Abstract
Müller glia (MG) are the principal glial cell type in the vertebrate retina. Recent work has identified the LIM homeodomain factor encoding gene Lhx2 as necessary for both Notch signaling and MG differentiation in late-stage retinal progenitor cells (RPCs). However, the extent to which Lhx2 interacts with other intrinsic regulators of MG differentiation is unclear. We investigated this question by investigating the effects of overexpression of multiple transcriptional regulators that are either known or hypothesized to control MG formation, in both wildtype and Lhx2-deficient RPCs. We observe that constitutively elevated Notch signaling, induced by N1ICD electroporation, inhibited gliogenesis in wildtype animals, but rescued MG development in Lhx2-deficient retinas. Electroporation of Nfia promoted the formation of cells with MG-like radial morphology, but did not drive expression of MG molecular markers. Plagl1 and Sox9 did not induce gliogenesis in wildtype animals, but nonetheless activated expression of the Müller marker P27Kip1 in Lhx2-deficient cells. Finally, Sox2, Sox8, and Sox9 promoted amacrine cell formation in Lhx2-deficient cells, but not in wildtype retinas. These findings demonstrate that overexpression of individual gliogenic factors typically regulates only a subset of characteristic MG markers, and that these effects are differentially modulated by Lhx2.
Müller glia (MG) are adult radial glial cells that function as the primary physiological support cells within the retina. MG are specified from the progenitor cells of the retinal neuroepithelium (RPCs), which also generate retinal neurons, and share many morphological and molecular features with RPCs1,2,3. Both cell types feature radial processes that delimit the apical and basal surfaces of the retina2,4 and both co-express many transcription factors, neurofilament proteins, and signaling pathway molecules3,5,6. Furthermore, in teleost fish, MG are capable of functioning as adult stem cells following injury where they activate RPC-specific genes, undergoe proliferative expansion and then differentiate into retinal neurons to efficiently regenerate missing cells7,8,9,10. In contrast, mammalian MG are essentially quiescent, showing very limited regenerative capacity in experimental injury models11,12,13,14,15.
The identification of transcription factors (TFs) that selectively control MG specification and drive MG differentiation has been a considerable challenge, largely due to the overlap of gene expression between RPCs and MG. Several studies show that TFs in the retina that control gliogenesis are often also required for RPC maintenance, survival, proliferation, and/or progression through stages of developmental competence16,17,18,19,20,21. This shared gene expression also makes discrimination between immature MG precursors and RPCs difficult. This is particularly problematic when analysis is performed at late embryonic or early postnatal timepoints, when both RPCs and MG precursors co-exist.
Classic studies of retinal cell lineage used morphological criteria to identify MG1,22,23,24. Several MG-specific molecular markers have also been identified, including GLUL, P27Kip1, and RLBP125,26,27. However, it is rare for both morphological criteria and multiple molecular markers to be tested for studies of any individual gene. It is also often unclear whether genes previously implicated as directing Müller gliogenesis are in fact truly sufficient to fully promote gliogenesis, or are required primarily for terminal differentiation and survival of MG. In this study we have used in vivo electroporation in neonatal mouse retina to misexpress genes encoding TFs implicated in MG differentiation in previous loss of function studies (Sox2, Sox8, Sox9, Plagl1)28,29,30, or whose expression has been detected in developing MG (Nfia, Rax)31,16.
Members of the Sry-related HMG-box (Sox) family are expressed in retinal progenitors and mature MG28,29. Inactivation of Sox2, Sox8, and Sox9 disrupt differentiation and survival of MG28,29, but the ability of Sox family members to specify MG remains unclear. Plagl1, previously named Zac1, encodes a C2H2 zinc finger transcription factor. Plagl1 has been shown to influence retinal cell fate decisions in Xenopus, with misexpression of Plagl1 promoting MG development30. The functional role of Plagl1 in the mammalian retina has not previously been characterized. The paired type homeodomain transcription factor Rax is expressed in developing MG, and retroviral transduction of RPCs with Rax induces expression of a subset of MG markers16. Nfia (Nuclear Factor I/A) was previously reported to be both necessary and sufficient to drive astrogliogenesis in cortex and spinal cord32,33. Intriguingly, Nfia expression is regulated by Lhx2 in the developing hippocampus34. We have previously shown Lhx2 to be an essential regulator of MG development21, but the role of Nfia during MG development has not previously been described.
We also analyzed the intracellular domain (ICD) of Notch1. Following ligand binding, the Notch intracellular domain (NICD) is cleaved and transported to the nucleus, where it forms an active transcriptional regulatory complex with the DNA-binding protein RBPJ and the co-activator MAML, thereby mediating its effects through regulation of target gene expression35. Early studies suggested that misexpression of Notch in the retina promoted Müller gliogenesis16. More recent analysis indicates that N1ICD is less directly instructive, instead functioning to maintain undifferentiated RPCs in a slowly proliferative state while blocking activation of neurogenic genes36,37.
In this report we assay whether these factors are sufficient to promote MG differentiation by analyzing expression of multiple MG specific markers, as well as cell morphology. Furthermore, we tested whether overexpression of these factors was sufficient to rescue Müller gliogenesis in cells lacking the LIM homeodomain TF Lhx2. We previously showed Lhx2 to both be essential for Müller gliogenesis and to act as a direct global regulator of expression of multiple gliogenic factors and MG-specific genes21. We show that none of the tested factors were sufficient to promote all aspects of MG differentiation when overexpressed. Several were sufficient to promote the formation of cells with MG-like radial morphology or to activate expression of P27Kip1, a marker of cellular quiescence in MG26. However, none could activate expression of GLUL, a selective marker of differentiated MG25, in wildtype tissue. Furthermore, none of the electroporated TFs could fully rescue MG development following Lhx2 loss of function. These results underscore the fact that few factors that are necessary for retinal gliogenesis are also sufficient to induce glial differentiation, and highlights the central role of Lhx2 in organizing and coordinating MG differentiation.
