Abstract
Alzheimer disease (AD) is becoming one of the most prevalent neurodegenerative conditions worldwide. Although the disease progression is becoming better understood, current medical interventions can only ameliorate some of the symptoms but cannot slow disease progression. Neuroinflammation plays an important role in the advancement of this disorder, and n–3 (ω-3) polyunsaturated fatty acids (PUFAs) are involved in both the reduction in and resolution of inflammation. These effects may be mediated by the anti-inflammatory and proresolving effects of bioactive lipid mediators (oxylipins) derived from n–3 PUFAs [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] in fish oil. Although interventions have generally used fish oil containing both EPA and DHA, several studies that used either EPA or DHA alone or specific oxylipins derived from these fatty acids indicate that they have distinct effects. Both DHA and EPA can reduce neuroinflammation and cognitive decline, but EPA positively influences mood disorders, whereas DHA maintains normal brain structure. Fewer studies with a plant-derived n–3 PUFA, α-linolenic acid, suggest that other n–3 PUFAs and their oxylipins also may positively affect AD. Further research identifying the unique anti-inflammatory and proresolving properties of oxylipins from individual n–3 PUFAs will enable the discovery of novel disease-management strategies in AD.
Keywords: Alzheimer disease, neuroinflammation, polyunsaturated fatty acids, oxylipins, resolution of inflammation, omega-3 fatty acids, class switching
Introduction
Alzheimer disease (AD)3 is the most common neurodegenerative disorder and constitutes ∼60–80% of all dementia (1–3). Age is the most common predictor, with 1 in 5 people aged >80 y and 1 in 3 people >90 y having AD (4, 5). With the life expectancy of the global population increasing, the prevalence of AD is set to escalate. From 1980 to 2010, when the life expectancy increased by ∼6 y for the US population, the age-adjusted death rates for AD increased 55-fold (6). Globally, 35.6 million people are living with dementia and this number is expected to reach 115.4 million by 2050 (7). An effective cure for the disease has yet to be discovered, and so lifestyle and nutritional factors are crucial in managing the disease. This review article aims to examine the role of n–3 PUFAs and PUFA-derived oxylipins in reducing neuroinflammation associated with AD.
Pathology of AD
AD is a disorder that has both familial and sporadic forms. Familial AD (FAD) has an onset <65 y of age and is found in <1% of the total cases (2). It is caused by autosomal dominant mutations in the amyloid precursor protein (APP) gene, the presenilin 1 (PS1) gene, and the presenilin 2 (PS2) gene (8). In the sporadic form of AD, the apolipoprotein E (ApoE) ε4 allele has the strongest association with the risk of developing AD (9). Homozygotes of the ApoE ε4 allele have 15 times more risk of developing AD than a noncarrier of this allele (10), and each copy of the allele lowers the age at onset by 10 y (9).
Despite the large amount of research in AD over the past 3 decades, the exact disease-related alterations in the AD brain and the order in which they occur remain to be understood (2). Like many other chronic diseases, AD develops due to multiple factors rather than a single cause, including several changes in the brain that begin up to 20 y before any symptoms appear (2). Current understanding of the pathology indicates that AD is characterized by progressive loss of synapses and neurons (8). As AD progresses, the capacity of synapses to transfer information starts to diminish, the number of synapses decreases, and subsequently death of the neurons occurs (2). Progressive dysfunction of the synapses and neurons is usually preceded by molecular events involving the accumulation of oligomeric assemblies of misfolded proteins. Among these molecular lesions identified in AD, amyloid plaques and neurofibrillary tangles (NFTs) are the most defining ones (11, 12). Amyloid plaques are formed in the extraneuronal space from aggregates of toxic amyloid β (Aβ), and NFTs are formed inside neurons by hyperphosphorylated tau protein.
A key protein involved in Aβ formation is APP, an integral membrane glycoprotein expressed in the brain that is involved in the regulation of synaptic function, neuronal activity, and brain cholesterol metabolism. APP can undergo sequential protein cleavage either through an α-pathway or a β-pathway (13). In the α-pathway, which is usually nonamyloidogenic, APP is cleaved first by α-secretase and then by γ-secretase. In the amyloidogenic β-pathway, APP is first cleaved by β-secretase [β-site APP cleaving enzyme 1(BACE1)], releasing the soluble peptide APPβ into the extracellular matrix and leaving a 99–amino acid C-terminal fragment within the membrane. This fragment is further processed by γ-secretase to form Aβ40 or Aβ42 and APP intracellular C-terminal domain (AICD) (14). AICD is further stabilized by Fe65, an intranuclear adapter protein, and binds to transcription factor Tip60, initiating the transcription of the enzyme neprilysin involved in Aβ degradation, thus regulating Aβ concentrations (15). In AD, impaired APP and subsequent Aβ degradation result in increased deposition and reduced clearance of Aβ40 and Aβ42 peptides in the extracellular matrix, eventually leading to the oligomerization of Aβ and plaque formation (16). Although initially it was thought that Aβ deposition happens only in the extracellular space, new data from transgenic mice and human patients point to the possibility of intracellular accumulation and its involvement in AD pathology (17).
The cascade of events starting from the cleavage of APP and leading to neuronal loss is referred to as the “amyloid cascade” hypothesis (18). This hypothesis, proposed in 1992, posits that Aβ deposition, the primary pathologic episode in AD causing the establishment of senile amyloid plaques, leads to abnormalities in tau protein causing NFT (18). Tau is a microtubule-associated protein (MAP) involved in regulating microtubule dynamics and stability. In AD, hyperphosphorylation of tau protein dissociates it from the microtubule structure, resulting in aggregation of tau protein into filamentous NFTs (19). NFTs cause a physical barrier to intracellular communication. In addition, dissociation of tau protein results in the destabilization of microtubules. The disruption of intracellular communication and the destabilization of microtubules lead to neuronal death and dementia (20, 21).
In the inherited form of AD (FAD), impairment of APP metabolism is caused due to mutations in APP, BACE1, PS1, and PS2. Although these genetic causes have been identified in FAD, what causes the aggregation of Aβ in the sporadic form of AD has yet to be completely understood. Histopathologic hallmarks are indistinguishable between FAD and sporadic forms of AD. Genomewide association studies have identified potential roles of genes involved in endosomal vesicle recycling, cholesterol metabolism, and in the innate immune system (22, 23). However, most of the risk and pathology of sporadic AD have been accounted for by the ApoE ε4 allele (24). ApoE is a 299–amino acid glycoprotein involved in the transport of lipids, including cholesterol, through ApoE receptors on the surface of the cell. Although neurons usually synthesize ApoE only under stress, microglia, astrocytes, choroid plexus, and vascular smooth muscle cells in the brain constitutively express ApoE (25). Although ApoE mainly functions as a lipid transporter in the brain, it also regulates Aβ metabolism, aggregation, and deposition. Due to genetic polymorphisms, there exist 3 major isoforms, ApoE ε2, ApoE ε3, and ApoE ε4 (26), with differing amino acids at positions 112 and 158 (27). The exact mechanism by which the product from ε4 allele stimulates AD pathology is not completely understood. Several possibilities, such as ApoE ε4 interaction with tau protein, ApoE ε4–mediated enhancement of Aβ production and oligomerization, involvement of ApoE ε4 at the level of cholesterol metabolism, and interaction of ApoE ε4 with bacterial pathogens have been suggested but remain controversial (28).
Involvement of ApoE ε4 in the metabolism of Aβ is widely studied and probably the most accepted of the above possibilities due to the observation that ApoE is co-deposited with Aβ in amyloid plaques (29). One argument is that because Aβ can interact with both the lipid-binding site and the receptor-binding site within ApoE, and because the Aβ-binding site on ApoE overlaps with the lipid-binding region, Aβ might compete with lipids for ApoE binding (30). The transport of lipids to neurons by ApoE is essential for synaptic maintenance and repair (31). Thus, the disruption of lipid binding in AD by Aβ and Aβ oligomers may compromise synaptic integrity and function. In addition, ApoE ε3 and ApoE ε2 have higher affinity for HDLs, whereas ApoE ε4 has high affinity for LDLs and VLDLs (32). The specific ApoE isoform and its lipidation status seem to dictate the nature of interaction between Aβ and ApoE. For example, ApoE ε4/Aβ complexes are far less stable than ApoE ε3/Aβ and ApoE ε2/Aβ complexes (33). This renders ApoE ε4 less efficient than ApoE ε3 or ApoE ε2 in clearing Aβ (34), resulting in increased concentrations of Aβ oligomers in an isoform-dependent manner (ApoE ε4 > ApoE ε3 > ApoE ε2) (35).
