Abstract
Cheap and abundant electrocatalysts for hydrogen evolution reactions (HER) have been widely pursued for their practical application in hydrogen-energy technologies. In this work, I present systematical study of the hydrogen evolution reactions on MXenes (Mo2X and W2X, X = C and N) based on density-functional-theory calculations. I find that their HER performances strongly depend on the composition, hydrogen adsorption configurations, and surface functionalization. I show that W2C monolayer has the best HER activity with near-zero overpotential at high hydrogen density among all of considered pure MXenes, and hydrogenation can efficiently enhance its catalytic performance in a wide range of hydrogen density further, while oxidization makes its activity reduced significantly. I further show that near-zero overpotential for HER on Mo2X monolayers can be achieved by oxygen functionalization. My calculations predict that surface treatment, such as hydrogenation and oxidization, is critical to enhance the catalytic performance of MXenes. I expect that MXenes with HER activity comparable to Pt in a wide range of hydrogen density can be realized by tuning composition and functionalizing, and promotes their applications into hydrogen-energy technologies.
As an important energy carrier, hydrogen is clean, abundant, and renewable, and has been extensively investigated for its practical applications in green-energy technologies. Series of hydrogen-related technologies have been developed for their practical applications, such as hydrogen production and utilization in fuel cell1,2,3,4,5,6. Hydrogen evolution reaction (HER) is involved in the electrochemical reactions of both these technologies and determines the efficiencies of hydrogen production and utilization. The electrocatalyst used in these technologies plays a key role on the efficient HER reactions. Currently, noble metals, such as platinum and palladium, are most common electrocatalysts in electrolysis of water and fuel cell because of their high catalytic performance in HER7,8,9,10,11,12. However, their ultra-high cost and very-low abundance are detrimental to the commercialization of these technologies on large scale. Extensive efforts have been carried out to reduce the amounts of noble metals by alloying with cheap metals and tuning the composition of the catalysts7,8,9,10,11,12. Alternatively, novel electrocatalysts with low cost and rich abundance have been widely investigated to replace noble-metal catalysts13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. Especially, two-dimensional (2D) monolayers as electrocatalysts, such as 2D transition-metal dichalcogenides monolayers (TMDs), have been attracting increasing interests because of their unique physical and chemical properties20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. Experimental and theoretical studies showed that the electrocatalytic activity strongly depended on their structure, conductivity, edge states, defects, tensile strain, etc20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. For example, the electrocatalytic activities of semiconducting TMDs in electrolysis of water were contributed to their metallic edges25,29. Further studies showed that the surfaces of metallic TMDs showed better HER performance than semiconducting counterparts26,28,31,33. However, most TMDs only showed electrocatalytic activity at low hydrogen coverage on surfaces or at edges because their conductivities were reduced or metallic TMDs changed to semiconducting ones as hydrogen coverage increases, which dramatically limits their practical applications33.
Recently, a new family of 2D monolayers, MXenes, were discovered36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52. The MXenes are transitional-metal carbides/nitrides monolayers and have a general formula, Mi+1Xi, where “M” is transition metal element, “X” is C or N, and i is a positive integer36. These monolayer with various thicknesses can be simply obtained by exfoliating layered ternary transition metal carbides/nitrides (Mi+1AXi), where “A” is main group element (group IIIA and IVA)36. Most recently, Xu et al. reported the growth of large-scale high quality, superconducting 2D Mo2C monolayer by chemical vapour deposition52. Theoretical and experimental studies showed that most of MXenes are metallic. As learnt from the noble metals and MX2 monolayers/nanoribbons, it is well-known that the high conductivity of electrocatalyst is the prerequisite to the excellent HER activity. It is, therefore, that MXenes may find applications as electrocatalysts in hydrogen-related green energy technologies. To date, the study on the HER performances of MXenes has not been available. In this work, the electrocatalytic performance of MXenes for their applications as catalysts in HER is investigated based on the calculations of density-functional theory (DFT). It is predicted that the HER performances of MXenes strongly depend on their composition, surface treatment, hydrogen coverage, and hydrogen adsorption sites. It is found that pure and hydrogenated W2C monolayers are excellent in HER in a wide range of hydrogen density, while oxidization results in the significant reduction of its HER ability. It is shown that oxidized Mo2X monolayer is much better than pure and hydrogenated counterparts in HER. It is suggested that surface treatment is crucial to the applications of MXenes as electrocatalysts in HER.
Results
Geometric structure
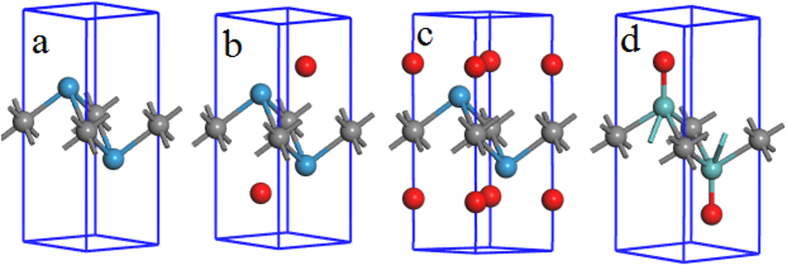
In the calculations, I focus on molybdenum- and tungsten-based MXenes, M2X (M = Mo and W; X = C and N). M2X monolayer is a three-atom-thick layer in a sequence of M1-X-M2 (Fig. 1a), and X-atom layer is enclaved in M-atom layers, leading to M6N octahedron. The unit cells of these MXenes are fully relaxed to obtain their lattice constants and study their electronic properties. It is noticed that the lattice constants of M2C MXenes are larger than those of M2N, and the lattice constants of Mo2X are larger than those of W2X (Table 1). The calculated total density of states (TDOSs) show that M2X monolayers are metallic (Supporting Data, S1), indicating their potential applications as electrocatalysts in HER.
Figure 1.
The representative structures of (a) pristine M2X and M2X with both sides covered by atoms (red) at different positions: (b) HC, (c) TX, and (d) TM. By removing red atoms from one side of the monolayer, one-side coverage is realized.
