALS
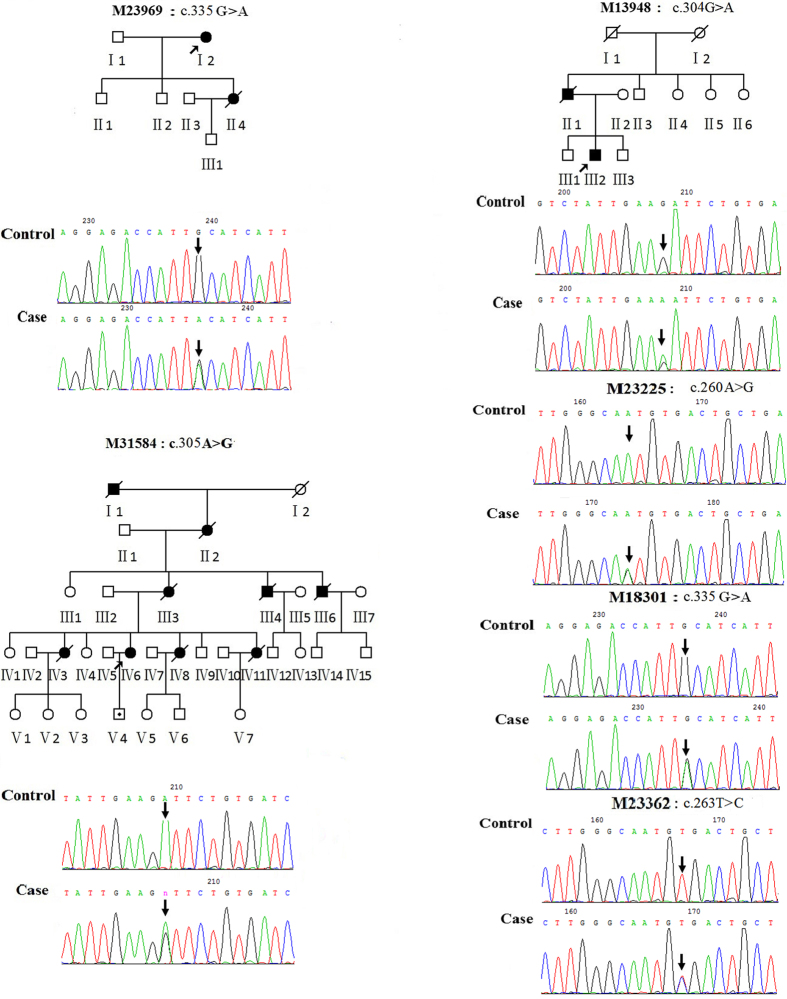
The c.335G>A, p.C111Y mutation was identified in a fALS proband (Fig. 1 M23969: I2). At age 68, she developed a slowly progressive distal weakness in her upper left limb, and one month later, the weakness spread to the lower left limb, while fasciculation and atrophy in the upper left limb were observed. A neurological examination revealed signs of upper motor neuron (UMN) damage, and an electromyogram indicated the presence of generalized chronic progressive neurogenic damage. Her daughter (Fig. 1 M23969: II4) developed progressive weakness in her lower left limb at age 46 and quickly developed weakness, dysarthria and dysphagia in all four extremities within one year. She then died of respiratory failure after18 months. Although her daughter carried the same mutation, she had an earlier age of onset and a seemingly worse clinical phenotype than her mother. Her sons did not carry this mutation or share this clinical phenotype.
Figure 1. Three pedigrees (M23969, M13948, and M31584) carried SOD1 mutations, and the corresponding forward sequencing chromatogram of mutations in both probands and controls is shown.
The arrow on the pedigree represents the proband. The circles denote females, and squares denote males. Affected individuals are noted by black symbols and unaffected individuals are noted by blank symbols. Carriers are noted by a black dot. Deceased individuals are noted by a slash symbol. M23225, M18301 and M23362 represent forward sequencing chromatogram of sALS patients with SOD1 mutations. M23969:c.335G>A (p.C111Y); M13948:c.304G>A (p.D101N); M31584:c.305A>G (p.D101G); M23225:c.260A>G (p.N86S); M18301:c.335G>A (p.C111Y); M23362:c.263T>C (p.V87A).