Potential Therapeutic Agents
The currently approved drugs for AD do not address the progressive neurodegeneration in AD and have yielded only mild symptomatic improvements (36). Most of these drugs target pathways in the amyloid cascade, focusing on diminishing Aβ generation from APP, preventing formation and facilitating removal of toxic Aβ aggregates, or preventing hyperphosphorylation and aggregation of tau protein (37). Immunization approaches that use anti-Aβ antibodies, although successful at clearing Aβ and reducing plaque load, have failed to improve cognitive abilities (38–40) and in some cases have caused serious side effects such as greater brain atrophy and meningoencephalitis (38). Compounds that inhibit Aβ aggregation or destabilize Aβ oligomeric species have to pass through the challenge of the blood-brain barrier to be effective (37). Drugs targeting inhibition of β-secretase (41), inhibition or modulation of γ-secretase (42), or upregulation of α-secretase (43) also are being investigated. However, although these enzymes are primarily involved in APP metabolism, they also have other substrates that are physiologically important, which makes inhibition a less viable option (37). Another approach is to modulate tau phosphorylation and aggregation by inhibiting glycogen synthase kinase 3 (GSK3), but a phase III clinical trial that examined this found no improvements in cognition and functional status (44, 45).
Inflammation in AD
In all of the amyloid cascade alterations that occur in AD, inflammation plays a crucial role in the clinical progression (46). Numerous studies have reported the association of elevated markers of inflammation and the accumulation of activated microglia with the pathologic lesions of AD (47). The deposition of Aβ causes activation of microglia, recruitment of astrocytes, and sustained production of proinflammatory cytokines (48, 49). These cytokines in turn accelerate Aβ production and amyloid formation, thus initiating a cycle of inflammation and amyloidogenesis (46, 50). In addition, inflammatory processes in cerebral vasculature also accelerate the progression of AD (51). The expression of inflammatory adhesion molecules is elevated in the endothelial cells of the AD brain (52, 53). In comparison to age-matched controls, AD brain microvessels released higher concentrations of inflammatory factors such as NO, TNF-α, TGF-β, IL-1β and IL-6 (52, 54, 55). Release of these inflammatory mediators contributes to the vicious cycle of amyloidogenesis and the resulting increase in inflammation. Although the involvement of inflammation in the development of disease is well documented, the cause of this inflammation is not yet understood. Revealing the causes of inflammation is crucial in developing preventative measures. In the meantime, strategies that target the resolution of inflammation represent an additional approach to treating this disease.
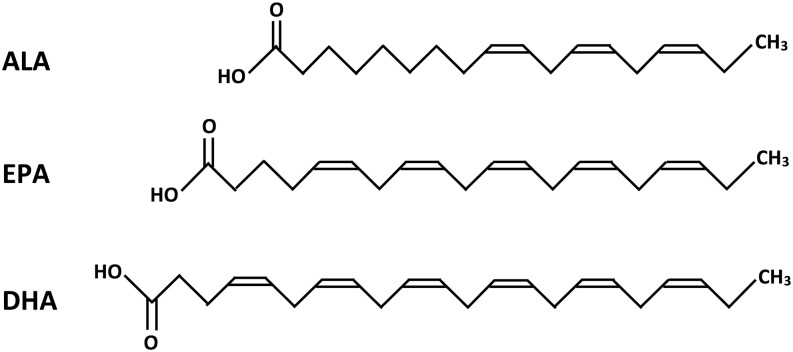
n–3 PUFAs are one such potential modulator of neuroinflammation (56). Increases in inflammation accelerate amyloidogenesis (46, 50) and n–3 PUFAs reduce the amyloid load and tau hyperphosphorylation by reducing neuroinflammation (57, 58). n–3 PUFAs are PUFAs with a double bond at the third carbon atom from their methyl end (Figure 1). Two major types of n–3 PUFAs, DHA and EPA, are abundantly present in fish oil. A plant-derived source of n–3 PUFA is α-linolenic acid (ALA; 18:3n–3), which is present in flaxseed oil and other plant oils.
FIGURE 1.
Structure of ALA, EPA, and DHA. ALA, α-linolenic acid.
In vitro experiments in immortalized BV-2 microglial cells, the main immune cell mediators in the brain, showed that EPA and/or DHA administration decreases the expression of proinflammatory factors, such as inducible NO synthase (iNOS), cyclo-oxygenase (COX) 2, IL-1β, IL-6, TNF-α, and NF-κB, and downregulates the cell-surface expression of the protein CD14 and Toll-like receptor 4 receptors involved in initiating the inflammatory response (59–61). In other types of brain cells (glial cultures and c6 glioma cells), the administration of EPA attenuates the increase in expression of proinflammatory cytokines such as IL-1β and IL-6 and promotes expression of the anti-inflammatory cytokine IL-4 (62–64). In rats, the consumption of a diet containing EPA for 4 wk before LPS-induced hippocampal inflammation prevented the reduction in hippocampal protein concentrations of anti-inflammatory cytokines IL-4 and IL-10 (65) and mitigated an increased expression of proinflammatory IL-1β (66). In humans, epidemiologic and observational studies have established an association of higher concentrations of n–3 PUFAs as well as lower n–6 to n–3 PUFA ratios with lower proinflammatory cytokine production (67–69). In elderly patients with chronic heart failure, n–3 PUFA supplementation resulted in reductions in plasma concentrations of TNF, IL-6, and intercellular adhesion molecule 1 (ICAM-1) (70), suggesting that similar effects may be observed in the brain. Indeed, there is an emerging literature that suggests the potential beneficial effect of n–3 PUFAs on inflammation in AD and other types of neurological disorders.
Role of Dietary n–3 PUFAs
It is estimated that primary intervention of known environmental risk factors in AD could prevent up to 20% of predicted new cases by 2025 (71, 72). In fact, the discordant occurrence of AD in monozygotic twins and differences in onset of up to 15 y in such patients show the role of modifiable environmental factors in disease progression (73–75). In particular, the potential role of n–3 PUFAs in modulating the risk of cognitive impairment has gained special attention due to the fact that observational studies reported a lower incidence of AD in populations who consume a high amount of fish (76–78). DHA, a predominant n–3 PUFA in fish oil, is a key component of membrane phospholipids in the brain (79); and oxidative products of PUFAs act as cellular mediators and may be involved in improving neuronal health, neurogenesis, and neuronal function through several mechanisms, resulting in the reduction in and resolution of inflammation. Interest in n–3 PUFAs for the treatment of AD is evidenced by guidelines from the 2013 International Conference on Nutrition and the Brain that included guidelines on modifications in dietary fat intake (80) and a recent letter from 109 scientists in 36 countries that urged the health ministers of the G8 countries to promote clinical trials for the prevention of AD, including those with n–3 FAs (72).
These expert recommendations with regard to n–3 PUFAs and AD come from the positive effects of n–3 FAs in cognition as elucidated by a large body of evidence from observational studies, randomized controlled trials, and animal studies (78, 81–91). For example, a cross-sectional population-based study in 1613 subjects reported that, with an increase of 137 mg n–3 PUFAs/d, the risk of cognitive decline was reduced by 19% (OR: 0.81; 95% CI: 0.66–1.00) (85). A recent meta-analysis by Wu et al. (92) indicated that a higher intake of fish was associated with a lower risk of AD. In a systematic review, Otaegui-Arrazola et al. (86) analyzed fish intake and the incidence of AD from population-based longitudinal observational studies. They identified and summarized 8 studies, of which 7 showed that, in the general population, the consumption of fish ≥1 time/wk significantly reduced AD risk and was associated with slower cognitive decline rates and better cognitive function. A recent retrospective cohort study in patients with mild cognitive impairment (MCI) and AD showed improvements in cognition and less atrophy with the long-term (6–48 mo) use of fish-oil supplements in ApoE ε4 participants (93). In addition, lower plasma and erythrocyte membrane n–3 PUFAs are linked to poorer cognition (94). Note also that some of the benefits observed with fish consumption might be derived from other ingredients in fish, such as vitamin D (95), because vitamin D deficiency has been linked to an increased risk of dementia and AD (96).
