Abstract
Globally, the popularity of energy drinks is steadily increasing. Scientific interest in their effects on cardiovascular and cerebrovascular systems in humans is also expanding and with it comes a growing number of case reports of adverse events associated with energy drinks. The vast majority of studies carried out in the general population report effects on blood pressure and heart rate. However, inconsistencies in the current literature render it difficult to draw firm conclusions with regard to the effects of energy drinks on cardiovascular and cerebrovascular variables. These inconsistencies are due, in part, to differences in methodologies, volume of drink ingested, and duration of postconsumption measurements, as well as subject variables during the test. Recent well-controlled, randomized crossover studies that used continuous beat-to-beat measurements provide evidence that cardiovascular responses to the ingestion of energy drinks are best explained by the actions of caffeine and sugar, with little influence from other ingredients. However, a role for other active constituents, such as taurine and glucuronolactone, cannot be ruled out. This article reviews the potentially adverse hemodynamic effects of energy drinks, particularly on blood pressure and heart rate, and discusses the mechanisms by which their active ingredients may interact to adversely affect the cardiovascular system. Research areas and gaps in the literature are discussed with particular reference to the use of energy drinks among high-risk individuals.
Keywords: blood pressure, sugar, caffeine, heart rate, public health
Introduction
One substantial subcategory of soft drinks that is considered to be one of the most popular is represented by energy drinks (EDs). Indeed, the popularity of EDs has substantially increased since their introduction around 1960 (1), and this subcategory been found to be one of the fastest growing segments in the beverage industry (2). The majority of EDs are targeted toward young adults aged between 18 and 34 y, with a reported consumption frequency of 1–4 EDs/mo (3); and approximately half of college student “ED users” consumed EDs while studying or working on a major project (3). To date, there exists an abundance and variety of EDs on the market, with >200 brands in the United States alone (4). However, only a few dominate the market, and there is not much difference in caffeine and sugar content when comparing the market leaders (Table 1). At the beginning of the 21st century, early concerns arose about the safety of EDs because they had been linked to cardiovascular complications (8), which led to sales restrictions and even bans in some European countries (9). Although these restrictions have since been lifted (10), Lithuania was the first European country to ban ED sales for minors (11) and, currently, the European Union is considering a sales ban of EDs for persons <18 y (12).
TABLE 1.
ED market leaders in the United States including their caffeine and sugar contents1
| Sugars (7), g/100 g |
||||||
| Market size in the United States (5), % | Caffeine (6), mg/100 g | Total | Fructose | Glucose | Sucrose | |
| Red Bull | 43 | 29 | 10.22 | 1.63 | 3.40 | 5.19 |
| Monster | 39 | 33 | 10.93 | 2.39 | 4.11 | 4.43 |
| Rockstar | 10 | 33 | 12.26 | 3.59 | 6.03 | 2.65 |
| NOS | 3 | 34 | 11.25 | — | — | — |
| Amp | 3 | 31 | 12.08 | — | — | — |
| Mean ± SD | NA | 32 ± 2 | 11.35 ± 0.84 | 2.54 ± 0.99 | 4.51 ± 1.36 | 4.09 ± 1.30 |
Red Bull, Red Bull GmbH; Monster, Monster Beverage; Rockstar, Rockstar, Inc.; NOS, Monster Beverage; Amp, PepsiCoA. ED, energy drink; NA, not applicable; —, content unknown, but ingredients include high-fructose corn syrup.
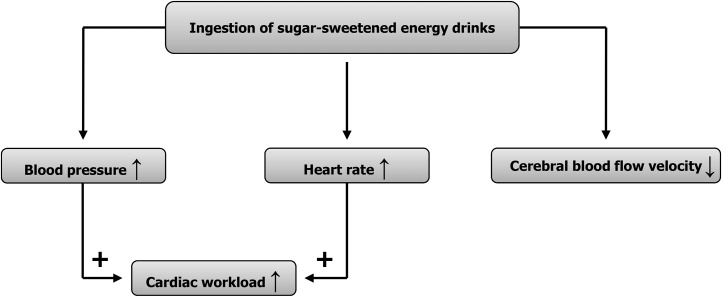
A recent clinical review focused on adverse health events in response to the ingestion of EDs and found that >50% of their included case reports were related to the cardiovascular system, followed by neurological issues (13). Moreover, there are 8 case reports in which large intakes of EDs were found to be associated with myocardial ischemia, with no additional triggers in the majority of cases (14). Increasing evidence of negative cardiovascular effects in response to ED consumption was recently highlighted by Sanchis-Gomar et al. (15), thereby providing specific recommendations for adolescents to prevent cardiac arrhythmias (16). Furthermore, with the use of beat-to-beat hemodynamic measurements in young healthy humans, work from our laboratory previously showed that blood pressure and other cardiovascular variables increased acutely in response to the ingestion of 1 can (355 mL) of a sugar-sweetened ED (17). This observation, which was confirmed by subsequent studies from our laboratory (18, 19), provided evidence that ED consumption negatively affected the hemodynamic system (Figure 1). The extent to which the various ingredients present in EDs contribute to their acute cardiovascular effects are reviewed and discussed in the following sections.
FIGURE 1.
Hemodynamic consequences of consumption of a commercially available, sugared energy drink. ↓, diminished; ↑, elevated; +, enhancing.
EDs and Hemodynamics: Overall Effects
A number of studies investigating the cardio- and cerebrovascular impact of EDs in young healthy subjects primarily showed myocardial effects, particularly on blood pressure and heart rate. Recent studies that used beat-to-beat measurements (17) showed that the ingestion of 1 can of a sugar-sweetened ED (355-mL drink volume) resulted in an augmented workload to the heart as evidenced by elevated blood pressure, heart rate, cardiac output, and double product values (17); the ingestion of the ED did not lead to a deterioration of microvascular endothelial function in response to acetylcholine, which suggests that impaired endothelial function, at least in the microvasculature, is unlikely to account for the blood pressure–elevating effect of the ED (17). An interesting and seemingly novel finding of this study was that the ED diminished cerebral blood flow velocity and increased the cerebrovascular resistance index, which could at least partly be explained by an increase in breathing frequency and the secondary reduction in end-tidal carbon dioxide (17). In a follow-up study, which also used beat-to-beat measurement techniques, it was found that the ingestion of the ED during a mental challenge imposed a cumulative cardiovascular load and reduced cerebral blood flow velocity (18). Moreover, under resting conditions, we confirmed our previous findings with regard to an overall adverse hemodynamic profile in response to the ingestion of an ED (18).