The c.304G>A, p.D101N mutation was detected in another fALS patient (Fig. 1 M13948: III2). He began undergoing gradually progressive weakness in his upper right limb at age 26. The distal weakness was predominant, preventing him from performing daily work. One year later, the weakness gradually spread to the lower limbs and upper left limb. Fasciculation and muscle atrophy was observed in all extremities. The tendon reflex was active, the Babinski reflex was detected, and the electromyogram demonstrated chronic reinnervation in all extremities. Two years later, he experienced worsening limb weakness and the development of dysarthria and dysphagia. The patient died of respiratory failure three years after the onset of symptoms. His father (Fig. 1 M13948: II2) began suffering from weakness of the upper limbs at the age of 38. Two years later, his remaining limbs and bulbar muscles were also weakened. Finally, he died of respiratory failure at age 41.
The third SOD1 mutation c.305A>G, p.D101G was identified in a 47-year-old female who first presented with progressive weakness and systemic fasciculations in her upper left limb (Fig. 1 M31584: IV6). Four months later, she experienced worsening weakness in her upper left limb, and she felt weak in her other limbs. Six months later, dysphagia and dysarthria occurred. Upon admission, a neurologic examination revealed the presence of atrophy, reduced muscle strength, areflexia, and the absence of bilateral Babinski and Hoffman signs in her left hand. The electromyogram revealed extensive chronic neurological changes. Her son was verified as a carrier of the c.305A>G mutation, but he did not present with clinically relevant ALS symptoms (Fig. 1 M31584: V4). Other members of the family experienced a similar, approximately two-year disease course and finally died of respiratory failure in a comparable manner to the proband. Unfortunately, the DNA samples of these family members were unavailable for genetic analysis. Moreover, IV1, IV4 and IV9 did not share this mutation.
SOD1 mutations in sALS
Three mutations (p.N86S, p.V87A and p.C111Y) were observed in three unrelated sALS patients. A 58-year-old female patient with the c.260A>G, p.N86S mutation (Fig. 1 M23225) was primarily characterized by progressive weakness and muscle atrophy in her lower limbs beginning at the age of 55. Her tendon reflex was normal and the Babinski reflex was absent. The electromyogram revealed chronic reinnervation in her lower limbs, distal left limb, and paravertebral muscles. The c.335G>A, p.C111Y mutation (Fig. 1 M18301) was identified in a juvenile-onset sALS patient suffering from an aggressive disease progression with muscle atrophy, worsening fasciculation and weakness in all limbs. His deep tendon reflexes were active in all limbs, and the Babinski reflex was present. One year later, he started suffering from occasional choking while drinking and mild dysarthria. The c.263T>C, p.V87A mutation (Fig. 1 M23362) was identified in a sALS patient who developed weakness and muscle atrophy in the upper left limb at 51 years of age. For 24 months, the muscle weakness gradually progressed to the upper right limb and the lower extremities. Physical examinations showed that the deep tendon reflexes of all limbs were brisk and the Hoffman sign was present. Electromyography revealed extensive chronic progressive neurological damage in all four extremities.
FUS mutations
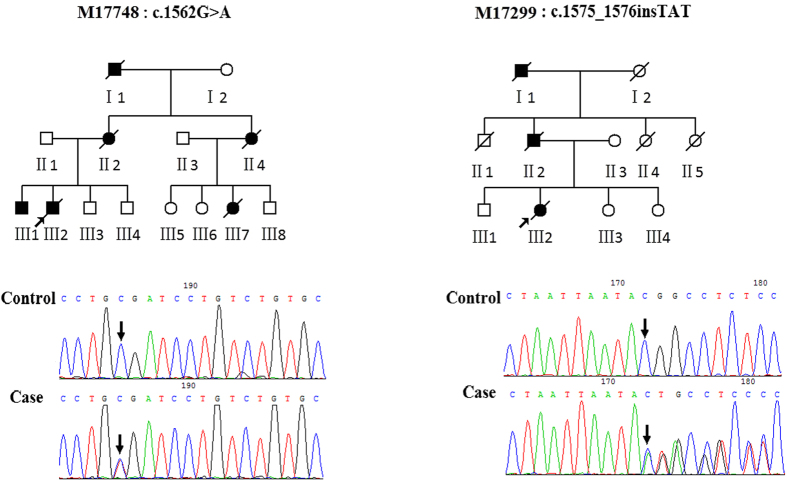
The c.1562G>A, p.R521H mutation (Fig. 2 M17748: III:2) was detected in a fALS patient who initially suffered from progressive weakness in the upper right limb at age 41. One year later, the weakness, fasciculation, and muscle atrophy progressed to other limbs. His symptoms were primarily characterized by proximal muscle weakness without signs of upper motor neuron damage or cognitive impairment. The electromyogram showed spontaneous denervation activity in all limbs. He presented with dysarthria and dysphagia after 13 months and died at age 44. His brother, who had the same mutation, also had a similar clinical phenotype and disease duration. III:3 and III:4 did not share this mutation. Therefore, the c.1562G>A, p.R521H mutation completely co-segregated with the disease phenotype within this family.
