Abstract
Vitamin B-12 is essential for brain development, neural myelination, and cognitive function. Inadequate vitamin B-12 status during pregnancy and early childhood has been associated with adverse child health outcomes, including impaired cognitive development. However, the underlying mechanisms have not been elucidated. This review was conducted to examine the evidence that links vitamin B-12 and cognition in children. The search strategy resulted in 17 studies: 3 cross-sectional, 1 case–control, and 12 cohort studies, and 1 randomized trial. Cognitive processes assessed included attention, memory, and perception. Developmental outcomes, academic performance, and intelligence quotient were also considered. Despite the high prevalence of vitamin B-12 insufficiency and associated risk of adverse cognitive outcomes in children, to our knowledge, no studies to date have been conducted to examine the effects of vitamin B-12 supplementation on cognition in children. The role of vitamin B-12 in the etiology of child cognitive outcomes needs to be elucidated to inform public health interventions.
Keywords: vitamin B-12, cobalamin, child, brain, cognition
Introduction
Vitamin B-12.
Vitamin B-12 is an essential B vitamin that plays an integral role in DNA synthesis, methylation reactions, and maintenance of genomic stability (1, 2). Cobalamin is a cofactor for methionine synthase, an enzyme that converts 5-methyltetrahydrofolate to tetrahydrofolate via remethylation of homocysteine (tHcy)7 to methionine (3). Methionine synthase is required for the production of S-adenosylmethionine, which is involved in >100 methylation reactions in the body and is an important methyl donor for neurotransmitter synthesis and maintenance of cellular integrity (4).
Vitamin B-12 is synthesized by microorganisms, and primary dietary sources are animal-source foods (5). Individuals with a low intake of meat and dairy products, such as vegans and vegetarians, are at high risk of vitamin B-12 deficiency (5). Several studies conducted in low- and middle-income countries also have reported a high prevalence of vitamin B-12 deficiency, particularly in pregnant women and young children (6), in part due to inadequate dietary vitamin B-12 intake (7, 8), increased requirements during pregnancy and lactation, and low vitamin B-12 content and bioavailability in breast milk (9). Micronutrient supplementation is a common strategy to prevent and treat cobalamin deficiency (10), and there is emerging interest in mandatory food fortification with vitamin B-12 (11).
The classic presentation of vitamin B-12 deficiency is hematologic, as pernicious anemia; inadequate vitamin B-12 intake or absorption impairs erythropoiesis (12). Intrinsic factor, a glycoprotein secreted by the gastric cells, is required for vitamin B-12 transport and absorption (13). In addition to its classic hematologic presentation, cobalamin deficiency also affects neuropsychiatric and cognitive function, particularly in young children and elderly populations (4, 14).
Vitamin B-12 biomarkers.
Vitamin B-12 assessment includes both circulating and functional vitamin B-12 biomarkers. Circulating biomarkers include total vitamin B-12 and holotranscobalamin, and functional biomarkers include methylmalonic acid (MMA) and tHcy (15–19). In this review, the following biomarkers of maternal or child vitamin B-12 status were included: plasma or serum total vitamin B-12, holotranscobalamin, MMA, and tHcy. Vitamin B-12 deficiency was defined as serum or plasma total vitamin B-12 concentrations <148 pmol/L, and vitamin B-12 insufficiency was defined as vitamin B-12 concentrations <221 pmol/L, unless otherwise specified.
Vitamin B-12 and brain development.
Central nervous system and brain development begins during week 3 of gestation and continues throughout infancy and early childhood (20, 21). During neurulation and cellular proliferation (22), cells begin to migrate and form the neural tube along the notochord, facilitating neuronal differentiation (20). Myelination and synaptogenesis begin in trimester 3 and continue to influence neuronal development in offspring during the first few years of life (22). The rate of brain development varies in the different regions of the brain and is influenced by various environmental and genetic factors. Inadequate nutrient availability in utero can affect brain development adversely during gestation and in postnatal stages (23). Vitamin B-12 is required for de novo DNA synthesis and methylation, which is critical for rapid cell division and growth, including in the developing fetus and brain (24). Vitamin B-12 plays an important role in neural myelination, synaptogenesis, myelination, and neurotransmitter synthesis, with potential effects on cognitive development and ultimate cognitive functioning (2, 22, 24–26). Inadequate vitamin B-12 status can impair these processes and lead to neural damage and brain atrophy (27, 28). Vitamin B-12 insufficiency in utero has been associated with impaired growth, psychomotor function, and brain development in epidemiologic studies, early deficits of which may be irreversible (24, 29).
Cognitive domains.
Cognition refers to the mental processes involved in memory, attention, learning, and executive functions, and cognitive development describes the maturation of these processes early in life (30). The main cognitive domains included in this review are indexes of memory, attention, and perception; global measures of cognitive development were also considered, including developmental outcomes, academic performance, and intelligence quotient (IQ). Cognitive assessment instruments include the Bayley Scales of Infant Development (BSID), Raven’s Colored Progressive Matrices, Wechsler Intelligence Scale for Children, Wide Range Assessment for Memory and Learning, Wide Range Assessment of Visual Motor Abilities, and Wide Range Achievement Test. The major cognitive domains and common cognitive assessment instruments are presented in Table 1.
TABLE 1.
Cognitive assessment instruments and respective domains
| Cognitive assessment instrument | Cognitive domains assessed |
| Bayley Scales of Infant Development | Mental and psychomotor development |
| Raven’s Colored Progressive Matrices | Intelligence, reasoning, problem-solving, learning, and abstract thinking |
| Wide Range Assessment Tests for Memory and Learning; Wide Range Assessment of Visual Motor Ability; Wide Range Assessment of Overall Achievement | Visual–spatial memory and skills, visual–motor skills, motor skills, reading abilities, and mathematical skills |
| Wechsler Intelligence Scales for Children; Wechsler Intelligence Scale for Adults | Intelligence quotient, attention span, coordination, concentration, short-term memory, perception, and execution |
| Digit span test | Short-term memory, attention, and speed |
| Intelligence quotient | Evaluation of information, conception, and observational skills |
| Kaufman Brief Intelligence Test | Verbal and nonverbal intelligence |
Nutrient–nutrient interactions: other B vitamins involved in cognition.
