Abstract
L-asparaginase is an important enzyme as therapeutic agents used in combination with other drugs in the treatment of acute lymphoblastic leukemia. A newly isolated actinomycetes strain, Streptomyces sp. NEAE-82, was potentially producing extracellular L-asparaginase, it was identified as Streptomyces fradiae NEAE-82, sequencing product was deposited in the GenBank database under accession number KJ467538. L-asparaginase was purified from the crude enzyme using ammonium sulfate precipitation, dialysis and ion exchange chromatography using DEAE Sepharose CL-6B. Further the kinetic studies of purified enzyme were carried out. The optimum pH, temperature and incubation time for maximum L-asparaginase activity were found to be 8.5, 40 °C and 30 min, respectively. The optimum substrate concentration was found to be 0.06 M. The Km and Vmax of the enzyme were 0.01007 M and 95.08 Uml−1min−1, respectively. The half-life time (T1/2) was 184.91 min at 50 °С, while being 179.53 min at 60 °С. The molecular weight of the subunits of L-asparaginase was found to be approximately 53 kDa by SDS–PAGE analysis. The purified L-asparaginase showed a final specific activity of 30.636 U/mg protein and was purified 3.338-fold. The present work for the first time reported more information in the production, purification and characterization of L-asparaginase produced by newly isolated actinomycetes Streptomyces fradiae NEAE-82.
L-asparaginase (L-asparagine aminohydrolase, E.C. 3.5.1.1) is the drug of choice used in combination therapy with other drugs in the treatment of acute lymphoblastic leukemia chemotherapy in children1. The demand for L-asparaginase will increase several fold in coming years due to its potential industrial applications as food processing aid in addition to its clinical applications2. L-asparaginase is highlighted as a key drug in the treatment of extranodal NK/T-cell lymphoma3.
Purification of a protein is an important step for characterization of its physical and biological properties. Moreover, for effective therapeutic use of a protein, it must be free of any contaminants and impurities. However, clinical employments of L-asparaginase are accompanied with fatal allergenic reactions to the patients4. These effects are mainly due to L-asparaginase associated L-glutaminase activity and bacterial endotoxins in enzyme preparations5. Several research groups have studied L-asparaginase production and purification in attempt to minimize impurities that produce allergenic reactions. The L-asparaginase enzyme was purified from Penicillium sp. that was grown on submerged fermentation. Different purification steps including salt precipitation, followed by separation on sephadex G-100-120 gel filtration and DEAE to obtain pure enzyme preparation. The purified enzyme showed 13.97 IU/mg specific activity. The polyacrylmide gel electrophoresis of the pure enzyme exhibited one protein of 66 kDa. The enzyme showed maximum activity at 7.0 pH and 37 °C and Km value 4 × 10−3 M6. Three marine soil isolates (S3, S4 and K8) synthesized asparaginase with yield ranging from 24.6 to 49.2 IU/ml. Isolate S3 showed the highest productivity of 49.2 IU/ml and optimum activity at pH 7.5 and 50 °C. The Km and Vmax of partially purified enzyme were approximately 24 μM and 51 IU/ml, respectively7.
Literature reports indicated that the enzyme’s biochemical and kinetic properties varies with the genetic nature of the microbial strain used, suggesting the need to search for other L-asparaginase sources8. Actinomycetes are a good source for the production of L-asparaginase9. Among the actinomycetes, Streptomyces species are distributed widely in marine and terrestrial habitats10 and are of commercial interest due to their unique capacity to produce novel metabolites. Several Streptomyces species such as Streptomyces gulbargensis11, Streptomyces olivaceus NEAE-11912, Streptomyces parvus NEAE-9513 and Streptomyces brollosae sp. nov., NEAE-11514 have been explored for L-asparaginase production. There are also reports of L-asparaginase production from Streptomyces noursei MTCC 10469, isolated from marine sponge Callyspongia diffusa15 and Streptomyces aurantiacus isolated from mangroves of Bhitarkanika16.
Most of the microbial L-asparaginase is intracellular in nature except few which are secreted outside the cell9. Extracellular L-asparaginase is more advantageous than intracellular type because of higher accumulation of enzyme in culture broth under normal conditions, easy extraction and downstream processing11, the extracellular L-asparaginase in bacteria is protease deficient and the liberated protein exported to the medium is mostly soluble, biologically active and has an authentic N-terminus, relatively free from endotoxins those results in minimization of adverse effects. Secretion also facilitates proper folding of proteins specially that requiring disulfide bridge formation, as it passes through a more favorable redox potential in the periplasmic space.
The present investigation deals with isolation, production, purification and characterization of an extracellular L-asparaginase, under submerged fermentation from newly isolated actinomycetes Streptomyces fradiae NEAE-82.
Results and Discussion
Morphology and cultural characteristics of the isolate no. NEAE-82
The colonial morphology of a 14 day culture of strain NEAE-82 grown on yeast extract/malt extract agar (ISP 2 medium) revealed that strain NEAE-82 had the typical characteristics of the genus Streptomyces17. It is aerobic, mesophilic, Gram-positive actinomycete that develops abundant and well-developed substrate and aerial mycelium. Strain NEAE-82 produced reddish brown aerial mycelium (Fig. 1) on yeast extract-malt extract agar, oatmeal agar, inorganic salts-starch agar, and peptone-yeast extract iron agar. Not-distinctive aerial mycelium on glycerol-asparagine agar and tyrosine agar. Substrate mycelium with no distinctive pigments on most tested medium and brown substrate mycelium was produced on peptone-yeast extract iron agar (Table 1). Substrate mycelium pigment is not a pH indicator. No pigment found in medium in yeast extract -malt extract agar, oatmeal agar inorganic salt-starch agar, glycerol–asparagine agar or tyrosine agar, faint brown pigments formed in peptone-yeast extract iron agar. Melanoid pigments not formed in peptone-yeast extract iron agar and tyrosine agar. Strain NEAE-82 grew well on yeast extract -malt extract agar (ISP medium 2), oatmeal agar (ISP medium 3), inorganic salt-starch agar (ISP medium 4), peptone-yeast extract iron agar (ISP medium 6) but poor growth on glycerol–asparagine agar (ISP medium 5) and tyrosine agar (ISP medium 7). Strain NEAE-82 formed an extensively branched substrate mycelium and aerial hyphae which differentiated into long straight spore chains. Spore chains in section Retinaculiaperti including open spiral spore chains (Fig. 2). Flexuous or spiral spore chains are seen on starch-nitrate agar. Mature spore chains generally have 10–50 spores per chain; longer chains are sometimes observed. Spore surface is smooth. Verticils are not present. The mycelium does not fragment.
Figure 1. Color of the aerial mycelium of Streptomyces sp. NEAE-82 grown on starch -nitrate agar medium for 7–14 days of incubation at 30 °C.
Table 1. Culture characteristics of the Streptomyces sp. strain NEAE-82.
| Medium | Aerial mycelium (spore-colour en masse) | Substrate mycelium | Diffusible pigment | Growth |
|---|---|---|---|---|
| ISP medium 2 (Yeast extract -malt extract agar) | Reddish brown | Not-distinctive | Non-pigmented | Excellent |
| ISP medium 3 (Oatmeal agar) | Reddish brown | Not-distinctive | Non-pigmented | Excellent |
| ISP medium 4 (Inorganic salt-starch agar) | Reddish brown | Not-distinctive | Non-pigmented | Excellent |
| ISP medium 5 (Glycerol asparagines agar) | Not-distinctive | Not-distinctive | Non-pigmented | Weak |
| ISP medium 6 (Peptone-yeast extract iron agar) | Reddish brown | Brown | Faint brown | Excellent |
| ISP medium 7 (Tyrosine agar) | Not-distinctive | Not-distinctive | Non-pigmented | Weak |
The substrate mycelium pigment was not pH sensitive when tested with 0.05 N NaOH or 0.05 N HCl.
The diffusible pigment was not pH sensitive when tested with 0.05 N NaOH or 0.05 N HCl.
Figure 2.
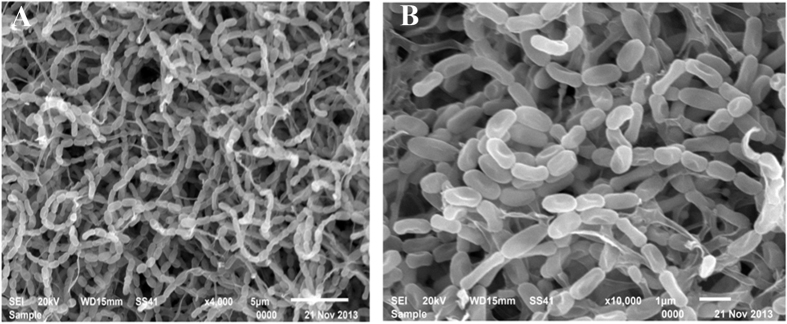
Scanning electron micrograph showing the spore-chain morphology and spore-surface ornamentation of strain NEAE-82 grown on starch nitrate agar medium for 14 days at 30 °C at magnification of 4000X (A) and 10000X (B).