Results from interventional studies with fish-oil supplementation in humans have been inconclusive. A study in participants with subjective memory complaints showed improvements in some cognitive functions with 6 wk of 37.5 mg EPA+DHA supplementation/d combined with phosphatidylserine (97). In a randomized, double-blind, placebo-controlled study, improvements in cognitive performance were reported with 1.8 g n–3 PUFA supplementation/d for 24 wk in patients with MCI but not in patients with AD (98). The OmegAD study also found similar results in which benefits of n–3 PUFAs (1.7 g DHA and 0.6 g EPA/d for 6 mo) were observed in very mild AD but not in advanced AD (99). This suggests that n–3 PUFA supplementation might not be beneficial in advanced stages of disease in which substantial neuronal loss has occurred. However, benefits in the earlier stages of disease suggest a potential role of n–3 PUFAs on primary prevention of AD.
Collectively, these results support the role of n–3 PUFAs in preventing and ameliorating AD-related symptoms. DHA and EPA, the major n–3 PUFAs in fish oil, are postulated to be the beneficial elements. However, the n–3 PUFA supplements used in the studies were usually a mixture of EPA and DHA (mainly fish oil), and individual effects of n–3 PUFAs are not well studied. The exact composition of the FAs tested in fish-oil preparations vary greatly depending on the source of the fish and method of preparation (100), and experiments evaluating the individual effects of EPA and DHA are rare due to the difficulty and higher cost associated with purifying EPA and DHA. However, understanding the specific roles of each of the major n–3 PUFAs will facilitate therapeutic interventions and possibly enhance the generation of structural analogs as treatment options. A longitudinal population-based study showed that the top quartile of plasma DHA concentrations was associated with a lower risk of developing all-cause dementia and AD than were the other 3 quartiles (101); furthermore, cholesteryl-ester DHA concentrations were low in both serum and brains of patients with AD (102). Improvements in some cognitive functions were reported in patients with subjective memory complaints with DHA supplementation (103). Another study in patients with AD and patients with MCI reported significant improvements in immediate memory and attention score with 240 mg DHA supplementation/d in MCI but not in advanced AD (104). No improvements were found in patients with advanced AD with DHA supplementation in one study (105), or with EPA supplementation in another (106).
Long-duration studies in human subjects are complex and therefore few. Animal models of AD, on the other hand, provide an opportunity to test long-term interventions aimed at primary prevention starting at an early age. Most studies with supplementation of n–3 PUFAs in animal models of AD have had positive results. Hooijmans et al. (107), examining animal studies reported up to April 2011, conducted a systematic review and meta-analysis of the effects of long-term n–3 PUFAs on AD. The study assessed 4 outcome measures (cognition, Aβ deposition, neuronal loss in the hippocampus, and cortical FA concentrations) from 15 animal studies that used supplementation for ≥10% of the animal’s life span. Three of these 15 studies used a mixed source of n–3 PUFAs, and other studies used DHA as the supplement. Analysis of the pooled data from the studies showed a substantial reduction in Aβ deposition with n–3 PUFA supplementation, as well as significant improvements in cognition and a striking reduction in hippocampal neuronal loss or neurodegeneration.
A PubMed search for publications between April 2011 and November 2015, which adopted the keywords and filters used by Hooijmans et al. in the review discussed above, retrieved 8 more animal studies that included n–3 PUFA supplementation in animal models of AD (Table 1) (108–115). All 8 of these studies showed improvements in cognitive and neuronal variables with n–3 PUFA supplementation. Three of these 8 studies used a mixed source of EPA and DHA. In aged mice, 2-mo EPA + DHA treatment increased these long-chain n–3 PUFAs in the brain and restored spatial memory deficits (113). Markers of neuroinflammation were also reduced significantly in the hippocampus of these aged mice. In the study by Kariv-Inbal et al. (114), a fish-oil diet mitigated the worsened neuronal markers and behavioral performance in ApoE ε4 mice. Oral administration of 300 mg EPA and DHA ⋅ kg–1 ⋅ d−1 to Aβ-infused rats for 12 wk before infusion resulted in a significantly lower number of reference and working memory errors, increased EPA and DHA concentrations and decreased arachidonic acid (AA; an n–6 PUFA, 20:4n–6) in the corticohippocampal region, and lower oxidative stress in the cerebral cortex and hippocampus (115).
TABLE 1.
Studies published after April 2011 that included n–3 PUFA supplementation in animal models of AD1
| Study, year (ref) | AD model | Supplement | Duration | Dose | Outcome |
| Teng et al., 2015 (108) | Transgenic APP/PS1 rats | DHA from algal sources | 4 mo | 0.6% (wt:wt) of the diet | Reduced Aβ plaque density |
| Improved behavioral testing | |||||
| Hosono et al., 2015 (109) | Tg2576 mice | DHA | 4 mo | 2.4 g/kg diet | Reduced Aβ(1–42)-to-Aβ(1–40) ratio |
| Torres et al., 2014 (110) | Transgenic 5xFAD mice | 2-Hydroxy DHA | 4 mo | 15 mg ⋅ kg–1 ⋅ d−1 | Reduction in Aβ accumulation |
| Improved cognitive scores | |||||
| Ma et al., 2014 (111) | Tau knockout mice | DHA alone or with α-lipoic acid | 5 mo | 0.6% DHA (or with 500 ppm α-lipoate) | Protected against hyperphosphorylation |
| Lower hippocampal synaptic deficits | |||||
| Improved Morris water maze deficits | |||||
| Fiol-deRoque et al., 2013 (112) | Transgenic 5xFAD mice | 2-Hydroxy DHA | 4 mo | 15 mg ⋅ kg–1 ⋅ d−1 | Improved radial arm maze test scores |
| Labrousse et al., 2012 (113) | Aged C57Bl6/J mice | EPA+DHA | 2 mo | 5.45 g EPA + 3.6 g DHA/kg diet | Reduced inflammatory markers |
| Improved spatial memory deficits | |||||
| Kariv-Inbal et al., 2012 (114) | ApoE-targeted replacement mice | Fish oil | 4 mo | 3.03 g fish oil/kg diet | Reduced hippocampal Aβ concentrations |
| Improved behavioral performance | |||||
| Hashimoto et al., 2011 (115) | Aβ-infused rats | Purified EPA+DHA | 12 wk | 300 mg ⋅ kg–1 ⋅ d−1 | Improved reference and working memory |
Aβ, amyloid β APP, amyloid precursor protein; ppm, parts per million; PS1, presenilin 1; ref, reference.
The remaining 5 animal studies used DHA supplementation alone. Hosono et al. (109) supplemented Tg2576 mice with DHA for 4 mo and reported a reduction in memory impairment. In another transgenic APP/PS1 rat model of AD, DHA supplementation reduced hippocampal Aβ plaque density and prefibrillar Aβ oligomers and improved cognition (108). Torres et al. (110) administrated a 2-hydroxy derivative of DHA (OH-DHA) orally to a double transgenic PS1/APP mouse model of AD for 4 mo at a dose of 15 mg ⋅ kg–1 ⋅ d−1. OH-DHA supplementation significantly downregulated Aβ concentrations (without affecting APP transgene expression) and normalized tau hyperphosphorylation. Fiol-deRoque et al. (112) reported significant improvements in memory recovery in the radial arm maze test in the above-mentioned mice. In another experiment with tau knockout mice, Ma et al. (111) reported that 5 mo of DHA supplementation improved microtubule stability by restoring phosphorylated and total GSK3β and mitigating hyperactivation of the tau C-Jun N-terminal kinases. These improvements in tau hyperphosphorylation also resulted in partial correction of hippocampal synaptic deficits.
Differential Effects of Individual n–3 PUFAs on AD
As indicated, although EPA and DHA are both neuroactive n–3 PUFAs, their effects on neuroinflammation and AD might be different from each other (see Table 2). EPA has a prominent effect on mood disorder–related symptoms. In studies in patients with depressive disorder, EPA administration significantly improved markers of depression (121, 122), and a meta-analysis of randomized controlled trials showed a greater antidepressant effect of EPA than DHA (123). Another clinical trial that compared EPA and DHA as monotherapy for major depressive disorder found no beneficial effect with either EPA or DHA (124). In addition, although both EPA and DHA have anti-inflammatory effects, EPA appears to be more effective than DHA. A study that investigated differential effects of purified EPA and DHA on stimulated peripheral blood mononuclear cells from patients with AD showed that EPA was more effective than DHA in reversing the proinflammatory profile of the AD patients’ cells (125). Stronger anti-inflammatory effects of EPA were reported in several other models as well (126, 127). In neuronal tissue, EPA acts as an anti-inflammatory agent by blocking the effects of IL-1 (128, 129), which is associated with age-related impairment in neuronal function (130). A study in older subjects discovered that higher plasma EPA, but not DHA, was associated with lower gray matter atrophy of the right hippocampal and parahippocampal area (131).