Vitamin B-12 deficiency may co-occur with other nutrient deficiencies, including other B vitamins involved in one-carbon metabolism (7). Micronutrients such as folate, riboflavin, and choline interact in one-carbon metabolism (31), and may influence vitamin B-12 biomarkers and cognitive function (32). For example, riboflavin and vitamin B-6 are important cofactors in folate metabolism (33). Riboflavin is an essential cofactor for methyltetrahydrofolate reductase, and riboflavin deficiency can impair conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, reducing tetrahydrofolate availability and subsequent nucleotide synthesis, homocysteine remethylation, and S-adenosylmethionine production for methylation reactions (34). Similarly, pyridoxal phosphate is required for the conversion of tetrahydrofolate to 5,10-methylenetetrahydrofolate and subsequent neurotransmitter synthesis (34). Choline is a precursor of the neurotransmitter acetylcholine, and it plays an important role in methylation reactions, myelination (35), and neurotransmitter synthesis (36). To date, most studies examining the effects of B vitamins on cognition have focused on multiple micronutrient supplementation and cognitive function or decline in adults. The specific effects of vitamin B-12 on cognitive development or potential mechanisms involved have not been elucidated.
Role of vitamin B-12 in neurodevelopmental and psychiatric conditions.
Vitamin B-12 deficiency has been implicated in an increased occurrence and severity of neurodevelopmental disorders (37) and psychiatric conditions such as Alzheimer disease, schizophrenia (38), autism (39), depression (40, 41), and epileptic conditions (42), based on neuroimaging and clinical and epidemiologic studies. Several systematic reviews have been conducted to examine the role of vitamin B-12 and other B vitamins in cognitive decline, dementia, and neuropsychiatric conditions. However, to our knowledge, there is limited evidence concerning the role of vitamin B-12 in cognitive development in healthy children.
Vitamin B-12 and cognition in children.
The objective of this review was to examine the evidence that links maternal or child vitamin B-12 status and cognitive development in children. We examined the evidence from observational studies and intervention trials on the role of vitamin B-12 in cognitive development. We then discussed research gaps and implications for the development of interventions to improve cognition in children.
Methods
We conducted a structured literature search with the use of the MEDLINE and PsycINFO electronic databases. Relevant MeSH and exact terms were used to identify published studies in MEDLINE and PsycINFO, respectively, through 6 September 2015. The search terms used are provided in Table 2, and the search strategy is summarized in Figure 1.
TABLE 2.
Search terms
| Database | Terms |
| PubMed | ((Vitamin B 12[MeSH] OR B12[tw] OR B 12[tw] OR Vitamin B 12 Deficiency[MeSH] OR cobalamin*[tw] OR transcobalamin*[tw] OR methylmalonic acid*[tw]) AND (cogniti*[tw] OR Mental Processes[MeSH] OR Cognition Disorders[MeSH] OR cognitive development[tw] OR attention[tw] OR memory[tw] OR comprehension[tw] OR problem solving[tw] OR cognitive function*[tw] OR neurocognitive[tw] OR reason*[tw] OR learn*[tw] OR mental status[tw] OR intelligence quotient [tw] OR mental development [tw])) AND (infant[MeSH] OR child[MeSH] ORadolescent[MeSH] OR baby[tw] OR babies[tw] OR newborn*[tw] OR neonat*[tw] OR toddler*[tw] OR child*[tw] OR preschool*[tw] OR pre-school*[tw]OR schoolchild*[tw] OR boy*[tw] OR girl*[tw] OR preteen* [tw] or teen*[tw] OR adolescen*[tw] OR youth*[tw] OR young person*[tw] OR young people[tw] OR Mothers[MeSH] OR maternal[tw] OR mother*[tw] OR Pregnant Women[MeSH] OR Pregnancy[MeSH] OR pregnan*[tw] OR Prenatal Care[MeSH] OR prenatal[tw] OR infant*[tw]) |
| PsycINFO | ((TX vitamin B 12) OR (TX B 12) OR (TX B12) OR (TX vitamin B 12 deficiency) OR (TX cobalamin*) OR (TX transcobalamin*) OR (TX methylmalonic acid*)) AND ((DE Cognitive ability) OR (DE Cognitive development) OR (TX Cognitive development) OR (DE attention) OR (DE memory) OR (DE comprehension) OR (DE problem solving) OR (TX cognitive function*) OR (TX neurocognitive) OR (DE reason*) OR (DE learn*) OR (TX mental status) OR (TX intelligence quotient) OR (TX mental development) OR (TX IQ)) AND ((TX infant) OR (TX child) OR (TX adolescent) OR (TX baby) OR (TX babies) OR (TX newborn) OR (DE neonat*) OR(TX toddler*) OR (TX child*) OR (TX preschool*) OR (TX pre-school*) OR (TX schoolchild*) OR (TX boy*) OR (TX girl*) OR (TX preteen*) OR (TX teen*) OR (TX adolescen*) OR (TX youth*) OR (TX young person*) OR (TX young people) OR (DE mothers) OR (TX maternal) OR (TX mother*) OR (TX pregnant women) OR (TX pregnancy) OR (TX pregnan*) OR (DE prenatal care) OR (TX prenatal) OR (TX infant*)) |
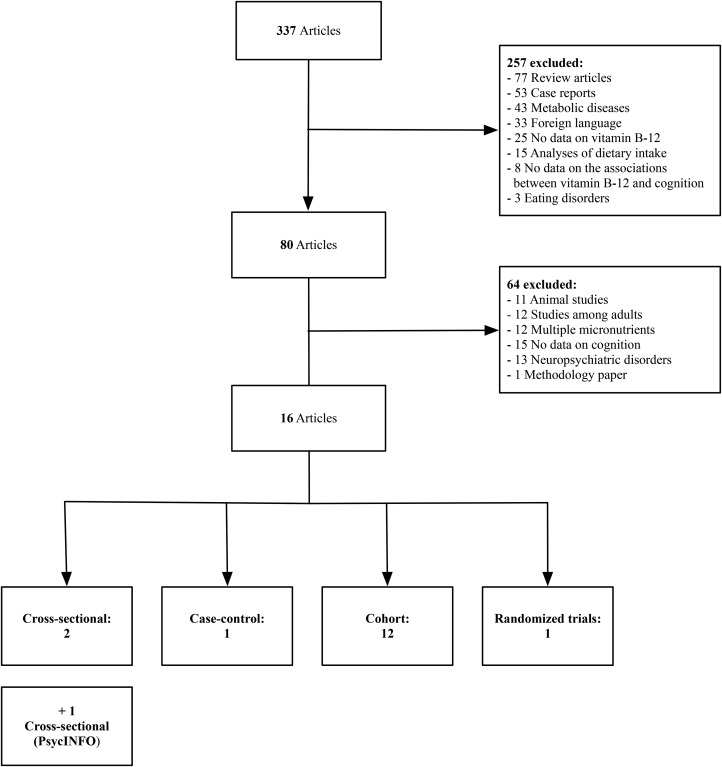
FIGURE 1.