Physiological characteristics
The physiological and biochemical reactions of strain NEAE-82 are shown in Table 2. It exhibited no antimicrobial activities against Staphylococcus aureus, Alternaria solani and Bipolaris oryzae. Sacchromyces cerevisiae, Candida albicans, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Rhizoctonia solani, Fusarium oxysporum, Aspergillus niger and Klebsiella pneumoniae. α–amylase (starch hydrolysis) (Fig. 3), protease (degradation of casein), cellulase (growth on cellulose), chitosanase and L-asparaginase of strain NEAE-82 were produced. Melanin production, lecithinase activity and uricase were not produced. Coagulation and peptonization of milk (Fig. 3) and gelatine liquefaction were positive. Maximum NaCl tolerance was 6% (w/v). D-fructose, D-xylose, D-galactose, D-Glucose, L-arabinose, ribose, D-mannose, sucrose, maltose, cellulose and trehalose are utilized for growth, only traces of growth on rhamnose. The optimal growth temperature of strain NEAE-82 was 30 °C and optimal pH was 8.5.
Table 2. Phenotypic properties that separate strain Streptomyces NEAE-82 from related Streptomyces species.
| Characteristic | Streptomyces sp. strain NEAE-82 | Streptomyces fradiae |
|---|---|---|
| Aerial mycelium on ISP mdeium 2 | Reddish brown | Red |
| Substrate mycelium on ISP medium 2 | Not-distinctive | Not-distinctive |
| Production of diffusible pigment | No diffusible pigment | No diffusible pigment |
| Spore chain morphology | Retinaculiaperti including open spirals | Retinaculiaperti morphology, including open spirals |
| Spore surface | Smooth | Smooth |
| Spore shape | Oval to oblong | |
| Melanin production on peptone-yeast extract iron agar (ISP medium 6) | − | − |
| Melanin production on tyrosine agar (ISP medium 7) | − | − |
| Max NaCl tolerance (%, w/v) | 6 | |
| Utilization of carbon sources (1%, w/v) | ||
| D(−) Fructose | + | + |
| D(+) Xylose | + | ± |
| D(+) Galactose | + | |
| D(+) Glucose | + | + |
| L-arabinose | + | + |
| Ribose | + | |
| D(+) Mannose | + | |
| Sucrose | + | ± |
| Maltose | + | |
| Rhamnose | + | ± |
| Raffinose | ± | ± |
| Cellulose | + | |
| Trehalose | + | |
Data for reference species were taken from Bergey’s Manual® of Systematic Bacteriology -volume five the actinobacteria (Goodfellow et al.21).
Abbreviations: +, Positive; −, Negative; ±, Doubtful; Blank cells, no data available.
The optimal growth temperature was 30 °C and optimal pH was 8.5. It exhibited no antimicrobial activities against Staphylococcus aureus, Alternaria solani, Bipolaris oryzae, Sacchromyces cerevisiae, Candida albicans, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Rhizoctonia solani, Fusarium oxysporum, Aspergillus niger and Klebsiella pneumoniae. α–amylase (starch hydrolysis), protease (degradation of casein), cellulase (growth on cellulose), chitosanase and L-asparaginase of strain NEAE-82 were produced while uricase, lecithinase were not produced. Coaggulation and peptonization of milk and gelatin liquefaction were positive.
Figure 3.
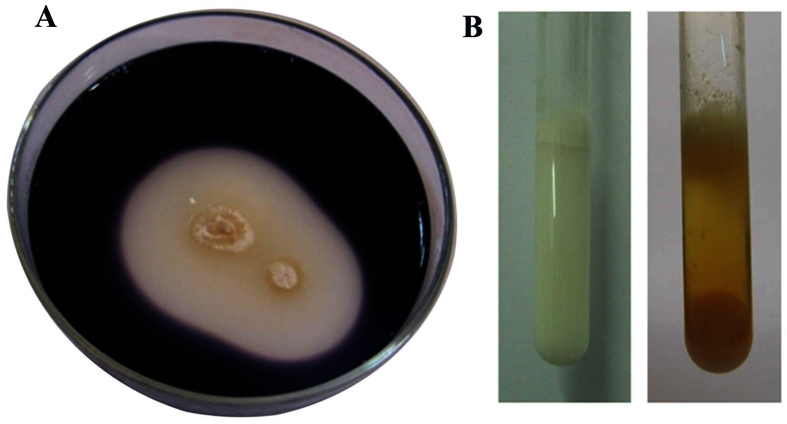
(A) Plate assay showing zone of hydrolysis of starch by strain NEAE 82. All the starch in the medium near the microbe has been hydrolyzed by extracellular amylases; (B) Coagulation and peptonization of milk by strain NEAE 82.
16S rRNA gene sequence comparisons and phylogenetic analysis
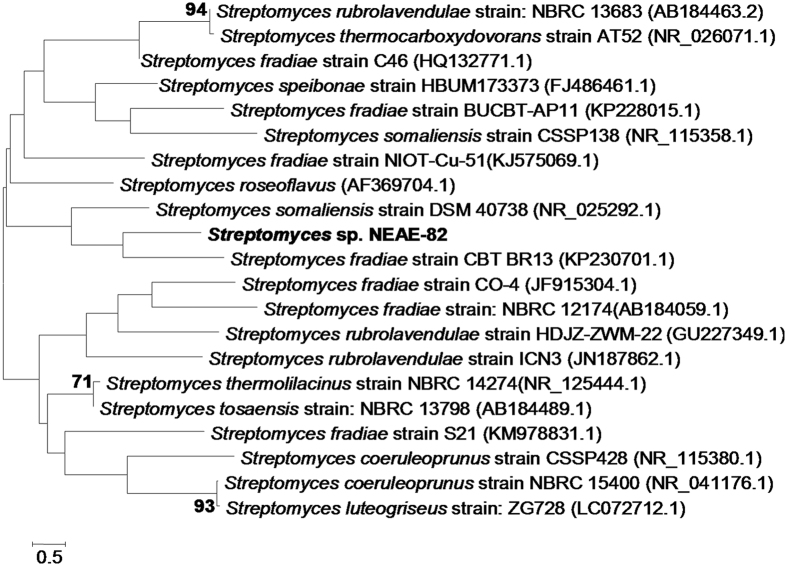
The 16S rRNA gene sequence (1508 bp) was determined for strain NEAE- 82. A BLAST search18 of the GenBank database using this sequence showed its similarity to that of many species of the genus Streptomyces. A phylogenetic tree (Fig. 4) based on 16S rRNA gene sequences of members of the genus Streptomyces was constructed according to the neighbour-joining method of Saitou and Nei19 with MEGA420. This tree shows the close phylogenetic association of strain NEAE-82 with certain other Streptomyces species. Phylogenetic analysis indicated that the strain NEAE-82 consistently falls into a clade together with Streptomyces somaliensis strain DSM 40738 (GenBank/EMBL/DDBJ accession No. NR_025292.1) and Streptomyces fradiae strain CBT BR13 (GenBank/EMBL/DDBJ accession No. KP230701.1).
Figure 4. Neighbour-joining phylogenetic tree based on 16S rRNA gene sequences, showing the relationships between strain NEAE-82 and related species of the genus Streptomyces.
Only bootstrap values above 50%, expressed as percentages of 1000 replications, are shown at the branch points. GenBank sequence accession numbers are indicated in parentheses after the strain names. Phylogenetic analyses were conducted in the software package MEGA4. Bar, 0.2 substitution per nucleotide position.
On the basis of the collected data and in view of the comparative study of the recorded properties of isolate No. NEAE-82 (Table 2) in relation to the closest related species of the genus Streptomyces, it is most closely related to the type strains of Streptomyces fradiae strain CBT BR13 (GenBank/EMBL/DDBJ accession No. KP230701.1) (99% sequence similarity)21. Therefore, this strain was identified as Streptomyces fradiae strain NEAE-82 and its sequencing product was deposited in the GenBank database under accession number KJ467538.