In agreement with the findings of our study (19) on the predominantly myocardial effects of sugar-sweetened EDs are the following: 1) a report that the consumption of a sugar-sweetened Monster ED (Monster Beverage; 2.0 mg caffeine/kg and 0.65 g sugar/kg) significantly elevated heart rate after 60 min compared with the placebo control (20), 2) a study in which blood pressure and heart rate responses to a sugar-sweetened ED (Rockstar, Rockstar, Inc.; 240 mg caffeine and ∼62 g sugar) resulted in significantly elevated blood pressure values (systolic blood pressure: +6.6 mm Hg; diastolic blood pressure: +4.2 mm Hg) compared with a sugar-matched placebo (21), and 3) findings from current studies evaluating the impact of sugar-free caffeinated energy shots [5-h Energy; 200 mg and 215 mg caffeine] on cardiovascular variables that showed that blood pressure variables significantly increased whereas heart rate did not change (22, 23). Moreover, repeated intakes of an ED [8.3 oz of a Red Bull ED (Red Bull GmbH) 4 times over 24 h; 1 oz = 30 mL] increased blood pressure variables when compared with a water drink with equivalent amounts of caffeine (8 oz water with 80 mg caffeine) (24). On the basis of the aforementioned literature, it seems evident that EDs adversely affect the hemodynamic system, the magnitude and side effects of which depend largely on their caffeine and sugar content.
EDs and Hemodynamics: Discrepancies between Studies
The effects of EDs on changes in blood pressure and heart rate are not always consistent, however (Table 2). A number of suggestions can be put forward to potentially explain these apparent discrepancies, including differences in the duration of postconsumption measurements, differences in methodologies (continuous compared with discontinuous blood pressure measurement), and differences in volume load, as well as in the subject’s condition before and during the test (posture). These factors are discussed below in more detail.
TABLE 2.
Studies investigating the cardiovascular impact of ED ingestion1
| Authors (ref) | ED | Caffeine content | Sugar/taurine content | Outcome measures and techniques | Duration of measurement | Study findings |
| Alford et al. (25) | RB (250 mL) | — | — | HR and BP: Omega 1400 monitor | — | HR ↑ 30 min postconsumption and after overnight caffeine fasting; (−) BP |
| Baum and Weiss (26) | RB (500 mL) | — | — | ECG; HR measured by using Polar Pulse tester; BP measured by using conventional cuff | Baseline and 40 min postconsumption | SV and fractional shortening ↑ in the resting period after exercise |
| Rush et al. (27) | “V” (New Zealand) | 1.2 mg/kg | — | Minute-by-minute HR by pulse oximetry | 30 min pre- and postconsumption | (−) HR |
| Bichler et al. (28) | Caffeine pill | 100 mg | 1000 mg taurine | HR and BP measured by using automatic digital monitor | Baseline and 75 and 100 min postconsumption | ↓ HR 45 min postconsumption; (−) mean BP |
| Rashti et al. (29) | “Meltdown RTD” (140 mL) | — | — | HR measured every 5 s by using wireless monitor; BP measured by applying Riva-Rocci | HR measured every 5 s; BP measured at baseline, 15, 30, 60, 90, 120 and 150 min postconsumption | (−) HR and BP |
| Steinke et al. (30) | 500-mL drink; type not specified | 200 mg | — | HR calculated from ECG; BP measured by manually applying Riva-Rocci | BP measured at baseline and at 30, 60, 120, 180, and 240 min postconsumption | Systolic BP and HR ↑ after ingestion |
| Ragsdale et al. (31) | RB original (250 mL) vs. low-calorie | — | — | HR measured by ECG; BP measured by automated sphygmomanometer | 0, 60, and 120 min postconsumption | (−) ≤120 min after ingestion |
| Worthley et al. (32) | 250-mL sugar-free | ∼80 mg | — | BP and HR not specified; peripheral arterial tonometry | — | Mean BP ↑ 60 min after ingestion; (–) HR; impaired endothelial function |
| Nelson et al. (20) | “Monster” standardized vs. flavor-matched placebo | 2 mg/kg | — | HR measured by using lead II via an automated tonometer | — | Resting HR ↑ 60 min postconsumption |
| An et al. (33) | “Hot6” vs. water control | 1.25 or 2.5 mg/kg | — | HR measured by Telemeter Heart Checker and BP measured by digital monitor | Baseline and 60 min postconsumption | (−) HR and BP |
| Phan and Shah (23) | Energy shot vs. decaffeinated shot (both sugar-free) | 215 mg | — | HR and central BP measured by using the SphygmoCor PWA system; automated peripheral BP device | ≤180 min postconsumption | ↑ peripheral and central systolic BP with the caffeinated energy shot; (−) HR |
| Marczinski et al. (22) | Energy shot (sugar-free) vs. placebo control vs. no drink | 200 mg | — | Automated HR and BP monitor | — | ↑ systolic and diastolic BP with caffeinated energy shot; (−) HR |
| Grasser et al. (17) | RB (355 mL) vs. water control | ∼114 mg | 39 g sugar; 1420 mg taurine | Continuous beat-to-beat hemodynamic measurements; transcranial Doppler; laser Doppler flowmetry | — | ↑ BP and HR ≤120 min postconsumption; ↓ CBFV |
| Grasser et al. (18) | RB (355 mL) vs. water control | ∼114 mg | 39 g sugar; 1420 mg taurine | Continuous beat-to-beat hemodynamic measurements; transcranial Doppler | — | ↑ BP and HR; ↓ CBFV; mental stress at 80 min postconsumption; ↑ BP and HR |
| Miles-Chan et al. (19) | RB (355 mL) vs. RB sugar-free vs. water control | ∼114 mg | 39 g sugar; 1420 mg taurine | Continuous beat-to-beat hemodynamic measurements | — | BP changes occur through different hemodynamic pathways |
| Olateju et al. (34) | RB vs. RB light vs. Suso Orange control (750 mL) | — | — | HR and BP measured by using Omron BP monitor | Baseline and 30, 60, 90, 120, 150, and 180 min postconsumption | Caffeine-containing EDs may elevate systolic BP in type 1 diabetes patients |
| Doerner et al. (35) | ED (326 mL) vs. caffeine-matched drink | 105 mg | 1304 mg taurine | Myocardial contractility and SV measured in the afternoon by using cardiac MRI; HR and BP not specified | — | ↑ left ventricular contractility and SV 60 min after ingestion with caffeine + taurine; ↑ diastolic BP with the caffeine only |
| Cavka et al. (36) | RB (500 mL) | — | 56 g | HR and BP measured by using a semiautomatic oscillometric monitor (Omron) | — | ↑ diastolic BP and HR 30 min after ingestion |
| Elitok et al. (37) | RB (355 mL) | — | 39 g | HR measured by ECG; BP measured at same intervals, technique not specified | Baseline and 60 and 120 min postconsumption | ↑ HR and BP for ≤2 h |
| Hajsadeghi et al. (38) | ED (250 mL) | 80 mg | — | BP measured in seated position with a mercury sphygmomanometer; HR by ECG in supine position | Baseline (resting period 5 min) and 30, 120, and 240 min postconsumption | ↓ HR; (−) BP |
| Svatikova et al. (21) | “Rockstar” (480 mL) vs. sugar-matched control | 240 mg | 62 g sugar; 2000 mg taurine | BP and HR monitored | Baseline and 30 min postconsumption | ↑ BP compared with sugar-matched placebo; (−) HR |
Publications were identified by using PubMed and Google Scholar databases for the period of January 1980 to June 2016. We used for our search the following keywords either individually or in combination: “Red Bull,” “Monster,” “ED,” “blood pressure,” “heart rate,” “hemodynamics,” and “cardiovascular.” Inclusion criteria were as follows: 1) English language, 2) original work in humans, and 3) HR and/or BP as primary or secondary outcomes. Exclusion criteria were as follows: 1) case studies, 2) reviews, 3) epidemiologic studies, 4) letters to the editor, and 5) media reports. We thoroughly screened the list of references in articles that met our inclusion criteria. Monster, Monster Beverage; Red Bull, Red Bull GmbH; Rockstar, Rockstar, Inc.; NOS, Monster Beverage; Amp, PepsiCoA; Omega 1400 monitor, Omega Engineering Inc.; Polar Pulse tester, Polar Electro Oy; Telemeter Heartchecker RS 100, Polar; SphygmoCor PWA, AtCor Medical Pty Ltd; Omron BP, Omron Healthcare, Inc. BP, blood pressure; CBFV, cerebral blood flow velocity; ECG, electrocardiogram; ED, energy drink; HR, heart rate; RB, Red Bull; ref, reference; SV, stroke volume; ↑, increased; (–), no change; ↓, decreased; —, variables not specified/provided by the reviewed articles.