TABLE 2.
Effect of EPA and DHA on markers of neuroinflammation1
| PUFA; study, year (ref) | Effect |
| DHA | |
| Rey et al., 2016 (116) | Resolvin D1, a DHA oxylipin, decreased LPS-induced expression of TNF-α, IL-6, and IL-1β |
| Zhao et al., 2011 (117) | NPD1, a DHA oxylipin, downregulated Aβ42-triggered expression of COX-2 and of B-94 (a TNF-α–inducible proinflammatory element) |
| Lu et al., 2010 (59) | Reduced expressions of TNF-α, IL-6, iNOS, and COX-2 |
| Pan et al., 2009 (118) | Mitigated increases in IL-6 |
| De Smedt-Peyrusse et al., 2008 (61) | Downregulated LPS-stimulated cell surface expression of CD14 and TLR4 |
| Lowered TNF-α protein expression, IL-1β protein expression, and activation of NF-κB | |
| Kawashima et al., 2008 (62) | Attenuated IL-1β–induced IL-6 production |
| Lukiw et al., 2005 (119) | NPD1, a DHA oxylipin, downregulated COX-2, TNF-α, and IL-1β expression |
| Marcheselli et al., 2003 (120) | NPD1, a DHA oxylipin, downregulated NF-κB activation and COX2 gene expression |
| EPA | |
| Rey et al., 2016 (116) | Resolvin E1, an EPA oxylipin, decreased LPS-induced expression of TNF-α, IL-6, and IL-1β |
| Lu et al., 2010 (59) | Inhibited iNOS and COX-2 expression and NO production |
| Kawashima et al., 2008 (62) | Inhibited IL-6 production, attenuated IL-1β–induced IL6 gene expression |
| Moon et al., 2007 (60) | Inhibited PGE2, IL-1β, IL-6, TNF-α, and release of NO |
| Downregulated the production of COX-2, iNOS, and proinflammatory cytokines at mRNA and/or protein levels | |
| Suppressed NF-κB activation by blocking IκB degradation, | |
| Lynch et al., 2007 (63) | Elevated IL-4 and blocked LPS-induced increases in IL-1β |
| Minogue et al., 2007 (64) | Lowered LPS- and Aβ-induced increases in IL-1β protein |
| Kavanagh et al., 2004 (65) | Prevented LPS-induced reduction in anti-inflammatory IL-4 and IL-10 |
Aβ, amyloid β COX, cyclooxygenase; IκB, inhibitor of κB; iNOS, inducible nitric oxide synthase; NPD1, neuroprotectin D1; PGE2, prostaglandin E2; ref, reference; TLR4, Toll-like receptor 4.
EPA also has been shown to improve cognition. In AD, Hashimoto et al. (132) studied the effect of preadministration of EPA in cognition and learning with the use of rats infused with Aβ and reported a decrease in the number of reference memory errors and working memory errors. DHA appears to be effective in cognitive variables and in reducing AD-related structural abnormalities. In triple transgenic (3xTg)-AD mice, DHA improved cognition and reduced entorhinal cortex neuron dysfunction (133); and in female APPswe/PS1ΔE9 transgenic mice, a DHA diet reduced plaque load (134). In the APP/PS1 transgenic rat model of AD, DHA supplementation reduced hippocampal Aβ plaque density, increased soluble Aβ oligomer concentrations, and improved behavioral aspects (108). DHA-containing phosphatidylcholine treatment improved learning and memory abilities, reduced phosphorylated tau concentrations, and partially corrected neuronal morphology in an Aβ23–35-induced AD rat model (135). Interestingly, DHA supplementation for 18 mo in human patients did not reduce cognitive decline (105). In conclusion, although both EPA and DHA showed benefits in inflammation and cognition in AD, EPA appears to be beneficial in mood disorders and DHA beneficial in preserving the structural integrity of the brain. In addition, active metabolites of EPA and DHA (i.e., oxylipins; see below) are different, which might play a major role in their inflammation-related mechanisms in AD (136–138).
Although the effects of fish-oil–based n–3 PUFAs on AD have been tested in animal and human trials, plant-based n–3 PUFAs such as ALA have received less attention. In contrast to other tissues (139), however, cerebral and cerebellar neurons appear to more efficiently convert ALA to DHA (140–142), and this can result in improvements in reference memory tasks (143). In addition, ALA conversion to EPA is efficient (141, 144), which could be important if oxygenated EPA metabolites (oxylipins) are the specific mediators of neuroprotection in AD. Neuroprotective effects of ALA in other forms of neurological diseases have been shown. In a rat model of spinal cord ischemia, ALA caused significant protection by reducing the loss of motor neurons and preventing apoptotic neuronal cell death (145). Yamamoto et al. (146) reported increased learning ability with supplementation of ALA-rich oil in rats. In an in vivo model of cerebral global ischemia, intracerebroventricular administration of ALA 30 min before induction of ischemia almost completely inhibited neuronal loss (147). ALA supplementation in rats improved cerebrovascular flow, which plays a pivotal role in the pathology of AD (148, 149), possibly by the activation of the tandem of pore domains in a weak inwardly rectifying K+ channel-1 related K+ channel (TREK-1) potassium channel, which is an important vasodilatation mediator. Specifically in AD, a prospective study conducted in participants aged 65–94 y found that ALA intake was strongly protective among persons with the ApoE ε4 allele (78). Spinal cord injury is associated with excitotoxicity, inflammation, and oxidative stress (150); and supplementation of ALA to spinal cord ischemic rats after injury reduced neuronal loss and improved functional outcome (145). The administration of ALA after this type of injury in adult rats showed significant neuroprotection by reducing neuronal cell loss, oligodendrocyte loss, and neuronal apoptosis and improving functional outcome (151, 152). In a brain ischemia rat model, the administration of ALA inhibited microglia activation, attenuated cell apoptosis, and improved behavioral function recovery (153).
Role of Oxylipins Derived from n–3 PUFAs in Their Anti-Inflammatory Effects
PUFAs participate in the process of causing or resolving inflammation through a class of lipid-derived mediators called oxylipins. These bioactive lipids are oxygenated FA metabolites biosynthesized by COX, lipoxygenase (LOX), and cytochrome P450 (CYP) enzymes (154). Oxylipins derived from n–3 PUFAs can potentially modulate neuroinflammation in 2 ways; first, through their anti-inflammatory effects, and second, through their proresolving effects. Oxylipins formed from n–3 PUFAs are generally anti-inflammatory, whereas those produced from n–6 PUFAs are generally proinflammatory (155, 156). n–3 PUFA–derived oxylipins act as anti-inflammatory compounds by reducing the concentration of and competing with proinflammatory oxylipins produced from n–6 PUFAs (157, 158). Inflammation is normally terminated by resolution, an active process involving a number of biochemical steps (159). The resolution of inflammation is important to achieve homeostasis in the tissue, but this process appears to be dysregulated in AD (160). Proresolving oxylipins produced from n–3 PUFAs are important mediators of this resolution phase of the inflammatory process (159). For example, resolvins, lipoxins, protectins, and maresins initiate pathways that signal the termination of an acute inflammatory phase (161). Understanding the role of specific oxylipins in neuroinflammation and its resolution may therefore shed light on how individual n–3 PUFAs mediate their beneficial effects in AD.
The most studied class of oxylipins is the eicosanoids produced from 20-carbon PUFAs, AA, and EPA. AA is the n–6 PUFA that is abundantly present on the membrane phospholipids of inflammatory cells (157) and generates proinflammatory oxylipins such as prostaglandin E (PGE) 2 and leukotriene B (LTB) 4. EPA exposure reduces the concentrations of AA oxylipins in inflammatory cells (157, 162, 163) in a dose-responsive manner (164). It can compete with AA for all 3 oxylipin pathways, resulting in the production of lower concentrations of AA-derived oxylipins and higher concentrations of EPA-derived oxylipins that are biologically less active (e.g., PGE3, LTB5) (165–167). In addition, EPA generates oxylipins such as E-series resolvins (RvEs) that mediate the resolution of inflammation (168, 169). For example, RvE1 (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid) competes with LTB4 (158), prevents the infiltration of neutrophils into sites of inflammation (169), decreases pulmonary polymorphonuclear neutrophil accumulation, reduces proinflammatory gene expression (170), reduces proinflammatory cytokine production (171), and inhibits leukocyte recruitment (172). Other EPA oxylipins such as 18-hydroxy-eicosapentaenoic acid (18-HEPE) also impart anti-inflammatory effects (173).