A diagrammatic representation of the search strategy used to identify and select studies for inclusion in the review.
Initial inclusion criteria for this review were the availability of an abstract and inclusion of data on maternal or child vitamin B-12 status (or intake) and child cognitive outcomes. The following vitamin B-12 biomarkers were included in this review: serum and plasma total vitamin B-12, MMA, tHcy, and holotranscobalamin. The cognitive domains included were memory, attention, and perception. Data on other aspects of cognitive functioning, including psychomotor and mental development, academic performance, and IQ also were extracted and summarized in this review.
Abstracts of all studies were searched, full-text articles were extracted and reviewed, and the following inclusion criteria were applied: 1) human studies, 2) availability of data on maternal or child vitamin B-12 status, dietary intake, or supplementation, and child cognitive outcomes, and 3) studies focusing on children and adolescents (<18 y). All observational cross-sectional, case–control, and cohort studies, as well as randomized trials, interventions, and quasi-randomized and uncontrolled trials that met participant and methodologic selection criteria were included. Sources were retrieved, collected, indexed, and assessed for vitamin B-12 exposure and child cognitive outcome data. Additional sources were identified from bibliographies of published studies, manual searches of related articles of interest in references, and scientific meeting abstracts. An additional search was used to find review articles, which were examined to crossreference other relevant studies. Abstracts and full-text articles were reviewed independently by 2 reviewers (SV and IEA) and evaluated for inclusion; data were extracted in duplicate, risk of bias was evaluated, and any discrepancies were resolved by a third reviewer (JLF). A standardized data extraction table was used to organize key information from studies that met the inclusion criteria. As part of this protocol, publication date, authors, study location, setting, target population, definitions of exposures and outcomes, main findings, and study limitations were recorded.
Results
Literature review
The structured literature search in PubMed resulted in 337 articles, which were reviewed for potential inclusion. After 257 studies were excluded (n = 77 review articles, n = 53 case reports, n = 43 metabolic disease studies, n = 33 studies in other languages, n = 25 articles missing data on vitamin B-12, n = 15 articles focusing on dietary assessment, n = 8 articles with no data on the association between vitamin B-12 and cognition, and n = 3 articles on eating disorders), 80 full-text articles were extracted for further review. After we excluded 64 studies that did not meet inclusion criteria (n = 11 animal studies, n = 12 multiple micronutrient studies, n = 12 studies in adult populations, n = 15 studies with no data on cognitive outcomes, n = 13 studies on neuropsychiatric conditions, and n = 1 methods paper), 16 studies were identified for inclusion in this review. The structured literature search in the PsycINFO database with the equivalent exact terms resulted in one additional cross-sectional study. A total of 17 studies were included in this review: 16 observational studies (3 cross-sectional, including 2 from PubMed and 1 from PsycINFO; 1 case-control; and 12 cohort) and 1 randomized trial. The structured literature search is presented in Figure 1. A summary of the main findings by maternal and child vitamin B-12 status is presented in Table 3, and findings from these studies are summarized in detail by study design in Supplemental Tables 1–4.
TABLE 3.
Summary of main findings by maternal and child vitamin B-12 status1
| Study (ref), location | Vitamin B-12 | Cognitive outcomes results |
| Maternal vitamin B-12 status | ||
| Bhate et al. (43), India | Plasma vitamin B-12, MMA, and tHcy | Higher maternal vitamin B-12 status was associated with improved child cognitive outcomes |
| Veena et al. (44), India | Plasma vitamin B-12 and tHcy | No consistent associations |
| Bhate et al. (45), India | Plasma vitamin B-12 | Higher maternal vitamin B-12 status was associated with improved child cognitive outcomes |
| Wu et al. (46), Canada | Plasma vitamin B-12 and holotranscobalamin | No significant associations |
| Bonilla et al. (47), United Kingdom | Dietary vitamin B-12 intake | No significant associations |
| Villamor et al. (48), United States | Dietary vitamin B-12 intake | Higher maternal vitamin B-12 intake was associated with poorer child cognitive outcomes |
| Boeke et al. (49), United States | Dietary vitamin B-12 intake | No significant associations |
| del Rio Garcia et al. (50), Mexico | Dietary vitamin B-12 intake | Higher maternal vitamin B-12 intake was associated with improved child cognitive outcomes |
| Child vitamin B-12 status | ||
| Eilander et al. (51), India | Plasma vitamin B-12 | Higher child vitamin B-12 status was associated with poorer cognitive outcomes |
| Louwman et al. (52), Netherlands | Serum vitamin B-12, MMA, tHcy, and holotranscobalamin | Higher child vitamin B-12 status was associated with improved cognitive outcomes |
| Ngyuyen et al. (53), United States | Serum vitamin B-12 | No significant associations |
| Strand et al. (54), India | Plasma vitamin B-12, tHcy, and MMA | Higher child vitamin B-12 status was associated with higher mental development scores |
| Kvestad et al. (55), India | Vitamin B-12 supplement | Vitamin B-12 supplementation was associated with higher gross motor development scores |
| Masalha et al. (56), Israel | Serum vitamin B-12 and dietary intake | Higher child vitamin B-12 dietary intake was associated with improved school performance |
| Duong et al. (57), Colombia | Plasma vitamin B-12 | Vitamin B-12 deficiency was associated with poorer school performance |
| Gewa et al. (58), Kenya | Dietary vitamin B-12 intake | Higher child vitamin B-12 dietary intake was associated with improved cognitive outcomes |
| Ahmadi et al. (59), Iran | Dietary vitamin B-12 intake | Higher child vitamin B-12 dietary intake was associated with improved school performance |
MMA, methylmalonic acid; tHcy, total homocysteine.
Maternal vitamin B-12 status and child cognitive outcomes
Vitamin B-12 biomarkers.