Duration of postconsumption measurements.
Although the literature is somewhat inconsistent with regard to duration, the importance of assessing the cardiovascular effects of EDs for a set duration postconsumption has been shown in numerous studies and is worth mentioning. An analysis of the time course of changes in blood pressure and heart rate in our study indicated that differences in response to a sugar-sweetened ED compared with a water control only became significant from 60 min postconsumption, with peak values being reached between 80 and 100 min (17). In line with these findings, a number of groups did not find blood pressure or heart rate to be increased by EDs for measurement periods of <60 min: Alford et al. (25) and Baum and Weiss (26) did not show a blood pressure–elevating effect of 250 or 500 mL of Red Bull at 30 and 40 min postconsumption, respectively, whereas another study by Bichler et al. (28) did not show a change in blood pressure or heart rate up to 45 min after ingesting capsules containing 100 mg caffeine and 1000 mg taurine (i.e., in amounts equivalent to those found in a 250-mL Red Bull ED).
In support of these studies are those that measured cardiovascular parameters for ≥60 min; for example, Elitok et al. (37) reported an increase in heart rate and blood pressure in response to the ingestion of 355 mL sugared Red Bull at 60 and 120 min postconsumption, whereas Worthley et al. (32) showed an increase in blood pressure at 60 min postconsumption of 250 mL of a sugar-free Red Bull–like drink with no change observed after carbonated water ingestion. Moreover, Steinke et al. (30) showed significant increases in heart rate (+5–7 beats/min), as well as in systolic and diastolic blood pressure (+4–8 mm Hg) between 60 and 240 min postconsumption of 500 mL of an ED that was similar in composition to Red Bull.
In contrast to the aforementioned studies, a recent study that investigated cardiovascular responses to a 480-mL Rockstar ED (240 mg caffeine and ∼62 g of sugar) observed significantly elevated blood pressure values 30 min after ingestion (21). This controversial observation could be due to differences between the EDs in terms of the amounts of caffeine (∼114 compared with 240 mg) and sugar (∼39 compared with ∼62 g) (i.e., 355 mL of a sugared Red Bull used in our study compared with 480 mL of a sugared Rockstar ED). However, taking into account that we observed a gradual blood pressure increase with peak responses between 80 and 100 min postconsumption (17), it is possible that peak blood pressure responses in the study by Svatikova et al. (21) could not be reached within the 30-min measurement period. Thus, unless the assessment of cardiovascular responses to EDs is conducted over periods of ≥60 min, there is a high risk of 1) false-negative results and 2) the possibility of underestimating their real cardiovascular impact (39, 40).
Methodologies: continuous compared with discontinuous blood pressure measurements.
The acute cardiovascular responses to sugar-sweetened EDs have been shown to differ between studies. In particular, blood pressure was shown by some to be increased in response to a sugar-sweetened ED, whereas others found no effect. Another possible explanation for these differences may relate to the fact that these studies used different methodologic approaches for measuring blood pressure. In work by our group, blood pressure was assessed by continuous beat-by-beat hemodynamic measurements (17–19), whereas other studies used an automated sphygmomanometer only occasionally throughout the experiment. It is possible that such infrequent measurements may lack the degree of accuracy required to detect significant, modest changes in blood pressure. For example, in a double-blind experiment, Ragsdale et al. (31) randomly assigned 68 participants to consume either 250 mL of an ED or a control drink, but they reported no changes in blood pressure over a 120-min postconsumption period with blood pressure assessed by arm cuff only at 0, 60, and 120 min postconsumption. Similarly, by using a mercury sphygmomanometer to assess blood pressure variables, Hajsadeghi et al. (38) investigated the cardiovascular impact of 250 mL of an ED in young healthy subjects and showed no significant changes in blood pressure at 30 min and at 120 min postconsumption. In light of our findings that both systolic and diastolic blood pressures peak at 80–100 min postconsumption, one cannot disregard the possibility that an increase in blood pressure at 60 min postconsumption of the ED in the study by Ragsdale et al. (31) and at 120 min postconsumption in the study by Hajsadeghi et al. (38) may have been detected as a significant increase if blood pressure had been measured continuously over the 120-min test period.
Differences in volume load.
Differential acute cardiovascular responses across the various studies could also result from differences in the volume of the ED ingested, thereby reflecting differences in the water load, as well as in the amount of active ingredients consumed (Table 2). Indeed, the 30% lesser volume of the EDs used in the study by Ragsdale et al. (31) and Hajsadeghi et al. (38) than that used in our study (250 compared with 355 mL) could have contributed to the lack of a significant increase in blood pressure measured over 120 min. Furthermore, although in the study by Worthley et al. (32) 250 mL of a sugar-free ED was found to elevate mean blood pressure at 60 min postconsumption, the increase in mean blood pressure (∼3.5 mm Hg) was less than that observed at the same time point in our study with 355 mL of Red Bull (∼5 mm Hg at 60 min postconsumption) (17). The potential importance of the volume of the ED ingested on the cardiovascular system may also be underscored by the observation that the increases in systolic and diastolic blood pressures in response to 355 mL of Red Bull in our study (17) were ∼2 mm Hg less than those reported in the study by Steinke et al. (30) in response to 500 mL of an ED with a composition similar to that of Red Bull. This contention is further substantiated by findings from Svatikova et al. (21) in which 480 mL of a Rockstar ED, containing 240 mg caffeine and ∼62 g sugar, substantially elevated blood pressure 30 min after ingestion. Furthermore, Passmore et al. (41), in their investigation in healthy male subjects of cardiovascular and renal effects of increasing oral doses of caffeine, observed a linear dose-response relation for systolic blood pressure, a finding that is in agreement with the aforementioned studies in which increasing volume loads, and therefore increasing amounts of caffeine, affected blood pressure.