Table 1. Lattice parameters of pure and hydrogenated MXene monolayers: M2X (M = Mo and W; X = C and N).
| a (Å) | c (Å) | X-M (Å) | H-M (Å) | |
|---|---|---|---|---|
| Mo2C | 2.978 | 2.368 | 2.088 | |
| Mo2CH-HC | 2.928 | 2.533 | 2.139/2.082 | 1.981 |
| Mo2CH-TX | 2.971 | 2.405 | 2.120/2.059 | 2.059 |
| Mo2CH-TM | 3.085 | 2.199 | 2.086/2.100 | 1.712 |
| Mo2CH2-HC | 2.960 | 2.498 | 2.112 | 2..002 |
| Mo2CH2-TX | 2.951 | 2.458 | 2.101 | 2.058 |
| Mo2CH2-TM | 3.063 | 2.300 | 2.109 | 1.716 |
| Mo2N | 2.798 | 2.799 | 2.137 | |
| Mo2NH-HC | 2.791 | 2.854 | 2.170/2.135 | 1.982 |
| Mo2NH-TX | 2.804 | 2.831 | 2.168/2.133 | 1.993 |
| Mo2NH-TM | 2.831 | 2.759 | 2.160/2.117 | 1.696 |
| Mo2NH2-HC | 2.791 | 2.876 | 2.159 | 1.978 |
| Mo2NH2-TX | 2.797 | 2.916 | 2.176 | 1.992 |
| Mo2NH2-TM | 3.103 | 2.168 | 2.095 | 1.705 |
| W2C | 2.874 | 2.657 | 2.125 | |
| W2CH-HC | 2.885 | 2.668 | 2.149/2.119 | 1.994 |
| W2CH-TX | 2.917 | 2.571 | 2.133/2.105 | 2.039 |
| W2CH-TM | 3.027 | 2.337 | 2.102/2.103 | 1.714 |
| W2CH2-HC | 2.909 | 2.654 | 2.141 | 2.003 |
| W2CH2-TX | 2.941 | 2.526 | 2.116 | 2.068 |
| W2CH2-TM | 3.048 | 2.368 | 2.119 | 1.717 |
| W2N | 2.787 | 2.897 | 2.165 | |
| W2NH-HC | 2.779 | 2.949 | 2.192/2.166 | 1.995 |
| W2NH-TX | 2.790 | 2.945 | 2.192/2.173 | 1.992 |
| W2NH-TM | 2.815 | 2.882 | 2.201/2.144 | 1.696 |
| W2NH2-HC | 2.778 | 2.982 | 2.189 | 2.000 |
| W2NH2-TX | 2.790 | 3.000 | 2.200 | 1.992 |
| W2NH2-TM | 2.846 | 2.856 | 2.177 | 1.701 |
a is lattice constant, c is the thickness of the monolayer in vertical direction, X-M is the bond length, and H-M is the hydrogen-metal distance.
Hydrogen adsorption
To investigate their HER abilities, the lattice parameters of hydrogen-covered MXenes need to be calculated. Generally, there are three possible sites for hydrogen atoms to be adsorbed on the monolayers, including top of hexagonal center (HC) (Fig. 1b), top of X atom (TX) (Fig. 1c), and top of M atom (TM) (Fig. 1d). To give a full understanding on the effect of hydrogen coverage on their HER abilities, two cases are considered, including one side and both sides of MXenes covered by hydrogen atoms. The unit cells of MXenes with various hydrogen coverages at different adsorption sites are fully relaxed to find the stable adsorption position and obtain lattice parameters. For simplicity, these MXenes with various hydrogen coverages at different adsorption sites are named as M2XHm-Ad, where m = 1 (H-coverage on one side of monolayer) and m = 2 (H-coverage on both sides of monolayer), and “Ad” is the adsorption site and can be HC, TX, and TM. The relaxed geometries show that the adsorption of hydrogen atoms on HC and TX of MXene unit cell has negligible effect on the lattice constants, while that on TM results in the lattice extension by 1~8% (Table 1). At the same time, the thicknesses of the monolayers are increased by ~5% when hydrogen atoms adsorb on HC and TX, but reduced by 10~20% for hydrogen adsorption on TM. As an indication of stable adsorption, the adsorption energy (Ead) is calculated as below:
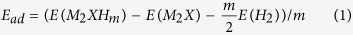 |
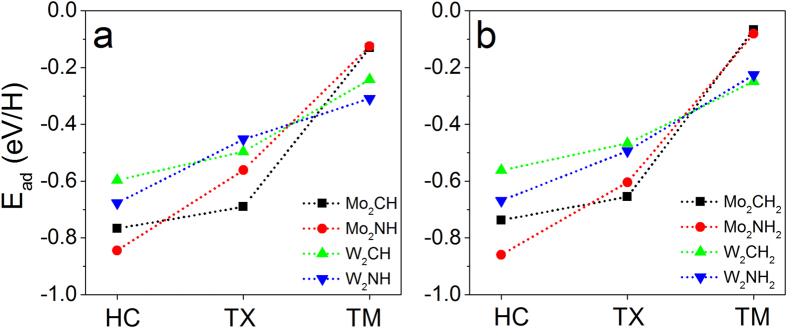
where E(M2XHm) and E(M2X) are the total energies of MXene unit cell with and without H atoms (m), and E(H2) is the energy of hydrogen molecule (H2). m is 1 for H-coverage on one side of monolayer or 2 for H-coverage on its both sides. Our calculations shows that the adsorption energies are negative at all of three possible sites (Fig. 2), indicating that all of the sites may be possible to host hydrogen atoms. It is found that HC is the most stable site to host hydrogen atom, followed by TX, and then by TM because the adsorption energy (negative) increases as the adsorption site changing from HC → TX → TM (Fig. 2). It is also found that the adsorption energy difference between two sites on W2C monolayer is the smallest among all of the considered MXenes. The variation of adsorption energy may affect the HER ability of MXenes.
Figure 2.
Calculated H-adsorption energy at different sites on M2X monolayers: (a) one-side H-coverage and (b) two-side H-coverage.