Purification of L-asparaginase from Streptomyces fradiae NEAE-82
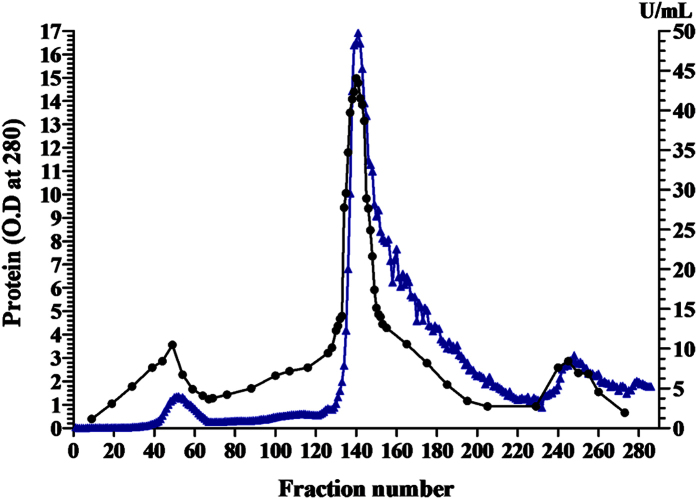
The crude culture filtrate of Streptomyces fradiae NEAE-82 had a total activity of 29507.688 U with protein content 3214.705 mg; the specific activity was 9.179 U/mg protein. The ammonium sulphate concentrated enzyme preparation had a protein content of 196.6 mg and the specific activity was 13.008 U/mg protein, showing purification fold of 1.417 with enzyme recovery at this step was 8.667 per cent. The fractions collected after ammonium sulphate precipitation were loaded on the column packed with DEAE Sepharose CL-6B and fractions of 2 ml were collected and analyzed for enzyme activity and protein content (Fig. 5). A total of 285 fractions were collected that showed one major L-asparaginase peak on the chromatogram. The total activity was 1442.393 with 47.081 mg protein after ion exchange chromatography through DEAE Sepharose CL-6B column. The specific activity of the purified enzyme was 30.636 U/mg of protein. Summary of the purification steps of the L-asparaginase produced by Streptomyces fradiae NEAE-82 is presented in Table 3.
Figure 5. Purification of L-asparaginase produced by Streptomyces fradiae NEAE-82 using ion exchange on DEAE Sepharose CL-6B.
(▲) refer to protein, (●) refer to L-asparaginase activity.
Table 3. Summary of the purification steps of the L-asparaginase produced by Streptomyces fradiae NEAE-82.
| Purification step | Total protein content (mg) | L-asparaginase activity |
|||
|---|---|---|---|---|---|
| Total activity (U) | Specific activity (U/mg protein) | Recovery (%) | Purification fold | ||
| Culture filtrate | 3214.705 | 29507.688 | 9.179 | 100 | 1 |
| (NH4)2SO4, post dialysis | 196.6 | 2557.325 | 13.008 | 8.667 | 1.417 |
| Ion exchange on DEAE Sepharose CL-6B | 47.081 | 1442.393 | 30.636 | 4.888 | 3.338 |
In Streptomyces albidoflavus, L-asparaginase has been purified in CM Sephadex C-50 column up to 99.3 folds9. In another report, 106 folds purified L-asparaginase was obtained from Pseudomonas aeroginosa 5007122 by the final CM Sephedex C-50 column chromatography. Recently L-asparaginase purity of about 98.23 folds was reported in Streptomyces noursei15 and 82.12 folds was reported in Streptomyces gulbergenesis11 in final purification.
Kinetics properties of the purified L-asparaginase
The activity of L-asparginase of Streptomyces fradiae NEAE-82 was evaluated at different levels of pH, temperature, effect of substrate concentration and incubation time.
Effect of pH on L-asparaginase activity
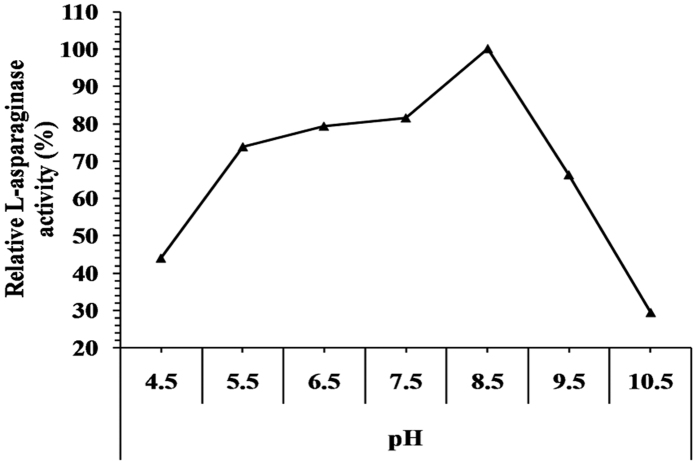
L-asparaginase activity was studied as a function of pH in range between 4.5–10.5 (Fig. 6). L-asparaginase was active over broad pH ranges (4.5–10.5). The enzyme activity increase gradually till pH 8.5 with maximum activity 20.932 IU (relative activity, 100%). At higher pH’s, enzyme activity was decreased. The enzyme retains up to 29.348% of activity at pH 10.5 compare to 44.021% at pH 4.5. It is known that L-asparaginase can completely lose its activity and also, recover it partially, depending on the exposure conditions. These results coincide with that of Dhevagi and Poorani23 who reported the maximal L-asparaginase activity of Streptomyces sp. PDK7 was between pH 8.0 and 8.5, and the optimal L-asparaginase activity extracted from Streptomyces gulbargensis was 9.011. L-asparaginase is one of the amidases that are generally active and stable at neutral and alkaline pH, whereas, pH 5.0 to 9.0 were reported earlier to be optimum for amidase activity24. L-asparaginase, purified from Streptomyces acrimycini NGP, exhibited maximum activity at pH 7.025; membrane bound L-asparaginase from Tetrahymena pyriformis acts optimally at pH 9.626 and the optimal L-asparaginase activity from Corynebacterium glutamicum was reported at pH 7.027. Maximum activity of purified L-asparaginase occurred at pH 9 was obtained for Pseudomonas aeruginosa 5007122.
Figure 6. Activity of L-asparaginase as a function of the pH of the reaction.
Effect of temperature on L-asparaginase activity
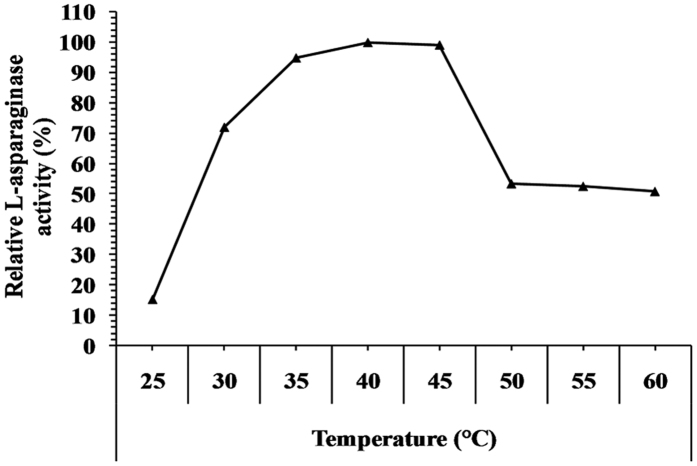
The temperature optimum of L-asparaginase from Streptomyces fradiae NEAE-82 is shown in Fig. 7. It was active at wide range of temperature condition from 25–60 °C. The maximum L-asparaginase activity of 26.848 IU was obtained at 40 °C. At higher temperature the L-asparaginase activity declined. The enzyme retains 50.85% of its activity at 60 °C. Our results were in agreement with a previous study which reported that the maximum activity of L-asparaginase purified from Streptomyces gulbargensis was at 40 °C11. Manna et al.28 have found 37 °C to be the optimum temperature for the enzyme activity obtained from Pseudomonas stutzeri MB-405. However, optimum temperature for L-asparaginase activity obtained from Erwinia sp. showed maximum activity at 35 °C29.
Figure 7. Effect of the temperature on L-asparaginase activity.
Effect of substrate concentration on the activity of L-asparaginase
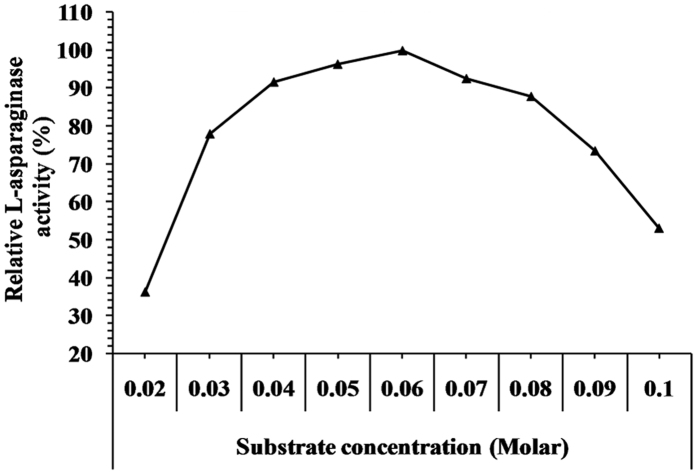
In this experiment, the influence of substrate concentration on L-asparaginase activity was examined by using different concentration of substrate ranging from 0.02 to 0.1 Molar to determine the optimum concentration of substrate required to give the highest L-asparaginase activity. The results in Fig. 8 showed a gradual increase in the enzyme activity with the increase in substrate concentration from 0.02 to 0.06 Molar. However, further increase in substrate concentration (0.07–0.1 Molar) lead to decrease in enzyme activity to 53.03% with 0.1 Molar substrate. The optimum substrate concentration for L-asparaginase activity was observed at 0.06 Molar.
Figure 8. Effect of the substrate concentration of the reaction on L-asparaginase activity.