Differences in posture during measurement.
It is well established that posture has a significant impact on blood pressure variables and heart rate in a resting condition (42–44), as well as in responses to specific cardiovascular maneuvers (44), an effect that was suggested to be caused by changes in the intrathoracic blood volume (44). A recent publication investigated the cardiovascular impact of an ED in young healthy subjects and observed that heart rate was significantly lower at 30 and 120 min postconsumption than at baseline levels (38). In this study, heart rate was measured discontinuously at 30, 120, and 240 min postconsumption by using a Cardioline AR1200 with the subject in a supine position, whereas blood pressure variables were measured in a sitting position after a resting period of 5 min, which indicates a change in body posture between heart rate and blood pressure measurements (38). Therefore, it is possible that a posture change during measurements as well as the position itself [i.e., a sitting position in our studies (17–19) compared with a supine position in the studies of Hajsadeghi et al. (38) for heart rate assessment] influence the heart rate response to ED ingestion.
EDs and Hemodynamic Effects: Role of Ingredients
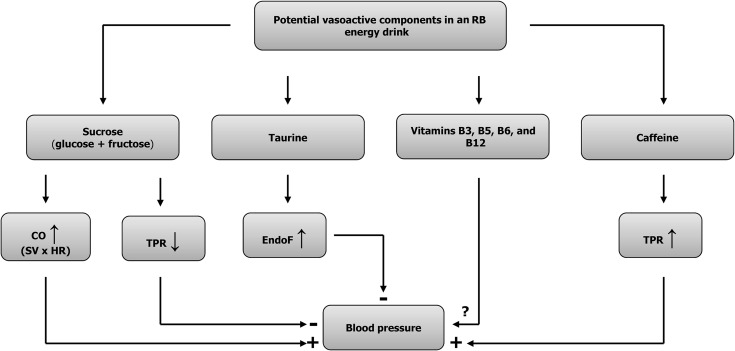
On the basis of a recent publication (19) it appears that caffeine and sugar are key ingredients underlying the hemodynamic impact of EDs on the human cardiovascular system (Figure 2), with other substances (i.e., taurine, vitamin B complex) playing only a minor role, if any. To further strengthen this hypothesis, we describe in more detail the cardio- and cerebrovascular impact of either caffeine or sugar, particularly for the heart rate response. It should also be noted that because hepatic cytochromes P450 (CYPs), in particular CYP1A2, are important for the metabolism of caffeine in the human body, it could be possible that CYP1A2 polymorphisms can influence cardiovascular responses to EDs.
FIGURE 2.
Potential vasoactive components contained in a commercially available, sugared RB energy drink and their hemodynamic impact. Interactions between distinct components are not included. CO, cardiac output; EndoF, endothelial function; HR, heart rate; RB, Red Bull (Red Bull GmbH); SV, stroke volume; TPR, total peripheral resistance; ↓, diminished; ↑, elevated; ?, not yet investigated; +, enhancing; –, attenuating.
Impact of caffeine
A major concern about the use of EDs relates to their caffeine content and its potential effect on blood pressure. Caffeine, the main active metabolite in EDs, is known to activate the sympathetic nervous system and to act as an antagonist of the neuromodulator adenosine (45). Because the functions of adenosine are largely inhibitory, caffeine has the potential to stimulate and potentiate sympathomimetic actions, leading to elevations in blood pressure (46). The content of caffeine in caffeinated beverages ranges from 2.8 mg/fluid ounce (fl oz) (9.5 mg/100 mL) in a classic Coca Cola [Pepsi Cola: 3.2 mg/fl oz (10.8 mg/100 mL)] to 51.3 mg/fl oz (173.4 mg/100 mL) in an espresso coffee, with ED caffeine contents ranging between these 2 extremes [e.g., Red Bull: 9.5 mg/fl oz (32.1 mg/100 mL); Rockstar: 9.4 mg/fl oz (31.8 mg/100 mL); Monster: 10.0 mg/fl oz (33.8 mg/100 mL)] (6). Acute ingestion of caffeine or caffeinated beverages has also been shown to increase blood pressure (47–49), although the outcome of these studies are heterogeneous because caffeine doses, method of dosage, subject population, and study design were variable. In particular, habitual caffeine intake of individuals as a factor in determining their differential blood pressure responses to the same dose of caffeine or caffeinated beverages has often been emphasized. Under resting conditions, caffeine causes an increase in blood pressure in caffeine-withdrawn subjects (50), whereas habitual coffee drinkers exhibited less or no effect of caffeine on blood pressure (51). Furthermore, caffeine-naive individuals may rapidly develop tolerance to its pressor effects over 2–3 d. However, other studies found that some people do not develop tolerance to the blood pressure–elevating effects of caffeine (52) and supplementation with caffeine alone has also been reported to evoke similar changes in blood pressure in habitual and nonhabitual caffeine users (53). Post hoc analyses conducted with the use of data from our studies (17, 18) also found no differences between habitual and nonhabitual caffeine users in the effect of the ED in increasing blood pressure after 24 h of caffeine avoidance.
In general, the ingestion of an amount of caffeine equivalent to 1–3 cups coffee (80–240 mg caffeine) appears to result in an increase in systolic blood pressure of 4–9 mm Hg and in diastolic blood pressure of 2–4 mm Hg. In a study by Hodgson et al. (49) in overnight-fasted, healthy adults who consumed 3 cups tea (∼150 mg caffeine) over a 3-h period, increases in systolic blood pressure (+9 mm Hg) were higher than those observed in our studies (+5–6 mm Hg) (17–19). The lower amount of caffeine in our ED (∼114 g) could account for some of these apparent differences in blood pressure responses between our study and the above-mentioned study that used tea because of a likely dose-response relation of caffeine on blood pressure variables (49). One possible mechanistic explanation for caffeine-induced increases in blood pressure could be related to its potential negative impact on endothelial function. Comparing caffeinated (80 mg caffeine) with decaffeinated coffee, Papamichael et al. (54) found, in young healthy subjects, an impaired endothelium-dependent, flow-mediated dilation in response to the caffeinated, but not to the decaffeinated, coffee. However, by using a microvascular endothelial function approach to assess endothelium-dependent vascular effects in response to an ED (∼114 mg caffeine), we did not find a change in microvascular endothelial function (17). This latter finding is in agreement with a recent study in which endothelial function was investigated in response to different EDs and coffee (55). Molnar and Somberg (55) observed improved endothelial function in 2 of 3 EDs, whereas 1 ED and the coffee did not affect endothelial function.