Results
Misexpression of N1ICD in the mammalian retina promotes RPC maintenance, and is sufficient to rescue MG development following loss of Lhx2 expression
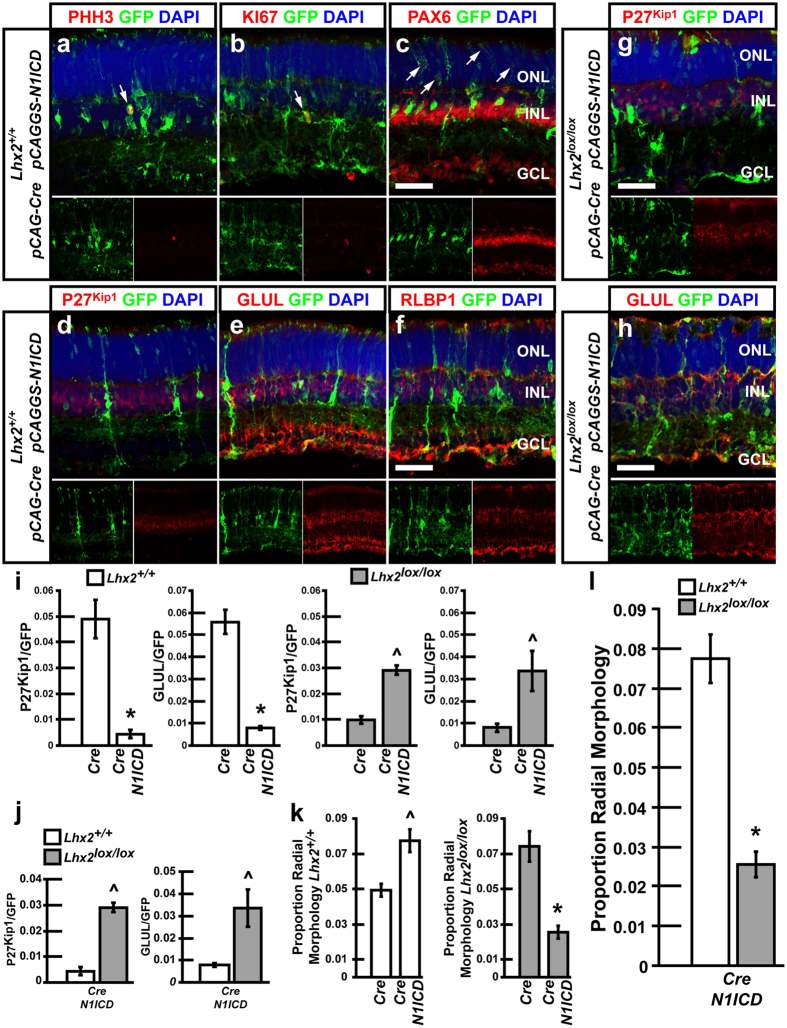
We electroporated neonatal mouse retinas with control pCAG-Cre/pCALNL-GFP (Cre) or pCAGGS-N1ICD/pCAG-Cre/pCALNL-GFP (N1ICD/Cre) constructs and analyzed the electroporated retinas at postnatal day (P)14. The pCAG-Cre plasmid constitutively expresses Cre recombinase, while pCALNL-GFP expresses the GFP fluorescent reporter following Cre mediated excision of a transcriptional stop site. The pCAGGS-N1ICD plasmid constitutively expresses the N1ICD domain. In wildtype (WT) retinas electroporated with Cre, we observed that approximately 5% of electroporated cells expressed the MG markers P27Kip1, GLUL, or displayed radial morphology characteristic of MG, where cell processes extended from the basal inner limiting membrane to the apical outer limiting membrane (4.9% P27Kip1 +ve; 5.6% GLUL +ve; 4.9% MG-like radial morphology) (Fig. 1a–f,i,k). Electroporation of N1ICD dramatically reduced the number of P27Kip1 and GLUL-expressing cells to 0.4 and 0.7% respectively (Fig. 1d,e,i). Furthermore, co-labeling with the MG marker RLBP1 was not detected (Fig. 1f). Conversely, the proportion of cells exhibiting radial morphology significantly increased to 7.7% (Fig. 1k).
Figure 1. Electroporation of N1ICD maintains radial RPCs and is sufficient to rescue loss of MG development resulting from Lhx2 loss of function.
(a–f) Lhx2+/+ retinas electroporated with Cre/GFP/N1ICD. (a–c) Fluorescent immunohistochemical labeling of electroporated retinas with GFP and the proliferation markers PHH3 and KI67, or the progenitor/amacrine/ganglion cell marker PAX6. Arrows indicate co-labeled cells. (d–f) fluorescent co-labeling with the MG markers P27Kip1, GLUL, and RLBP1. (g,h) Lhx2lox/lox retinas electroporated with Cre/GFP/N1ICD and analyzed by fluorescent immunohistochemical labeling with P27Kip1 and GLUL. (i,j) Quantification of GFP/P27Kip1 and GFP/GLUL co-labeled cells in Lhx2+/+ and Lhx2lox/lox mice following Cre/GFP or Cre/GFP/N1ICD. (k,l) Quantification of radial cells in Lhx2+/+ or Lhx2lox/lox mice following Cre/GFP or Cre/GFP/N1ICD electroporation. *Indicates significant decrease while ^ indicates significant increases (P < 0.05, N = 6 for marker counts, N = 12 for radial morphology counts). ONL, outer nuclear layer; INL inner nuclear layer; GCL, ganglion cell layer. Scale bars: 50 μm (c,f,g,h).
The generation of cells with radial morphology, but not MG marker expression, suggested that electroporation of N1ICD/Cre promoted the maintenance of undifferentiated radial RPCs at P14, validating previous reports36. We immunostained N1ICD/Cre electroporated retinas for two markers of actively proliferating cells, Phosphohistone H3 (PHH3) and KI67, as well as the RPC-expressed transcription factor PAX6. We found that subsets of electroporated cells were labeled with both PHH3 and KI67, whereas co-labeling was never observed in Cre controls (Fig. 1a,b). Furthermore, ectopic co-labeling of PAX6 was detected in the outer nuclear layer (ONL) of the retina, where PAX6 is not normally expressed at P14 (Fig. 1c).
We previously reported that Lhx2, which encodes a LIM homeodomain transcription factor, is necessary for MG development21. Lhx2 drives MG development in part by directly activating expression of Notch signaling pathway genes21. Here we electroporated Cre or N1ICD/Cre into Lhx2lox/lox retinas to determine whether N1ICD could rescue the loss of MG resulting from Lhx2 knockout. Electroporation of Cre into Lhx2lox/lox retinas resulted in a dramatically reduced proportion of P27Kip1 and GLUL-labeled MG, as reported previously (Fig. 1i)21. Interestingly, we observed an increase in the number of radial cells following electroporation of Cre into Lhx2lox/lox retinas (Fig. 1k). We previously showed that Lhx2 loss of function does not result in an increase of proliferating RPCs21. Electroporation of N1ICD/Cre into Lhx2lox/lox rescued the number of cells expressing both P27Kip1 and GLUL, while significantly reducing the number of cells featuring MG-like radial morphology (Fig. 1g–l). The proportion of cells expressing either MG markers or showing radial morphology was similar (P27Kip1 +ve 2.9%, GLUL +ve 3.4%, radial morphology 2.6%). The number of cells expressing MG markers was comparable to controls, where Cre was electroporated into WT retinas, though the number of radial cells remained statistically reduced (p = 0.05, N = 6 each condition, 12 total eyes, P27Kip1; P > 0.05, N = 6 each condition, 12 total eyes, GLUL; P < 0.05, N = 12 each condition, 24 total eyes, MG-like radial morphology) (Fig. 1i,k).