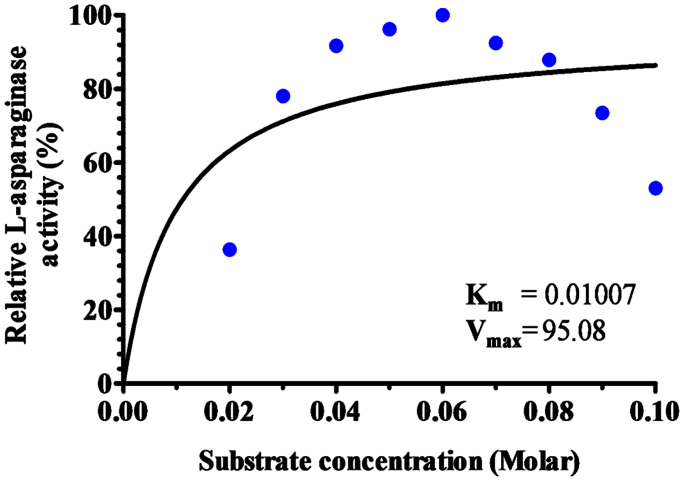
The typical Michaelis-Menten relationship was obtained between the substrate concentrations and the initial velocity of the reaction. Michaelis-Menten plot showed in Fig. 9 illustrated the Km and Vmax values for L-asparaginase enzyme. The plot gave Km value of 0.01007 M and Vmax of 95.08 Uml−1min−1 for the hydrolysis of L-asparagine. Km value is defined as the substrate concentrations that result in half maximal velocity for the enzymatic reaction, or an equivalent way of stating substrate concentration at which half of the enzyme active sites in the sample are filled (i.e. saturated) by substrate molecules in the steady state. It can be used as a relative measure of substrate affinity with the studied enzyme. Km reflects the affinity of the enzyme for its substrate30. The lower the Km is the stronger binding ability the enzyme has. Vmax is the limiting velocity as substrate concentrations get very large. Vmax is expressed in units of product formed per unit of time. If the molar concentration of enzyme is known, Vmax be expressed as moles of product formed per second per mole of enzyme sites31. This is the turnover number, the number of molecules of substrate converted to product by one enzyme site per second. However, there are many factors that affect on kinetic parameters of the enzymes (Km and Vmax) such as; type of enzyme, different forms of enzyme (crude, modified or purified), changes in enzyme conditions (pH, temperature, etc.), source of the enzyme (different microorganisms), type of used substrates, and the assay procedures32.
Figure 9. Michaelis-Menten plot for L-asparaginase produced by Streptomyces fradiae NEAE-82.
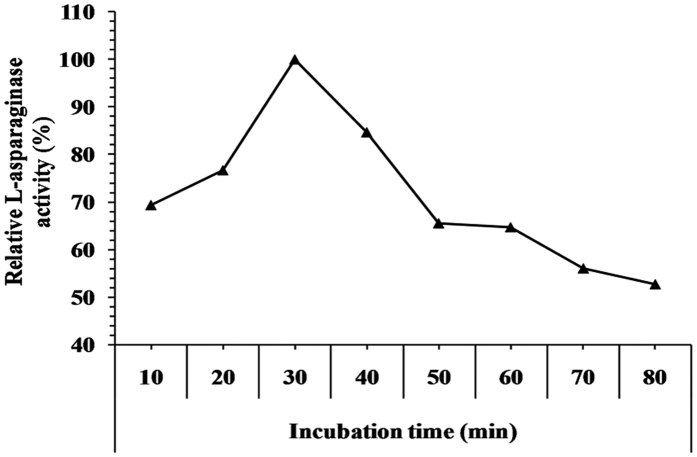
Effect of incubation time on enzyme activity
The L-asparaginase activity (Fig. 10), increased as the incubation time increased up to 30 min (L-asparaginase activity of 34.128 IU). After which only a slight decrease in L-asparaginase activity was observed. El-Bessoumy et al.22 reported that, maximum activity of L-asparaginase purified from Pseudomonas aeruginosa 50071 was at 30 min. In addition, the effect of incubation time on the activity of purified L-asparaginase from Streptomyces noursei showed that the activity reached its maximum at 35 min15. The decrease in L-asparaginase activity was observed after longer period of incubation with substrate. The decrease may due to the product inhibition.
Figure 10. Effect of different incubation periods on L-asparaginase activity.
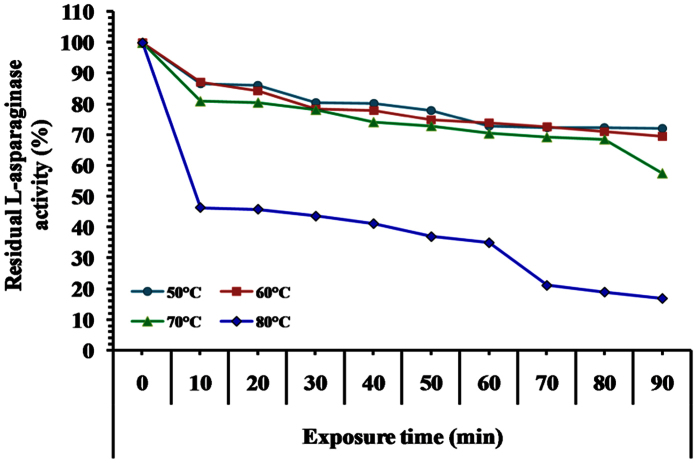
Thermal stability
The effect of temperature on the stability of L-asparaginase showed maximum enzyme activity at 50 °C (Fig. 11). Around 86.126% of the initial activity was retained by the enzyme after 20 min of incubation at 50 °C. About 72.072% of L-asparaginase activity was lost after incubation at 50 °C for 90 min, while a rapid decrease in the enzyme activity (16.874%) was observed after incubation at 80 °C for 90 min. Thermal inactivation process of the enzyme follows the theoretical curve of a sample first order reaction. However, linear regression of the obtained data was assayed to determine half life time (T1/2) as shown in Table 4. The half-life time (T1/2) was 184.91 min at 50 °С, while being 179.53 min at 60 °С. On the other hand, destruction of enzyme activity was observed at 80 °C with low half-life time (75.36 min). It can be concluded from the previous results that the higher thermal stability behavior of L-asparaginase was at 50 °C. Results presented in Table 4 were normalized to activity of un-incubated enzyme (time 0 = 100%) for the percentage of activity remaining. Heat inactivation half-life (T1/2) and heat deactivation constant (k) were determined by fitting the data to a first-order decay curve using Graph-Pad Prism software. An earlier study reported no significant loss of L-asparaginase activity purified from Streptomyces radiopugnans MS1, when the enzyme was pre-incubated at 40 °C for 60 min33. Similar results were recorded with Streptomyces noursei15, E. carotovora34, Pseudomonas stutzeri MB 40528.
Figure 11. Thermal stability of L-asparaginase as a function of the time of the reaction.
Table 4. Half life time (T1/2) and heat deactivation constant (k) of L-asparaginase produced by Streptomyces fradiae NEAE-82.
|
Half life time (min) | |
| 50 °C | 184.91 |
| 60 °C | 179.53 |
| 70 °C | 150.29 |
| 80 °C | 75.36 |
| Thermal inactivation rate constant kd1(min−1)* | |
| 50 °C | 0.0037 |
| 60 °C | 0.0039 |
| 70 °C | 0.0046 |
| 80 °C | 0.0092 |
| D-value (min) | |
| 50 °C | 332.84 |
| 60 °C | 323.16 |
| 70 °C | 270.51 |
| 80 °C | 135.64 |
| Thermal inactivation rate constant kd 2(min−1)** | |
| 50 °C | 0.0021 |
| 60 °C | 0.0021 |
| 70 °C | 0.0026 |
| 80 °C | 0.0051 |
*kd1 is the deactivation constant after losing 50% of initial activity (at T1/2).
**kd2 is the deactivation constant after losing 90% of initial activity (at D value).
A source-dependent variation of physicochemical and biochemical properties, like optimum pH, temperature, substrate specificity, inhibition pattern, etc., of microbial enzymes is well documented8. Comparative evaluation of L-asparaginase for its potential activity from different microbial sources revealed that biochemical and therapeutic properties differ with source of strain in addition to enzyme properties. L-asparaginases from Erwinia chrysanthemi and E. coli are currently in clinical use as effective drugs in the treatment of the acute lymphoblastic leukemia. Comparison of the two enzymes, lead to the conclusion that most patients allergic to the E. coli- L-asparaginase, Erwinia asparaginase is considered less toxic and is frequently employed compared with allergic reactions to E. coli- L-asparaginase. However, Erwinia asparaginase had a shorter half life than E. coli4. The optimum temperature for L-asparaginase activity obtained from E. coli showed maximum activity at 37 °C and pH 7–835. The maximum L-asparaginase activity obtained from Erwinia aroideae36 occurred between pH 7 and 8 and optimum temperature for L-asparaginase activity obtained from Erwinia sp. showed maximum activity at 35 °C29. However, the characterization of the enzyme produced by newly isolated actinomycetes Streptomyces fradiae NEAE-82 revealed that the optimum pH, temperature and incubation time for maximum L-asparaginase activity were found to be 8.5, 40 °C and 30 min, respectively. It was active at wide range of temperature condition from 25–60 °C and active over broad pH ranges (4.5–10.5). The physiological pH is one of the perquisites for antitumor activity37 under alkaline pH condition, L-asparaginase becomes a competitive inhibitor38. This property of the enzyme is most suitable for complete elimination of asparagines from the body when tumor patient is treated with L-asparaginase in-vivo and clarified that the enzyme produced by Streptomyces fradiae NEAE-82 under the present study (optimum pH 8.5) has effective antitumor activity.