HER activity of pure MXenes
Basically, an advanced catalyst for the enhanced electrochemical hydrogen evolution reaction should reduce the HER reaction overpotential and consequently increase the HER efficiency, which can be quantified by the reaction Gibbs free energy of hydrogen adsorption (ΔGH)9,10,11,53. To investigate the hydrogen-coverage (H-coverage) dependent HER activity of pure MXene, a supercell with 2 × 2 × 1 unit cells is constructed based on the unit of MXene with one surface fully covered by hydrogen atoms at different adsorption sites (M2XH-Ad). Partial H-coverage is realized by removing H atom one by one from the H-covered surface of M2XH-Ad and the calculation on H-coverage-dependent HER performance is carried out accordingly. The H-coverage is defined as  (n = 0~4). The H-coverage dependent ΔGH can be calculated as below10,11,31,53:
(n = 0~4). The H-coverage dependent ΔGH can be calculated as below10,11,31,53:
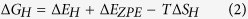 |
where ΔEH is the hydrogen chemisorption energy. It can be differential chemisorption energy as calculated from:
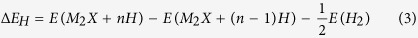 |
or, be average chemisorption energy as calculated from:
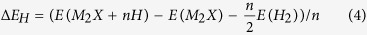 |
where n ( = 0~4) is the number of H atoms adsorbed on one side of a M2X monolayer. The H-coverage-dependent ΔGH can be obtained by changing n. E(M2X + nH) and E(M2X) in Eqs (3) and (4) are the energies of monolayer supercell with variable hydrogen atoms (n) and pure M2X supercell, respectively. ΔSH is the difference in entropy and T is room temperature. ΔEZPE is the difference in zero point energy between the adsorbed and the gas phase. ΔEZPE − TΔSH is about 0.24 eV. Therefore, Eq. (2) can be simplified to ΔGH = ΔEH + 0.24. According to the two methods (Eqs (3) and (4)) for the calculation of hydrogen chemisorption energy, the Gibbs free energies are defined as differential ΔGH (d-ΔGH) and average ΔGH (a-ΔGH), which can be used to express the HER activities in the individual and collective processes, respectively. The individual process describes that hydrogen is produced one by one, while the collective process shows that all of hydrogen atoms on the surface are simultaneously converted to molecules. In principle, an electrocatalyst with optimal HER performance should have a ΔGH near 0 eV.
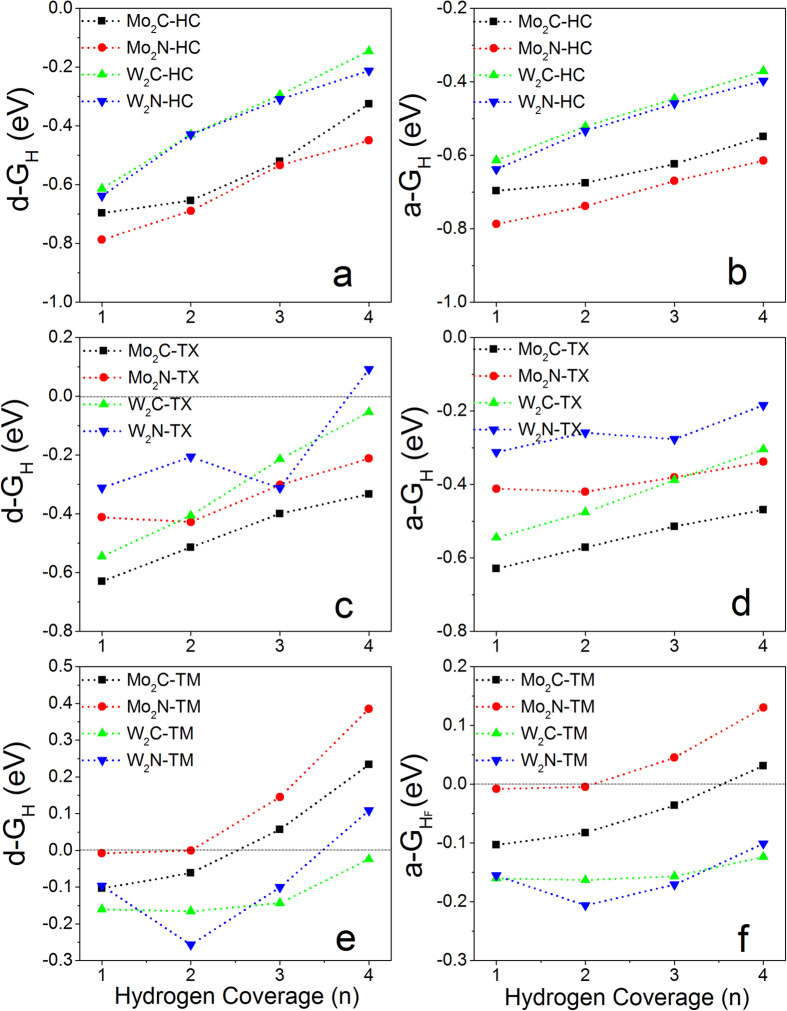
The calculated ΔGH shows that the HER activities of M2X monolayers strongly depend on the H-coverage and adsorption sites (Fig. 3). For M2X monolayers with H adsorbed on HC (M2X-HC), the calculated Gibbs free energies are negative and increase with the increment of H-coverage in both individual and collective processes (Fig. 3a,b). The W2X monolayers are better than Mo2X in the HER activity because of their relatively lower overpotentials (absolute values of ΔGH). Especially, the d-ΔGH of W2C-HC is about −0.15 eV at full H-coverage (n = 4) (Fig. 3a), indicating good HER performance at high H-density in individual process. The calculated average Gibbs free energies (a-ΔGH) for M2X-HC are less than −0.3 eV (Fig. 3b). The collective processes are more difficult to take place than individual processes because the total energy in the collective process needs to multiply the number hydrogen atoms removed from the surface, which are far away from 0 eV (Fig. 3b).
Figure 3.
Calculated differential Gibbs free energy as a function of H-coverage on pure M2X monolayers (M2X-Ad) with H atoms adsorbed on: (a) HC, (c) TX, and (e) TM; Calculated average Gibbs free energy as a function of H-coverage on pure M2X monolayers with H atoms adsorbed on: (b) HC, (d) TX, and (f) TM.
If the hydrogen atoms adsorb on TX sites of M2X monolayers (M2X-TX), it is found that the HER activities are improved because of relatively lower overpotentials (Fig. 3c,d) at the same H-coverage compared to M2X-HC (Fig. 3a,b). In particular, the differential Gibbs free energies of W2C-TX and W2N-TX monolayers at full H-coverage (n = 4) are −0.05 and 0.09 eV (Fig. 3c), respectively, which are much near zero than those with H atoms adsorbed on HC sites (−0.14 eV for W2C and −0.21 eV for W2N, Fig. 3a). Similarly, the collective processes are difficult because their overpotentials are larger than those in individual processes (Fig. 3c,d).