Several studies have examined the associations between maternal or child vitamin B-12 status and cognitive outcomes in children, and have reported contradictory findings. Higher maternal vitamin B-12 status during pregnancy has been associated with improved measures of cognition in children later in life. A prospective analysis was conducted within the Pune Maternal Nutrition Study in India to examine the associations between mothers’ plasma vitamin B-12 concentrations at 28 wk of gestation (plasma vitamin B-12 <77.0 pM compared with >224.0 pM) and cognitive outcomes in their children at 9 y of age (43). Children born to mothers with higher plasma vitamin B-12 concentrations during pregnancy performed significantly better in short-term memory tasks and Color Trail A and digit span backward tests than did children born to mothers with lower plasma vitamin B-12 concentrations (plasma vitamin B-12 >224.0 compared with <77.0 pM; P < 0.05) in multivariate analyses adjusting for familial socioeconomic status and child sex, age, weight, head circumference, and plasma vitamin B-12 concentrations (43). There were no differences between children in the lower vitamin B-12 and higher vitamin B-12 groups on scores on intelligence tests or visual agnosia in multivariate analyses adjusting for familial socioeconomic status and child sex, age, and anthropometric measures. The authors also reported associations between maternal plasma vitamin B-12 concentrations during pregnancy and attention in their children at 9 y of age (43). Lower maternal plasma vitamin B-12 concentrations during pregnancy were associated with a significantly lower attention span in children at 9 y of age than were higher maternal plasma vitamin B-12 concentrations (plasma vitamin B-12 <77.0 compared with >224.0 pM; mean ± SD: 159.0 ± 41.2 compared with 182.0 ± 55.2 s; P < 0.05) in multivariate analyses adjusting for familial socioeconomic status and child sex, age, length, and weight (43). Another prospective cohort study from India studied the associations between mothers’ plasma vitamin B-12 concentrations during pregnancy and cognitive outcomes in their children at 10 y of age (44). Children born to women with adequate or deficient plasma vitamin B-12 concentrations during pregnancy (plasma vitamin B-12 ≥150 compared with <150 pmol/L) had similar attention scores, as measured by the Wechsler Intelligence Scale for Children-III (mean ± SD: 32.3 ± 8.0 compared with 33.2 ± 8.2; P > 0.05) (44). No significant associations were observed between maternal plasma vitamin B-12 concentrations and child cognitive outcomes. However, lower maternal plasma vitamin B-12 concentrations during pregnancy (plasma vitamin B-12 <150 pmol/L) were associated with improved verbal fluency scores in children compared with adequate vitamin B-12 status during pregnancy (plasma vitamin B-12 ≥150 compared with <150 pmol/L; β = 0.19; 95% CI: 0.01, 0.37; P < 0.05) (retrieval ability: number of animal names listed in 1 min) (44). This study did not report any significant associations between maternal vitamin B-12 status and other cognitive test components (44). In another cohort study in India, higher maternal plasma vitamin B-12 concentrations (plasma vitamin B-12 ≥150 pmol/L) during pregnancy (28 wk gestation) were associated with significantly higher mental development quotients (101 compared with 98; P = 0.035) and social development quotients (93 compared with 91; P = 0.029) in children at 2 y of age than those in children born to mothers with lower plasma vitamin B-12 concentrations (plasma vitamin B-12 <150 pmol/L) (45). In contrast, in a cohort study in Canada, mothers’ plasma vitamin B-12 concentrations during pregnancy (16–36 wk gestation) were not significantly associated with neurocognitive outcomes in their children at 18 mo of age, as measured by the BSID-III (46).
Maternal vitamin B-12 dietary intake.
Several observational studies have been conducted to examine the associations between mothers’ dietary vitamin B-12 intake and cognitive outcomes in their children later in life. A prospective cohort study was conducted in England to evaluate the associations between mothers’ vitamin B-12 intake during pregnancy (with an FFQ at 32 wk gestation) and IQ in their children at 8 y of age (n = 6259) (47). Maternal vitamin B-12 intake during pregnancy was associated with a higher IQ in children (2.0 IQ points; 95% CI: 1.3, 2.8 IQ points; P < 0.001) in multivariate analyses that adjusted for maternal daily energy intake, child age, and child sex (47). However, findings were no longer statistically significant in multivariate analyses after additionally adjusting for maternal education, age, parity, alcohol intake, folic acid supplementation, and infection (0.7 IQ points; 95% CI: −0.04, 1.4 IQ points; P = 0.06), or after additionally adjusting for length of gestation, duration of breastfeeding, and infant birth weight (0.5 IQ points; 95% CI: −0.2, 1.2 IQ points; P = 0.15).
In a prospective analysis within Project Viva in Massachusetts, investigators examined the associations between maternal dietary intake (with an FFQ) of B vitamins during the first and second trimesters of pregnancy and cognitive outcomes in children at 3 y of age, including overall intelligence, visual motor and spatial skills, and fine motor skills (48). Higher maternal dietary vitamin B-12 intake during the second trimester of pregnancy was associated with lower child vocabulary scores as measured by the Peabody Picture Vocabulary Test III, with a 0.4 point decrease for every 2.6 μg increase in maternal vitamin B-12/d (−0.4 points; 95% CI: −0.8, −0.01 points; P = 0.01) (48). Findings remained statistically significant after adjusting for socioeconomic, demographic, and dietary factors (48). In another analysis in the same cohort, cognitive tests were administered to children at 7 y of age (n = 895) (49). Maternal vitamin B-12 dietary intake during pregnancy was not significantly associated with child IQ (49).
In a prospective cohort study in Mexico, investigators examined the associations between maternal dietary vitamin B-12 intake (with an FFQ) in the first trimester of pregnancy and child development at 1, 3, 6, and 12 mo of age (50). Inadequate maternal vitamin B-12 intake (<2.0 μg/d) was associated with poorer mental development scores (β = −1.6; 95% CI: −2.8, −0.3; P < 0.05) in children at 12 mo of age as measured by the BSID-II (50).
Child vitamin B-12 status and cognitive outcomes
Vitamin B-12 biomarkers.
Several observational studies have been conducted to examine the associations between vitamin B-12 status and cognitive outcomes in children. A cross-sectional study was conducted to examine the associations between child plasma vitamin B-12 concentrations and the mental processing index, which included measures of fluid reasoning, short-term memory, retrieval ability, and speed in school-aged children (6–10 y) in India (51). In this study, higher child plasma total vitamin B-12 concentrations [median (IQR): 197.0 pmol/L (151, 266 pmol/L)] were associated with poorer number recall and word order, indicators of short-term memory [log (vitamin B-12), β = −0.19; 95% CI: −0.36, −0.03; P < 0.05] (51). Higher child plasma vitamin B-12 concentrations were also associated with lower mental processing index scores [log (vitamin B-12), β = −0.12; 95% CI: −0.22, −0.02; P = 0.02] in multivariate analyses after adjusting for child hemoglobin, serum folate concentrations, and WHO height-for-age z scores (51).