DHA supplementation also reduces the production of AA-derived oxylipins. For example, glial cells in culture produce less thromboxane B2 (TXB2) and 6-keto-prostaglandin F1α (6-k-PGF1α) and 12-hydroxy-eicosatetraenoic acid (12-HETE) when supplemented with DHA (174). In addition, many novel oxylipins derived from DHA via the LOX and CYP pathways have recently been identified, with many of these appearing to play a particular role in the resolution of inflammation. These include the D-series resolvins, protectins, and maresins (56, 169), which are elevated in the murine brain with DHA-rich fish-oil feeding (175). Reduced concentrations of DHA-derived neuroprotectin D1 (NPD1) have been reported in the AD brain (119, 160), and low concentrations of NPD1 are inversely related to cellular markers of neuroinflammation (176). NPD1 has been shown to be neuroprotective (119, 160, 177). In human neural progenitor cells stimulated with IL-1β, NPD1 downregulates NF-κB activation and COX-2 expression (120). The infusion of NPD1 in the mouse brain reduces infarct volume, leukocyte infiltration, NF-κB activation, and COX-2 expression with greater potency than does DHA itself (120). The infusion of the aspirin triggered epimer of NPD1 (AT-NPD1; 10,17R-dihydroxy-DHA) also reduces neuroinflammation similar to NPD1 (178).
Although the body of literature for ALA is much smaller, it has been shown to reduce inflammation in various models (179, 180) and to provide neuroprotection (149). ALA also produces metabolically active oxylipins (181), and although much less is known about these, they may mediate its beneficial effects in AD. In older adults, 4 wk of ALA feeding normalized the proinflammatory oxylipin profile in blood (182), suggesting that it also could have effects on the brain. The ALA-derived oxylipin 13-hydroxy-octadecatrienoic acid (13-HOTrE) significantly suppressed IL-1β–induced expression of matrix metalloproteinase (MMP) 1, 3, and 9 proteins in chondrocytes, suggesting that it is an anti-inflammatory substance (183). This oxylipin also is associated with less glomerulomegaly, indicating that it may have an anti-inflammatory effect (184).
Conclusions
There is a pressing demand for more research in preventing, delaying, and diminishing the effects of AD (185). In this regard, n–3 PUFAs can help reduce and resolve inflammation, which plays a major role in the progression of AD. However, although the use of mixed sources of EPA and DHA in most studies prevents an understanding of the individual effects of these PUFAs, several studies do indicate that they may have distinct effects such as EPA’s more prominent effects in mood disorders and DHA’s ability to alleviate structural abnormalities. This may, in part, be due to their different effects on the oxylipin profiles they generate, and their effects on the reduction in and resolution of neuroinflammation. In addition, oxylipins synthesized from ALA also may have antineuroinflammatory effects. Future research delineating the unique anti-inflammatory and proresolving properties of oxylipins from individual n–3 PUFAs will help the development of novel disease management strategies in AD.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; Aβ, amyloid β AD, Alzheimer disease; AICD, amyloid precursor protein intracellular C-terminal domain; ALA, α-linolenic acid; APP, amyloid precursor protein; AT-NPD1, aspirin triggered epimer of neuroprotectin D1; BACE1, β-site amyloid precursor protein cleaving enzyme 1; COX, cyclo-oxygenase; CYP, cytochrome P450; FAD, familial Alzheimer disease; GSK3, glycogen synthase kinase 3; ICAM-1, intercellular adhesion molecule 1; iNOS, inducible nitric oxide synthase; LOX, lipoxygenase; LTB, leukotriene B; MAP, microtubule-associated protein; MCI, mild cognitive impairment; MMP, matrix metalloproteinase; NFT, neurofibrillary tangle; NPD1, neuroprotectin D1; PGE, prostaglandin E; PS1, presenilin 1; PS2, presenilin 2; RvE, E-series resolvin; TREK-1, tandem of pore domains in a weak inwardly rectifying K+ channel-1 related K+ channel; TXB2, thromboxane B2; 6-k-PGF1α, 6-keto-prostaglandin F1α; 12-HETE, 12-hydroxy-eicosatetraenoic acid; 13-HOTrE, 13-hydroxy-octadecatrienoic acid; 18-HEPE, 18-hydroxy-eicosapentaenoic acid.
References
- 1.Philipson O, Lord A, Gumucio A, O’Callaghan P, Lannfelt L, Nilsson LN. Animal models of amyloid-beta-related pathologies in Alzheimer’s disease. FEBS J 2010;277:1389–409. [DOI] [PubMed] [Google Scholar]
- 2.Thies W, Bleiler L; Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement 2013;9:208–45. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer's Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dementi 2015;11:332–84. [DOI] [PubMed] [Google Scholar]
- 4.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80:1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, Kukull WA. National estimates of the prevalence of Alzheimer's disease in the United States. Alzheimers Dement 2011;7:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports- deaths: final data for 2010: from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital Stat Rep 2013;61:1–117. [PubMed] [Google Scholar]
- 7.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75e2. [DOI] [PubMed] [Google Scholar]
- 8.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014;30:421–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261:921–3. [DOI] [PubMed] [Google Scholar]
- 10.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM; APOE and Alzheimer Disease Meta Analysis Consortium. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA 1997;278:1349–56. [PubMed] [Google Scholar]
- 11.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet 2006;368:387–403. [DOI] [PubMed] [Google Scholar]
- 12.Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J Clin Invest 2002;110:1375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong S, Duan Y, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer’s disease: a re-evaluation of amyloid cascade hypothesis. Transl Neurodegener 2012;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selkoe DJ. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci 1994;17:489–517. [DOI] [PubMed] [Google Scholar]
- 15.Pardossi-Piquard R, Petit A, Kawarai T, Sunyach C, Alves da Costa C, Vincent B, Ring S, D’Adamio L, Shen J, Muller U, et al. Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron 2005;46:541–54. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297:353–6. [DOI] [PubMed] [Google Scholar]
- 17.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 2007;8:499–509. [DOI] [PubMed] [Google Scholar]
- 18.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992;256:184–5. [DOI] [PubMed] [Google Scholar]
- 19.Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta 2005;1739:216–23. [DOI] [PubMed] [Google Scholar]
- 20.Himmelstein DS, Ward SM, Lancia JK, Patterson KR, Binder LI. Tau as a therapeutic target in neurodegenerative disease. Pharmacol Ther 2012;136:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 1999;24:751–62. [DOI] [PubMed] [Google Scholar]
- 22.Hardy J, Bogdanovic N, Winblad B, Portelius E, Andreasen N, Cedazo-Minguez A, Zetterberg H. Pathways to Alzheimer’s disease. J Intern Med 2014;275:296–303. [DOI] [PubMed] [Google Scholar]
- 23.Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, Pocklington A, Abraham R, Hollingworth P, Sims R, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One 2010;5:e13950 Erratum in: PLoS One 2011;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 2004;25:641–50. [DOI] [PubMed] [Google Scholar]
- 25.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang YD. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 2006;26:4985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zannis VI, Breslow JL. Human very low-density lipoprotein apolipoprotein-E isoprotein polymorphism is explained by genetic-variation and posttranslational modification. Biochemistry 1981;20:1033–41. [DOI] [PubMed] [Google Scholar]
- 27.Weisgraber KH, Rall SC, Mahley RW. Human-E apoprotein heterogeneity - cysteine-arginine interchanges in the amino-acid-sequence of the apo-E isoforms. J Biol Chem 1981;256:9077–83. [PubMed] [Google Scholar]
- 28.Balin BJ, Hudson AP. Etiology and pathogenesis of late-onset Alzheimer’s disease. Curr Allergy Asthma Rep 2014;14:417. [DOI] [PubMed] [Google Scholar]
- 29.Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res 1991;541:163–6. [DOI] [PubMed] [Google Scholar]
- 30.Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron 2014;81:740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahley RW, Rall SC, Apolipoprotein E. Far more than a lipid transport protein. Annu Rev Genomomics Hum Genet 2000;1:507–37. [DOI] [PubMed] [Google Scholar]
- 32.Weisgraber KH. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res 1990;31:1503–11. [PubMed] [Google Scholar]