Different from H atoms adsorbed on HC and TX, all of considered MXenes with H atoms adsorbed on TM show good HER activities at certain H-coverage because of near zero overpotentials in both individual and collective processes (Fig. 3e,f). For example, Mo2N monolayer (Mo2N-TM) shows the best HER performance at low H-coverage in both individual and collective processes due to near-zero d-ΔGH (−0.008 eV for n = 1 and −0.001 eV for n = 2) (Fig. 3e) and a-ΔGH (−0.008 eV for n = 1 and −0.004 eV for n = 2) (Fig. 3f). W2C monolayer (W2C-TM) still shows the best HER activity with d-ΔGH = −0.02 eV at high H-coverage (n = 4) in individual process (Fig. 3e). Mo2C-TM is excellent for HER reactions at low hydrogen density in individual process (d-ΔGH = −0.1 for n = 1 and −0.06 for n = 2) (Fig. 3e) and at high hydrogen density in collective process (a-ΔGH = −0.04 eV for n = 3 and 0.03 eV for n = 4) (Fig. 3f). Clearly, the weak chemical-adsorption leads to the enhancement of HER activity (Fig. 3).
HER activity of MXenes with one side hydrogenated
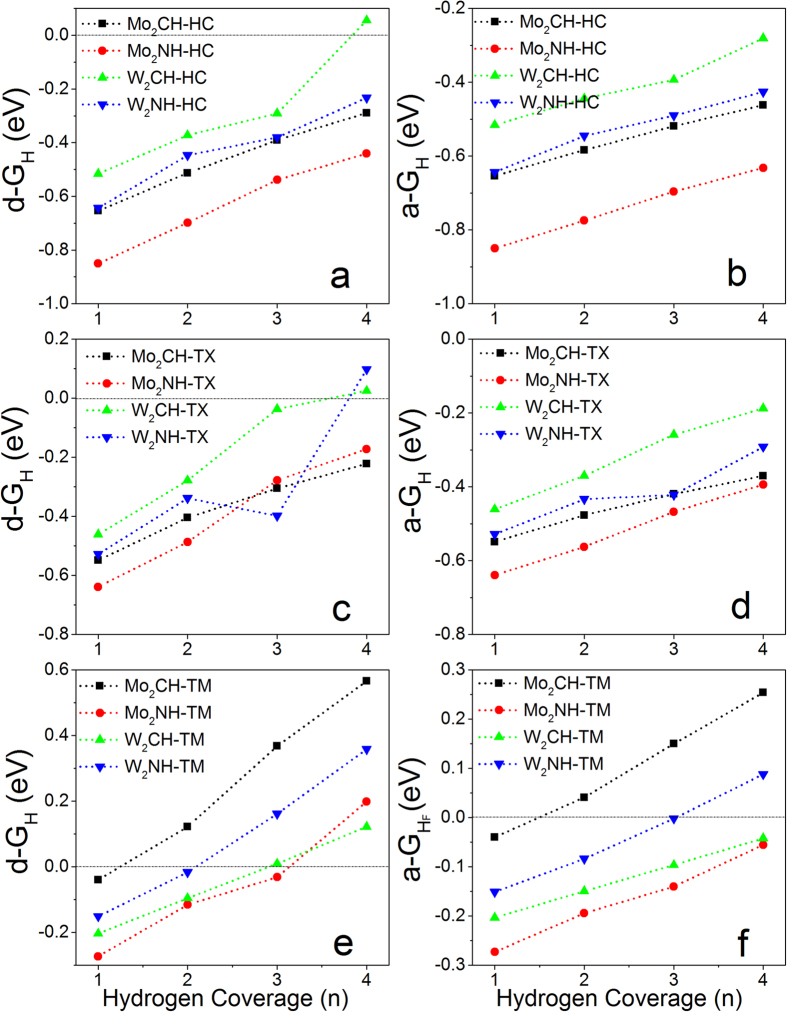
It had been reported that MXene can be easily functionalized by H, OH, and F, which affect their performance in energy storage40,41,43. To study the effect of hydrogenation on HER activity, a supercell with 2 × 2 × 1 unit cells is constructed based on the unit of MXene with both surfaces fully covered by hydrogen atoms at different adsorption sites (M2XH2-Ad). By removing H atom one by one from one of its surfaces, the effect of hydrogenation on the H-coverage dependent HER activity can be evaluated. To calculate the Gibbs free energies, it only needs to replace M2X in Eqs (3) and (4) by M2XH. Therefore, E(M2XH + nH) is the energy of monolayer with one side covered with variable hydrogen atoms (n) and another side fully covered by hydrogen atoms (M2XH-Ad-nH), and E(M2XH) is the energy of M2X monolayer with one side fully covered by hydrogen atoms (M2XH-Ad). The calculated overpotentials show that hydrogenation can efficiently improve the HER activities of M2X monolayers (Fig. 4). For M2X monolayer with hydrogenation at HC sites on one of its surfaces (M2XH-HC), the HER activities are improved because of the reduced overpotentials in individual processes (Fig. 4a). In particular, the d-ΔGH of W2C monolayer is about 0.05 eV at full H-coverage (n = 4), which just satisfies the basic requirement for an efficient electrocatalyst with ΔGH = 0 eV. Although the average Gibbs free energies (a-ΔGH < 0) for M2XH-HC in collective processes are increased (Fig. 4b), they are still less than −0.2 eV, indicating that the collective processes are difficult to take place.
Figure 4.
Calculated differential Gibbs free energy as a function of H-coverage on one-side hydrogenated M2X monolayers (M2XH-Ad) with H atoms adsorbed on: (a) HC, (c) TX, and (e) TM; Calculated average Gibbs free energy as a function of H-coverage on one-side hydrogenated M2X monolayers with H atoms adsorbed on: (b) HC, (d) TX, and (f) TM.
Interestingly, the hydrogenation on one side of W2C monolayer with H atoms at TX sites (W2CH-TX) can efficiently improve its catalytic performance at high H-density (Fig. 4c). The calculated differential Gibbs free energies of W2CH-TX are close to zero at high H-coverage (−0.04 and 0.02 eV for n = 3 and 4), indicating excellent HER performance. The calculated average Gibbs free energies (negative) (Fig. 4d) are less than differential ones (Fig. 4c), indicating that collective process is also difficult to happen.