A nested case–control study was conducted in the Netherlands to examine measures of cognitive function in adolescents who had consumed a macrobiotic (vegan) diet (n = 93; 10–16 y) from birth until 6 y of age compared with adolescents who had consumed an omnivorous diet (control; n = 102; 10–18 y) during the same time period (52). This study compared measures of intelligence in adolescents who had consumed vegan or omnivorous diets earlier in life; individuals who consumed the macrobiotic diet had significantly lower fluid intelligence scores than did adolescents in the omnivorous diet group (52). Vitamin B-12 biomarkers were evaluated in individuals at 16 y of age, including serum total vitamin B-12, plasma holotranscobalamin, serum MMA, and plasma tHcy. In analyses in adolescents (16 y), elevated serum MMA (>0.29 μmol/L), an indicator of impaired vitamin B-12 status, was associated with poorer short-term memory as measured by the digit span test (β = −0.24; 95% CI: −0.46, −0.02; P < 0.05) (52). Individuals who had consumed a vegan diet were then classified as having a normal or deficient vitamin B-12 status on the basis of serum vitamin B-12 and MMA concentrations (normal: serum vitamin B-12 ≥229 pmol/L or MMA ≤0.29 μmol/L compared with deficient: serum vitamin B-12 <229 pmol/L or MMA >0.29 μmol/L). Adolescents with lower serum vitamin B-12 concentrations had significantly lower fluid intelligence scores than did individuals with normal serum vitamin B-12 concentrations (r = −0.28; 95% CI: −0.48, −0.08; P = 0.003) (52).
In a secondary analysis of data from the NHANES-III in children (6–16 y), serum vitamin B-12 concentrations were not associated with any of the cognitive outcomes assessed (i.e., reading and math measured by the Wide Range Assessment of Overall Achievement–Revised, and block design and digit span measured by Wechsler Intelligence Scale for Children–Revised) (53). However, higher child serum vitamin B-12 concentrations were correlated with higher math (r = 0.06; P < 0.05) and digit span (r = 0.08; P < 0.05) test scores, which reflect attention and short-term memory (53).
In a prospective cohort study in India, investigators examined the associations between vitamin B-12 status (i.e., plasma total vitamin B-12, MMA, and tHcy) and neurocognitive development (BSID-II) in children 12–18 mo of age (54). Higher child plasma vitamin B-12 concentrations were associated with significantly higher BSID-II mental development scores (1.3; 95% CI: 0.2, 2.4; P = 0.02) (54). Similarly, higher plasma MMA (1.1; 95% CI: 0.3, 1.8; P = 0.004) and tHcy (2.0; 95% CI: 0.5, 3.4; P = 0.007) concentrations were associated with lower BSID-II mental development scores (54). The effects of vitamin B-12 and/or folic acid supplementation on early child development were assessed in a recent randomized controlled trial in India (55). In this study, 422 children aged 6–30 mo were randomly assigned to 1 of 4 groups stratified by age (<12 compared with ≥12 mo) as follows: 1) vitamin B-12, 2) folic acid, 3) vitamin B-12 and folic acid, or 4) placebo daily for a duration of 6 mo. Children who received both vitamin B-12 and folic acid had 0.45 (95% CI: 0.19, 0.73; P = 0.0001) and 0.28 (95% CI: 0.02, 0.54; P < 0.05) higher SD units in gross motor and problem-solving functioning domains, respectively than did those in the placebo group (55). Children in the vitamin B-12 group had significantly higher gross motor developmental scale scores at end line than did those in the placebo group (4 points; 95% CI 0.3, 7.8 points; P < 0.05) as measured by the Ages and Stages Questionnaire 3 (55).
A cross-sectional study was conducted in Israel to examine the associations between child serum vitamin B-12 concentrations (serum vitamin B-12 <200 pg/mL) and academic performance in 67 elementary school children (9–11 y) (56). Child serum vitamin B-12 concentrations were evaluated, and an FFQ was administered to assess the number of meals containing meat consumed per week (as a proxy for vitamin B-12 intake); parental employment status, academic achievement index, and grades in school (numeric grades from 0–100) were also evaluated (56). A total of 25% of children had low serum vitamin B-12 concentrations (serum vitamin B-12 <200 pg/mL); a higher frequency of meat consumption was associated with improved school performance (r = 0.3; P = 0.006) (56). Higher serum vitamin B-12 concentrations (serum vitamin B-12 ≥200 pg/mL; mean ± SE: 0.49 ± 0.10; 95% CI: 0.29, 0.70; P = 0.0001) and paternal employment status (mean ± SE: 0.25 ± 0.10; 95% CI: 0.04, 0.46; P = 0.02) were significantly associated with greater academic achievement (>80%). Similar findings were reported from another cohort study in Colombia; this study was conducted to examine the associations between plasma vitamin B-12 concentrations and academic performance in school-aged children (5–12 y; n = 3156) (57). Children who were vitamin B-12 deficient (plasma vitamin B-12 <148 pmol/L) had a significantly higher risk of school absenteeism (RR: 1.89; 95% CI: 1.53, 2.34; P < 0.0001) and grade repetition (i.e., failing ≥3 subjects, including mathematics and language; RR: 2.36; 95% CI: 1.03, 5.41; P = 0.04) than did nondeficient children (plasma vitamin B-12 ≥148 pmol/L) (57).
Child vitamin B-12 dietary intake.
A randomized controlled feeding trial was conducted in Kenya to examine the effects of consuming food-based interventions on various indexes of cognitive function in school-aged children (median age: 7.4 y), including problem-solving tasks, a verbal reasoning test, and a digit span test (58). Children were randomly assigned to 1 of 4 groups: 1) vegetables with maize and beans, 2) milk plus maize and beans, 3) meat (60 g beef) plus vegetables, and 4) a control group with no food supplement. In an ancillary analysis conducted within this trial, higher dietary vitamin B-12 intake in children was associated with improved memory, as measured by the digit span test (0.24 points; β ± SEM: 0.01 ± 0.005 points; P < 0.05) (58). In a cohort study in Iran in school-aged children, total dietary vitamin B-12 intake during breakfast meals was not significantly associated with short-term memory (r = 1.0; P = 0.50) as measured by number recall on the Wechsler Scale (59). In the same study, dietary vitamin B-12 intake from breakfast meals was associated with improved school performance (i.e., average annual grades) (P = 0.02) (59). However, child vitamin B-12 status was not assessed in this study (59).