Nfia promotes incomplete MG development, and partially rescues MG differentiation following Lhx2 loss of function
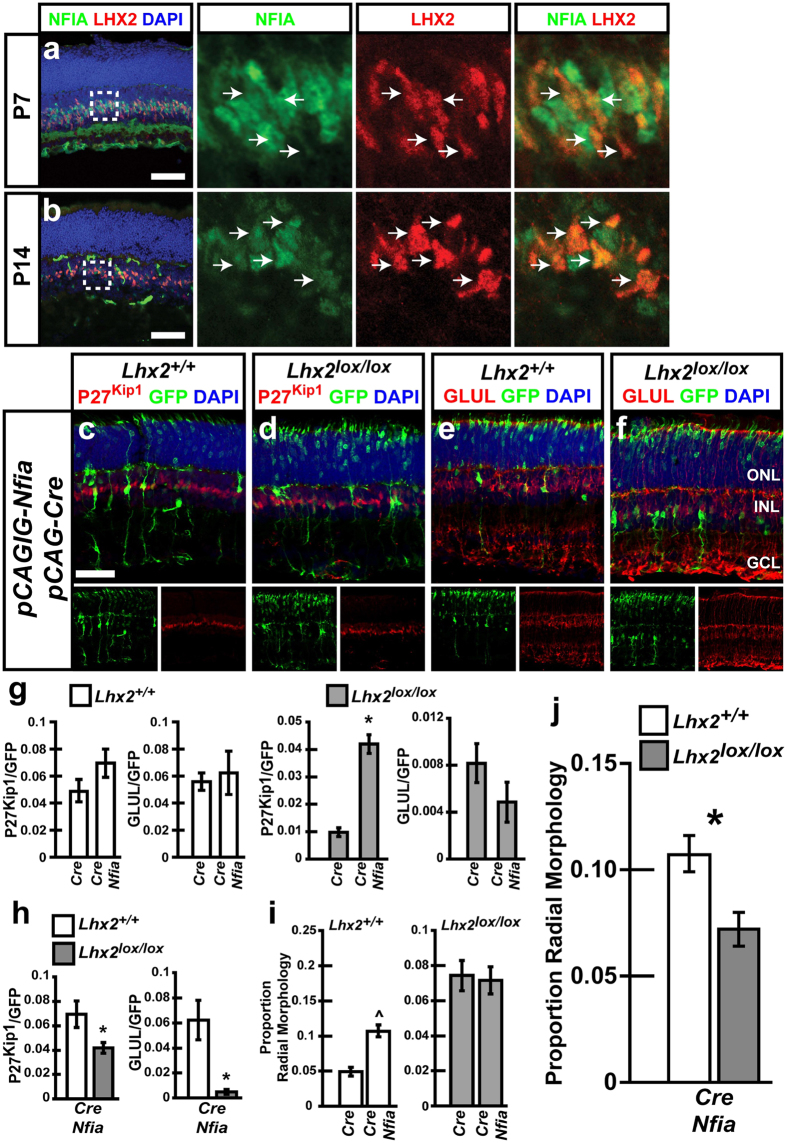
The retinal localization and function of Nfia has not previously been established. We utilized a monoclonal antibody specific for NFIA to assess retinal expression and localization. NFIA protein expression became restricted to the medial inner nuclear layer (INL) of the retina by P7 and co-localized with LHX2 in MG (Fig. 2a). NFIA +ve cells that did not co-express LHX2 were also detected at P7. At P14, NFIA co-localized with LHX2 in MG with relatively fewer NFIA +ve/LHX2 − ve cells present (Fig. 2b). We did not detect any LHX2 +ve cells that did not co-label with NFIA at either timepoint, indicating that all MG express NFIA.
Figure 2. NFIA is expressed in retinal MG and electroporation of Nfia promotes the formation of radial cells and is sufficient to rescue loss of P27Kip1 expression resulting from Lhx2 loss of function.
(a,b) Immunohistochemical co-labeling of NFIA with LHX2 at P7 and P14, arrows indicate co-labeled cells. (c–f) Electroporation of Lhx2+/+ and Lhx2lox/lox retinas with Cre/GFP/Nfia and analyzed by immunohistochemical co-labeling of GFP with the MG markers P27Kip1 and GLUL. (g,h) Quantification of GFP/P27Kip1 and GFP/GLUL co-labeled cells in Lhx2+/+ and Lhx2lox/lox mice following Cre/GFP or Cre/GFP/Nfia. (i,j) Quantification of radial cells in Lhx2+/+ or Lhx2lox/lox mice following Cre/GFP or Cre/GFP/Nfia electroporation. *Indicates significant decrease (P < 0.05, N = 6 for marker counts, N = 12 for radial morphology counts). Scale bars: 50 um (a–c).
We next electroporated pCAG-Nfia/pCAG-Cre/pCALNL-GFP (Nfia/Cre) into neonatal retinas, and assessed MG marker expression at P14 (Fig. 2c,e). The pCAG-Nfia plasmid constitutively expresses NFIA. Electroporation of Nfia/Cre did not significantly alter the proportion of P27Kip1 or GLUL expressing cells in the retina as compared to Cre in control experiments, though non-significant increases were observed for both markers (Fig. 2g). Electroporation of Nfia/Cre resulted in a significant increase in the proportion of MG-like radial cells, with twice as many detected compared to Cre controls (Fig. 2i). Together, these results suggest that misexpression of Nfia/Cre may be sufficient to promote morphological, but not molecular, characteristics of MG in WT retina.
Previous studies observed that endogenous expression of Lhx2 in hippocampal progenitor cells may override the gliogenic activity of Nfia, and that misexpression of Nfia with concurrent Lhx2 loss of function promoted hippocampal astrogliogenesis34. We tested whether Nfia was sufficient to promote MG development with Lhx2 loss of function by electroporating Nfia/Cre into Lhx2lox/lox mice. Nfia/Cre was sufficient to rescue the proportion of P27Kip1 +ve cells (Fig. 2g). The proportion of cells expressing GLUL was unchanged from that observed following electroporation of Cre into Lhx2lox/lox mice, and remained significantly reduced compared to wild type animals (Fig. 2g). The proportion of MG-like radial cells seen in Lhx2lox/lox mice electroporated with Nfia/Cre was unchanged from Lhx2lox/lox mice electroporated with Cre (Fig. 2i). Furthermore, the proportion of radial cells generated in Lhx2lox/lox retinas represented a significant decrease from levels seen following electroporation of Nfia/Cre into wild type animals (Fig. 2j).
Rax and Plagl1 are not sufficient to promote MG development, but Plagl1 partially rescues MG development following Lhx2 loss of function
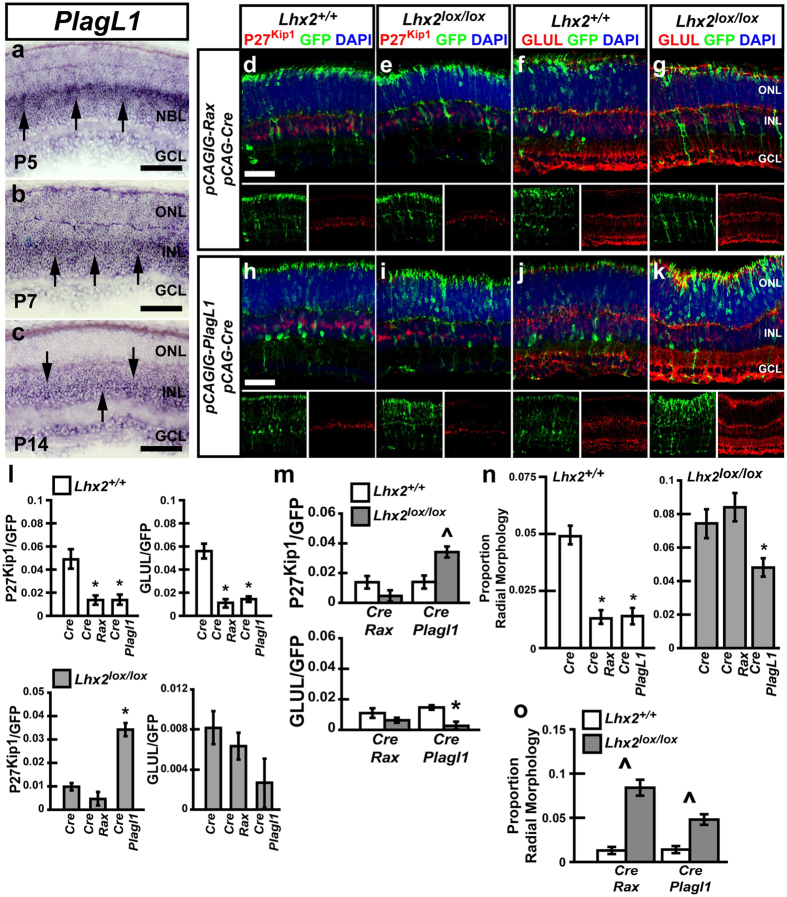
The retinal expression pattern of Plagl1 in the mouse has not been reported. We performed in situ hybridization to detect Plagl1 RNA expression in the retina, and found expression consistent with MG localization (Fig. 3a–c). RNA expression was identified in the medial neuroblastic layer (NBL) at P5 (Fig. 3a), consistent with the location of differentiating MG. Plagl1 was similarly enriched in the medial INL, where MG are located at P7 (Fig. 3b). By P14, Plagl1 expression is less clearly concentrated in the medial INL, although expression remains generally enriched in the INL (Fig. 3c). Cumulatively, these results indicate that Plagl1 expression is enriched in developing MG.