Molecular weight determination by Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS–PAGE)
The molecular weight of the extracted enzyme was determined by performing SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) according to the method of Laemmli39, with a separating acrylamide gel of 10% and stacking gel 5% containing 0.1% SDS. After the electrophoresis, the gel was stained in 0.025 Coomassie brilliant blue R-250 and destained with a solution of methanol- acetic acid and water in the ratio of 4:1:5. The molecular weight of the purified L-asparaginase was determined in comparison with standard molecular weight markers (molecular mass range: 9–178 kDa). SDS–PAGE of the enzyme preparation revealed only a single distinctive protein band for the pure preparation of L-asparaginase with an apparent molecular weight of 53 kDa (Fig. 12).
Figure 12. SDS-polyacrylamide gel electrophoresis of the purified L-asparaginase from Streptomyces fradiae NEAE-82.
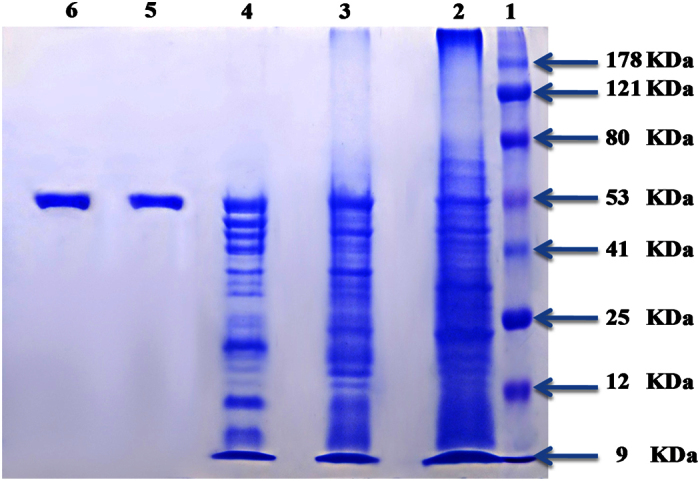
Lane 1: Protein marker; Lane 2–4: Ammonium sulphate fractions (50, 60, 70% respectively); Lane 5, 6: Purified protein.
L-asparaginase is known as a homotetramer. Four active sites are located at the interface between two subunits forming an intimate dimer, and hence the asparaginase is more accurately described as a dimer of dimers with a molecular mass of approximately 120 to 160 kDa possessing antitumor activity40. The molecular weight of the L-asparaginase was found to be varied according to the source of enzyme like 80 kDa in Corynebacterium glutamicum27, 140 kDa in Streptomyces sp. PDK223 and 116 kDa in S. albidoflavus9. Purified L-asparaginase from S. tendae checked by SDS-PAGE revealed a distinct protein band near 97.4 kDa41. In this respect, L-asparaginases purified from Pseudomonas stutzeir MB-405, Thermus thermophilus and Escherichia coli were with smaller molecular weight values ranging from 33–34 kDa28,42. Other reports on production and purification of L-asparaginase from E. coli revealed that the molecular weight was determined as 153 KDa with the help of SDS-PAGE43. The functional L-asparaginase from E. coli is a homotetramer with a molecular weight of about 142 kDa44. Purified L-asparaginase from Streptomyces gulbargensis11, Streptomyces albidoflavus9, Streptomyces PDK223 and Streptomyces noursei15 exhibited a molecular weight of 85, 112, 140 kDa and 102 kDa, respectively. Reports on production and purification of L-asparaginase from Pseudomonas aeruginosa 50071 by SDS- PAGE revealed a peptide chain with molecular weight of 160 kDa22.
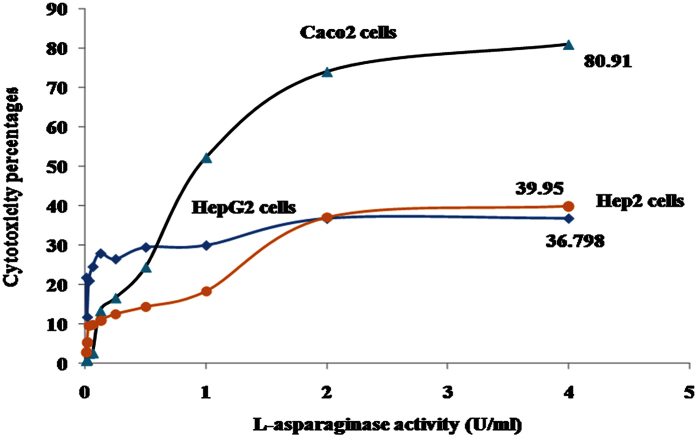
Cytotoxicity and anticancer activities of L-asparaginase on non-cancerous and cancer cells
The safety pattern of purified L-asparaginase was scanned on human fibroblast cells. Generally, the obtained data revealed that, the treatment IC50 on all cells ranged from 2 to 4 U/ml (Fig. 13). The anti-proliferative activity of L-asparaginase on cancer cells was quantitatively estimated on HepG2, Hep2 and Caco2 cells. The obtained data generally indicated that, the activity of the extract against Caco2 was superior to that with either HepG2 or Hep2 cells (Figs 14 and 15, Table 5). Up on cancer cells treatment, the selected recommended dose (4 U/ml) showed anti-proliferation activities against Caco2 cells with percentages 80.9 with cancer cell selectivity index reached 4. On the other hand, the anticancer activity of the purified on Hep2 and Caco2 cells reached 39.95 and 36.79%, respectively with selectivity index 0.88 and 0.64, respectively. Sialyl LewisX (sLex) is a tetrasaccharide carbohydrate that is often linked to cell-surface glycoproteins. Sialic acids in general play important roles in biological characteristics of cancer and other cells because it overexpressed in some tumor cell types and that are implicated in cellular invasiveness, differentiation and tumourigenecity45,46. Sialyl Lex is a major E-selectin ligand, mediating both homotypic and heterotypic cell adhesion of endothelial cells47. Endothelial cell surface E-selectin was shown to be necessary for endothelial cell (EC) binding to colon cancer cells47. L-asparaginase has been reported to prevent glycosylation several forms as sialylation, of newly synthesized proteins48. So, we suggest that as almost the same reports of Yu et al.49 in ovarian cancer, L-asparaginase could significantly alter the interactions between microvascular ECs, colon cancer cells, and ECM components, resulting incolon cancer cell injury.
Figure 13. Cytotoxicity and safety assay of the purified L-asparaginase on non-cancerous human fibroblast cells.
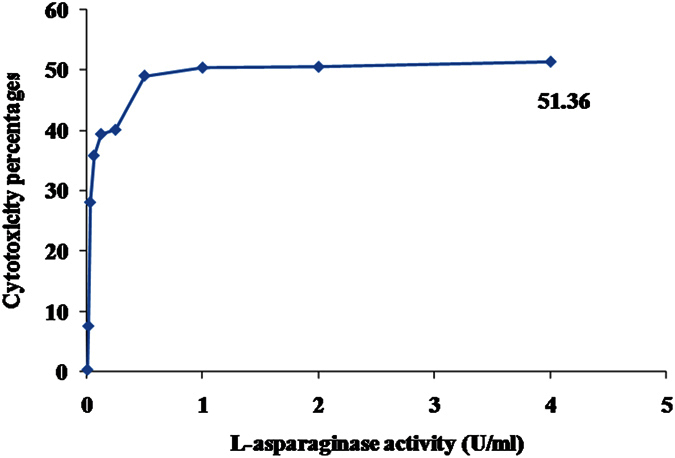
Figure 14. The anticancer effects of the purified L-asparaginase on Caco2, Hep2 and HepG2 cells.
Figure 15. The anticancer effect of the purified L-asparaginase on Caco2 cells after 48 of treatment, cells undergoing apoptosis are characterized by cellular rounding up, shrinkage, membrane blebbing and loss of cell adhesion.