Discussion
Emerging evidence suggests that inadequate supply of vitamin B-12 during pregnancy and early childhood can have lasting effects on infant growth and brain development, which may be irreversible. However, most studies to date have been observational in design, and included a single measurement of vitamin B-12 status or intake during pregnancy (or early childhood) and global measures of cognition in children later in life. These observational studies have several methodologic limitations, including small sample size, limited assessment of biomarkers of vitamin B-12 status, and nonspecific cognitive assessment tools, which constrain interpretation of the findings. To our knowledge, there is relatively limited evidence regarding the role of vitamin B-12 in specific child cognitive outcomes, and only one randomized trial has been conducted to date to examine the effects of child vitamin B-12 supplementation on cognitive outcomes.
Findings from observational studies that examined the associations between maternal vitamin B-12 status or intake and child cognitive outcomes were heterogeneous. Two studies from India noted that a higher maternal vitamin B-12 status during pregnancy was associated with improved child cognitive outcomes and developmental indexes (43, 45). One study from Mexico reported that inadequate maternal dietary intake of vitamin B-12 during pregnancy was associated with poorer developmental outcomes in their infants (50). Four observational studies noted no significant associations between maternal vitamin B-12 status and child cognitive outcomes, although specific cognitive outcomes assessed varied, and the ages of children ranged from 1 to 10 y of age (44, 46, 47, 49). One study noted that higher maternal dietary vitamin B-12 intake during gestation was associated with lower vocabulary skills in children at 3 y of age (48).
Findings from observational studies that evaluated the associations between child vitamin B-12 status or intake and cognitive outcomes were more consistent: higher child vitamin B-12 status was associated with improved cognitive performance (52, 58), school performance (56, 57, 59), and developmental indexes (54, 55) in several studies. However, one cross-sectional study suggested that higher child vitamin B-12 status was associated with poorer cognitive measures (51), and a large population-based study did not report any significant associations between vitamin B-12 status and cognition in children (53).
Research gaps: study design
Most studies to date on vitamin B-12 and cognition in children have been observational in design, which constrains the ability to establish temporality and causal inference. The 3 cross-sectional studies in this review had conflicting findings: one study noted associations between vitamin B-12 intake from meat and improved school performance (grade point average) (56), whereas another study noted an inverse association between child vitamin B-12 status and memory (51), and a third study found no significant associations between child serum vitamin B-12 concentrations and cognitive outcomes (53). Several cohort studies have reported associations between maternal dietary vitamin B-12 intake during pregnancy and child cognitive outcomes (47, 49, 58, 59). These studies used a variety of dietary assessment methods and cutoff points for vitamin B-12 intake, which limits comparability of findings between studies. In other cohort studies (50, 54), plasma vitamin B-12 concentrations were measured in either the mother or child only at one time point. The time integration or the relevant etiologic period of vitamin B-12 in the development of cognitive outcomes later in childhood is unknown. The randomized controlled trial in India examined the effects of vitamin B-12 and/or folic acid supplementation on improved gross motor development and suggested a beneficial role for vitamin B-12 on motor and problem-solving skills (55).
Vitamin B-12 biomarkers
The variety of vitamin B-12 biomarkers assessed in different studies (including serum and plasma total vitamin B-12, MMA, tHcy, and holotranscobalamin), laboratory methods, timing of assessment, and cutoffs for vitamin B-12 deficiency and insufficiency pose challenges when interpreting findings across studies. In addition to total vitamin B-12, other circulating (e.g., holotranscobalamin) and functional (e.g., MMA) biomarkers are needed to comprehensively assess vitamin B-12 status. Further research is needed to evaluate appropriate vitamin B-12 biomarkers prospectively during pregnancy and early childhood.
Role of additional nutrients
One-carbon metabolism is influenced by a variety of nutrients that interact and can compensate for one another when there is a deficiency of a single nutrient. The potential role of other micronutrients in one-carbon metabolism, such as folate, riboflavin, pyridoxine, and choline, need to be examined, as they may influence both vitamin B-12 metabolism and cognitive development (60). Additional factors, including maternal nutritional status, educational levels, and infant and young child feeding practices, also need to be considered in prospective analyses, because they may be associated with both vitamin B-12 status and cognitive development in offspring (61). In addition to inclusion of these variables as covariates in analyses, the interactions between these nutrients in the etiology of cognitive outcomes need to be elucidated.
Cognitive outcomes
The range of cognitive domains and instruments used in different studies constrains comparability of findings. Although many of these instruments are standardized, many have not been validated in different age groups, populations, or other cultural contexts with high sensitivity and specificity (62). For example, the BSID, as a screening tool, is one of the most widely used neurocognitive assessment instruments, is administered to children from birth to 2.5 y of age, and focuses on psychomotor and mental development. However, for children at older ages, a wide range of cognitive assessment instruments have been developed that examine different cognitive functions, as well as tests that assess global intelligence and various perceptual abilities (63). Since different aspects of cognitive functioning (e.g., explicit memory, sustained attention, selective attention, inhibitory control, and learning) depend on distinct neural systems, they can be affected differentially by a nutritional alteration. For this reason, it is suggested that future studies select tasks that examine biologically distinct aspects of cognitive functioning, in addition to including indexes of global cognitive development (64–66). In addition, when examining the effects of a nutritional alteration on cognitive function in children, it is optimal to assess each aspect of cognitive functioning at various time points in order to determine if the development of each of these processes is delayed or permanently altered (66). It is also necessary to examine the role of potential confounders in the assessment of cognitive development, such as factors in the home environment in which the child was reared, by using the Home Observation for Measurement of the Environment scores (67). Cognitive assessment tools that examine specific aspects of brain function (including neuroimaging techniques) and are sensitive to nutritional interventions also are needed urgently, particularly for young children.
Translational research
Findings to date have identified a potential role for vitamin B-12 status in child cognitive functioning and perhaps ultimate cognitive potential. However, there is limited prospective data, and the causal and biological mechanisms are unknown. To our knowledge, only one randomized controlled trial has been conducted to date to determine the effects of vitamin B-12 and/or folic acid on early child development indexes. Studies in animal and cellular models provide valuable insight into causal mechanisms and pathways of vitamin B-12 in the etiology of adverse cognitive outcomes. However, there is limited prospective and causal data in human populations. The biological mechanisms of vitamin B-12 in cognition need to be elucidated experimentally in relevant animal models to inform translational research in at-risk human populations.