Figure 3. Electroporation of Rax both blocks MG development and fails to rescue the loss of MG resulting from Lhx2 loss of function, whereas Plagl1 blocks MG development but is sufficient to rescue P27Kip1 expression resulting from Lhx2 loss of function.
(a–c) In situ hybridization of Plagl1 at P5, P7, and P14. Arrows show regions of Plagl1 RNA enrichment. (d–g) Electroporation of Lhx2+/+ and Lhx2lox/lox retinas with Cre/GFP/Rax and analyzed by immunohistochemical co-labeling of GFP with the MG markers P27Kip1 and GLUL. (h–k) Electroporation of Lhx2+/+ and Lhx2lox/lox retinas with Cre/GFP/Plagl1 and analyzed by immunohistochemical co-labeling of GFP with P27Kip1 and GLUL. (l,m) Quantification of GFP/P27Kip1 and GFP/GLUL co-labeled cells in Lhx2+/+ and Lhx2lox/lox mice following Cre/GFP, Cre/GFP/Rax or Cre/GFP/PlagL1 electroporation. (n,o) Quantification of radial cells in Lhx2+/+ or Lhx2lox/lox mice following Cre/GFP, Cre/GFP/Rax or Cre/GFP/PlagL1 electroporation. *Indicates significant decrease while ^ indicates significant increases (P < 0.05, N = 6 for marker counts, N = 12 for radial morphology counts). NBL, neuroblastic layer. Scale bars: 100 μm (a–c), 50 μm (d,h).
To determine whether Rax or Plagl1 were sufficient promote MG development we electroporated neonatal mice with either pCAG-Rax/pCAG-Cre/pCALNL-GFP (Rax/Cre) or pCAG-Plagl1/pCAG-Cre/pCALNL-GFP (Plagl1/Cre), and assayed for MG formation. The pCAG-Rax plasmid constitutively expresses RAX, while pCAG-Plagl1 constitutively expresses PLAGL1. Contradicting previous reports, misexpression of Rax into WT mice resulted in significant decreases in expression of the MG markers P27 Kip1 and GLUL, as well as decreases in MG-like radial cells (Fig. 3d,f,l,n). Electroporation of Rax/Cre also notably altered the position of rod photoreceptors, with photoreceptor soma concentrating near the apical ONL adjacent to the retinal outer limiting membrane, instead of positioning randomly throughout the ONL (Fig. 3d,f). Similarly, WT retinas electroporated with Plagl1/Cre showed significantly reduced expression of both MG markers (Fig. 3h,j,l), as well as reduced numbers of MG-like radial cells (Fig. 3n).
Though neither factor proved sufficient to promote MG development, we tested whether either Rax/Cre or Plagl1/Cre could rescue the loss of MG development seen following Lhx2 loss of function. Electroporation of Rax/Cre into neonatal Lhx2lox/lox retinas resulted in no significant change in the number of cells expressing either P27 Kip1 or GLUL (Fig. 3e,g,l) as compared to Cre controls. Interestingly, the proportion of MG generated following electroporation of Rax/Cre into WT and Lhx2lox/lox retinas does not significantly differ, indicating that misexpression of Rax blocked MG development to a similar extent as loss of Lhx2 function. The number of cells featuring MG-like radial morphology was also unchanged following electroporation of Rax/Cre into Lhx2lox/lox mice compared to electroporation of Cre.
Electroporation of Plagl1/Cre into Lhx2lox/lox retinas, however, resulted in a significant rescue of expression of P27 Kip1 but not GLUL (Fig. 3i,k,l). Despite increased P27 Kip1 expression, the number of MG-like radial cells generated was reduced compared to Lhx2lox/lox retinas electroporated with Cre (Fig. 3n). Cumulatively, our results indicate that neither Rax nor Plagl1 is sufficient to promote MG development in the mouse retina. Plagl1 however may be functionally redundant for Lhx2 in the activation of P27Kip1.
Sry-related HMG-box (Sox) family members Sox2, Sox8, Sox9 are insufficient to promote MG development, but drive amacrine cell differentiation in the absence of Lhx2
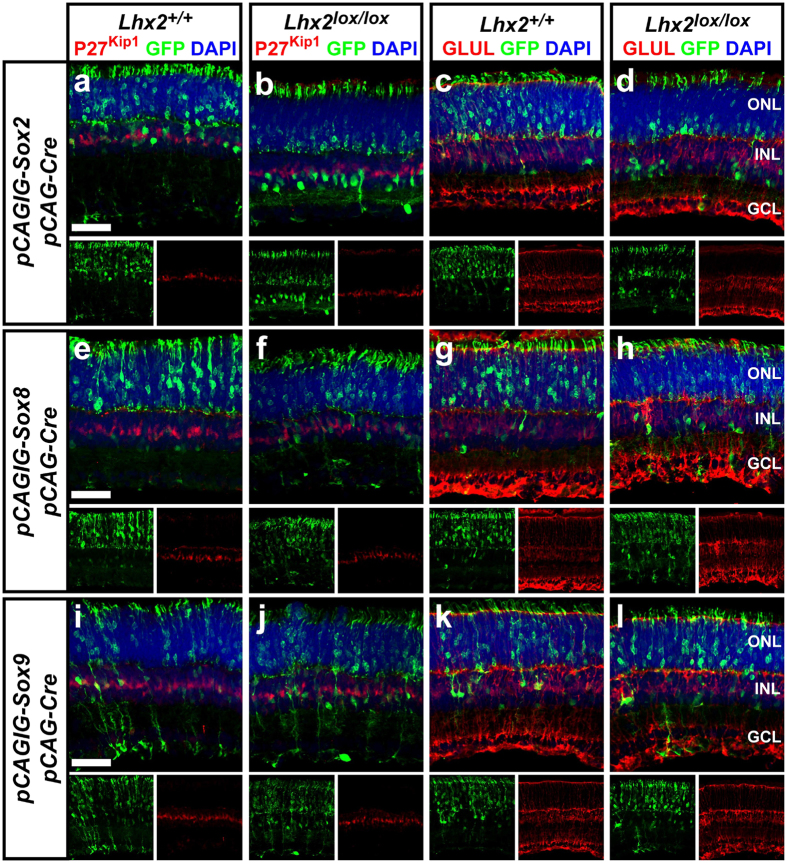
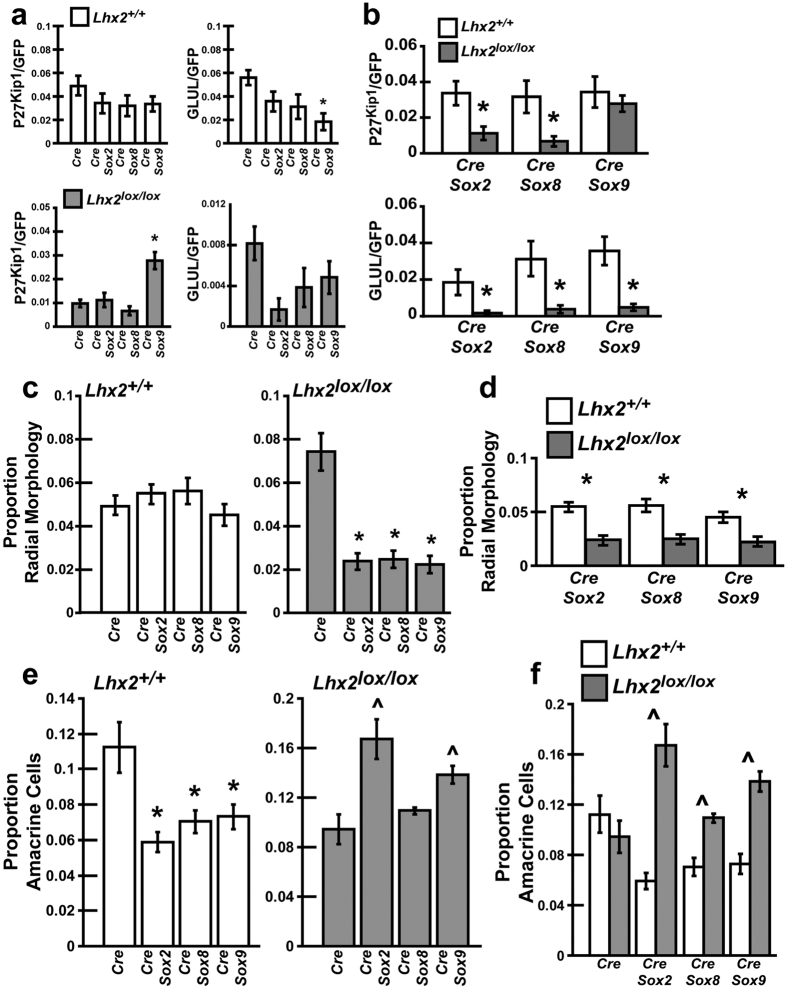
To test the sufficiency of Sox2, Sox8, and Sox9 to promote MG development we electroporated neonatal WT retinas with pCAG-Sox2/pCAG-Cre/pCALNL-GFP, pCAG-Sox8/pCAG-Cre/pCALNL-GFP, or pCAG-Sox9/pCAG-Cre/pCALNL-GFP, herein referred to as Sox2/Cre, Sox8/Cre, or Sox9/Cre, and assayed for MG. The pCAG-Sox2, -Sox8, -Sox9 plasmids constitutively expresses SOX2, SOX8, and SOX9 respectively. Electroporation of Sox2/Cre or Sox8/Cre did not result in significant changes in the number of MG as determined by marker expression or MG-like radial morphology at P14 (Figs 4a,c,e,g and 5a,c). Electroporation of Sox9/Cre also did not affect expression of P27Kip1 or the proportion of radial cells, but did result in significantly decreased GLUL expression (Figs 4i,k and 5a,c).
Figure 4. Electroporation of Sox2, Sox8 and Sox9 in the mouse retina.