Figure 2. Two corresponding reverse sequencing chromatograms depicting FUS mutations in ALS patients and controls.
M17748:c.1562G>A (p.R521H); M17299:c.1575_1576insTAT (p.P525_Y526insY).
The novel mutation c.1575_1576insTAT, p.P525_Y526insY was identified in a female proband (Fig. 2 M17299: III2). Her initial symptoms, beginning at age 43, were progressive weakness of the upper left limb and slow progression of weakness into the upper right limb and lower limbs. Meanwhile, muscle atrophy of the upper left limb occurred. Due to the proximal weakness, she could not lift her arms or go down stairs. A neurological examination showed that the tendon reflexes in her upper limbs were disappearing, while the tendon reflexes in her lower limbs were pathologically brisk. The electromyogram showed general spontaneous denervation activity and a loss of motor units in the tibialis anterior. Approximately 28 months later, the weakness in her limbs, mild dysarthria and dysphagia worsened, and she finally died of respiratory failure. Her father presented with a similar clinical phenotype and disease duration. Unluckily, his DNA sample was not obtained. III:1, III:3, and III:4 did not carry this mutation and share this clinical phenotype.
Discussion
In this study, we systematically described the frequencies of SOD1, FUS and TARDBP mutations in patients with ALS from central southern China. Mutations in SOD1 were present in 20.0% of fALS patients and 1.9% of sALS patients, which is consistent with previous studies in western populations8, and indicates that SOD1 mutations play a key role in the pathology of ALS patients from different ethnicities. In addition, the frequency of FUS mutation was approximately 13.3% in fALS patients, which is consistent with reports from several other populations11,12,13,14, but is higher than the frequencies found in Catalan (8%)15, German (2.4–6.9%)16,17, Italian (4.4%)12, and Belgian (2.9%)18 populations. This difference may be due to small sample sizes and the differing ethnicities of fALS patients. Although FUS mutations were not found in sALS patients, this is probably due to both the selective detection of exons in mutational hotspot regions and the use of small sample sizes. Zou ZY reported that FUS mutations could be present in approximately 1.0% of northern Chinese sALS patients, which is similar to the 1.6% mutation frequency found in Korean patients and the 1.25% mutation frequency found Italian patients12,14,19,20. With regards to the frequency of TARDBP mutation, it appears that the frequency of TARDBP mutations in Caucasian populations (Italy: 2.7%; France and Quebec: 4.5%) was higher than that in Asian populations21,22,23,24. The prevalence was different, even within the same population. Zou ZY reported that TARDBP mutations were only present in 0.73% of northern Chinese sALS patients25, but Soong’s research showed that TARDBP mutations have a higher frequency (4.3%), second only to SOD1 mutations (7.5%), in Taiwanese ALS patients, especially fALS patients26. Undeniably, our study did not identify TARDBP mutations, which is probably due to our small sample sizes and the ethnic differences of our study population. In addition, we previously reported that C9orf72 expansion was present in approximately 8.33% of fALS patients, which was lower than the frequency of FUS and SOD1 mutation27. This study indicates that in Chinese ALS patients, we should screen SOD1 mutations first, then FUS mutations, and then C9orf72 and TARDBP mutations.