The presence of sugar as a calorie-containing ingredient in the ED may also play a role by virtue of the fact that glucose-induced insulin release, possibly by its effects on reducing total peripheral resistance, may limit the blood pressure–elevating effects of the sugar-sweetened ED. Such an assumption is in line with the findings of the above-mentioned study of smaller increases in blood pressure when tea was consumed with a meal than when consumed without the meal (49). We previously investigated the hemodynamic impact of caffeine and sugar in amounts similar to those found in Red Bull EDs (19). It was observed that a comparable quantity of caffeine alone exerts the same effect on blood pressure as the sugar-sweetened ED, but the increase occurs through different hemodynamic pathways—with the sugar-sweetened EDs’ effects being primarily myocardial, whereas caffeine elicited primarily vascular effects (19). There is need for caution in comparing studies of a sugar-sweetened ED with tea or coffee beverages because they all differ in a variety of other compounds that could contribute to the overall outcome of the actions of caffeine on the cardiovascular system. One might speculate that the polyphenolic compounds in tea or coffee and the high taurine content of a Red Bull ED could interfere with the actions of caffeine through pharmacodynamic and/or pharmacokinetic interactions (e.g., alterations in caffeine absorption, metabolism, and/or its elimination).
Impact of sugar
It can be speculated that the sugars contained in EDs could affect the cardiovascular system on the basis that the ingestion of food calories is generally accompanied by increases in heart rate (56), cardiac output (56), and pulmonary ventilation rate (57) (all of which contribute to the thermogenic effect of the calorie load). Indeed, in a randomized crossover study, our group recently showed that the ingestion of 500 mL water containing fructose (60 or 30 g), glucose (60 g), or sucrose (60 g) increased heart rate 60 min after ingestion; sucrose and glucose reduced total peripheral resistance and increased cardiac output, whereas, in contrast, fructose tended to increase total peripheral resistance (58). Moreover, we observed that changes in heart rate were time-dependent, with a significant increase only after 45 min postconsumption and the highest values at 120 min (59), therefore underscoring the importance of the chosen postconsumption measurement duration. A limitation of the contention that the sugar content in an ED will significantly affect heart rate and cardiac output is the amounts of sugar used in these aforementioned studies (58, 59), which were higher than in the 355-mL Red Bull ED.
Potential mechanisms underlying differential effects of sugars on postprandial blood pressure.
Differential insulin release and impact on blood pressure regulation.
In response to glucose ingestion, the resulting increase in blood glucose concentrations induces a rapid increase in plasma insulin, but this is markedly lower in response to fructose (60–64), which is slowly converted to glucose in the liver and only partly released as glucose into the circulation (65, 66). With the use of a hyperinsulinemic-euglycemic approach to investigate the impact of insulin on vascular resistance, Baron and Brechtel (67) observed that insulin dose-dependently increased cardiac output by stimulating the heart, but decreased systemic vascular resistance. In this context, systemic insulin resistance could be due to adrenergic stimulation (68), which can be partly explained by an enhanced sympathetic drive in response to increases in plasma insulin concentrations (69, 70). Although fructose elevates heart rate, which is more pronounced than with glucose (59), the main hemodynamic difference between glucose and fructose could be attributed to their impact on changes in total peripheral resistance and cardiac contractility (58). The differential effect of sugars on plasma insulin and vascular resistance through enhanced sympathetic activation may be a mechanism to explain the observed disparity in blood pressure responses.
Endothelial dysfunction.
A further explanation for the observed differential effects of fructose and glucose with regard to changes in total peripheral resistance and blood pressure could be due to differences in endothelial function. Augmentation of skeletal muscle blood flow is an important physiologic function of insulin (71). In a seminal study, Steinberg et al. (72) observed that endothelial function is impaired in human obesity and type 2 diabetes, both of which are conditions associated with insulin resistance. Due to limited insulin release, the ingestion of fructose might be expected to lead to microvascular endothelial dysfunction, which could contribute to its effect in increasing blood pressure. One study compared the hemodynamic effects of fructose, glucose, and sucrose and found no reduction in acetylcholine-mediated microvascular endothelial function (58). Similarly, Bidwell et al. (73) observed that forearm blood flow was not lower in response to a drink containing both glucose and fructose (ratio of 45:55) than in a drink containing glucose only. It is important to bear in mind, however, that both of these studies were conducted in young healthy subjects and so it remains to be seen whether endothelial dysfunction would occur under similar circumstances in glucose-intolerant subjects.
Caffeine-sugar interactions
Although millions of people would not savor their coffee without a spoonful or 2 of sugar, and despite the enormous research interest in the health consequences of sugar-sweetened EDs, little is known about whether the caffeine and sugar may interact to affect the cardiovascular system. As pointed out earlier, caffeine alone and sugar alone evoke distinct cardiovascular changes in healthy humans (Figure 2). However, a recent study in healthy subjects investigating the effects of caffeine, sugar, and a combination of both (drink volume kept equivalent to a standard 250-mL ED for a 70-kg person) (74) reported that combination treatment did not increase heart rate in comparison to either caffeine or sugar alone (74). These findings therefore contrast with our observations that heart rate and cardiac output substantially increased in response to a sugar-sweetened caffeinated ED and that these effects were not seen in response to a sugar-free version of the ED nor to a caffeine-equivalent water control (19). One possible explanation for the discrepancy between our study (19) and that of Rush et al. (74) could reside in the somewhat short post-treatment observation period of 30 min in which to detect a response to the combination treatment. This notion is strengthened by our observation of increasing heart rate and cardiac output only after 60–80 min after ingesting the sugar-sweetened ED (19). Further research is required to determine the effect on hemodynamic variables of an interaction, if any, between caffeine and sugar in EDs.
Impact of taurine
Although taurine administration, in amounts far greater than found in EDs, has been shown to reduce blood pressure (75), some studies concluded that when taurine, in amounts similar to that found in EDs, is provided in combination with caffeine, it may contribute to the acute increases in blood pressure. In one such study (28), the ingestion of capsules containing 100 mg caffeine and 1000 mg taurine did not alter mean arterial blood pressure at 45 min postconsumption, but increased blood pressure after subjects underwent a memory test at 70 min postconsumption. In another study that compared the impact of 500 mL Red Bull ED with or without taurine on heart rate and stroke volume before and after exercise, Baum and Weiss (26) showed that the ED led to significant increases in the contractility of the left atrium during a postexercise recovery period. The fact that these cardiac effects leading to increased stroke volume were not observed with the drinks that lacked taurine led the authors to suggest that taurine, either alone or in combination with caffeine, was responsible for the increase in stroke volume (26). A limitation of this interpretation and conclusion, however, is that another ingredient in Red Bull EDs, glucuronolactone, was also absent in the drinks without taurine, such that a role for glucuronolactone in this putative interaction with caffeine cannot be completely ruled out. However, to date, there is no information, to our knowledge, about the potential role of glucuronolactone on any component of the cardiovascular system. Moreover, current available versions of Red Bull EDs seem to no longer contain glucuronolactone (76). Similarly, the extent to which other active metabolites (including vitamins and minerals) present in Red Bull or other EDs may interact with caffeine and taurine to influence their blood pressure–elevating effect remains at present unknown. In previous work by our group, cardiovascular changes in response to sugar-free Red Bull were found to be largely the same as those of a caffeine-equivalent water control (19). Therefore, it is likely that these effects were due to caffeine alone, with little or no influence of the auxiliary components (taurine, glucuronolactone, and B-group vitamins) (19). In summary, it appears that caffeine and a possible combination of caffeine and taurine may negatively influence hemodynamic variables. Further research is required to determine the exact role, if any, of the other components, such as glucuronolactone, on the observed cardiovascular changes in response to EDs.