- 33.Tai LM, Bilousova T, Jungbauer L, Roeske SK, Youmans KL, Yu C, Poon WW, Cornwell LB, Miller CA, Vinters HV, et al. Levels of soluble apolipoprotein E/amyloid-beta (Abeta) complex are reduced and oligomeric Abeta increased with APOE4 and Alzheimer disease in a transgenic mouse model and human samples. J Biol Chem 2013;288:5914–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 2000;97:2892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto T, Serrano-Pozo A, Hori Y, Adams KW, Takeda S, Banerji AO, Mitani A, Joyner D, Thyssen DH, Bacskai BJ, et al. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid beta peptide. J Neurosci 2012;32:15181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oboudiyat C, Glazer H, Seifan A, Greer C, Isaacson RS. Alzheimer’s disease. Semin Neurol 2013;33:313–29. [DOI] [PubMed] [Google Scholar]
- 37.Reitz C. Alzheimer's disease and the amyloid cascade hypothesis: a critical review. Int J Alzheimers Dis 2012;2012:369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553–62. [DOI] [PubMed] [Google Scholar]
- 39.Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M, et al. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res 2009;6:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 2009;73:2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamagi U, Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord 2010;30:131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imbimbo BP, Giardina GA. Gamma-secretase inhibitors and modulators for the treatment of Alzheimer’s disease: disappointments and hopes. Curr Top Med Chem 2011;11:1555–70. [DOI] [PubMed] [Google Scholar]
- 43.Desire L, Drouin D, Sol O, Lambeng N, Schweighoffer F. EHT 0202: a neuroprotective and procognitive alpha-secretase stimulator targeted towards Alzheimer’s disease therapy. Eur J Neurol 2008;15:37. [Google Scholar]
- 44.Tariot PN, Aisen P, Cummings J, Jakimovich L, Schneider L, Thomas R, Becerra L, Loy R. The ADCS valproate neuroprotection trial: primary efficacy and safety results. Alzheimers Dement 2009;5(4, Suppl):84–5. [Google Scholar]
- 45.Tariot PN, Raman R, Jakimovich L, Schneider L, Porsteinsson A, Thomas R, Mintzer J, Brenner R, Schafer K, Thal L, et al. Divalproex sodium in nursing home residents with possible or probable Alzheimer Disease complicated by agitation: a randomized, controlled trial. Am J Geriatr Psychiatry 2005;13:942–9. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging 2000;21:383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 2006;12:1005–15. [DOI] [PubMed] [Google Scholar]
- 48.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci 2006;24:167–76. [DOI] [PubMed] [Google Scholar]
- 49.Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int J Dev Neurosci 2006;24:157–65. [DOI] [PubMed] [Google Scholar]
- 50.Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Todarello O, Pellicani V, Capurso SA, Pietrarossa G, Santamato V, Capurso A, et al. Circulating biomarkers of cognitive decline and dementia. Clin Chim Acta 2006;364:91–112. [DOI] [PubMed] [Google Scholar]
- 51.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation 2011;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging 2001;22:837–42. [DOI] [PubMed] [Google Scholar]
- 53.Frohman EM, Frohman TC, Gupta S, de Fougerolles A, van den Noort S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J Neurol Sci 1991;106:105–11. [DOI] [PubMed] [Google Scholar]
- 54.Dorheim MA, Tracey WR, Pollock JS, Grammas P. Nitric-oxide synthase activity is elevated in brain microvessels in alzheimers-disease. Biochem Biophys Res Commun 1994;205:659–65. [DOI] [PubMed] [Google Scholar]
- 55.Grammas P, Ovase R. Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer’s disease brain. Am J Pathol 2002;160:1583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orr SK, Trepanier MO, Bazinet RP. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot Essent Fatty Acids 2013;88:97–103. [DOI] [PubMed] [Google Scholar]
- 57.Vom Berg J, Prokop S, Miller KR, Obst J, Kalin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters O, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med 2012;18:1812–9. [DOI] [PubMed] [Google Scholar]
- 58.Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, et al. Extended results of the Alzheimer's disease anti-inflammatory prevention trial. Alzheimers Dement 2011;7:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 2010;35:2238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moon DO, Kim KC, Jin CY, Han MH, Park C, Lee KJ, Park YM, Choi YH, Kim GY. Inhibitory effects of eicosapentaenoic acid on lipopolysaccharide-induced activation in BV2 microglia. Int Immunopharmacol 2007;7:222–9. [DOI] [PubMed] [Google Scholar]
- 61.De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S, Laye S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem 2008;105:296–307. [DOI] [PubMed] [Google Scholar]
- 62.Kawashima A, Harada T, Imada K, Yano T, Mizuguchi K. Eicosapentaenoic acid inhibits interleukin-6 production in interleukin-1beta-stimulated C6 glioma cells through peroxisome proliferator-activated receptor-gamma. Prostaglandins Leukot Essent Fatty Acids 2008;79:59–65. [DOI] [PubMed] [Google Scholar]
- 63.Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, Roche OJ, O’Connell F, Lynch MA. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging 2007;28:845–55. [DOI] [PubMed] [Google Scholar]
- 64.Minogue AM, Lynch AM, Loane DJ, Herron CE, Lynch MA. Modulation of amyloid-beta-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid. J Neurochem 2007;103:914–26. [DOI] [PubMed] [Google Scholar]
- 65.Kavanagh T, Lonergan PE, Lynch MA. Eicosapentaenoic acid and gamma-linolenic acid increase hippocampal concentrations of IL-4 and IL-10 and abrogate lipopolysaccharide-induced inhibition of long-term potentiation. Prostaglandins Leukot Essent Fatty Acids 2004;70:391–7. [DOI] [PubMed] [Google Scholar]
- 66.Lonergan PE, Martin DS, Horrobin DF, Lynch MA. Neuroprotective actions of eicosapentaenoic acid on lipopolysaccharide-induced dysfunction in rat hippocampus. J Neurochem 2004;91:20–9. [DOI] [PubMed] [Google Scholar]
- 67.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010;303:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006;91:439–46. [DOI] [PubMed] [Google Scholar]
- 69.Kalogeropoulos N, Panagiotakos DB, Pitsavos C, Chrysohoou C, Rousinou G, Toutouza M, Stefanadis C. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clini Chim Acta 2010;411:584–91. [DOI] [PubMed] [Google Scholar]
- 70.Zhao YT, Shao L, Teng LL, Hu B, Luo Y, Yu X, Zhang DF, Zhang H. Effects of n-3 polyunsaturated fatty acid therapy on plasma inflammatory markers and N-terminal pro-brain natriuretic peptide in elderly patients with chronic heart failure. J Int Med Res 2009;37:1831–41. [DOI] [PubMed] [Google Scholar]
- 71.Han JY, Han SH. Primary prevention of Alzheimer’s disease: is it an attainable goal? J Korean Med Sci 2014;29:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith AD, Yaffe K. Dementia (including Alzheimer’s disease) can be prevented: statement supported by international experts. J Alzheimers Dis 2014;38:699–703. [DOI] [PubMed] [Google Scholar]
- 73.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 2006;63:168–74. [DOI] [PubMed] [Google Scholar]
- 74.Järvenpää T, Raiha I, Kaprio J, Koskenvuo M, Laine M, Kurki T, Viljanen T, Rinne OA. 90-Year-old monozygotic female twin pair discordant for Alzheimer’s disease. Neurobiol Aging 2003;24:941–5. [DOI] [PubMed] [Google Scholar]
- 75.Rapoport SI, Pettigrew KD, Schapiro MB. Discordance and concordance of dementia of the Alzheimer type (Dat) in monozygotic twins indicate heritable and sporadic forms of Alzheimers-disease. Neurology 1991;41:1549–53. [DOI] [PubMed] [Google Scholar]
- 76.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 1997;42:776–82. [DOI] [PubMed] [Google Scholar]
- 77.Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002;325:916–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–6. [DOI] [PubMed] [Google Scholar]
- 79.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 2001;40:1–94. [DOI] [PubMed] [Google Scholar]
- 80.Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, Fraser G, Kesler S, Levin SM, Lucey B, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol Aging 2014;35(Suppl 2):S74–8. [DOI] [PubMed] [Google Scholar]
- 81.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 2007;69:1921–30. [DOI] [PubMed] [Google Scholar]
- 82.Jensen MM, Skarsfeldt T, Hoy CE. Correlation between level of (n-3) polyunsaturated fatty acids in brain phospholipids and learning ability in rats: a multiple generation study. Biochim Biophys Acta 1996;1300:203–9. [DOI] [PubMed] [Google Scholar]
- 83.Lim SY, Suzuki H. Effect of dietary docosahexaenoic acid and phosphatidylcholine on maze behavior and fatty acid composition of plasma and brain lipids in mice. Int J Vitam Nutr Res 2000;70:251–9. [DOI] [PubMed] [Google Scholar]