In current study, four mutations of SOD1 (p.N86S, p.V87A, p.D101N, p.D101G) were identified firstly in Chinese ALS patients. p.N86S mutation was previously first identified in a juvenile onset case of fALS, all reported affected family members present worse phenotype and ephemeral duration28, while in our study, the sALS patients with this same mutation showed only mild clinical phenotypes. Although Andersen had previously reported the p.V87A mutation, the clinical features of the patients were not detailed29. It was noted that the patients with the p.V87A mutation were prone to experience relatively typical ALS-related clinical symptoms. The third mutation we identified, p.D101N, has been associated with a rapid disease course, with patients exhibiting a low propensity towards aggregate formation. In our study, the patients with the D101N mutation presented a similar clinical phenotype, with a relatively early age of onset and rapid ALS disease progression30. The fourth mutation we identified, p.D101G, is located in same site as p.D101N and was first detected in an ALS family from the UK31. In our study, all patients with the D101G mutation experienced a short disease duration. Both the D101N and D101G mutations have been shown to be associated with a rapidly progressing disease lasting approximately 2.5 years. This rapid disease progression may be caused by mutant SOD1 aggregation30,32,33. The final mutation we identified, p.C111Y, has been identified in North American, Japanese and Chinese families. The most common initial symptoms were primarily located in the spinal cord, and the disease progressed relatively slowly34,35. According to our results, all five of these mutations were located in exon 4, which appears to be a hotspot of variation in comparison to other exons.
With regards to FUS mutations, to our knowledge, the p.P525_Y526insY mutation is the first reported in an ALS patient. This discovery broadens the spectrum of known genetic mutations in patients with ALS. The p.P525_Y526insY mutation is located in an Arg/Gly-rich region of the C-terminal domain of FUS that contains the nuclear localization signal (NLS)36. C-terminal fALS-associated FUS mutations affect the major NLS of the protein and thus impair its nuclear import37. The P525L mutation at the same site has shown the strongest degree of cytosolic mislocalization and was reported to cause especially aggressive forms of fALS38,39. Thus, it is possible that the p.P525_Y526insY mutation disrupts nuclear import. The previously reported p.R521H mutation, located in a mutational hotspot region of the FUS gene, may result in aberrant localization and cytoplasmic accumulation of the mutant FUS protein40,41. Patients with the p.R521H mutation were prone to notice spinal symptoms first. Moreover, the p.R521H mutation was shown to correlate with a longer disease course42. In fact, several FUS mutations cause disease characteristics distinctive of ALS patients. The p.P525L variant, frameshift mutations and gene deletions were identified to associate with juvenile disease onset and aggressive disease duration2,43. The p.R521C mutation were particularly associated with phenotypes of neck and proximal muscle weakness44,45.
In summary, this study not only verified the high frequency of SOD1 mutations in Chinese fALS or sALS patient groups, but it also highlighted the importance of FUS mutations in Chinese fALS groups and revealed that TARDBP mutations are an uncommon cause of sALS or fALS in Chinese populations. In addition, we identified one novel mutation and broadened the spectrum of known FUS mutations contributing to ALS. Meanwhile, we provided a basis for the further study of genotype-phenotype correlations.
Methods
Subjects
A total of 173 unrelated patients with ALS were recruited from the Department of Neurology, Xiangya Hospital. All patients were of Han nationality, and were from the central southern China region, including the provinces of Hunan, Hubei, Jiangxi, Henan, and Guangxi. All patients, including 158 sALS patients (male: 69.4%; age at onset: 49.4 ± 12.1 years) and 15 fALS patients (male: 66.7%; age of onset: 47.5 ± 12.2 years), met the EI Escorial criteria for ALS diagnosis46. The 500 healthy individuals that were matched by gender, age and geographic region (male: 67.0%; age at onset: 48.5 ± 12.5 years). They were from the Xiangya Hospital Health Center. This study was approved by the Ethics Committee of Xiangya Hospital of the Central South University in China (equivalent to an Institutional Review Board) and carried out in accordance with the approved guidelines. Written informed consent was obtained from all participants.
Mutation screening
Genomic DNA was extracted from peripheral blood leukocytes using standard methods. The quality and quantity of DNA were assessed with a fluorometer. All DNA samples were diluted to 50 ng/uL. Using Sanger sequencing, all patient DNA was screened for mutations in all exons of SOD1 (NM_000454.4) and TARDBP (NM_007375.3) and in exons 5–6 and exons 14–15 of FUS (NM_004960.3). All primers were designed by Primer 5 software to amplify coding regions of SOD1, TARDBP and FUS using polymerase chain reaction (PCR) and to flank non-coding regions. Primers and PCR reaction conditions are listed in Supplementary Data 1. PCR products were sequenced using identical forward and reverse primers with BigDye terminator v3.1 sequencing chemistry on an ABI 3730xl DNA analyzer (Applied Biosystems). The DNA sequences were analyzed using Sequencher software, version 4.2. When a novel mutation was found, we first confirmed whether it was a novel mutation using the HGMD database (http://www.hgmd.cf.ac.uk/ac) and the dbSNP database (http://www.ncbi.nlm.nih.gov/snp). We then screened for the presence of the novel mutations in healthy controls. Finally, we used SIFT online software to predict the pathogenicity of the novel variants.