Hydrogenation dramatically affect the HER performances of M2XH-TM monolayers in both individual and collective processes (Fig. 4e,f). It is found that all of M2XH-TM monolayers show excellent HER activities at certain H-coverage in both processes. The calculated d-ΔGH and a-ΔGH on Mo2CH-TM are −0.04 eV at n = 1. The Gibbs free energies in individual processes are −0.03 eV for Mo2NH-TM and −0.02 eV for W2NH-TM at n = 3, respectively. Although the HER activity of W2CH-TM is reduced at n = 4 (d-ΔGH = 0.12 eV), it is strongly improved at n = 3 (d-ΔGH = −0.01 eV) in individual process (Fig. 4e). Importantly, the calculated a-ΔGH for Mo2NH-TM and W2CH-TM are about −0.04 eV at full H-coverage (n = 4) and that for W2NH-TM is about −0.02 eV at n = 3 (Fig. 4f), indicating the collective processes can take place. Compared to pure MXenes (Fig. 3), it is found that hydrogenation can efficiently enhance their HER performance, especially in the collective processes.
HER activity of oxidized MXenes
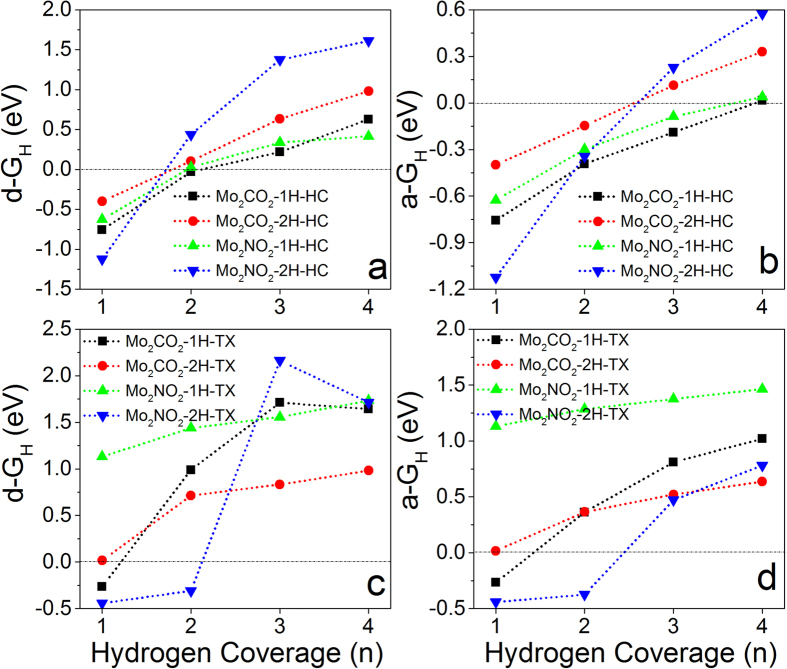
Beside hydrogenation, the MXenes are also easily oxidized. In the section, I focus on the effect of oxygen-functionalization on their HER activities. The MXenes with two sides oxidized are named as M2XO2 (M = Mo and W, and X = C and N). There are three possible sites for oxygen to cover the surfaces M2X monolayers (Fig. 1b–d). The unit cells of M2XO2-Ad monolayers (Ad = HC, TX, and TM) are fully relaxed to obtain the stable adsorption site. It is found that M2XO2-HC and M2XO2-TX are more stable than M2XO2-TM because their energies are low by ~3–4 eV/unit, and M2XO2-TX is more stable than M2XO2-HC by an energy of 0.7–1.2 eV/unit (Fig. 5). To investigate their applications in HER reactions, both M2XO2-HC and M2XO2-TX are studied because the adsorption of hydrogen may lead to phase transition. The unit cells of M2XO2 monolayers with H atoms adsorbed on the tops of oxygen atoms (M2XO2-mH-Ad, m = 1 and 2, Ad = HC and TX) are optimized (insets in Fig. 5). Our calculations show that the energy differences between M2XO2-1H-TX and M2XO2-1H-HC was reduced to 0.05~0.26 eV when one side is fully covered by hydrogen atoms, except Mo2NO2-1H (Fig. 5). Mo2NO2-1H-HC is more stable than Mo2NO2-1H-TX by an energy of 0.6 eV (Fig. 5), indicating phase transition from Mo2NO2-TX to Mo2NO2-HC. When both sides of M2XO2 monolayers are covered by hydrogen atoms (M2XO2-2H), phase transitions take place in all systems because M2XO2-2H-HC is more stable than M2XO2-2H-TX by an energy of 0.02~0.82 eV (Fig. 5). The calculated lattice constants of M2XO2-Ad and M2XO2-mH-Ad are larger than those of M2X (Supporting data, Table T1). Because of possible phase transition during hydrogen evolution reaction, both adsorption sites (HC and TX) are considered when calculating Gibbs free energies.
Figure 5. Calculate energy differences of M2X monolayers with oxidization at different sites and H-adsorption.
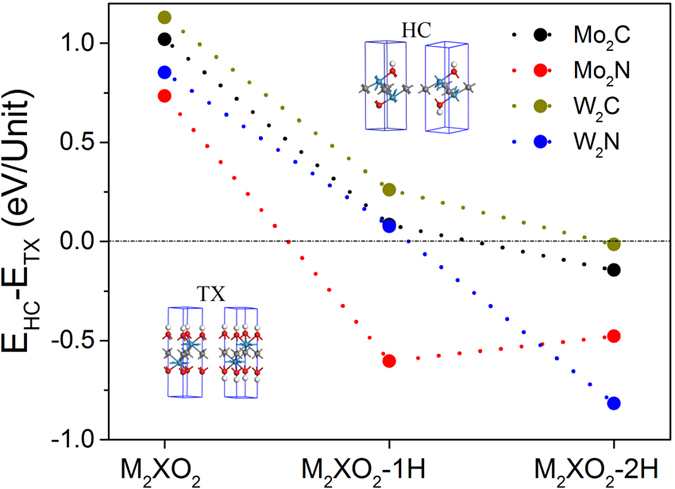
The insets show the oxidized M2X monolayers with H atoms at HC and TX sites, respectively.