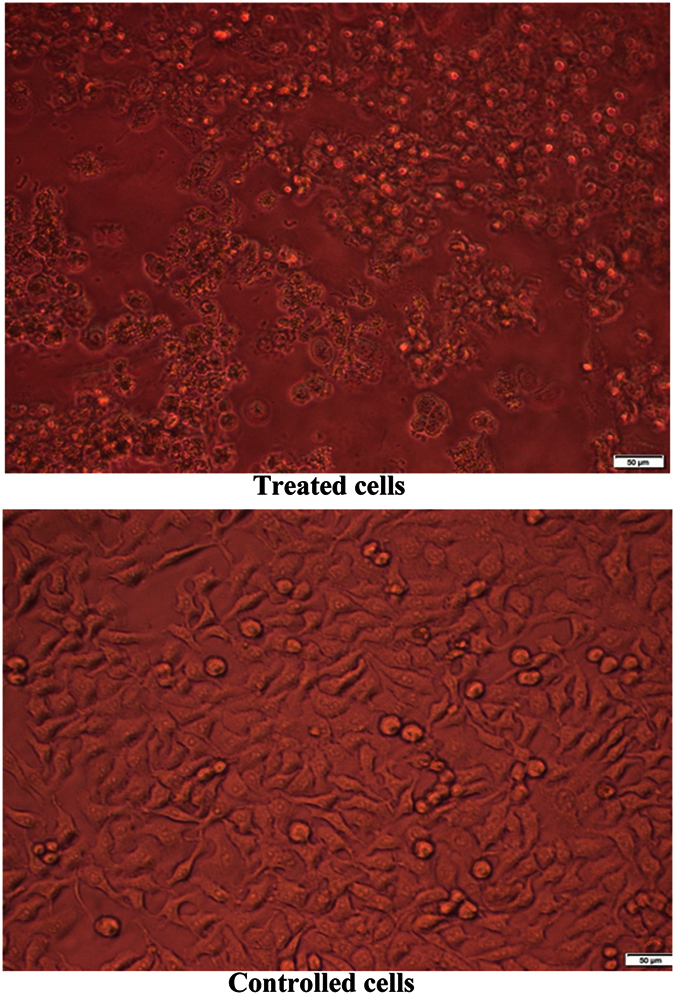
Table 5. The Cancer cell selectivity index of the purified L-asparaginase.
| Cell type | Cancer cells selectivity index (SI) |
|---|---|
| HepG2 cells | 0.6472 |
| Caco2 cells | 4 |
| Hep2 cells | 0.8843 |
Materials and Methods
Microorganisms and cultural conditions
Streptomyces spp. used in this study were isolated from various soil samples collected from different localities of Egypt and Saudi Arabia. Actinomycetes had been isolated from the soil using standard dilution plate method procedure on Petri plates containing starch nitrate agar medium of the following composition (g/L): Starch, 20; KNO3, 2; K2HPO4, 1; MgSO4.7H2O, 0.5; NaCl, 0.5; CaCO3, 3; FeSO4.7H2O, 0.01; agar, 20 and distilled water up to 1 L; then plates were incubated for a period of 7 days at 30 °C. Streptomyces isolates were purified and maintained as spore suspensions in 20% (v/v) glycerol at −20 °C for subsequent investigation.
Screening of L-asparaginase production by plate assay
It is generally observed that L-asparaginase production is accompanied by an increase in pH of the culture filtrates50. The plate assay was based on Gulati et al.51 method with the incorporation of pH indicator phenol red (prepared in ethanol) in medium containing L-asparagine (sole nitrogen source). Phenol red at acidic pH is yellow and at alkaline pH turns pink, thus a pink zone is formed around microbial colonies producing L-asparaginase. Screening of potential L-asparaginase producing actinomycetes was carried out with the use of asparagine dextrose salts agar (ADS Agar) (asparagine 1.0%, dextrose 0.2%, K2HPO4 0.1%, MgSO4 0.05%, agar 1.5%), pH was adjusted to 6.8 and supplemented with phenol red as a pH indicator (0.009% final concentration)52 and sterilized at 1.5 atmospheric pressure for 20 min. Inoculated plates were incubated at 30 °C for 7 days. Plates were examined for change in color of medium from yellowish to pink due to change of pH indicating the positive asparaginase activity. Colonies with pink zones were considered as L-asparaginase-producing strains. Isolates exhibiting L-asparaginase activity were selected for further study. Control plates were prepared as uninoculated medium and medium without dye.
Inoculum preparation
250 ml Erlenmeyer flasks containing 50 ml of asparagine dextrose salts broth (L-asparagine 1.0%, dextrose 0.2%, K2HPO4 0.1%, MgSO4 0.05%) were inoculated with three disks of 8 mm diameter taken from the 7 days old stock culture grown on starch nitrate agar medium. The flasks were incubated for 48–72 h in a rotatory incubator shaker at 30 °C and 150 rpm and were used as inoculum for subsequent experiments.
Production of L-asparaginase by submerged fermentation
The selected strain was cultured in fifty ml of asparagine dextrose salts broth medium (at a specified pH) dispensed in 250 ml Erlenmeyer conical flasks. The inoculated flasks were incubated on a rotatory incubator shaker at 30–37 °C with shaking at 150–250 rpm. After the specified incubation time for each set of experimental trials, the mycelium of the tested isolate was collected by centrifugation at 5000 × g for 30 min at 4 °C.
Assay of L-asparaginase activity
L-asparaginase activity was determined by measuring the amount of ammonia released by nesslerization according to the method described by Wriston and Yellin52. The reaction mixture containing 1.5 ml of 0.04 M L-asparagine prepared in 0.05 M Tris-HCl buffer, pH 8.6 and 0.5 ml of an enzyme to make up the total volume to 2 ml. The tubes were incubated at 37 °C for 30 minutes. The reaction was stopped by adding 0.5 ml of 1.5 M trichloroacetic acid (TCA). The blank was prepared by adding enzyme after the addition of TCA. The precipitated protein was removed by centrifugation at 10,000 × g for 5 min and the liberated ammonia in the supernatant was determined colorimetrically by direct nesslerization by adding 1 ml Nessler’s reagent into tubes containing 0.5 ml of clear supernatant and 7 ml distilled water and incubated at room temperature for 20 min. A yellow coloration indicates the presence of ammonia: at higher concentrations, a brown precipitate may form. The yellow color was read using a UV-visible spectrophotometer (Optizen Pop –UV/Vis spectrophotometer) at 480 nm. The amount of ammonia liberated was calculated using ammonium (ammonium chloride) standard curve. One unit (U) of L-asparaginase is defined as the amount of enzyme which catalyzed the formation of 1 μmole of ammonia from L-asparagine per minute at 37 °C and pH 8.6. The enzyme activity was expressed in terms of units per gram dry fermented substrate (U/gds).
Morphology and cultural characteristics
The morphology of the spore chain and the spore surface ornamentation of strain NEAE-82 were examined on inorganic salt/starch agar after 14 days at 30 °C. The gold-coated dehydrated specimen can be examined at different magnifications with Analytical Scanning Electron Microscope Jeol JSM-6360 LA operating at 20 Kv at the Central Laboratory, City for Scientific Research and Technological Applications, Alexandria, Egypt. Aerial spore-mass color, substrate mycelial pigmentation and the production of diffusible pigments were observed on ISP medium 1–7 as described by Shirling and Gottlieb53.
Physiological characteristics
Physiological characteristics were performed following the methods of Shirling and Gottlieb53. The ability of the organism to inhibit the growth of four bacterial (Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, or Klebsiella), and five fungal strains (Rhizoctonia solani, Alternaria solani, Bipolaris oryzae, Fusarium oxysporum, Fusarium solani) was determined.
16S rRNA sequencing
The PCR amplification reaction and sequencing were performed in accordance with the methods described by El-Naggar et al.12. Sequencing product was deposited in the GenBank database under accession number KJ467538.
Sequence alignment and phylogenetic analysis
The partial 16S rRNA gene sequence of strain NEAE-82 was aligned with the corresponding 16S rRNA sequences of the type strains of representative members of the genus Streptomyces retrieved from the GenBank, EMBL, DDBJ and PDB databases by using BLAST program (www.ncbi.nlm.nih.gov/blst)18 and the software package MEGA4 version 2.120 was used for multiple alignment and phylogenetic analysis. The phylogenetic tree was constructed via the neighbor-joining algorithm19 based on the 16S rRNA gene sequences of strain NEAE-82 and related organisms.
Purification of L-asparaginase from Streptomyces fradiae NEAE-82
All purification steps were carried out at 4 °C using crude enzyme extract. The extracellular crude enzyme was prepared at the end of the fermentation period by centrifugation at 11,000 × g for 30 min. The cell free supernatant was used as the crude enzyme preparation. Finely powdered ammonium sulfate was slowly added to the clear supernatant obtained after centrifugation to reach 45% saturation and incubated overnight. The precipitate was collected by centrifugation at 11000 × g for 30 min, while the supernatant was brought to 55–85% saturation with ammonium sulphate. Then the precipitates were collected separately by centrifugation, dissolved in a minimal amount of 50 mM Tris-HCl buffer pH 8.4 and dialyzed overnight against the same buffer. After dialysis, the samples were used for protein estimation and enzyme assay by the method of Lowry et al.54 and direct Nesslerization method52, respectively and stored at 4 °C for further purification.