Statistical analysis
Statistical analysis of clinical data was performed using SPSS 18.0 (SPSS, Inc., Chicago, IL). To compare the differences among the two groups, the Mann-Whitney U test was used for continuous variables and Chi-square test was used for categorical variables. The threshold of statistical significance was set at p < 0.05.
Additional Information
How to cite this article: Hou, L. et al. Screening of SOD1, FUS and TARDBP genes in patients with amyotrophic lateral sclerosis in central-southern China. Sci. Rep. 6, 32478; doi: 10.1038/srep32478 (2016).
Supplementary Material
Acknowledgments
We are grateful to all subjects for participation in our study. This study was supported through the National Basic Research Program (973 Program) (No. 2011CB510000 to Lu Shen) and the National Natural Science Foundation of China (No. 81471295 to Lu Shen and No. 81100845 to Juan Du).
Footnotes
Author Contributions L.H., B.J. and L.S. designed the study. L.H., B.J., T.X., L.Z., Z.Z. and J.W. conducted the experiments, analyzed and interpreted the data. L.H. and L.S. wrote the manuscript. J.W., X.Y., B.T. and L.S. supervised the study. J.D. and L.S. provided financial support.
References
- Koppers M. et al. VCP mutations in familial and sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 33, doi: 837.e710.1016/j.neurobiolaging.2011.10.006 (2012). [DOI] [PubMed] [Google Scholar]
- Sabatelli M., Conte A. & Zollino M. Clinical and genetic heterogeneity of amyotrophic lateral sclerosis. Clin. Genet. 83, 408–416, doi: 10.1111/cge.12117 (2013). [DOI] [PubMed] [Google Scholar]
- Lillo P. et al. Overlapping features of frontotemporal dementia and amyotrophic lateral sclerosis. Rev. Medica Chile 142, 867–879 (2014). [DOI] [PubMed] [Google Scholar]
- Trojsi F., Monsurro M. R., Esposito F. & Tedeschi G. Widespread Structural and Functional Connectivity Changes in Amyotrophic Lateral Sclerosis: Insights from Advanced Neuroimaging Research. Neural. Plast., doi: 47353810.1155/2012/473538 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Sayana P., Zhang X. J. & Le W. D. Genetics of amyotrophic lateral sclerosis: an update. Mol. Neurodegener. 8, doi: 2810.1186/1750-1326-8-28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freischmidt A. et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631−+, doi: 10.1038/nn.4000 (2015). [DOI] [PubMed] [Google Scholar]
- Lin K.-P. et al. Mutational analysis of MATR3 in Taiwanese patients with amyotrophic lateral sclerosis. Neurobiol. Aging 36, 2005.e2001–2004, doi: 10.1016/j.neurobiolaging.2015.02.008 (2015). [DOI] [PubMed] [Google Scholar]
- Pasinelli P. & Brown R. H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 7, 710–723, doi: 10.1038/nrn1971 (2006). [DOI] [PubMed] [Google Scholar]
- Tarlarini C. et al. Novel FUS mutations identified through molecular screening in a large cohort of familial and sporadic amyotrophic lateral sclerosis. Eur J Neurol, doi: 10.1111/ene.12772 (2015). [DOI] [PubMed] [Google Scholar]
- Zou Z. Y. et al. De novo FUS gene mutations are associated with juvenile-onset sporadic amyotrophic lateral sclerosis in China. Neurobiology of aging 34, 1312.e1311-1318, doi: 10.1016/j.neurobiolaging.2012.09.005 (2013). [DOI] [PubMed] [Google Scholar]
- Belzil V. V. et al. Identification of novel FUS mutations in sporadic cases of amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. 12, 113–117, doi: 10.3109/17482968.2010.536840 (2011). [DOI] [PubMed] [Google Scholar]
- Corrado L. et al. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. Journal of medical genetics 47, 190–194, doi: 10.1136/jmg.2009.071027 (2010). [DOI] [PubMed] [Google Scholar]
- Tsai C. P. et al. FUS, TARDBP, and SOD1 mutations in a Taiwanese cohort with familial ALS. Neurobiol. Aging 32, doi: 553.e1310.1016/j.neurobiolaging.2010.04.009 (2011). [DOI] [PubMed] [Google Scholar]