Similarly, a supercell with 2 × 2 × 1 unit cells is constructed based on the unit of M2XO2-mH-Ad (m = 1 and 2, Ad = HC and TX). By removing H atom one by one from a H-covered surface, the H-coverage dependent HER activity can be evaluated. The DFT-calculated Gibbs free energies show that Mo2X monolayers with oxidization at HC sizes are better than those at TX sites in HER activities (Fig. 6). Mo2XO2-1H-HC (X = C and N) shows the best HER performance at n = 2 (d-ΔGH = −0.03~0.03 eV), and the HER activities of Mo2XO2-2H-HC are better if 1 < n < 2 where the d-ΔGH cross the reference line (0 eV) in individual processes (Fig. 6a). Importantly, the a-ΔGH of Mo2XO2-1H-HC (X = C and N) at full H-coverage (n = 4) are near-zero (0.01~0.04 eV, Fig. 6b), indicating the collective processes can take place. It is expected that the collective HER process on Mo2XO2-1H-HC is dominant at high H-density, and the individual processes may take over the role when H-density is low. Similarly, the collective processes of Mo2XO2-2H-HC can take place within the H-coverage from 2 to 3 (Fig. 6b). For Mo2X monolayers with oxidization at TX, only Mo2CO2-2H-TX shows high HER activity at low H-coverage (n = 1, ΔGH = 0.02 eV) (Fig. 6c,d). Although the oxidization results in the improvement of the HER performances of W2CO2 monolayers with O atoms at HC sites at low H-coverage, but their HER activities at high H-coverage are greatly reduced (Supporting data, S-2a,b). W2CO2 monolayers with O atoms at TX sites shows poor HER performances (S-2c,d). Our calculations also show that W2NO2 monolayers with partial H-coverage (0 < n < 4) are unstable and their Gibbs free energies are not calculated.
Figure 6.
Calculated overpotentials as a function of H-coverage on oxidized Mo2X monolayers: (a) differential Gibbs free energy for H atoms adsorbed on HC sites, (b) average Gibbs free energy for H atoms adsorbed on HC sites, (c) differential Gibbs free energy for H atoms adsorbed on TX sites, (b) average Gibbs free energy for H atoms adsorbed on TX sites.
Discussion
By systematically analysing the HER activities of pure and functionalized M2X monolayers with various H-adsorption configurations, it is found that the HER activity of MXene strongly depends on its composition, hydrogen adsorption configuration, and surface functionalization. Generally, the less stable adsorption, the better HER activity. It is found that pure and hydrogen-functionalized W2C monolayers shows the best HER performance at high H-coverage among all of considered systems, and is comparable to Pt because of near-zero overpotential. The overpotentials of pure W2C monolayer at full H-coverage in individual process decreases from 0.15 eV to 0.05 eV, then to 0.02 eV as the H-adsorption site changing from HC → TX → TM (Fig. 3a,c,e). The hydrogen functionalization can efficiently improve the HER activity of W2C monolayer. Almost zero Gibbs free energies at high H-coverage in individual processes can be achieved on W2CH regardless of H-adsorption sites (Fig. 4a,c,e). Importantly, both differential and average Gibbs free energies of W2C-TM and W2CH-TM are within a range of −0.2 to 0.1 eV in the whole H-coverage (n = 1~4) (Figs 3e,f and 4e,f), indicating its excellent performance in hydrogen evolution reaction. Their HER activities are much better than MX2 monolayers and nanoribbons when considering the H-coverage density and oeverpotentials, where 2D MX2 only showed high activities at certain H-density23,26,29,30,31,32,33,34,35,54,55 (Supporting data, Table S-2). A recent work on V2CO2 was noticed during revision, which showed that pure monolayer is worse on HER, while metal-decoration on its surface could improve its HER performance at certain H-coverage56. My calculations predict that pure W2C, W2CH and Mo2XO2 (X = C and N) monolayers show better HER performances with near-zero Gibbs free energies in wide range of H-coverages than Ni-V2CO2 (Table S-2). Although H atoms prefer to occupy HC or TX sites on W2C monolayer (Fig. 2), the H adsorption on TM is also exothermic and the energy is only higher than those on HC and TX by 0.20~0.35 eV/unit. In experiments, H atoms may first approach to TM sites because metal layers are outmost, and then HER reactions occurs accordingly. Interestingly, the oxidized W2C monolayers show better HER activities with near-zero Gibbs free energy at low H-coverage, but worse at high H-coverage (S-2a,b). It is, therefore, suggested that acid solution is required in HER reactions to avoid oxidization and keep the HER performance of W2C monolayer. Different from oxidized W2C monolayers, the oxygen functionalized Mo2X monolayers shows excellent HER catalytic activities at medium H-coverage (n = 2) in individual processes (Fig. 6a) and at high H-coverage in collective processes (Fig. 6b). Especially, the average Gibbs free energies of Mo2XO2-1H-HC at full H-coverage (n = 4) in collective processes are 0.02~0.04 eV (Fig. 6b). Compared with pure and H-functionalized Mo2X monolayers (Figs 3 and 4), it is found that oxidization can dramatically improve their HER activities at full H-coverage in collective processes (Fig. 6b), but their HER performances at low H-coverage are greatly reduced.
Conclusions
I carries out first-principles calculations to investigate the catalytic activities of MXenes for applications into HER. It is found that pure and hydrogenated W2C monolayers are better than other MXenes without oxygen functionalization and show the best HER performances at high H-density in HER reactions because of near-zero overpotential. In particular, excellent HER performance in the whole H-coverage from zero to full can be achieved on W2C monolayers with H atoms adsorbed on the top of W atoms, which can be realized by controlling experimental conditions. However, the optimal HER activity of W2C monolayer is degraded if it is oxidized or functionalized by oxygen atoms, especially at high H-coverage. It is further shown that the oxidization can dramatically improve the HER activities of Mo2X monolayers. The oxidized Mo2X monolayers exhibit best HER performances in individual processes at medium H-coverage and in collective processes at high H-coverage due to near-zero Gibbs free energies. For practical applications in experiments, I suggest that oxidization should be avoided to keep the advanced HER activity of W2C monolayer used as electrocatalyst, while Mo2X monolayers may need to be functionalized by oxygen to enhance their activities. The MXenes with activity comparable to novel metals may be obtained by tuning compositions and functionalizing, and find applications into HER reactions.