- 84.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 2008;71:430–8. [DOI] [PubMed] [Google Scholar]
- 85.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62:275–80. [DOI] [PubMed] [Google Scholar]
- 86.Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, Martinez-Lage P. Diet, cognition, and Alzheimer’s disease: food for thought. Eur J Nutr 2014;53:1–23. [DOI] [PubMed] [Google Scholar]
- 87.Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ 2002;325:932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, Burke GL, Carlson MC. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005;65:1409–14. [DOI] [PubMed] [Google Scholar]
- 89.Devore EE, Grodstein F, van Rooij FJ, Hofman A, Rosner B, Stampfer MJ, Witteman JC, Breteler MM. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am J Clin Nutr 2009;90:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol 2005;62:1849–53. [DOI] [PubMed] [Google Scholar]
- 91.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n–3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr 2007;85:1142–7. [DOI] [PubMed] [Google Scholar]
- 92.Wu S, Ding Y, Wu F, Li R, Hou J, Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci Biobehav Rev 2015;48:1–9. [DOI] [PubMed] [Google Scholar]
- 93.Daiello LA, Gongvatana A, Dunsiger S, Cohen RA, Ott BR. Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement 2015;11:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n–3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr 2007;85:1103–11. [DOI] [PubMed] [Google Scholar]
- 95.Lu Z, Chen TC, Zhang A, Persons KS, Kohn N, Berkowitz R, Martinello S, Holick MF. An evaluation of the vitamin D3 content in fish: is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol 2007;103:642–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, Fried L, Kestenbaum BR, Kuller LH, Langa KM, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014;83:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richter Y, Herzog Y, Cohen T, Steinhart Y. The effect of phosphatidylserine-containing omega-3 fatty acids on memory abilities in subjects with subjective memory complaints: a pilot study. Clin Interv Aging 2010;5:313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1538–44. [DOI] [PubMed] [Google Scholar]
- 99.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease. OmegAD study: a randomized double-blind trial. Arch Neurol 2006;63:1402–8. [DOI] [PubMed] [Google Scholar]
- 100.Gruger EH, Stansby ME, Nelson RW. Fatty acid composition of oils from 21 species of marine fish freshwater fish + shellfish. J Am Oil Chem Soc 1964;41:662. [Google Scholar]
- 101.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 2006;63:1545–50. [DOI] [PubMed] [Google Scholar]
- 102.Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: a case-control study. Br J Nutr 2003;89:483–9. [DOI] [PubMed] [Google Scholar]
- 103.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N Jr, Stedman M; MIDAS Investigators. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement 2010;6:456–64. [DOI] [PubMed] [Google Scholar]
- 104.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T, Guo J, Yamashima T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res 2006;56:159–64. [DOI] [PubMed] [Google Scholar]
- 105.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR Jr, Weiner M, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304:1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boston PF, Bennett A, Horrobin DF, Bennett CN. Ethyl-EPA in Alzheimer’s disease—a pilot study. Prostaglandins Leukot Essent Fatty Acids 2004;71:341–6. [DOI] [PubMed] [Google Scholar]
- 107.Hooijmans CR, Pasker-de Jong PC, de Vries RB, Ritskes-Hoitinga M. The effects of long-term omega-3 fatty acid supplementation on cognition and Alzheimer’s pathology in animal models of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2012;28:191–209. [DOI] [PubMed] [Google Scholar]
- 108.Teng E, Taylor K, Bilousova T, Weiland D, Pham T, Zuo X, Yang F, Chen PP, Glabe CG, Takacs A, et al. Dietary DHA supplementation in an APP/PS1 transgenic rat model of AD reduces behavioral and Abeta pathology and modulates Abeta oligomerization. Neurobiol Dis 2015;82:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosono T, Mouri A, Nishitsuji K, Jung CG, Kontani M, Tokuda H, Kawashima H, Shibata H, Suzuki T, Nabehsima T, et al. Arachidonic or docosahexaenoic acid diet prevents memory impairment in Tg2576 mice. J Alzheimers Dis 2015;48:149–62. [DOI] [PubMed] [Google Scholar]
- 110.Torres M, Price SL, Fiol-Deroque MA, Marcilla-Etxenike A, Ahyayauch H, Barcelo-Coblijn G, Teres S, Katsouri L, Ordinas M, Lopez DJ, et al. Membrane lipid modifications and therapeutic effects mediated by hydroxydocosahexaenoic acid on Alzheimer’s disease. Biochim Biophys Acta 2014;1838:1680–92. [DOI] [PubMed] [Google Scholar]
- 111.Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, Kiosea NC, Nazari S, Chen PP, Nothias F, et al. Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris water maze with aging. J Neurosci 2014;34:7124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fiol-deRoque MA, Gutierrez-Lanza R, Teres S, Torres M, Barcelo P, Rial RV, Verkhratsky A, Escriba PV, Busquets X, Rodriguez JJ. Cognitive recovery and restoration of cell proliferation in the dentate gyrus in the 5XFAD transgenic mice model of Alzheimer’s disease following 2-hydroxy-DHA treatment. Biogerontology 2013;14:763–75. [DOI] [PubMed] [Google Scholar]
- 113.Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Gregoire S, Bretillon L, Laye S. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One 2012;7:e36861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kariv-Inbal Z, Yacobson S, Berkecz R, Peter M, Janaky T, Lutjohann D, Broersen LM, Hartmann T, Michaelson DM. The isoform-specific pathological effects of apoE4 in vivo are prevented by a fish oil (DHA) diet and are modified by cholesterol. J Alzheimers Dis 2012;28:667–83. [DOI] [PubMed] [Google Scholar]
- 115.Hashimoto M, Tozawa R, Katakura M, Shahdat H, Haque AM, Tanabe Y, Gamoh S, Shido O. Protective effects of prescription n-3 fatty acids against impairment of spatial cognitive learning ability in amyloid beta-infused rats. Food Funct 2011;2:386–94. [DOI] [PubMed] [Google Scholar]
- 116.Rey C, Nadjar A, Buaud B, Vaysse C, Aubert A, Pallet V, Laye S, Joffre C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun 2016;55:249–59. [DOI] [PubMed] [Google Scholar]
- 117.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS One 2011;6:e15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW, et al. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem 2009;20:715–25. [DOI] [PubMed] [Google Scholar]
- 119.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 2005;115:2774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem 2003;278:43807–17. [DOI] [PubMed] [Google Scholar]
- 121.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477–9. [DOI] [PubMed] [Google Scholar]
- 122.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002;59:913–9. [DOI] [PubMed] [Google Scholar]
- 123.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 2009;28:525–42. [DOI] [PubMed] [Google Scholar]
- 124.Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA, Hyman Rapaport M. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry 2015;76:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Serini S, Bizzarro A, Piccioni E, Fasano E, Rossi C, Lauria A, Cittadini AR, Masullo C, Calviello G. EPA and DHA differentially affect in vitro inflammatory cytokine release by peripheral blood mononuclear cells from Alzheimer’s patients. Curr Alzheimer Res 2012;9:913–23. [DOI] [PubMed] [Google Scholar]
- 126.Mickleborough TD, Tecklenburg SL, Montgomery GS, Lindley MR. Eicosapentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin Nutr 2009;28:71–7. [DOI] [PubMed] [Google Scholar]
- 127.Sierra S, Lara-Villoslada F, Comalada M, Olivares M, Xaus J. Dietary eicosapentaenoic acid and docosahexaenoic acid equally incorporate as decosahexaenoic acid but differ in inflammatory effects. Nutrition 2008;24:245–54. [DOI] [PubMed] [Google Scholar]
- 128.Martin DS, Lonergan PE, Boland B, Fogarty MP, Brady M, Horrobin DF, Campbell VA, Lynch MA. Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem 2002;277:34239–46. [DOI] [PubMed] [Google Scholar]
- 129.Babcock T, Helton WS, Espat NJ. Eicosapentaenoic acid (EPA): an antiinflammatory omega-3 fat with potential clinical applications. Nutrition 2000;16:1116–8. [DOI] [PubMed] [Google Scholar]
- 130.Layé S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot Essent Fatty Acids 2010;82:295–303. [DOI] [PubMed] [Google Scholar]
- 131.Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, Amieva H, Allard M, Dartigues JF, Cunnane SC, et al. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology 2012;79:642–50. [DOI] [PubMed] [Google Scholar]