- Zou Z. Y. et al. Screening of the FUS gene in familial and sporadic amyotrophic lateral sclerosis patients of Chinese origin. Eur. J. Neurol. 19, 977–983, doi: 10.1111/j.1468-1331.2012.03662.x (2012). [DOI] [PubMed] [Google Scholar]
- Syriani E., Morales M. & Gamez J. FUS/TLS gene mutations are the second most frequent cause of familial ALS in the Spanish population. Amyotrophic lateral sclerosis: official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 12, 118–123, doi: 10.3109/17482968.2010.539235 (2011). [DOI] [PubMed] [Google Scholar]
- Drepper C., Herrmann T., Wessig C., Beck M. & Sendtner M. C-terminal FUS/TLS mutations in familial and sporadic ALS in Germany. Neurobiol. Aging 32, doi: 548.e110.1016/j.neurobiolaging.2009.11.017 (2011). [DOI] [PubMed] [Google Scholar]
- Waibel S., Neumann M., Rabe M., Meyer T. & Ludolph A. C. Novel missense and truncating mutations in FUS/TLS in familial ALS. Neurology 75, 815–817, doi: 10.1212/WNL.0b013e3181f07e26 (2010). [DOI] [PubMed] [Google Scholar]
- Van Damme P. et al. The occurrence of mutations in FUS in a Belgian cohort of patients with familial ALS. Eur. J. Neurol. 17, 754–756, doi: 10.1111/j.1468-1331.2009.02859.x (2010). [DOI] [PubMed] [Google Scholar]
- Kwon M. J. et al. Screening of the SOD1, FUS, TARDBP, ANG, and OPTN mutations in Korean patients with familial and sporadic ALS. Neurobiol. Aging 33, doi: 1017.e1710.1016/j.neurobiolaging.2011.12.003 (2012). [DOI] [PubMed] [Google Scholar]
- Sproviero W. et al. FUS mutations in sporadic amyotrophic lateral sclerosis: Clinical and genetic analysis. Neurobiol. Aging 33, doi: 837.e110.1016/j.neurobiolaging.2011.10.005 (2012). [DOI] [PubMed] [Google Scholar]
- Corrado L. et al. High Frequency of TARDBP Gene Mutations in Italian Patients With Amyotrophic Lateral Sclerosis. Hum. Mutat. 30, 688–694, doi: 10.1002/humu.20950 (2009). [DOI] [PubMed] [Google Scholar]
- Iida A. et al. Large-scale screening of TARDBP mutation in amyotrophic lateral sclerosis in Japanese. Neurobiol. Aging 33, 786–790, doi: 10.1016/j.neurobiolaging.2010.06.017 (2012). [DOI] [PubMed] [Google Scholar]
- Kabashi E. et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genet. 40, 572–574, doi: 10.1038/ng.132 (2008). [DOI] [PubMed] [Google Scholar]
- Ye C. H. et al. Absence of Mutations in Exon 6 of the TARDBP Gene in 207 Chinese Patients with Sporadic Amyotrohic Lateral Sclerosis. PLoS One 8, doi: e6810610.1371/journal.pone.0068106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z. Y. et al. Screening of the TARDBP gene in familial and sporadic amyotrophic lateral sclerosis patients of Chinese origin. Neurobiol. Aging 33, doi: 2229.e1110.1016/j.neurobiolaging.2012.03.014 (2012). [DOI] [PubMed] [Google Scholar]
- Soong B. W. et al. Extensive molecular genetic survey of Taiwanese patients with amyotrophic lateral sclerosis. Neurobiology of aging 35, 2423.e2421-2426, doi: 10.1016/j.neurobiolaging.2014.05.008 (2014). [DOI] [PubMed] [Google Scholar]
- Jiao B. et al. Identification of C9orf72 repeat expansions in patients with amyotrophic lateral sclerosis and frontotemporal dementia in mainland China. Neurobiol. Aging 35, doi: 936.e1910.1016/j.neurobiolaging.2013.10.001 (2014). [DOI] [PubMed] [Google Scholar]
- Hayward C., Brock D. J., Minns R. A. & Swingler R. J. Homozygosity for Asn86Ser mutation in the CuZn-superoxide dismutase gene produces a severe clinical phenotype in a juvenile onset case of familial amyotrophic lateral sclerosis. Journal of medical genetics 35, 174, doi: 10.1136/jmg.35.2.174 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P. M. et al. Sixteen novel mutations in the Cu/Zn superoxide dismutase gene in amyotrophic lateral sclerosis: a decade of discoveries, defects and disputes. Amyotroph. Lateral. Scler. Mot. Neuron Disord. 4, 62–73, doi: 10.1080/14660820310011700 (2003). [DOI] [PubMed] [Google Scholar]