Methods
The first-principles calculations are carried out to investigate the hydrogen evolution reaction of MXenes. The calculations are based on the density functional theory (DFT)57 and the Perdew-Burke-Eznerhof generalized gradient approximation (PBE-GGA)58. The projector augmented wave (PAW) scheme59,60 as incorporated in the Vienna ab initio simulation package (VASP)61 is used in the study. The Monkhorst and Pack scheme of k-point sampling is used for integration over the first Brillouin zone62. A 15 × 15 × 1 grid for k-point sampling for geometry optimization of unit cells, and an energy cut-off of 500 eV are consistently used in our calculations. A sufficiently large supercell is used so that the monolayers in neighbouring cells in the vertical direction are separated by a vacuum region of at least 20 Å. Dipole correction is not included. Good convergence is obtained with these parameters and the total energy was converged to 2.0 × 10−5 eV/atom. The error bar (or uncertainty) of the DFT calculation is less than 5 meV.
Additional Information
How to cite this article: Pan, H. Ultra-high electrochemical catalytic activity of MXenes. Sci. Rep. 6, 32531; doi: 10.1038/srep32531 (2016).
Supplementary Material
Acknowledgments
Hui Pan thanks the support of the Science and Technology Development Fund from Macau SAR (FDCT-068/2014/A2, FDCT-132/2014/A3, and FDCT-110/2014/SB) and Multi-Year Research Grant (MYRG2014-00159-FST and MYRG2015-00017-FST) from Research & Development Office at University of Macau. The DFT calculations were performed at High Performance Computing Cluster (HPCC) of Information and Communication Technology Office (ICTO) at University of Macau.
Footnotes
Author Contributions H.P. conceived the idea, performed the calculations, and wrote the paper.
References
- Mallouk T. E. Water electrolysis: Divide and conquer. Nat. Chem. 5, 362–363 (2013). [DOI] [PubMed] [Google Scholar]
- Dincer I. & Acar C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 40, 11094–11111 (2015). [Google Scholar]
- Wang J., Zhang Y., Capuano C. B. & Ayers K. E. Ultralow charge-transfer resistance with ultralow Pt loading for hydrogen evolution and oxidation using Ru@Pt core-shell nanocatalysts. Sci. Rep. 5, 12220 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zheng H. F., Sun Z. J., Han A. & Du P. W. Earth-abundant copper-based bifunctional electrocatalyst for both catalytic Hydrogen Production and water oxidation. ACS Catal. 5, 1530–1538 (2015). [Google Scholar]
- Strmcnik D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 1, 466–472 (2009). [DOI] [PubMed] [Google Scholar]
- Patel P. P. et al. Nanostructured robust cobalt metal alloy based anode electro-catalysts exhibiting remarkably high performance and durability for proton exchange membrane fuel cells. J. Mate. Chem. A 3, 14015–14032 (2015). [Google Scholar]
- Koh S. & Strasser P. Electrocatalysis on bimetallic surfaces: modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J. Am. Chem. Soc. 129, 12624–12625 (2007). [DOI] [PubMed] [Google Scholar]
- Gressley J., Jarmaillo T. F., Bonde J., Chorkendorff I. & Nørskov J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 5, 909–913 (2006). [DOI] [PubMed] [Google Scholar]
- Norskov J. K., Bligaard T., Rossmeisl J. & Christensen C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009). [DOI] [PubMed] [Google Scholar]
- Karlberg G. S. et al. Cyclic voltammograms for H on Pt(111) and Pt(100) from first principles. Phys. Rev. Lett. 99, 126101 (2007). [DOI] [PubMed] [Google Scholar]
- Pan H., Feng Y. P. & Lin J. Y. Enhancement of hydrogen evolution on tungsten doped platinum. J. Comput. Theor. Nanosci. 7, 547–551 (2010). [Google Scholar]
- Xia B. Y. et al. One-pot synthesis of Pt-Co alloy nanowire assemblies with tunable composition and enhanced electrocatalytic properties. Angew. Chem. Int. Ed. 54, 3797–3801 (2015). [DOI] [PubMed] [Google Scholar]
- Lim C. S., Tan S. M., Sofer Z. & Pumera M. Impact electrochemistry of layered transition metal dichalcogenides. ACS Nano 9, 8474–8583 (2015). [DOI] [PubMed] [Google Scholar]
- Yu L., Xia B. Y., Wang X. & Lou X. W. General formation of M-MoS3 (M = Co, Ni) hollow structures with enhanced electrocatalytic activity for hydrogen evolution. Adv. Mater. 28, 92–97 (2016). [DOI] [PubMed] [Google Scholar]
- Falkowski J. M. & Surendranath Y. Metal chalcogenide nanofilms: platforms for mechanistic studies of electrolysis. ACS Catal. 5, 3411–3416 (2015). [Google Scholar]
- Wu H. B., Xia B. Y., Yu L., Yu X. Y. & Lou X. W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 6, 6512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. F., Muckerman J. T. & Fujita E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 49, 8896–8909 (2013). [DOI] [PubMed] [Google Scholar]
- Ma F. X., Wu H. B., Xia B. Y., Xu C. Y. & Lou X. W. Hierarchical β-Mo2C nanotubes organized by ultrathin nanosheets as a highly efficient electrocatalyst for hydrogen production. Angew. Chem. Int. Ed. 54, 15395–15399 (2015). [DOI] [PubMed] [Google Scholar]
- Xu M. et al. Porous CoP concave polyhedron electrocatalysts synthesized from metal-organic frameworks with enhanced electrochemical properties for hydrogen evolution. J. Mater. Chem. A 3, 21471–21477 (2015). [Google Scholar]
- Yu X. Y., Yu Y., Wu H. B. & Luo X. W. Formation of nickel sulfide nanoframes from MOFs with enhanced eseudocapacitive and electrocatalytic properties. Angew. Chem. Int. Ed. 54, 5331–5335 (2015). [DOI] [PubMed] [Google Scholar]
- Jaramillo T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007). [DOI] [PubMed] [Google Scholar]
- Karunadasa H. I. et al. Molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 335, 698–702 (2012). [DOI] [PubMed] [Google Scholar]
- Tsai C., Chan K., Nørskov J. K. & Abild-Pedersen F. Rational design of MoS2 catalysts: Tuning the structure and activity via transition metal doping. Catal. Sci. Technol. 5, 246–253 (2015). [Google Scholar]
- Yu X. Y., Hu H., Wang Y. W., Chen H. Y. & Luo X. W. Ultrathin MoS2 nanosheets supported on N-doped carbon nanoboxes with enhanced Lithium storage and electrocatalytic properties. Angew. Chem. Int. Ed. 54, 7395–7398 (2015). [DOI] [PubMed] [Google Scholar]