- 132.Hashimoto M, Hossain S, Tanabe Y, Kawashima A, Harada T, Yano T, Mizuguchi K, Shido O. The protective effect of dietary eicosapentaenoic acid against impairment of spatial cognition learning ability in rats infused with amyloid beta(1–40). J Nutr Biochem 2009;20:965–73. [DOI] [PubMed] [Google Scholar]
- 133.Arsenault D, Julien C, Tremblay C, Calon F. DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice. PLoS One 2011;6:e17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perez SE, Berg BM, Moore KA, He B, Counts SE, Fritz JJ, Hu YS, Lazarov O, Lah JJ, Mufson EJ. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: possible gender effects. J Neurosci Res 2010;88:1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qu MH, Yang X, Wang Y, Tang Q, Han H, Wang J, Wang GD, Xue C, Gao Z. Docosahexaenoic acid-phosphatidylcholine improves cognitive deficits in an Abeta23–35-induced Alzheimer’s disease rat model. Curr Top Med Chem 2016;16:558–64. [DOI] [PubMed] [Google Scholar]
- 136.Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, Aid S, Poumes-Ballihaut C, Champeil-Potokar G, Lavialle M. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev 2004;44:509–38. [DOI] [PubMed] [Google Scholar]
- 137.Roberts LJ II, Fessel JP, Davies SS. The biochemistry of the isoprostane, neuroprostane, and isofuran Pathways of lipid peroxidation. Brain Pathol 2005;15:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gorjão R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J, Curi R. Comparative effects of DHA and EPA on cell function. Pharmacol Ther 2009;122:56–64. [DOI] [PubMed] [Google Scholar]
- 139.Williams CM, Burdge G. Long-chain n-3 PUFA: plant v. marine sources. Proc Nutr Soc 2006;65:42–50. [DOI] [PubMed] [Google Scholar]
- 140.Kaduce TL, Chen YC, Hell JW, Spector AA. Docosahexaenoic acid synthesis from n-3 fatty acid precursors in rat hippocampal neurons. J Neurochem 2008;105:1525–35. [DOI] [PubMed] [Google Scholar]
- 141.Alessandri JM, Extier A, Langelier B, Perruchot MH, Heberden C, Guesnet P, Lavialle M. Estradiol favors the formation of eicosapentaenoic acid (20: 5n-3) and n-3 docosapentaenoic acid (22: 5n-3) from alpha-linolenic acid (18: 3n-3) in SH-SY5Y neuroblastoma cells. Lipids 2008;43:19–28. [DOI] [PubMed] [Google Scholar]
- 142.Domenichiello AF, Chen CT, Trepanier MO, Stavro PM, Bazinet RP. Whole body synthesis rates of DHA from alpha-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J Lipid Res 2014;55:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baumgartner J, Smuts CM, Zimmermann MB. Providing male rats deficient in iron and n-3 fatty acids with iron and alpha-linolenic acid alone affects brain serotonin and cognition differently from combined provision. Lipids Health Dis 2014;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care 2002;5:127–32. [DOI] [PubMed] [Google Scholar]
- 145.Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg 2003;38:564–75. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto N, Saitoh M, Moriuchi A, Nomura M, Okuyama H. Effect of dietary alpha-linolenate/linoleate balance on brain lipid compositions and learning ability of rats. J Lipid Res 1987;28:144–51. [PubMed] [Google Scholar]
- 147.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J 2000;19:1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ Res 2007;101:176–84. [DOI] [PubMed] [Google Scholar]
- 149.Nguemeni C, Gouix E, Bourourou M, Heurteaux C, Blondeau N. Alpha-linolenic acid: a promising nutraceutical for the prevention of stroke. PharmaNutrition 2013;1:1–8. [Google Scholar]
- 150.Michael-Titus AT. Omega-3 fatty acids and neurological injury. Prostaglandins Leukot Essent Fatty Acids 2007;77:295–300. [DOI] [PubMed] [Google Scholar]
- 151.King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci 2006;26:4672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996;139:244–56. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y, Sun Q, Chen X, Jing L, Wang W, Yu Z, Zhang G, Xie M. Linolenic acid provides multi-cellular protective effects after photothrombotic cerebral ischemia in rats. Neurochem Res 2014;39:1797–808. [DOI] [PubMed] [Google Scholar]
- 154.Massey KA, Nicolaou A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radic Biol Med 2013;59:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 2008;47:147–55. [DOI] [PubMed] [Google Scholar]
- 156.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care 2007;10:136–41. [DOI] [PubMed] [Google Scholar]
- 157.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013;75:645–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 2007;178:3912–7. [DOI] [PubMed] [Google Scholar]
- 159.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000 2013;63:149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X, Zhu M, Hjorth E, Cortes-Toro V, Eyjolfsdottir H, Graff C, Nennesmo I, Palmblad J, Eriksdotter M, Sambamurti K, et al. Resolution of inflammation is altered in Alzheimer's disease. Alzheimers Dement 2014;11(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2001;2:612–9. [DOI] [PubMed] [Google Scholar]
- 162.Peterson LD, Jeffery NM, Thies F, Sanderson P, Newsholme EA, Calder PC. Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E-2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids 1998;33:171–80. [DOI] [PubMed] [Google Scholar]
- 163.von Schacky C, Kiefl R, Jendraschak E, Kaminski WE. n-3 Fatty acids and cysteinyl-leukotriene formation in humans in vitro, ex vivo, and in vivo. J Lab Clin Med 1993;121:302–9. [PubMed] [Google Scholar]
- 164.Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, Calder PC. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr 2006;83:331–42. [DOI] [PubMed] [Google Scholar]
- 165.Chapkin RS, Akoh CC, Miller CC. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J Lipid Res 1991;32:1205–13. [PubMed] [Google Scholar]
- 166.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA 2006;103:11276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem 2007;282:22254–66. [DOI] [PubMed] [Google Scholar]
- 168.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA 2005;102:7671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol 2010;184:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 2005;201:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weylandt KH, Krause LF, Gomolka B, Chiu CY, Bilal S, Nadolny A, Waechter SF, Fischer A, Rothe M, Kang JX. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-alpha. Carcinogenesis 2011;32:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Petroni A, Salami M, Blasevich M, Papini N, Galli C. Inhibition by n-3 fatty acids of arachidonic acid metabolism in a primary culture of astroglial cells. Neurochem Res 1994;19:1187–93. [DOI] [PubMed] [Google Scholar]
- 175.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 2003;278:14677–87. [DOI] [PubMed] [Google Scholar]
- 176.Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG. Docosahexaenoic acid therapy of experimental ischemic stroke. Transl Stroke Res 2011;2:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci 2009;29:3875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, Zhang C, Jun B, Obenaus A, Fredman G, et al. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp Neurol 2012;236:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hassan A, Ibrahim A, Mbodji K, Coeffier M, Ziegler F, Bounoure F, Chardigny JM, Skiba M, Savoye G, Dechelotte P, et al. An alpha-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-kappaB in rats with TNBS-induced colitis. J Nutr 2010;140:1714–21. [DOI] [PubMed] [Google Scholar]
- 180.Winnik S, Lohmann C, Richter EK, Schafer N, Song WL, Leiber F, Mocharla P, Hofmann J, Klingenberg R, Boren J, et al. Dietary alpha-linolenic acid diminishes experimental atherogenesis and restricts T cell-driven inflammation. Eur Heart J 2011;32:2573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr 2015;6:513–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Caligiuri SP, Aukema HM, Ravandi A, Pierce GN. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp Gerontol 2014;59:51–7. [DOI] [PubMed] [Google Scholar]
- 183.Schulze-Tanzil G, de Souza P, Behnke B, Klingelhoefer S, Scheid A, Shakibaei M. Effects of the antirheumatic remedy hox alpha—a new stinging nettle leaf extract—on matrix metalloproteinases in human chondrocytes in vitro. Histol Histopathol 2002;17:477–85. [DOI] [PubMed] [Google Scholar]
- 184.Caligiuri SP, Love K, Winter T, Gauthier J, Taylor CG, Blydt-Hansen T, Zahradka P, Aukema HM. Dietary linoleic acid and alpha-linolenic acid differentially affect renal oxylipins and phospholipid fatty acids in diet-induced obese rats. J Nutr 2013;143:1421–31. [DOI] [PubMed] [Google Scholar]
- 185.Cooper JK. Nutrition and the brain: what advice should we give? Neurobiol Aging 2014;35(Suppl 2):S79–83. [DOI] [PubMed] [Google Scholar]