Abstract
This manuscript describes the development of a culture system whereby mature contracting myotubes were formed from adult rat derived satellite cells. Satellite cells, extracted from the Tibialis Anterior (TA) of adult rats, were grown in defined serum-free growth and differentiation media, on a non-biological substrate, N-1[3-trimethoxysilyl propyl] diethylenetriamine. Myotubes were evaluated morphologically and immunocytochemically, using MyHC specific antibodies, as well as functionally using patch clamp electrophysiology to measure ion channel activity. Results indicated the establishment of the rapid expression of adult myosin isoforms that contrasts to their slow development in embryonic cultures. This culture system has applications in the understanding and treatment of age related muscle myopathy, muscular dystrophy, and for skeletal muscle engineering by providing a more relevant phenotype for both in vitro and in vivo applications.
Keywords: muscle, satellite cells, in vitro, serum-free, electrophysiology
Introduction
Adult tissue-derived in vitro systems provide a unique modality to study physiologic and age related diseases. The study of adult skeletal muscle physiology and myopathies has especially benefitted from the ease of isolation of satellite cells which are adult muscle progenitor cells. Satellite cells are situated between the sarcolemma and basal lamina of muscle fibers. These normally quiescent cells, activated in response to exercise and pathological injuries, migrate to the site of damage where they proliferate, elongate, and fuse to each other, as well as to existing myofibers, to replace lost or damaged cellular material.1
Currently, the prevailing dogma for the study of muscle related diseases and physiological phenomenon in vitro focuses on cells derived from adult animals grown in serum-containing medium formulations. Generally, animal-sera have been used in adult and embryonic muscle culture due to their ability to enable the rapid proliferation of muscle cells.2–4 Once the cells have reached a confluent monolayer, the removal, or drastic reduction, of serum concentration triggers extensive myotube formation in the cultured cells. This has been the long established protocol due to cost efficiency, relative ease of use, and general effectiveness.5 The drawback of this culture system is that the contents of animal sera have not been fully elucidated nor well characterized; there is also the batch to batch variability that occurs during serum production.6, 7 Therefore, one cannot rule out the possibility of adverse stimulatory or inhibitory effects occurring as a result of its use. Consequently, the clinical applications of an in vitro model system that uses serum containing medium formulations are limited.
Previous work has detailed the development of a serum-free system that mimics the in vivo process of myotube formation and the physiological contractile properties of myotubes derived from fetal hind-limb satellite cells.8–10 However, the use of embryonic muscle limits the applicability of these systems to the study of age related diseases and adult physiological processes such as Myosin Heavy Chain (MyHC) class switching.
To address this issue a culture system was developed where all aspects of the system were defined and controlled to provide a new model for the study of adult muscle physiology and disease. The process utilized satellite cells derived from adult rat hind-limbs, a defined serum-free media formulation, as well as a non-biological cell growth promoting substrate N-1[3-trimethoxysilyl propyl] diethylenetriamine (DETA). The triamine functionality of DETA is also a structural analog to spermidine, a growth factor known to promote cellular survival.11, 12 This system exhibited physiological maturation (adult MyHC expression) and responded to physiological stimuli to provide a suitable model for the study of age related disease and muscle myopathies. Combined with previous work modeling neuromuscular junction formation,13–16 new functional assays could be developed from the adult cells derived from transgenics or disease related animals for more relevant in vitro disease models. Recent FDA/NIH initiatives for regulatory science require the use of serum-free media and as such, a new serum-free system for the study of adult derived skeletal muscle myotubes will be of paramount importance in the advancement of muscle biology and in vitro drug discovery.
Materials and Methods
Surface Modification and Characterization
Glass coverslips (VWR cat. nr. 48366067, 22×22 mm2 No. 1) arranged in ceramic staining racks (Thomas Scientific, Swedesboro, NJ) were chemically cleaned and dried prior to surface modification. First, the coverslips were soaked in a solution of 50/50 methanol (VWR cat. nr. BJLP230-4) / hydrochloric acid (VWR cat. nr. EM1.00314.2503) for 2 hours and then rinsed thoroughly with DI water. The coverslips were then immersed in concentrated sulfuric acid (VWR cat. nr. BDH3072) for a minimum of 2 hours, rinsed as before and then boiled in deionized water for at least 30 minutes. After boiling the glass slides were placed in an oven set to 110°C and allowed to dry overnight. The 3-Trimethoxysilyl propyl diethylenetriamine (DETA), (United Chemical Technologies Inc. T2910) film was formed by the reaction of the cleaned and dried surfaces with a 0.1% (v/v) solution of the organosilane in freshly distilled toluene (VWR cat. nr. BDH1151). The DETA-toluene solution was prepared in a glove box (MBraun, Stratham, NH) under nitrogen atmosphere, and the reaction was carried out in the lab atmosphere in a dish covered with an inverted beaker. The dish containing the clean slides was heated to approximately 100°C for 30 minutes, and then allowed to cool to room temperature, when the slides were taken out of DETA solution, rinsed carefully three times with dry toluene and reheated in a fourth toluene rinse to approximately 100°C for 30 additional minutes. The DETA-coated slides were oven dried at 110°C for at least 2 hours prior to use or storage. Surfaces were characterized by static water contact angle measurements using a Rame-Hart Model 250 goniometer, and by X-ray Photoelectron Spectroscopy (XPS) using a VG ESCALAB 220i-XL spectrometer equipped with an aluminum anode and a quartz monochromator. The spectrometer was calibrated against the reference binding energies of a clean Ag sample. XPS survey scans were recorded in order to determine the relevant elements (pass energy of 50 eV, step size of 1 eV). Si 2p, C 1s, N 1s, and O 1s high resolution spectra were recorded in order to determine the quality of the surfaces (pass energy of 20 eV, step size of 0.1 eV). The fitting of the peaks was performed with Avantage version 3.25 software, provided by Thermo Electron Corporation.