EDs and Hemodynamic Effects: What Is Missing?
From 1980 through 2014, there have been 43 case reports related to the ingestion of EDs (13). The majority of these reports were evaluated and published after 2010, and it can be argued that this could be due to a greater awareness among clinicians of the symptoms of caffeine and ED overconsumption. More than 80% of the aforementioned cases referred to cardiovascular and neurological events, with the former accounting for 52% and the latter for 29% (13). This observation is in line with the findings of Goldfarb et al. (77), who reviewed acute cardiovascular events in response to ED consumption. They reported that between 1980 and 2013 there were a total of 17 cases, with the majority presenting atrial and ventricular arrhythmias (n = 10), whereas 2 cases even presented with cardiac arrest (77). This suggests a higher risk of cardiac arrhythmias in predisposed individuals, those undergoing treatment with substances known to influence the cardiac propagation system (77), and in individuals with undiagnosed cardiac conditions. Moreover, the aforementioned review by Ali et al. (13) reported cases with coronary vasospasm and hypertension in response to ED consumption. On the basis of these publications, a possible adverse influence of ED consumption should not be disregarded, in particular in patients suffering from cardiac illnesses. Furthermore, taking into consideration our previous findings of elevated blood pressure and diminished cerebrovascular blood flow velocity in response to 1 can of an ED (17, 18), it can be speculated that in persons at risk of hypertension or those with impaired cerebral blood flow (e.g., atherosclerosis), EDs might even potentiate their cardio- and cerebrovascular risk. Our speculation is substantiated by recent studies in which increasing evidence of negative cardiovascular effects in response to ED consumption was presented (14, 15).
However, the scientific literature with regard to the impact of coffee or caffeine on the risk of stroke is controversial, ranging from findings that showed a transient increase in risk of ischemic stroke onset, particularly among infrequent drinkers (78), to a recent observation that heavier daily coffee consumption (≥3 cups/d) is associated even with decreased stroke prevalence (79). It is of note that the latter study observed an increasing risk in stroke prevalence in coffee-naive persons within the first 2 h after consumption (79). Moreover, according to James (80), even modest elevations in blood pressure by 4 and 2 mm Hg (systolic and diastolic, respectively) in response to the intake of dietary caffeine “could account for premature deaths in the region of 14% for coronary heart disease and 20% for stroke” (80). There is therefore a need for future research to focus on the impact of EDs in persons at risk of cardio- and cerebrovascular diseases, which should be undertaken as short- and long-term randomized controlled studies.
Conclusions
Recent research discussed here suggests that ED consumption can lead to an acute adverse hemodynamic profile with an augmented cardiac workload and diminished cerebral blood flow velocity, even during a mental stress test. These adverse changes are most likely caused by caffeine or by the effect of an interaction between caffeine and sugar on the cardiovascular system, whereas auxiliary substances play just a minor role. These cardio- and cerebrovascular changes in response to EDs have only been studied in healthy young humans but not in those at risk of cardiovascular events or those with pre-existing hypertension and/or impaired cerebral circulation. To standardize methods to allow for more accurate data comparison, future studies should consider the following: 1) the use of continuous blood pressure measurements, when possible; 2) measuring hemodynamic responses for a minimum of 60 min ED postconsumption; and 3) maintaining a standard posture throughout the test. Given their global popularity and estimated market value of >$40 billion, accurately assessing the potential adverse effects of EDs has important implications for the prevention and management of obesity, type 2 diabetes, and cardiovascular disease.
Acknowledgments
All authors read and approved the final manuscript.
References
- 1.Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks—a growing problem. Drug Alcohol Depend 2009;99:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heckman MA, Sherry K, de Mejia EG. Energy drinks: an assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Compr Rev Food Sci Food Saf 2010;9:303–17. [DOI] [PubMed] [Google Scholar]
- 3.Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutr J 2007;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lal GG. Getting specific with functional beverages. Food Technol 2007;61:24–31. [Google Scholar]
- 5.CaffeineInformer. Top selling energy drink brands. 2015 [cited 2016 Jun 15]. Available from: http://www.caffeineinformer.com/the-15-top-energy-drink-brands.
- 6.CaffeineInformer. Caffeine content of drinks. 2016 [cited 2016 Jun 15]. Available from: http://www.caffeineinformer.com/the-caffeine-database.
- 7.CaffeineInformer. Sugar in drinks. 2015 [cited 2016 Jun 15]. Available from: http://www.caffeineinformer.com/sugar-in-drinks.
- 8.Birchard K. Irish concerned about health effects of stimulant soft drinks. Lancet 2000;356:1911. [DOI] [PubMed] [Google Scholar]
- 9.Independent Print Ltd. European court backs ban on Red Bull over health concerns. 2004 [cited 2016 Jun 15]. Available from: http://www.independent.co.uk/life-style/health-and-families/health-news/european-court-backs-ban-on-red-bull-over-health-concerns-68019.html.
- 10.Reuters. France ends 12-year ban on energy drink Red Bull. 2008 [cited 2016 Jun 15]. Available from: http://www.reuters.com/article/2008/07/15/us-france-redbull-idUSL1576964720080715.
- 11.Stoll JD, Esterl M, Robinson F. Lithuania bans energy drinks sales to minors. The Wall Street Journal. May 15, 2014 [cited 2016 Jun 15]. Available from: http://www.wsj.com/articles/SB10001424052702304908304579563690934380648.
- 12.Newstalk.com. Is the EU going to ban the sale of energy drinks to under-18s? 2015 [cited 2016 Jun 15]. Available from: https://www.newstalk.com/Is-the-EU-going-to-ban-the-sale-of-energy-drinks-to-under18s.