Conclusions
Vitamin B-12 deficiency is a major public health problem, and is associated with an increased risk of adverse child health outcomes. Observational studies to date have demonstrated associations between vitamin B-12 status or dietary intake and cognitive outcomes in children. The importance of adequate vitamin B-12 status, particularly during pregnancy and early childhood, cannot be overemphasized in light of the role of vitamin B-12 in neural myelination, brain development, and fetal and child growth. Further research is urgently needed to elucidate the role of vitamin B-12 in cognitive development in order to inform public health approaches in at-risk populations.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BSID, Bayley Scales of Infant Development; IQ, intelligence quotient; MMA, methylmalonic acid; tHcy, total homocysteine.
References
- 1.Scott JM, Molloy AM. The discovery of vitamin B(12). Ann Nutr Metab 2012;61:239–45. [DOI] [PubMed] [Google Scholar]
- 2.Rush EC, Katre P, Yajnik CS. Vitamin B12: one carbon metabolism, fetal growth and programming for chronic disease. Eur J Clin Nutr 2014;68:2–7. [DOI] [PubMed] [Google Scholar]
- 3.Morris MS. The role of B vitamins in preventing and treating cognitive impairment and decline. Adv Nutr 2012;3:801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briani C, Dalla Torre C, Citton V, Manara R, Pompanin S, Binotto G, Adami F. Cobalamin deficiency: clinical picture and radiological findings. Nutrients 2013;5:4521–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawlak R, Lester SE, Babatunde T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr 2014;68:541–8. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and perinatal health. Adv Nutr 2015;6:552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaminathan S, Thomas T, Kurpad AV. B-vitamin interventions for women and children in low-income populations. Curr Opin Clin Nutr Metab Care 2015;18:295–306. [DOI] [PubMed] [Google Scholar]
- 8.Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr 2004;24:299–326. [DOI] [PubMed] [Google Scholar]
- 9.Dror DK, Allen LH. Interventions with vitamins B6, B12 and C in pregnancy. Paediatr Perinat Epidemiol 2012;26 Suppl 1:55–74. [DOI] [PubMed] [Google Scholar]
- 10.Carmel R. Efficacy and safety of fortification and supplementation with vitamin B12: biochemical and physiological effects. Food Nutr Bull 2008; 29(2, Suppl)S177–87. [DOI] [PubMed] [Google Scholar]
- 11.Allen LH, Rosenberg IH, Oakley GP, Omenn GS. Considering the case for vitamin B12 fortification of flour. Food Nutr Bull 2010; 31(1, Suppl)S36–46. [DOI] [PubMed] [Google Scholar]
- 12.Kumar N. Neurologic aspects of cobalamin (B12) deficiency. Handb Clin Neurol 2014;120:915–26. [DOI] [PubMed] [Google Scholar]
- 13.Alpers DH, Russell-Jones G. Gastric intrinsic factor: the gastric and small intestinal stages of cobalamin absorption. a personal journey. Biochimie 2013;95:989–94. [DOI] [PubMed] [Google Scholar]
- 14.Molloy AM, Kirke PN, Brody LC, Scott JM, Mills JL. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr Bull 2008;29(2 Suppl):S101–11; discussion S12–5. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Wright Z, Hvas AM, Moller J, Sanders TA, Nexo E. Holotranscobalamin as an indicator of dietary vitamin B12 deficiency. Clin Chem 2003;49:2076–8. [DOI] [PubMed] [Google Scholar]
- 16.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94:348S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizu RA, et al. . Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94:313S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. Am J Clin Nutr 2011;94:297S–302S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr 2011;94:322S–31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85:614S–20S. [DOI] [PubMed] [Google Scholar]
- 21.Kretchmer N, Beard JL, Carlson S. The role of nutrition in the development of normal cognition. Am J Clin Nutr 1996;63:997S–1001S. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol 2001;56:5–15. [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishna T. Vitamins and brain development. Physiol Res 1999;48:175–87. [PubMed] [Google Scholar]
- 24.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull 2008; 29(2, Suppl)S126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006;5:949–60. [DOI] [PubMed] [Google Scholar]
- 26.Lövblad K, Ramelli G, Remonda L, Nirkko AC, Ozdoba C, Schroth G. Retardation of myelination due to dietary vitamin B12 deficiency: cranial MRI findings. Pediatr Radiol 1997;27:155–8. [DOI] [PubMed] [Google Scholar]
- 27.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 2008;66:250–5. [DOI] [PubMed] [Google Scholar]
- 28.Fernàndez-Roig S, Lai SC, Murphy MM, Fernandez-Ballart J, Quadros EV. Vitamin B12 deficiency in the brain leads to DNA hypomethylation in the TCblR/CD320 knockout mouse. Nutr Metab (Lond) 2012;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepper MR, Black MM. B12 in fetal development. Semin Cell Dev Biol 2011;22:619–23. [DOI] [PubMed] [Google Scholar]
- 30.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol 2006;13:158–65. [DOI] [PubMed] [Google Scholar]
- 31.Bertolo RF, McBreairty LE. The nutritional burden of methylation reactions. Curr Opin Clin Nutr Metab Care 2013;16:102–8. [DOI] [PubMed] [Google Scholar]
- 32.Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr 2000;71:614S–20S. [DOI] [PubMed] [Google Scholar]
- 33.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging 2002;6:39–42. [PubMed] [Google Scholar]
- 34.Strain JJ, Dowey L, Ward M, Pentieva K, McNulty H. B-vitamins, homocysteine metabolism and CVD. Proc Nutr Soc 2004;63:597–603. [DOI] [PubMed] [Google Scholar]
- 35.Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 2013;5:3481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollenbeck CB. An introduction to the nutrition and metabolism of choline. Cent Nerv Syst Agents Med Chem 2012;12:100–13. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, Tassone F, Hertz-Picciotto I. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 2011;22:476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ssonko M, Ddungu H, Musisi S. Low serum vitamin B12 levels among psychiatric patients admitted in Butabika mental hospital in Uganda. BMC Res Notes 2014;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Farsi YM, Waly MI, Deth RC, Al-Sharbati MM, Al-Shafaee M, Al-Farsi O, Al-Khaduri MM, Gupta I, Ali A, Al-Khalili M, et al. . Low folate and vitamin B12 nourishment is common in Omani children with newly diagnosed autism. Nutrition 2013;29:537–41. [DOI] [PubMed] [Google Scholar]