The dialyzed enzyme solution obtained from the previous step was loaded into a diethylaminoethyl (DEAE) Sepharose CL-6B column (2.3 cm × 12 cm) that was pre-equilibrated with 50 mM Tris-HCl buffer (pH 8.4). The column was washed with two column volume of the above buffer and the adsorbed protein was isocratic eluted using a 0.5 M NaCl in 50 mM Tris–HCl (pH 8.4). Fractions were collected at a flow rate of 1 ml min−1 (each fraction containing 2 ml) using a microfractionator (Bio-Red). All chromatographic runs were monitored for protein at 280 nm. The fraction were collected and examined for enzyme activity and protein content by procedures described earlier52,54. Fractions showing high L-asparaginase activity were collected for further use.
Characterization of L-asparaginase enzyme
The effect of the incubation time on L-asparginase activity was studied by incubating the reaction mixture for different times (10, 20, 30, 40, 50, 60, 70 and 80 min), then the activity of the enzyme was determined. The optimum pH of L-asparaginase activity was studied; L-asparaginase enzyme was pre-incubated with 0.05 M buffers over a range of pH 4–10 under assay conditions, and the amount of ammonia liberated was determined. The buffers used were citric acid- Na2HPO4 (pH 4.5–7.5), Tris-HCl (pH 8.5), and glycine-NaOH (pH 9.5–10.5). The optimum temperature for L-asparaginase activity was determined by incubating the assay mixture at different temperatures ranging from 25 to 60 °C in 0.05 M Tris-HCl buffer under assay conditions. Effect of different substrate concentration on L-asparaginase activity was determined by incubating 0.1 ml from the purified enzyme with different concentration of the specific substrate (0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, and 1 M) and then the activity of the enzyme was determined under assay conditions.
Determination of kinetic properties (Km, Vmax)
The reaction kinetics of the purified enzyme was determined from Lineweaver-Burk plots55 with L-asparagine as substrate under defined assay conditions. The Michaelis–Menten constant (Km) and maximal velocity (Vmax) were determined for the enzyme at each of the measured temperatures using the Michaelis–Menten equation:
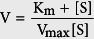 |
where V is the reaction velocity (a function of enzyme concentration), [S] is the substrate concentration, Km is the substrate concentration at half-maximal velocity, and Vmax is the maximal velocity. Vmax and Km values were determined using nonlinear regression56.
Enzyme thermal stability
Thermal stability of the L-asparaginase was carried by pre-incubating the buffered enzyme prepared in absence of its substrate for different time interval ranging from 0.0 to 90 min at different temperatures (50, 60, 70 and 80 °С). After incubation, the enzyme was cooled then the residual activities were assayed under the standard conditions.
Molecular weight determination and checking of enzyme homogeneity
The molecular weight of the subunit and the purity of enzyme was checked by performing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE was performed following the method of Laemmli39, with separating acrylamide gel 10% (w/v) and stacking gel 5% (w/v) containing 0.1% (w/v) SDS. After the electrophoresis, the gel was stained with coomassie brilliant blue R-250 and de-stained with a solution of methanol, acetic acid and water in the ratio of 4:1:5. Approximate subunit molecular weight of L-asparaginase was determined using standard wide range molecular weight marker (9–178 kDa).
Cell lines
Cancerous cells
Human epithelial colorectal adenocarcinoma cells (caco2 cells), liver hepatocellular carcinoma cells (HepG2 cells) and human laryngeal carcinoma cells (Hep2 cells).
Non-cancerous cells
Human fibroblast cells (FB).
Cytotoxicity and anticancer assay
In order to determine both safety and the anticancer activities of the purified L-asparaginase on both cancerous (CaCo2, HepG2 and Hep2 cells) and non-cancerous cells (Human fibroblast cells), neutral red assay protocol was used. Briefly, 100 μl of each of serially diluted purified enzymes in either RPMI or DMEM media were incubated with about 6 × 104 cell/ml of each cell type on 96-well plates. After 48 hours, the cellular cytotoxic effects were quantified using neutral red assay57.
Selectivity index (SI)
The degree of anticancer selectivity of the tested enzyme was expressed as the previous report58 with a minor modification59, SI = LC50 of pure compound in a normal cell line/LC50 of the same pure compound in cancer cell line, where LC50 is the concentration required to kill 50% of the cell population.
Conclusion
Production of L-asparaginase using different microbial systems has attracted much attention, owing to the cost-effective and ecofriendly nature. The present study revealed an efficient production of an extracellular L-asparaginase throughout various purification steps from isolate Streptomyces fradiae NEAE-82 under submerged fermentation. From the culture filtrate of this strain, L-asparaginase enzyme was purified by column chromatography and molecular weight was determined by SDS-PAGE. The enzyme substrate specificity, kinetic parameters were also determined. Furthermore, high catalytic activity of the enzyme over a wide range of pH and temperature, and its considerable stability, makes it highly favorable for use as a potent anticancer agent, and for other applications in healthcare industry.
Additional Information
How to cite this article: El-Naggar, N. E. et al. Purification, characterization, cytotoxicity and anticancer activities of L-asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82. Sci. Rep. 6, 32926; doi: 10.1038/srep32926 (2016).
Footnotes
The authors declare no competing financial interests.
Author Contributions N.E.E. proposed the research concept, designed the experiments, providing necessary tools for experiments, experimental instructions, conducted most of the experiments, analyzed and interpreted the data and wrote the manuscript. S.F.D. designed and conducted some of the experiments, provided some instruments, experimental instructions, as well as contributed to reviewing process. H.M.S. assisted in some scientific experiments, contributed to the manuscript reviewing and had given final approval of the version to be published. N.M.E. designed, conducted and writing cytotoxicity and anticancer activities. S.M.E. performed experiments. All authors read and approved the manuscript.
References
- Verma N., Kumar K., Kaur G. & Anand S. E. coli K-12 asparaginase-based asparagine biosensor for leukemia. Artif Cells Blood Substit Immobil Biotechnol 35, 449–456 (2007). [DOI] [PubMed] [Google Scholar]
- Pedreschi F., Kaack K. & Granby K. The effect of asparaginase on acrylamide formation in French fries. Food chem 109, 386–392 (2008). [DOI] [PubMed] [Google Scholar]
- Yong W. Clinical study of l-asparaginase in the treatment of extranodal NK/T-cell lymphoma, nasal type. Hematol Oncol (2015). 10.1002/hon.2207. [DOI] [PubMed] [Google Scholar]
- Duval M. et al. Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group phase 3 trial. Blood 99, 2734–2739 (2002). [DOI] [PubMed] [Google Scholar]
- Kotzia G. A. & Lbrou N. E. Cloning, expression and characterisation of Erwinia carotovora L-asparaginase. J Biotechnol 119, 309–323 (2005). [DOI] [PubMed] [Google Scholar]
- Patro K. R. & Gupta N. Extraction, purification and characterization of L- asparaginase from Penicillium sp. by submerged fermentation. Int J Biotechnol Mol Biol Res 3, 30–34 (2012). [Google Scholar]
- Basha N. S., Rekha R., Komala M. & Ruby S. Production of extracellular anti-leukemic enzyme L-asparaginase from marine actinomycetes by solid state and submerged fermentation: Purification and characterization. Tropical J Pharm Res 8, 353–360 (2009). [Google Scholar]
- Eden O. B., Shaw M. P., Lilleyman J. S. & Richards S. Non-randomized study comparing toxicity of Escherichia coli and Erwinia asparaginase in children with leukaemia. Med Pediat Oncol 18, 497–502 (1990). [DOI] [PubMed] [Google Scholar]
- Narayana K., Kumar K. & Vijayalakshmi M. L-asparaginase production by Streptomyces albidoflavus. Indian J Microbiol 48, 331–336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathom-Aree W. et al. Diversity of actinomycetes isolated from Challenger Deep sediment (10,898 m) from the Mariana Trench. Extremophiles 10, 181–189 (2006). [DOI] [PubMed] [Google Scholar]