- 13.Ali F, Rehman H, Babayan Z, Stapleton D, Joshi DD. Energy drinks and their adverse health effects: a systematic review of the current evidence. Postgrad Med 2015;127:308–22. [DOI] [PubMed] [Google Scholar]
- 14.Lippi G, Cervellin G, Sanchis-Gomar F. Energy drinks and myocardial ischemia: a review of case reports. Cardiovasc Toxicol 2016;6:207–12. [DOI] [PubMed] [Google Scholar]
- 15.Sanchis-Gomar F, Leischik R, Lippi G. Energy drinks: increasing evidence of negative cardiovascular effects. Int J Cardiol 2016;206:153. [DOI] [PubMed] [Google Scholar]
- 16.Sanchis-Gomar F, Pareja-Galeano H, Cervellin G, Lippi G, Earnest CP. Energy drink overconsumption in adolescents: implications for arrhythmias and other cardiovascular events. Can J Cardiol 2015;31:572–5. [DOI] [PubMed] [Google Scholar]
- 17.Grasser EK, Yepuri G, Dulloo AG, Montani JP. Cardio- and cerebrovascular responses to the energy drink Red Bull in young adults: a randomized cross-over study. Eur J Nutr 2014;53:1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasser EK, Dulloo AG, Montani JP. Cardiovascular and cerebrovascular effects in response to Red Bull consumption combined with mental stress. Am J Cardiol 2015;115:183–9. [DOI] [PubMed] [Google Scholar]
- 19.Miles-Chan JL, Charriere N, Grasser EK, Montani JP, Dulloo AG. The blood pressure-elevating effect of Red Bull energy drink is mimicked by caffeine but through different hemodynamic pathways. Physiol Rep 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson MT, Biltz GR, Dengel DR. Cardiovascular and ride time-to-exhaustion effects of an energy drink. J Int Soc Sports Nutr 2014;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svatikova A, Covassin N, Somers KR, Somers KV, Soucek F, Kara T, Bukartyk J. A randomized trial of cardiovascular responses to energy drink consumption in healthy adults. JAMA 2015;314:2079–82. [DOI] [PubMed] [Google Scholar]
- 22.Marczinski CA, Stamates AL, Ossege J, Maloney SF, Bardgett ME, Brown CJ. Subjective state, blood pressure, and behavioral control changes produced by an “energy shot”. J Caffeine Res 2014;4:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan JK, Shah SA. Effect of caffeinated versus noncaffeinated energy drinks on central blood pressures. Pharmacotherapy 2014;34:555–60. [DOI] [PubMed] [Google Scholar]
- 24.Franks AM, Schmidt JM, McCain KR, Fraer M. Comparison of the effects of energy drink versus caffeine supplementation on indices of 24-hour ambulatory blood pressure. Ann Pharmacother 2012;46:192–9. [DOI] [PubMed] [Google Scholar]
- 25.Alford C, Cox H, Wescott R. The effects of Red Bull energy drink on human performance and mood. Amino Acids 2001;21:139–50. [DOI] [PubMed] [Google Scholar]
- 26.Baum M, Weiss M. The influence of a taurine containing drink on cardiac parameters before and after exercise measured by echocardiography. Amino Acids 2001;20:75–82. [DOI] [PubMed] [Google Scholar]
- 27.Rush E, Schulz S, Obolonkin V, Simmons D, Plank L. Are energy drinks contributing to the obesity epidemic? Asia Pac J Clin Nutr 2006;15:242–4. [PubMed] [Google Scholar]
- 28.Bichler A, Swenson A, Harris MA. A combination of caffeine and taurine has no effect on short term memory but induces changes in heart rate and mean arterial blood pressure. Amino Acids 2006;31:471–6. [DOI] [PubMed] [Google Scholar]
- 29.Rashti SL, Ratamess NA, Kang J, Faigenbaum AD, Chilakos A, Hoffman JR. Thermogenic effect of meltdown rtd energy drink in young healthy women: a double blind, cross-over design study. Lipids Health Dis 2009;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinke L, Lanfear DE, Dhanapal V, Kalus JS. Effect of “energy drink” consumption on hemodynamic and electrocardiographic parameters in healthy young adults. Ann Pharmacother 2009;43:596–602. [DOI] [PubMed] [Google Scholar]
- 31.Ragsdale FR, Gronli TD, Batool N, Haight N, Mehaffey A, McMahon EC, Nalli TW, Mannello CM, Sell CJ, McCann PJ, et al. Effect of Red Bull energy drink on cardiovascular and renal function. Amino Acids 2010;38:1193–200. [DOI] [PubMed] [Google Scholar]
- 32.Worthley MI, Prabhu A, De Sciscio P, Schultz C, Sanders P, Willoughby SR. Detrimental effects of energy drink consumption on platelet and endothelial function. Am J Med 2010;123:184–7. [DOI] [PubMed] [Google Scholar]
- 33.An SM, Park JS, Kim SH. Effect of energy drink dose on exercise capacity, heart rate recovery and heart rate variability after high-intensity exercise. J Exerc Nutrition Biochem 2014;18:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olateju T, Begley J, Green DJ, Kerr D. Physiological and glycemic responses following acute ingestion of a popular functional drink in patients with type 1 diabetes. Can J Diabetes 2015;39:78–82. [DOI] [PubMed] [Google Scholar]
- 35.Doerner JM, Kuetting DL, Luetkens JA, Naehle CP, Dabir D, Homsi R, Nadal J, Schild HH, Thomas DK. Caffeine and taurine containing ED increases left ventricular contractility in healthy volunteers. Int J Cardiovasc Imaging 2015;31:595–601. [DOI] [PubMed] [Google Scholar]
- 36.Cavka A, Stupin M, Panduric A, Plazibat A, Cosic A, Rasic L, Debeljak Z, Martinovic G, Drenjancevic I. Adrenergic system activation mediates changes in cardiovascular and psychomotoric reactions in young individuals after Red Bull (®) energy drink consumption. Int J Endocrinol 2015;2015:751530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elitok A, Öz F, Panc C, Sarıkaya R, Sezikli S, Pala Y, Bugan ÖS, Ateş M, Parıldar H, Ayaz MB, et al. Acute effects of Red Bull energy drink on ventricular repolarization in healthy young volunteers: a prospective study. Anatol J Cardiol 2015;15:919–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajsadeghi S, Mohammadpour F, Manteghi MJ, Kordshakeri K, Tokazebani M, Rahmani E, Hassanzadeh M. Effects of energy drinks on blood pressure, heart rate, and electrocardiographic parameters: an experimental study on healthy young adults. Anatol J Cardiol 2016;16:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grasser EK, Miles-Chan JL, Montani JP. Hemodynamic responses to energy drink consumption. JAMA 2016;315:2018. [DOI] [PubMed] [Google Scholar]
- 40.Svatikova A, Covassin N, Somers KR. Hemodynamic responses to energy drink consumption—reply. JAMA 2016;315:2018–9. [DOI] [PubMed] [Google Scholar]
- 41.Passmore AP, Kondowe GB, Johnston GD. Renal and cardiovascular effects of caffeine: a dose-response study. Clin Sci (Lond) 1987;72:749–56. [DOI] [PubMed] [Google Scholar]
- 42.Netea RT, Lenders JW, Smits P, Thien T. Both body and arm position significantly influence blood pressure measurement. J Hum Hypertens 2003;17:459–62. [DOI] [PubMed] [Google Scholar]