(a–d) Electroporation of Lhx2+/+ and Lhx2lox/lox retinas with Cre/GFP/Sox2 and analyzed by immunohistochemical co-labeling of GFP with the MG markers P27Kip1 and GLUL. (e–h) Electroporation of Lhx2+/+ and Lhx2lox/lox retinas with Cre/GFP/Sox8 and analyzed by immunohistochemical co-labeling of GFP with the MG markers P27Kip1 and GLUL. (i–j) Electroporation of Lhx2+/+ and Lhx2lox/lox retinas with Cre/GFP/Sox9 and analyzed by immunohistochemical co-labeling of GFP with the MG markers P27Kip1 and GLUL. Scale bars: 50 um (a,e,i).
Figure 5. Electroporation of Sox2, Sox8, or Sox9 are insufficient to promote MG development and largely fail to rescue the loss of MG resulting from Lhx2 loss of function, instead promoting amacrine cell development.
(a,b) Quantification of GFP/P27Kip1 and GFP/GLUL co-labeled cells in Lhx2+/+ and Lhx2lox/lox mice following Cre/GFP, Cre/GFP/Sox2, Cre/GFP/Sox8, or Cre/GFP/Sox9 electroporation. (c,d) Quantification of radial cells in Lhx2+/+ or Lhx2lox/lox mice following Cre/GFP, Cre/GFP/Sox2, Cre/GFP/Sox8, or Cre/GFP/Sox9 electroporation. (e,f) Quantification of amacrine cells by morphology in Lhx2+/+ or Lhx2lox/lox mice following Cre/GFP, Cre/GFP/Sox2, Cre/GFP/Sox8, or Cre/GFP/Sox9 electroporation. *Indicates significant decrease while ^ indicates significant increases (P < 0.05, N = 6 for marker counts and amacrine cell counts, N = 12 for radial morphology counts).
We next tested whether electroporation of Sox genes could rescue the loss of MG resulting from Lhx2 loss of function. We electroporated Lhx2lox/lox mice with Sox2/Cre, Sox8/Cre, or Sox9/Cre and quantified the numbers of MG generated at P14. Sox2 and Sox8 were insufficient to rescue MG marker expression, with the proportion of P27Kip1 or GLUL expressing cells identical to that of Lhx2lox/lox animals electroporated with Cre (Figs 4b,d,f,h and 5a,b). Sox9 did rescue P27Kip1 expression, but not GLUL expression (Figs 4j,l and 5a,b). The proportion of P27Kip1 labeled cells was not significantly different from that of WT mice electroporated with Cre or Cre/Sox9. Interestingly, the number of MG-like radial cells was significantly reduced following electroporation of Cre/Sox2, Cre/Sox8, and Cre/Sox9 into Lhx2lox/lox animals, compared with Lhx2lox/lox animals electroporated with Cre (Figs 4b,d,f,h,j,l and 5c). The number of MG-like radial cells generated was also significantly reduced compared to WT mice electroporated with the respective Sox genes (Fig. 5d).
The loss of radial cells in Lhx2lox/lox mice was coupled with an increase of cells localized in the basal INL, consistent with amacrine cells. To characterize the role of Sox genes in the regulation of amacrine cell development we electroporated WT and Lhx2lox/lox mice and scored the number of amacrine cells generated. Electroporation of Sox2/Cre, Sox8/Cre, or Sox9/Cre in WT mice yielded significantly decreased numbers of amacrine cells as compared to Cre controls (Fig. 5e). Conversely, electroporation of Sox2/Cre and Sox9/Cre but not Sox8/Cre into Lhx2lox/lox animals resulted in a notable increase in amacrine cells, compared to Cre electroporated controls (Fig. 5e). Sox2/Cre, Sox8/Cre, and Sox9/Cre electroporations all generated significantly more amacrine cells in Lhx2lox/lox than WT animals (Fig. 5f). Cumulatively, these results suggest that Sox2, Sox8, and Sox9 are insufficient to promote MG development, but all strongly promote amacrine cell development in the absence of Lhx2 expression.
Discussion
This study offers a reevaluation of the phenotypes seen following overexpression of retinal transcriptional regulators that have been previously implicated in MG development. Our observations corroborate many previously observed phenotypes, while qualifying and adding context to others. Overexpression of individual glial-enriched TFs had varying effects on MG generation and differentiation, and were likewise differentially regulated by Lhx2. These results are summarized in Table 1.
Table 1. Differential regulation of MG differentiation following overexpression of individual transcriptional regulators.
| Gene | Wildtype |
Lhx2-deficient |
||||
|---|---|---|---|---|---|---|
| Radial | P27Kip1 | GLUL | Radial | P27Kip1 | GLUL | |
| Hes5 | + | + | + | 0 | 0 | 0 |
| N1ICD | + | − | − | − | + | + |
| Nfia | + | 0 | 0 | 0 | + | 0 |
| Rax | − | − | − | 0 | 0 | 0 |
| Plagl1 | − | − | − | − | + | 0 |
| Sox2 | 0 | 0 | 0 | − | 0 | 0 |
| Sox8 | 0 | 0 | 0 | − | 0 | 0 |
| Sox9 | 0 | 0 | − | − | + | 0 |
Changes in the fraction of radial cells and both P27Kip1 and GLUL-positive following overexpression of the indicated factor are shown in both wildtype and Lhx2-deficient backgrounds. Increases (+), decreases (−), or unchanged (0) fractions of cells are relative to Cre/GFP controls in the indicated genetic background. Hes5 data for P27Kip1 and GLUL is from ref. 21.