- Amena S., Vishalakshi N., Prabhakar M., Dayanand A. & Lingappa K. Production, purification and characterization of L-asparaginase from Streptomyces gulbargensis. Brazil J Microbiol 41, 173–178 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar N. E., Moawad H., El-Shweihy N. M. & El-Ewasy S. M. Optimization of culture conditions for production of the anti-leukemic glutaminase free L-asparaginase by newly isolated Streptomyces olivaceus NEAE-119 using response surface methodology. BioMed Res Int (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar N. E. Extracellular production of the oncolytic enzyme, L-asparaginase, by newly isolated Streptomyces sp. strain NEAE-95 as potential microbial cell factories: Optimization of culture conditions using response surface methodology. Curr pharm biotechnol 16, 162–178 (2015). [DOI] [PubMed] [Google Scholar]
- El-Naggar N. E. & Moawad H. Streptomyces brollosae sp. nov., NEAE-115, a novel L-asparaginase producing actinomycete isolated from Brollos Lake at the Mediterranean coast of Egypt. J Pure Appl Microbiol 9, 11–20 (2015). [Google Scholar]
- Dharmaraj S. Study of L-asparaginase production by Streptomyces noursei MTCC 10469, isolated from marine sponge Callyspongia diffusa. Iran J Biotechnol 9, 102–108 (2011). [Google Scholar]
- Gupta N., Mishra S. & Basak U. Occurrence of Streptomyces aurantiacus in mangroves of Bhitarkanika. Malaysian J Microbiol 3, 7–14 (2007). [Google Scholar]
- Williams S. Genus Streptomyces waksman and henrici 1943. BERGEY’s Manual of Syntematic Bacteriology 4, 2452–2492 (1989). [Google Scholar]
- Altschul S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic acids res 25, 3389–3402 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N. & Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406–425 (1987). [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M. & Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24, 1596–1599 (2007). [DOI] [PubMed] [Google Scholar]
- Goodfellow M. et al. Bergey’s manual of systematic bacteriology, second edition, The Actinobacteria Part A. Second edition. Vol. 5. Springer, New York Dordrecht Heidelberg London. Library of Congress Control Number: 2012930836 (2012). [Google Scholar]
- El-Bessoumy A. A., Sarhan M. & Mansour J. Production, isolation, and purification of L-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. J Biochem Mol Biol 37, 387–393 (2004). [DOI] [PubMed] [Google Scholar]
- Dhevagi P. & Poorani E. Isolation and characterization of L-asparaginase from marine actinomycetes. Ind J Biotechnol 5, 514–520 (2006). [Google Scholar]
- Ohshima M., Yamamoto T. & Soda K. Further characterization of glutaminase isozymes from Pseudomonas aeruginosa. Agri Biologi Chem 40, 2251–2256 (1976). [Google Scholar]
- Selvam K. & Vishnupriya B. Partial purification and cytotoxic activity of L-asparaginase from Streptomyces acrimycini NGP. Int J Res Pharma Biomed 4, 859–69 (2013). [Google Scholar]
- Triantafillou D., Georgatsos J. & Kyriakidis D. Purification and properties of a membrane-bound L-asparaginase of Tetrahymena pyriformis. Mol Cell Biochem 81, 43–51 (1988). [DOI] [PubMed] [Google Scholar]
- Mesas J. M., Gil J. A. & Martín J. F. Characterization and partial purification of L-asparaginase from Corynebacterium glutamicum. J Gen Microbiol 136, 515–519 (1990). [DOI] [PubMed] [Google Scholar]
- Manna S., Sinha A., Sadhukhan R. & Chakrabarty S. Purification, characterization and antitumor activity of L-asparaginase isolated from Pseudomonas stutzeri MB-405. Curr Microbiol 30, 291–298 (1995). [DOI] [PubMed] [Google Scholar]
- Borkotaky B. & Bezbaruah R. Production and properties of asparaginase from a new Erwinia sp. Folia Microbiol 47, 473–476 (2002). [DOI] [PubMed] [Google Scholar]
- Wulff G. Enzyme-like catalysis by molecularly imprinted polymers. Chem Reviews 102, 1–28 (2002). [DOI] [PubMed] [Google Scholar]
- Bisswanger H. Multiple Equilibria. Enzyme Kinetics: Principles and Methods 5–50 (2002). [Google Scholar]
- Copeland R. A. Enzymes: a practical introduction to structure, mechanism, and data analysis. John Wiley & Sons (2004). [Google Scholar]
- Kumar S. & Selvam K. Isolation and purification of high efficiency L-asparaginase by quantitative preparative continuous-elution SDS PAGE electrophoresis. Microb Biochem Technol 3, 73–83 (2011). [Google Scholar]
- Maladkar N. K., Singh V. K. & Naik S. R. Fermentative production and isolation of L-asparaginase from Erwinia carotovora, EC-113. Hindustan antibiot bull 35, 77–86 (1992). [PubMed] [Google Scholar]
- Cedar H. & Schwartz J. H. Production of L-asparaginase II by Escherichia coli. J Bacteriol 96, 2043–2048 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. E. & Ciegler A. Production by Erwinia aroideae. Appl Microbiol 18, 64–67 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddalingeshwara K. G. & Lingappa K. Production and characterization of L-asparaginase-a tumor inhibitor. Int J Pharm Tech Res 3, 314–319 (2011). [Google Scholar]
- Stecher A. L., de Deus P. M., Polikarpov I. & Abrahao-Neto J. Stability of L-asparaginase: An enzyme used in leukemia treatment. Pharm Acta Helv 74, 1–9 (1999). [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970). [DOI] [PubMed] [Google Scholar]
- Aghaiypour K., Wlodawer A. & Lubkowski J. Structural basis for the activity and substrate specificity of Erwinia chrysanthemi L-asparaginase. Biochem 40, 5655–5664 (2001). [DOI] [PubMed] [Google Scholar]
- Kavitha A. & Vijayalakshmi M. Optimization and purification of L-asparaginase produced by Streptomyces tendae TK-VL_333. Z. Naturforsch 65, 528–531 (2010). [DOI] [PubMed] [Google Scholar]
- Soares A. L., Guimarães G. M., Polakiewicz B., de Moraes Pitombo R. N. & Abrahão-Neto J. Effects of polyethylene glycol attachment on physicochemical and biological stability of E. coli L-asparaginase. Int J Pharm 237, 163–170 (2002). [DOI] [PubMed] [Google Scholar]
- Borah D., Yadav R. N. S., Sangra A., Shahin L. & Chaubey A. K. Production, purification and process optimization of asparaginase. (an anticancer enzyme) from E. coli, isolated from sewage water. Asian J Pharm Clin Res 5, 202–204 (2012). [Google Scholar]
- Sanches M., Barbosa J. A., de Oliveira R. T., Abrahao Neto J. & Polikarpov I. Structural comparison of E. coli L-asparaginase in two monoclinic space groups. Acta Crystallogr Sect D 59, 416–422 (2003). [DOI] [PubMed] [Google Scholar]
- Morgenthaler J., Kemmner W. & Brossmer R. Sialic acid dependent cell adhesion to collagen IV correlates with in vivo tumourigenicity of the human colon carcinoma sublines HCT116, HCT116a and HCT116b. Biochem Biophys Res Commun 171, 860–866 (1990). [DOI] [PubMed] [Google Scholar]
- Matsushita Y., Nakamori S., Seftor E. A., Hendrix M. J. C. & Irimura T. Human colon carcinoma cells with increased invasive capacity obtained by selection for sialyl-dimericLeX antigen. Exp Cell Res 196, 20–5 (1991). [DOI] [PubMed] [Google Scholar]
- Flugy A. M. et al. E-selectin modulates the malignant properties of T84 colon carcinoma cells. Biochem Biophys Res Commun 293, 1099–1106 (2002). [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. & Kessel D. Inhibition of glycoprotein synthesis in L5178Y mouse leukaemic cells by L-asparaginase in vitro. Nature 226, 850–1 (1970). [DOI] [PubMed] [Google Scholar]
- Yu M. et al. L-asparaginase inhibits invasive and angiogenic activity and induces autophagy in ovarian cancer. J Cell Mol Med 16(10), 2369–2378 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejong P. J. L-Asparaginase production by Streptomyces griseus. Appl Microbial 23, 1163–1164 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati R., Saxena R. & Gupta R. A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett Appl Microbiol 24, 23–26 (1997). [DOI] [PubMed] [Google Scholar]
- Wriston J. & Yellin T. L-asparaginase: a review. Adv Enzymol Relat Areas Mol Biol 39, 185–248 (1973). [DOI] [PubMed] [Google Scholar]
- Shirling E. B. & Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16, 313–340 (1966). [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L. & Randall R. J. Protein measurement with the Folin phenol reagent. J biol Chem 193, 265–275 (1951). [PubMed] [Google Scholar]
- Lineweaver H. & Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 56, 658–666 (1934). [Google Scholar]
- Stone M. M. et al. Temperature sensitivity of soil enzyme kinetics under N‐fertilization in two temperate forests. Global Change Biol 18, 1173–1184 (2012). [Google Scholar]
- Weyermann J., Lochmann D. & Zimmer A. A practical note on the use of cytotoxicity assays. Int J Pharm 288, 369–76 (2005). [DOI] [PubMed] [Google Scholar]
- Koch A., Tamez P., Pezzuto J. & Soejarto D. Evaluation of plants used for antimalarial. 241 treatment by the Massai of Kenya. J Ethnopharmacol 101, 95–99 (2005). [DOI] [PubMed] [Google Scholar]
- Yassin A. M., Elnouby M., El-Deeb N. M. & Hafez E. E. Tungsten oxide nanoplates; the novelty in targeting metalloproteinase-7 gene in both cervix and colon cancer cells. Appl Biochem Biotechnol 10.1007/s12010-016-2120-xpp 1–15 [DOI] [PubMed] [Google Scholar]