- 43.Netea RT, Elving LD, Lutterman JA, Thien T. Body position and blood pressure measurement in patients with diabetes mellitus. J Intern Med 2002;251:393–9. [DOI] [PubMed] [Google Scholar]
- 44.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Low PA. Influence of posture on the valsalva manoeuvre. Clin Sci 2001;100:433–40. [PubMed] [Google Scholar]
- 45.Biaggioni I, Paul S, Puckett A, Arzubiaga C. Caffeine and theophylline as adenosine receptor antagonists in humans. J Pharmacol Exp Ther 1991;258:588–93. [PubMed] [Google Scholar]
- 46.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 1999;51:83–133. [PubMed] [Google Scholar]
- 47.Karatzis E, Papaioannou TG, Aznaouridis K, Karatzi K, Stamatelopoulos K, Zampelas A, Papamichael C, Lekakis J, Mavrikakis M. Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: should we consider monitoring central blood pressure? Int J Cardiol 2005;98:425–30. [DOI] [PubMed] [Google Scholar]
- 48.Hartley TR, Lovallo WR, Whitsett TL. Cardiovascular effects of caffeine in men and women. Am J Cardiol 2004;93:1022–6. [DOI] [PubMed] [Google Scholar]
- 49.Hodgson JM, Burke V, Puddey IB. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J Hypertens 2005;23:47–54. [DOI] [PubMed] [Google Scholar]
- 50.Robertson D, Frölich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, Oates JA. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med 1978;298:181–6. [DOI] [PubMed] [Google Scholar]
- 51.Corti R, Binggeli C, Sudano I, Spieker L, Hänseler E, Ruschitzka F, Chaplin WF, Lüscher TF, Noll G. Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content—role of habitual versus nonhabitual drinking. Circulation 2002;106:2935–40. [DOI] [PubMed] [Google Scholar]
- 52.Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens 2005;23:921–8. [DOI] [PubMed] [Google Scholar]
- 53.Sudano I, Spieker L, Binggeli C, Ruschitzka F, Lüscher TF, Noll G, Corti R. Coffee blunts mental stress-induced blood pressure increase in habitual but not in nonhabitual coffee drinkers. Hypertension 2005;46:521–6. [DOI] [PubMed] [Google Scholar]
- 54.Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond) 2005;109:55–60. [DOI] [PubMed] [Google Scholar]
- 55.Molnar J, Somberg JC. Evaluation of the effects of different energy drinks and coffee on endothelial function. Am J Cardiol 2015;116:1457–60. [DOI] [PubMed] [Google Scholar]
- 56.Sidery MB, Macdonald IA. The effect size on the cardiovascular responses to food ingestion. Br J Nutr 1994;71:835–48. [DOI] [PubMed] [Google Scholar]
- 57.Zwillich CW, Sahn SA, Weil JV. Effects of hypermetabolism on ventilation and chemosensitivity. J Clin Invest 1977;60:900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grasser EK, Dulloo A, Montani JP. Cardiovascular responses to the ingestion of sugary drinks using a randomised cross-over study design: does glucose attenuate the blood pressure-elevating effect of fructose? Br J Nutr 2014;112:183–92. [DOI] [PubMed] [Google Scholar]
- 59.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R730–7. [DOI] [PubMed] [Google Scholar]
- 60.Tappy L, Randin JP, Felber JP, Chiolero R, Simonson DC, Jequier E, DeFronzo RA. Comparison of thermogenic effect of fructose and glucose in normal humans. Am J Physiol 1986;250:E718–24. [DOI] [PubMed] [Google Scholar]
- 61.Blaak EE, Saris WHM. Postprandial thermogenesis and substrate utilization after ingestion of different dietary carbohydrates. Metabolism 1996;45:1235–42. [DOI] [PubMed] [Google Scholar]
- 62.Fukagawa NK, Veirs H, Langeloh G. Acute effects of fructose and glucose ingestion with and without caffeine in young and old humans. Metabolism 1995;44:630–8. [DOI] [PubMed] [Google Scholar]
- 63.Schwarz JM, Schutz Y, Froidevaux F, Acheson KJ, Jeanprêtre N, Schneider H, Felber JP, Jéquier E. Thermogenesis in men and women induced by fructose vs glucose added to a meal. Am J Clin Nutr 1989;49:667–74. [DOI] [PubMed] [Google Scholar]
- 64.Rebello T, Hodges RE, Smith JL. Short-term effects of various sugars on antinatriuresis and blood-pressure changes in normotensive young men. Am J Clin Nutr 1983;38:84–94. [DOI] [PubMed] [Google Scholar]
- 65.Chong MFF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 2007;85:1511–20. [DOI] [PubMed] [Google Scholar]
- 66.Hallfrisch J. Metabolic effects of dietary fructose. FASEB J 1990;4:2652–60. [DOI] [PubMed] [Google Scholar]
- 67.Baron AD, Brechtel G. Insulin differentially regulates systemic and skeletal muscle vascular resistance. Am J Physiol 1993;265:E61–7. [DOI] [PubMed] [Google Scholar]
- 68.Rocchini AP, Yang JQ, Gokee A. Hypertension and insulin resistance are not directly related in obese dogs. Hypertension 2004;43:1011–6. [DOI] [PubMed] [Google Scholar]
- 69.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res 2010;33:386–93. [DOI] [PubMed] [Google Scholar]
- 70.Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med 1986;61:1081–90. [PubMed] [Google Scholar]
- 71.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 1995;96:786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. implications for the syndrome of insulin resistance. J Clin Invest 1996;97:2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bidwell AJ, Holmstrup ME, Doyle RP, Fairchild TJ. Assessment of endothelial function and blood metabolite status following acute ingestion of a fructose-containing beverage. Acta Physiol (Oxf) 2010;200:35–43. [DOI] [PubMed] [Google Scholar]
- 74.Rush E, Long X, Obolonkin V, Ding J, Lucas P. Caffeine with and without sugar: individual differences in physiological responses during rest. Journal of Caffeine Research 2014;4:127–30. [Google Scholar]
- 75.Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987;75:525–32. [DOI] [PubMed] [Google Scholar]
- 76.RedBull.com. Red Bull energy drink ingredients. 2016 [cited 2016 May 17]. Available from: http://energydrink.redbull.com/ingredients-red-bull.
- 77.Goldfarb M, Tellier C, Thanassoulis G. Review of published cases of adverse cardiovascular events after ingestion of energy drinks. Am J Cardiol 2014;113:168–72. [DOI] [PubMed] [Google Scholar]
- 78.Mostofsky E, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Coffee and acute ischemic stroke onset: the Stroke Onset Study. Neurology 2010;75:1583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liebeskind DS, Sanossian N, Fu KA, Wang HJ, Arab L. The coffee paradox in stroke: increased consumption linked with fewer strokes. Nutr Neurosci 2015. Jun 22 (Epub ahead of print; DOI: 10.1179/1476830515Y.0000000035). [DOI] [PubMed] [Google Scholar]
- 80.James JE. Critical review of dietary caffeine and blood pressure: a relationship that should be taken more seriously. Psychosom Med 2004;66:63–71. [DOI] [PubMed] [Google Scholar]