- Kibsgaard J., Chen Z., Reinecke B. N. & Jaramillo T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 11, 963–969 (2012). [DOI] [PubMed] [Google Scholar]
- Voiry D. et al. Covalent functionalization of monolayered transition metal dichalcogenides by phase engineering. Nat. Chem. 7, 45–49 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu H. B., Yan Y., Wang X. & Luo X. W. Hierarchical MoS2 microboxes constructed by nanosheets with enhanced electrochemical properties for Lithium storage and water splitting. Energy Environ. Sci. 7, 3302–3306 (2014). [Google Scholar]
- Ambrosi A., Sofer Z. & Pumera M. 2H → 1T phase transition and hydrogen evolution activity of MoS2, MoSe2, WS2 and WSe2 strongly depends on the MX2 composition. Chem. Commun. 51, 8450–8453 (2015). [DOI] [PubMed] [Google Scholar]
- Tsai C., Chan K., Abild-Pedersen F. & Nørskov J. K. Active edge sites in MoSe2 and WSe2 catalysts for the hydrogen evolution reaction: a density functional study. Phys. Chem. Chem. Phys. 16, 13156–13164 (2014). [DOI] [PubMed] [Google Scholar]
- Qu Y., Pan H., Kwok C. T. & Wang Z. A first-principles study on the hydrogen evolution reaction of VS2 nanoribbons. Phys. Chem. Chem. Phys. 17, 24820–24825 (2015). [DOI] [PubMed] [Google Scholar]
- Voiry D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 13, 850–855 (2013). [DOI] [PubMed] [Google Scholar]
- Tsai C., Abild-Pedersen F. & Nørskov J. K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 14, 1381–1387 (2014). [DOI] [PubMed] [Google Scholar]
- Pan H. Metal dichalcogenides monolayers: novel catalysts for electrochemical hydrogen production. Sci. Rep. 4, 5438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. Z. et al. MoS2 nanosheets: a designed structure with high active site density for the hydrogen evolution reaction. ACS Catal. 3, 2101–2107 (2013). [Google Scholar]
- Chen T. Y. et al. Comparative study on MoS2 and WS2 for electrocatalytic water splitting. Int. J. Hydro. Energy 38, 12302–12309 (2013). [Google Scholar]
- Naguib M., Mochalin V. N., Barsoum M. W. & Gogotsi Y. MXenes: a new family of two-dimensional materials. Adv. Mater. 26, 992–1005 (2014). [DOI] [PubMed] [Google Scholar]
- Wang X. F. et al. Atomic-scale recognition of surface structure and intercalation mechanism of Ti3C2X. J. Am. Chem. Soc. 137, 2715–2721 (2015). [DOI] [PubMed] [Google Scholar]
- Ji X. et al. Different charge-storage mechanisms in disulfide vanadium and vanadium carbide monolayer. J. Mater. Chem. A. 3, 9909–9914 (2015). [Google Scholar]
- Ling Z. et al. Flexible and conductive MXene films and nanocomposites with high capacitance. J. Am. Chem. Soc. 111, 16676–16681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames C. & Islam M. S. Ion intercalation into two-dimensional transition-metal carbides: global screening for new high-capacity battery materials. J. Am. Chem. Soc. 136, 16270–16276 (2014). [DOI] [PubMed] [Google Scholar]
- Hu J. P., Xu B., Ouyang C. Y., Yang S. Y. A. & Yao Y. G. Investigations on V2C and V2CX2 (X = F, OH) mono layer as a promising anode material for Li ion batteries from first-principles calculations. J. Phys. Chem. C 118, 24274–24281 (2014). [Google Scholar]
- Pan H. Electronic properties and lithium storage capacities of two-dimensional transition-metal nitrides monolayers. J. Mater. Chem. A. 3, 21486–21493 (2015). [Google Scholar]
- Xie Y. et al. Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem. Soc. 136, 6385–6394 (2014). [DOI] [PubMed] [Google Scholar]
- Jing Y., Zhou Z., Cabrera C. R. & Chen Z. F. Graphene, inorganic graphene analogs and their composites for lithium ion batteries. J. Mater. Chem. A 2, 12104–12122 (2014). [Google Scholar]
- Lukatskaya M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013). [DOI] [PubMed] [Google Scholar]
- Tang Q., Zhou Z. & Shen P. W. Are MXenes promising anode materials for Li ion batteries? computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 134, 16909–16916 (2012). [DOI] [PubMed] [Google Scholar]
- Mashtalir O. et al. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 4, 1716 (2013). [DOI] [PubMed] [Google Scholar]
- Lei J. C., Zhang X. & Zhou Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 10, 276–286 (2015). [Google Scholar]
- Tang Q. & Zhou Z. Graphene-analogous low-dimensional materials. Prog. Mater. Sci. 2013 58, 1244–1315 (2013). [Google Scholar]
- Zhang X., Ma Z. N., Zhao X. D., Tang Q. & Zhou Z. Computational studies on structural and electronic properties of functionalized MXene monolayers and nanotubes. J. Mater. Chem. A 3, 4960–4966 (2015). [Google Scholar]
- Xie Y. & Kent P. R. C. Hybrid density functional study of structural and electronic properties of functionalized Tin+1Xn (X = C, N) monolayers. Phys. Rev. B 87, 235441 (2013). [Google Scholar]
- Xu C. et al. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 14, 1135–1141 (2015). [DOI] [PubMed] [Google Scholar]
- Nørskov J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005). [Google Scholar]
- Pan H. Tension-enhanced hydrogen evolution reaction on vanadium disulfide monolayer. Nanoscale Res. Lett. 11, 113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. L., Wang S. Y., An Y. R. & Lau W. M. Catalytic activity of MS2 monolayer for electrochemical hydrogen evolution. J. Phys. Chem. C 120, 1623–1632 (2016). [Google Scholar]
- Ling C. Y., Shi L., Ouyang Y. X., Chen Q. & Wang J. L. Transition metal-promoted V2CO2 (MXenes): A new and highly active catalyst for hydrogen evolution reaction. Adv. Sci. 1600180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenberg P. & Kohn W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964). [Google Scholar]
- Blöchl P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Burke K. & Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). [Google Scholar]
- Kresse G. & Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- Monkhorst H. J. & Pack J. Special points for brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.