- Ayers J. et al. Distinctive features of the D101N and D101G variants of superoxide dismutase 1; two mutations that produce rapidly progressing motor neuron disease. J. Neurochem. 128, 305–314, doi: 10.1111/jnc.12451 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulug I. G., Katsanis N., de Belleroche J., Collinge J. & Fisher E. M. An improved protocol for the analysis of SOD1 gene mutations, and a new mutation in exon 4. Human molecular genetics 4, 1101–1104 (1995). [DOI] [PubMed] [Google Scholar]
- Prudencio M., Hart P. J., Borchelt D. R. & Andersen P. M. Variation in aggregation propensities among ALS-associated variants of SOD1: Correlation to human disease. Human molecular genetics 18, 3217–3226, doi: 10.1093/hmg/ddp260 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio M., Durazo A., Whitelegge J. P. & Borchelt D. R. An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1. Human molecular genetics 19, 4774–4789, doi: 10.1093/hmg/ddq408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A. et al. SOD1 gene mutations in ALS patients from British Columbia, Canada: Clinical features, neurophysiology and ethical issues in management. Amyotroph. Lateral. Scler. 9, 108–119, doi: 10.1080/17482960801900073 (2008). [DOI] [PubMed] [Google Scholar]
- Suzuki M. et al. Familial amyotrophic lateral sclerosis with Cys111Tyr mutation in Cu/Zn superoxide dismutase showing widespread Lewy body-like hyaline inclusions. J. Neurol. Sci. 300, 182–184, doi: 10.1016/j.jns.2010.09.007 (2011). [DOI] [PubMed] [Google Scholar]
- Zakaryan R. P. & Gehring H. Identification and characterization of the nuclear localization/retention signal in the EWS proto-oncoprotein. Journal of molecular biology 363, 27–38, doi: 10.1016/j.jmb.2006.08.018 (2006). [DOI] [PubMed] [Google Scholar]
- Dormann D. et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. The EMBO journal 29, 2841–2857, doi: 10.1038/emboj.2010.143 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A. et al. Two Italian kindreds with familial amyotrophic lateral sclerosis due to FUS mutation. Neurobiology of aging 30, 1272–1275, doi: 10.1016/j.neurobiolaging.2009.05.001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski T. J. Jr. et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science (New York, N.Y.) 323, 1205–1208, doi: 10.1126/science.1166066 (2009). [DOI] [PubMed] [Google Scholar]
- Vance C. et al. Mutations in FUS, an RNA Processing Protein, Cause Familial Amyotrophic Lateral Sclerosis Type 6. Science 323, 1208–1211, doi: 10.1126/science.1165942 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski T. J. & Vanderburg C. R. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis (vol 323, pg 1205, 2009). Science 324, 465–465 (2009). [DOI] [PubMed] [Google Scholar]
- Millecamps S. et al. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype-phenotype correlations. Journal of medical genetics 47, 554–560, doi: 10.1136/jmg.2010.077180 (2010). [DOI] [PubMed] [Google Scholar]
- Conte A. et al. P525L FUS mutation is consistently associated with a severe form of juvenile amyotrophic lateral sclerosis. Neuromuscular disorders: NMD 22, 73–75, doi: 10.1016/j.nmd.2011.08.003 (2012). [DOI] [PubMed] [Google Scholar]
- Blair I. P. et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. Journal of neurology, neurosurgery, and psychiatry 81, 639–645, doi: 10.1136/jnnp.2009.194399 (2010). [DOI] [PubMed] [Google Scholar]
- Yamashita S. & Ando Y. Genotype-phenotype relationship in hereditary amyotrophic lateral sclerosis. Translational neurodegeneration 4, 13, doi: 10.1186/s40035-015-0036-y (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B. R., Miller R. G., Swash M., Munsat T. L. & World Federation Neurology Res, G. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Mot. Neuron Disord. 1, 293–299, doi: 10.1080/146608200300079536 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.