In general, overexpression of these factors in a WT background did not promote MG formation. Only N1ICD and Nfia overexpression lead to an increase in cells with MG-like radial morphology, but failed to induce expression of either P27Kip1 or GLUL, with N1ICD reducing expression of both markers. Indeed, Rax and Plagl1 both significantly decreased the fraction of cells showing MG-like radial morphology and expressing MG markers. These results stand in sharp contrast to the potently gliogenic Notch target Hes5, which robustly drives expression of both markers21. Electroporation of PlagL1, Sox2, Sox8, and Sox9 had no affect on the population density (cells/μm2) of surviving electroporated cells at P14 (Fig. S1). Electroporation of N1ICD and Nfia resulted in a general reduction of the number of electroporated cells/μm2 while Rax resulted in an increase in cells/μm2 (Fig. S1). Overexpression of several different individual factors in Lhx2-deficient cells lead to rescued expression of P27Kip1, but only N1ICD was able to rescue expression of GLUL. Finally, we uncover a previously unknown role for Lhx2 in suppressing Sox-dependent formation of amacrine cells. These findings highlight the complex combinatorial molecular relationships controlling MG differentiation.
In the specific case of N1ICD, constitutive Notch signaling induced electroporated cells to adopt a RPC-like state, with increased numbers of proliferative radial cells displaying decreased MG marker expression. This stands in sharp contrast to overexpression of the Notch pathway effector Hes5, which induced dramatic increases in the fraction of cells with MG-like radial morphology (Cre = 0.049 ± .0011; Hes5/Cre = 0.182 ± .0040, n = 12.), as well as P27Kip1 and GLUL labeling21. Ectopic expression of the RPC marker PAX6, and the proliferation markers KI67 and PHH3, was observed following N1ICD electroporation. These results fit well with previous reports that indicate that ectopic activation of Notch in the retina promotes progenitor maintenance and blocks cell cycle exit and terminal differentiation36,37.
We have previously demonstrated that Lhx2 function was required for Müller gliogenesis, and that MG development was disrupted in Lhx2 knockouts21. Lhx2 directly activates expression of Notch pathway genes in RPCs, consequently conditional inactivation of Lhx2 results in rapid down-regulation of Notch signaling. Whether Lhx2 mediated its effects on Müller development through direct regulation of MG gene expression, or indirectly via regulation of Notch pathway gene expression, was unclear. Interestingly, Lhx2 also regulates RPC progression through successive competence states38. Loss of function of Lhx2 leads to cell cycle exit, resulting in progenitor depletion and restricted neurogenesis, effects similar to loss of Notch function37,38,39. These studies suggest that Lhx2 controls MG differentiation in large part by regulating Notch pathway activation. Indeed, rapid RPC dropout coupled with failed gliogenesis may account for the increased number of radial cells which lack expression of MG markers following Lhx2 loss of function in this study.
Here, we demonstrate that misexpression of N1ICD was sufficient to rescue the effects of Lhx2 loss of function. Expression of both P27Kip1 and GLUL were restored, although the proportion of cells expressing both remained slightly reduced compared to WT controls. The fraction of cells with MG-like radial morphology was also reduced, indicating N1ICD may rescue RPC dropout resulting from Lhx2 loss of function. These data indicate that Lhx2 promotes Müller gliogenesis largely through regulation of the Notch signaling pathway, and is at least partially dispensable for regulation of MG gene expression. Similar observations have been made regarding the role of Sox2 in MG development, wherein ectopic activation of Notch signaling was sufficient to rescue MG development in Sox2 knockouts40. Interestingly, we previously reported that overexpression of Hes5, a potently Müller gliogenic Notch transcriptional effector, was insufficient to rescue the effects of Lhx2 loss of function, with the fraction of cells expressing P27Kip1, GLUL21 or displaying radial morphology (Cre/Lhx2lox/lox = 0.074 ± .0029; Hes5/Cre/Lhx2lox/lox = 0.087 ± .0026, n = 12), unchanged. Rescue of MG differentiation with N1ICD but not Hes5 in Lhx2-deficient cells suggests that concurrent activation of multiple Notch pathway target genes is required for MG differentiation, and that the gliogenic function of individual Notch pathway effectors such as Hes5 is instructive only in the context of functional Notch signaling.
Nfia, which strongly promotes astrogliogenesis elsewhere in the CNS, showed mixed effects on generation of MG, despite its strong and selective expression in late-stage RPCs and mature MG. Though electroporation of Nfia triggered a significant increase in the fraction of MG-like radial cells in WT retina, with twice the fraction of electroporated cells showing clear radial morphology, it did not induce any significant change in MG marker expression. Furthermore, no effect on the fraction of cells with MG-like radial morphology was seen following electroporation of Nfia/Cre into Lhx2lox/lox mice. Despite not activating MG marker expression in WT mice, Nfia overexpression was sufficient to rescue P27Kip1 expression in Lhx2 knockouts. The gliogenenic effects of Nfia have previously been shown to be Lhx2-dependent in the hippocampus, where Nfia misexpression blocks neurogenesis and promotes astrogliogenesis34. We see a similar phenomenon, wherein electroporation of Nfia into WT mice seemingly blocks neurogenesis in the retina, resulting in a proportional increase of MG-like radial cells. This increase in radial cells was similar to that seen following electroporation of N1ICD, as was the rescue of P27Kip1 expression in the Lhx2 knockouts. Nfia can function as a downstream transcriptional effector of the Notch pathway41, and our data suggests that this remains true of Nfia in the retina. Rescue of expression of the Cdk inhibitor P27Kip1 indicates that Nfia may play a key role in regulating MG quiescence, a known role of Notch in MG42, and we speculate that Notch may regulate MG quiescence in part through Nfia.
We found that electroporation of both Rax and Plagl1 potently inhibited Müller gliogenesis. Previous studies reported that retroviral transduction of neonatal rat retinas with Rax resulted in 90% of transduced cells displaying radial morphology and expressing MG markers such as CRALBP16, while injection of Plagl1 into Xenopus embryos resulted in a 4-fold increase in MG30. In the case of Plagl1, the divergence in phenotypes observed may simply reflect evolutionary differences between amphibians and mammals in the transcriptional circuitry controlling gliogenesis. However, we did observe that electroporation of Plagl1/Cre in Lhx2lox/lox mice did partially rescue P27Kip1 expression, though the proportion of P27Kip1expressing cells remained notably lower than that in WT controls. Plagl1 may therefore contribute to MG quiescence in the mammalian retina.
While electroporation of Rax potently inhibited gliogenesis, it also led to observable photoreceptor phenotypes, where photoreceptor cell nuclei were shifted to the outer region of the outer nuclear layer. That Rax electroporation affects photoreceptor development is consistent with a previously demonstrated role of Rax in later stages of photoreceptor differentiation43. The complete lack of gliogenic effects resulting from Rax electroporation may also be due to high levels of Rax expression induced by electroporation of CAG-based expression plasmids in this study44,45, compared to the relatively weak retroviral promoters used to drive Rax expression in previous reports16. RAX competes directly with the Notch effectors HES1, HES5, and HEY1 for binding of the embryonic enhancer locus for photoreceptor Otx2 transcription (EELPOT), with RAX activating Otx2 and promoting photoreceptor specification via EELPOT while HES1, HES5, and HEY1 repress Otx246. The blockade of MG development in our study may thus result from constitutive activation of Otx2 expression following Rax electroporation.