- 40.Hintikka J, Tolmunen T, Tanskanen A, Viinamaki H. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry 2003;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry 2002;159:2099–101. [DOI] [PubMed] [Google Scholar]
- 42.Di Rosa G, Lenzo P, Parisi E, Neri M, Guerrera S, Nicotera A, Alibrandi A, Germano E, Caccamo D, Spano M, et al. . Role of plasma homocysteine levels and MTHFR polymorphisms on IQ scores in children and young adults with epilepsy treated with antiepileptic drugs. Epilepsy Behav 2013;29:548–51. [DOI] [PubMed] [Google Scholar]
- 43.Bhate V, Deshpande S, Bhat D, Joshi N, Ladkat R, Watve S, Fall C, de Jager CA, Refsum H, Yajnik C. Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food Nutr Bull 2008;29:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Muthayya S, Kurpad AV, Yajnik CS, Fall CH. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10-year-old children in South India. J Nutr 2010;140:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhate VK, Joshi SM, Ladkat RS, Deshmukh US, Lubree HG, Katre PA, Bhat DS, Rush EC, Yajnik CS. Vitamin B12 and folate during pregnancy and offspring motor, mental and social development at 2 years of age. J Dev Orig Health Dis 2012;3:123–30. [DOI] [PubMed] [Google Scholar]
- 46.Wu BT, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One 2012;7:e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonilla C, Lawlor DA, Taylor AE, Gunnell DJ, Ben-Shlomo Y, Ness AR, Timpson NJ, St Pourcain B, Ring SM, Emmett PM, et al. . Vitamin B-12 status during pregnancy and child’s IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children. PLoS One 2012;7:e51084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villamor E, Rifas-Shiman SL, Gillman MW, Oken E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol 2012;26:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol 2013;177:1338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.del Río Garcia C, Torres-Sanchez L, Chen J, Schnaas L, Hernandez C, Osorio E, Portillo MG, Lopez-Carrillo L. Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutr Neurosci 2009;12:13–20. [DOI] [PubMed] [Google Scholar]
- 51.Eilander A, Muthayya S, van der Knaap H, Srinivasan K, Thomas T, Kok FJ, Kurpad AV, Osendarp SJ. Undernutrition, fatty acid and micronutrient status in relation to cognitive performance in Indian school children: a cross-sectional study. Br J Nutr 2010;103:1056–64. [DOI] [PubMed] [Google Scholar]
- 52.Louwman MW, van Dusseldorp M, van de Vijver FJ, Thomas CM, Schneede J, Ueland PM, Refsum H, van Staveren WA. Signs of impaired cognitive function in adolescents with marginal cobalamin status. Am J Clin Nutr 2000;72:762–9. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen CT, Gracely EJ, Lee BK. Serum folate but not vitamin B-12 concentrations are positively associated with cognitive test scores in children aged 6–16 years. J Nutr 2013;143:500–4. [DOI] [PubMed] [Google Scholar]
- 54.Strand TA, Taneja S, Ueland PM, Refsum H, Bahl R, Schneede J, Sommerfelt H, Bhandari N. Cobalamin and folate status predicts mental development scores in North Indian children 12–18 mo of age. Am J Clin Nutr 2013;97:310–7. [DOI] [PubMed] [Google Scholar]
- 55.Kvestad I, Taneja S, Kumar T, Hysing M, Refsum H, Yajnik CS, Bhandari N, Strand TA. Folate, vitamin BSG. Vitamin B12 and folic acid improve gross motor and problem-solving skills in young North Indian children: a randomized placebo-controlled trial. PLoS One 2015;10:e0129915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masalha R, Afawi Z, Mahajnah M, Mashal A, Hallak M, Ibrahim A, Bolotin A, Ifergane G, Wirguin I. The impact of nutritional vitamin B12, folate and hemoglobin deficiency on school performance of elementary school children. J Pediatr Neurol 2008;6:6. [Google Scholar]
- 57.Duong MC, Mora-Plazas M, Marin C, Villamor E. Vitamin B-12 deficiency in children is associated with grade repetition and school absenteeism, independent of folate, iron, zinc, or vitamin a status biomarkers. J Nutr 2015;145:1541–8. [DOI] [PubMed] [Google Scholar]
- 58.Gewa CA, Weiss RE, Bwibo NO, Whaley S, Sigman M, Murphy SP, Harrison G, Neumann CG. Dietary micronutrients are associated with higher cognitive function gains among primary school children in rural Kenya. Br J Nutr 2009;101:1378–87. [DOI] [PubMed] [Google Scholar]
- 59.Ahmadi A, Sohrabi Z, Eftekhari MH. Evaluating the relationship between breakfast pattern and short-term memory in junior high school girls. Pak J Biol Sci 2009;12:742–5. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg IH, Miller JW. Nutritional factors in physical and cognitive functions of elderly people. Am J Clin Nutr 1992; 55(6, Suppl)1237S–43S. [DOI] [PubMed] [Google Scholar]
- 61.Pasricha SR, Shet AS, Black JF, Sudarshan H, Prashanth NS, Biggs BA. Vitamin B-12, folate, iron, and vitamin A concentrations in rural Indian children are associated with continued breastfeeding, complementary diet, and maternal nutrition. Am J Clin Nutr 2011;94:1358–70. [DOI] [PubMed] [Google Scholar]
- 62.de Jager CA, Dye L, de Bruin EA, Butler L, Fletcher J, Lamport DJ, Latulippe ME, Spencer JP, Wesnes K. Criteria for validation and selection of cognitive tests for investigating the effects of foods and nutrients. Nutr Rev 2014;72:162–79. [DOI] [PubMed] [Google Scholar]
- 63.Isaacs E, Oates J, ILSI Europe a.i.s.b.l. Nutrition and cognition: assessing cognitive abilities in children and young people. Eur J Nutr 2008;47 Suppl 3:4–24. [DOI] [PubMed] [Google Scholar]
- 64.Strupp BJ, Diamond A. Assessing cognitive function in animal models of mental retardation. Ment Retard Dev Disabil Res Rev 1996;2:216–26. [Google Scholar]
- 65.Strupp BJ, Levitsky DA. Enduring cognitive effects of early malnutrition: a theoretical reappraisal. J Nutr 1995; 125(8, Suppl)2221S–32S. [DOI] [PubMed] [Google Scholar]
- 66.Wainwright PE, Colombo J. Nutrition and the development of cognitive functions: interpretation of behavioral studies in animals and human infants. Am J Clin Nutr 2006;84:961–70. [DOI] [PubMed] [Google Scholar]
- 67.Patel SA, Murray-Kolb LE, LeClerq SC, Khatry SK, Tielsch JM, Katz J, Christian P. Household wealth and neurocognitive development disparities among school-aged children in Nepal. Paediatr Perinat Epidemiol 2013;27:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]