One of the more surprising findings of this study was the general failure of Sox family transcription factors to promote MG development. Sox2, Sox8, and Sox9 have all been shown to be required for the development of MG in the mammalian retina28,29. Previous work also indicated that Sox8 and Sox9 loss of function resulted in compensatory increases in the fraction of photoreceptors, complementing the reduction of MG29. That study did not observe any increase in the fraction of MG following overexpression of Sox8 and Sox9 in late embryonic RPCs. We also found that electroporation of Sox family members failed to promote Müller gliogenesis, and largely failed to rescue MG formation following Lhx2 loss of function, although Sox9 did restore P27Kip1 expression. Cumulatively, our data indicates that while Sox2, Sox8 and Sox9 may be necessary for MG development, none appears sufficient for retinal gliogenesis.
Interestingly, we found that Lhx2 seemed to constrain the ability of Sox2, Sox8 and Sox9 to drive amacrine cell differentiation. Sox2-dependent promotion of amacrine specification was previously reported in E17 explants following retroviral overexpression28. Sox2 has also been shown to be necessary for differentiation of individual AC subtypes47. We found that electroporation of Sox2/Cre, Sox8/Cre, and Sox9/Cre into Lhx2lox/lox mice resulted in a substantially increased proportion of amacrine cells compared with electroporation of WT mice. Electroporation of Sox2/Cre and Sox9/Cre both significantly boosted amacrine cell numbers in Lhx2lox/lox mice compared to electroporation of Cre alone. This suggests that in addition to being necessary for retinal gliogenesis, Lhx2 may also play a more general role in modulating and constraining levels of neurogenesis in late-stage RPCs, as has been described in the cortex and hippocampus34,48,49. Whether this modulation is achieved indirectly through regulation of Notch or directly through competition and interaction at common target loci is unclear. Ectopic activation of Notch via misexpression of N1ICD rescued gliogenesis in Sox2 knockouts40, and similarly rescued gliogenesis resulting from Lhx2 loss of function in this study. These observations indicate that Notch pathway regulation by Lhx2 and Sox family members contributes significantly to controlling the balance of amacrine cell and MG generation.
Methods
Animals
Timed pregnant female CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and pups of either sex were used for electroporation. Lhx2lox/lox mice (obtained from Dr. Edwin Monuki, University of California, Irvine) have been described50. Timed pregnancies of Lhx2lox/lox mice were initiated and pups of either sex were used for electroporation. All procedures were approved by and carried out in accordance with guidelines approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine, in accordance with NIH guidelines (protocol #MO16M62).
Cell counts
All counts were performed blindly on whole retinal sections as previously described51. The total number of cells counted for each condition is presented in Table S1. Differences between two means were assessed by unpaired two-tailed Student’s t-test. Radial MG morphology was scored by tracing GFP labeling of individual cells from the inner limiting membrane through the inner plexiform and nuclear layers of the retina, past the outer plexiform layer and into the outer nuclear layer. Amacrine cells were scored based on morphology visualized by GFP labeling using the following criteria: cell soma positioned in the inner (basal) inner nuclear layer, dendrite extension into the inner plexiform layer but not extending beyond the retinal ganglion cell layer, and absence of an apical process extending to the outer plexiform layer. The number of electroporated cells/μm2 was determined for each condition in WT animals by counting GFP labeled cells and dividing by the area of the confocal imaging field (49506.25 μm2).
Electroporation
Electroporation of neonatal mice of either sex was performed at P0 as previously described52. Electroporated retinas were harvested at P14. DNA constructs used for electroporation in this study are as follows: pCAG-Cre (Addgene plasmid 13775, deposited by C. Cepko53), pCALNL-GFP (Addgene plasmid 13770, deposited by C. Cepko53), pCAGIG-Nfia (Gateway cloned from Ultimate Human ORF Collection (Life Technologies)), pCAGIG-Plagl1 (Gateway cloned from Ultimate Human ORF Collection (Life Technologies)), pCAGIG-Rax (Gateway cloned from Ultimate Human ORF Collection (Life Technologies)), pCAGIG-Sox2 (Gateway cloned from Ultimate Human ORF Collection (Life Technologies)), pCAGIG-Sox8 (Gateway cloned from Ultimate Human ORF Collection (Life Technologies)), pCAGIG-Sox9 (Gateway cloned from Ultimate Human ORF Collection (Life Technologies)), pCAGGS-N1ICD (Addgene plasmid 26891, deposited by N. Gaiano54). All plasmids activate expression of their respective genes of interest, Cre recombinase, or GFP from the broadly active CAG promoter/enhancer.
Immunohistochemistry
Fluorescent immunohistochemistry was performed on cryosectioned tissue as previously described51. Antibodies utilized for fluorescent immunohistochemistry are as follows: goat anti-GFP (1:500; Rockland Immunochemicals, Limerick, PA), rabbit anti-GFP (1:1000; Invitrogen, Waltham, MA), mouse anti-Glutamine synthase (GLUL) (1:200; BD Biosciences, San Jose, CA), mouse anti-KI67 (1:200; BD Biosciences, San Jose, CA), rabbit anti-LHX2 (1:1500; generated in house with Covance, Princeton, NJ), mouse anti-NFIA (1:200; CDI Laboratories), mouse anti-P27Kip1 (1:200; Invitrogen), mouse anti-PAX6 (1:200; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), rabbit anti-phosphohistone H3 (PHH3) (1:200; Millipore, Billerica, MA). Secondary antibodies used were as follows: AlexaFluor488 conjugated donkey anti-goat IgG (1:500; Jackson Immunoresearch, West Grove, PA), AlexaFluor488 conjugated donkey anti-rabbit IgG (1:500; Jackson Immunoresearch, West Grove, PA), AlexaFluor594 conjugated donkey anti-rabbit IgG (1:500; Jackson Immunoresearch, West Grove, PA), AlexaFluor594 conjugated donkey anti-mouse IgG (1:500; Jackson Immunoresearch, West Grove, PA). All section immunohistochemical data shown was imaged and photographed on a Zeiss Meta 510 LSM confocal microscope.
In Situ Hybridization
Single color in situ hybridization was performed as previously described31. The RNA probe for Plagl1 was generated from the following EST sequence: GenBank accession number BE995637.
Additional Information
How to cite this article: de Melo, J. et al. Multiple intrinsic factors act in concert with Lhx2 to direct retinal gliogenesis. Sci. Rep. 6, 32757; doi: 10.1038/srep32757 (2016).
Supplementary Material
Acknowledgments
This works was supported by NIH R01EY020560 and R01EY017015 (to S.B), a grant from the Knights Templar Pediatric Ophthalmology Foundation (J.d.M), and NIH F32EY024201 (to B.S.C). S.B. was a W.M. Keck Distinguished Young Scholar in Medical Research.
Footnotes
Author Contributions J.d.M. and S.B. designed the experiments. J.d.M. and B.S.C. performed the experiments and J.d.M. reviewed and prepared the figures. J.d.M. and S.B. wrote the main manuscript text and all authors reviewed the manuscript.
References
- Turner D. L. & Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature 328, 131–136 (1987). [DOI] [PubMed] [Google Scholar]
- Magalhaes M. M. & Coimbra A. The rabbit retina Muller cell. A fine structural and cytochemical study. J. Ultrastruct. Res. 39, 310–326 (1972). [DOI] [PubMed] [Google Scholar]
- Reichenbach A. & Bringmann A. New functions of Muller cells. Glia 61, 651–678 (2013). [DOI] [PubMed] [Google Scholar]
- Goldman D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 15, 431–442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K. et al. The transcriptome of retinal Muller glial cells. J. Comp. Neurol. 509, 225–238 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav A. P., Roesch K. & Cepko C. L. Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog. Retin. Eye Res. 28, 249–262 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett B. V. & Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 26, 6303–6313 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos R. L., Barthel L. K., Meyers J. R. & Raymond P. A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 27, 7028–7040 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel S. M., Montgomery J. E., Burket C. T. & Hyde D. R. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 27, 1712–1724 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassen S. C. et al. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev. Neurobiol. 67, 1009–1031 (2007). [DOI] [PubMed] [Google Scholar]
- Ooto S. et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 101, 13654–13659 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F. et al. Wnt signaling promotes regeneration in the retina of adult mammals. J. Neurosci. 27, 4210–4219 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl M. O. et al. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 105, 19508–19513 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J. et al. Preferential regeneration of photoreceptor from Muller glia after retinal degeneration in adult rat. Vision Res. 48, 223–234 (2008). [DOI] [PubMed] [Google Scholar]
- Del Debbio C. B. et al. Notch and Wnt signaling mediated rod photoreceptor regeneration by Muller cells in adult mammalian retina. Plos One 5, e12425 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Mukherjee S., Bao Z. Z., Morrow E. M. & Cepko C. L. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron 26, 383–394 (2000). [DOI] [PubMed] [Google Scholar]
- Lee H. Y. et al. Multiple requirements for Hes 1 during early eye formation. Dev. Biol. 284, 464–478 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa R. & Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 1192, 90–98 (2008). [DOI] [PubMed] [Google Scholar]
- Wall D. S. et al. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J. Cell Biol. 184, 101–112 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. J. et al. Lhx2 balances progenitor maintenance with neurogenic output and promotes competence state progression in the developing retina. J. Neurosci. 33, 12197–12207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J. et al. Lhx2 Is an Essential Factor for Retinal Gliogenesis and Notch Signaling. J. Neurosci. 36, 2391–2405 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D. & Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc. Natl. Acad. Sci. USA 84, 156–160 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R. & Fraser S. E. Multipotent precursors can give rise to all major cell types of the frog retina. Science 239, 1142–1145 (1988). [DOI] [PubMed] [Google Scholar]
- Turner D. L., Snyder E. Y. & Cepko C. L. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4, 833–845 (1990). [DOI] [PubMed] [Google Scholar]
- Riepe R. E. & Norenburg M. D. Muller cell localisation of glutamine synthetase in rat retina. Nature 268, 654–655 (1977). [DOI] [PubMed] [Google Scholar]
- Levine E. M., Close J., Fero M., Ostrovsky A. & Reh T. A. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev. Biol. 219, 299–314 (2000). [DOI] [PubMed] [Google Scholar]
- Sheedlo H. J., Jaynes D., Bolan A. L. & Turner J. E. Mullerian glia in dystrophic rodent retinas: an immunocytochemical analysis. Brain Res. Dev. Brain Res. 85, 171–180 (1995). [DOI] [PubMed] [Google Scholar]
- Lin Y. P., Ouchi Y., Satoh S. & Watanabe S. Sox2 plays a role in the induction of amacrine and Muller glial cells in mouse retinal progenitor cells. Invest. Ophthalmol. Vis. Sci. 50, 68–74 (2009). [DOI] [PubMed] [Google Scholar]
- Muto A., Iida A., Satoh S. & Watanabe S. The group E Sox genes Sox8 and Sox9 are regulated by Notch signaling and are required for Muller glial cell development in mouse retina. Exp. Eye Res. 89, 549–558 (2009). [DOI] [PubMed] [Google Scholar]
- Ma L., Hocking J. C., Hehr C. L., Schuurmans C. & McFarlane S. Zac1 promotes a Muller glial cell fate and interferes with retinal ganglion cell differentiation in Xenopus retina. Dev. Dyn. 236, 192–202 (2007). [DOI] [PubMed] [Google Scholar]
- Blackshaw S. et al. Genomic analysis of mouse retinal development. Plos Biol 2, E247 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneen B. et al. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52, 953–968 (2006). [DOI] [PubMed] [Google Scholar]
- Kang P. et al. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74, 79–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian L. et al. Transcription factor Lhx2 is necessary and sufficient to suppress astrogliogenesis and promote neurogenesis in the developing hippocampus. Proc. Natl. Acad. Sci. USA 108, E265–E274 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierfelice T., Alberi L. & Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69, 840–855 (2011). [DOI] [PubMed] [Google Scholar]
- Bernardos R. L., Lentz S. I., Wolfe M. S. & Raymond P. A. Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina. Dev. Biol. 278, 381–395 (2005). [DOI] [PubMed] [Google Scholar]
- Jadhav A. P., Cho S. H. & Cepko C. L. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc. Natl. Acad. Sci. USA 103, 18998–19003 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. J. et al. Lhx2 balances progenitor maintenance with neurogenic output and promotes competence state progression in the developing retina. J. Neurosci. 33, 12197–12207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C. P., Feldman D. E., Ida Jr J. A. & Cepko C. L. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development 121, 3637–3650 (1995). [DOI] [PubMed] [Google Scholar]
- Surzenko N., Crowl T., Bachleda A., Langer L. & Pevny L. SOX2 maintains the quiescent progenitor cell state of postnatal retinal Muller glia. Development 140, 1445–1456 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. et al. NFIA controls telencephalic progenitor cell differentiation through repression of the Notch effector Hes1. J. Neurosci. 30, 9127–9139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner C., Ackerman K. M., Lahne M., Hobgood J. S. & Hyde D. R. Repressing notch signaling and expressing TNFalpha are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J. Neurosci. 34, 14403–14419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie S. et al. Rax Homeoprotein Regulates Photoreceptor Cell Maturation and Survival in Association with Crx in the Postnatal Mouse Retina. Mol. Cell. Biol. 35, 2583–2596 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Q. et al. Evaluation of three different promoters driving gene expression in developing chicken embryo by using in vivo electroporation. Genet. Mol. Res. 13, 1270–1277 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Y., Fu S., Niu R., Yang C. & Lin J. Transcriptional activity assessment of three different promoters for mouse in utero electroporation system. Plasmid 74, 52–58 (2014). [DOI] [PubMed] [Google Scholar]
- Muranishi Y. et al. An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. J. Neurosci. 31, 16792–16807 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney I. E. et al. Sox2 regulates cholinergic amacrine cell positioning and dendritic stratification in the retina. J. Neurosci. 34, 10109–10121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. J. & O’Leary D. D. Role for Lhx2 in corticogenesis through regulation of progenitor differentiation. Mol. Cell. Neurosci. 56, 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. C. et al. Lhx2 regulates the timing of beta-catenin-dependent cortical neurogenesis. Proc. Natl. Acad. Sci. USA 112, 12199–12204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangale V. S. et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science 319, 304–309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J. et al. Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2. Proc. Natl. Acad. Sci. USA 109, 4657–4662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J. & Blackshaw S. In vivo electroporation of developing mouse retina. J. Vis. Exp. (52). pii: 2847, doi: 10.3791/2847 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T. & Cepko C. L. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. USA 104, 1027–1032 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L., Yoon K., Wang M. & Gaiano N. Notch3 signaling promotes radial glial/progenitor character in the mammalian telencephalon. Dev. Neurosci. 28, 58–69 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.