Skeletal Muscle Isolation and Serum-Free Medium
The left and right Tibialis Anterior (TA) were dissected from Adult Sprague-Dawley rats aged 6–12 months (Charles-River Laboratories). Briefly, rats were euthanized by inhalation of an excess of CO2. This procedure complies with IACUC standards laid out by the Animal Research Council of University of Central Florida. The muscle was collected in a 15 ml conical tube and washed briefly in PBS to remove debris. The tissue was then minced into fine pieces and enzymatically dissociated in a 25 mg collagenase II (Worthington Biochemicals, LS004176), 1 mg dispase (Worthington Biochemicals, LS02104) solution in DMEM (Gibco, 11965) for 1 hour in a 37°C water bath at 100 rpm. Muscle was removed and triturated using fire polished glass pipettes. The suspension was plated on 100 mm uncoated dishes for 1 hour at 37°C, 100% relative humidity. The unattached cell suspension was collected and centrifuged at 300 g for 5 min at 4°C. The supernatant was removed and the pellet was re-suspended in proliferation medium (Table 1) and plated on DETA coverslips. The suspension was allowed to incubate for 45 minutes before additional medium was added. The cells were then serial plated twice on DETA coverslips to obtain a more pure population of myoblasts on the third coverslip. After 8 days the proliferation medium was slowly removed and replaced with differentiation medium (Table 2) to promote cell alignment and fusion. The cells were maintained in a 5% CO2 incubator and one half the differentiation media was changed every 3–4 days.
Table 1.
Composition of serum-free growth medium for a 500 ml sample.
| Component | Company | Catalogue # | Quantity |
|---|---|---|---|
| Neurobasal | Invitrogen | 21103049 | 250 ml |
| L15 | Invitrogen | 11415064 | 250 ml |
| aFGF | Invitrogen | 13241-013 | 10 ug |
| Antibiotic-antimycotic | Invitrogen | 15240062 | 5 ml |
| Calcium chloride | Fisher | 10035-04-8 | 250 ug |
| VEGF | RND systems | 293-ve-010 | 10 ug |
| bFGF | RND Systems | 3339-FB-025 | 20 ug |
| CNTF | Cell Sciences | CRC 401B | 20 ug |
| NT-3 | Cell Sciences | CRN 500B | 10 ug |
| Nt-4 | Cell Sciences | CRN 501B | 10 ug |
| GDNF | Cell Sciences | CRG 400B | 10 ug |
| BDNF | Cell Sciences | CRB 600B | 10 ug |
| CT-1 | Cell Sciences | CRC 700B | 10 ug |
| LIF | Sigma | L5158 | 10 ug |
| Vitronectin | Sigma | V0132 | 50 ug |
Table 2.
Composition of serum free differentiation medium for 500 ml.
| Component | Company | Catalogue # | Quantity |
|---|---|---|---|
| Neurobasal | Invitrogen | 21103049 | 250 ml |
| L15 | Invitrogen | 11415064 | 250 ml |
| EGF | Invitrogen | 53003018 | 50 ug |
| IGF | Sigma | I2656 | 5 ug |
Immunocytochemistry
Myosin Heavy Chain
Coverslips were rinsed with PBS and fixed in 4% paraformaldehyde for 10 min. They were then washed in PBS, incubated in PBS supplemented with 1% bovine serum albumin and 0.1% triton X-100 (permeabilization solution) for 20 min, before being blocked for 30 min in the permeabilization solution + 5% donkey serum (blocking solution). The fixed cells were incubated in blocking solution overnight at 4°C with either a primary antibody against MyHC all classes (A4.1025, IgG, Developmental Studies Hybridoma Bank), diluted 1:10, a MyHC slow antibody (Sigma M8421), diluted 1:500, or a MyHC fast antibody (abcam ab91506), diluted 1:1000. Coverslips were washed with PBS and incubated with Alexafluor secondary antibodies (Invitrogen) diluted (1:400) in PBS for 2 hours at ambient temperature in the dark. After rinsing in PBS, the coverslips were mounted on glass slides using VectaShield + DAPI mounting media (Vector Laboratories H1200) and viewed on a confocal microscope (UltraVIEW™ LCI, PerkinElmer).
Double Staining with Pax-7 and Myo-D
Cultures were processed for immunocytochemistry as described above. Next, cells were incubated overnight at 4°C with primary antibodies against Myo-D (abcam ab16148), diluted 1:1500, and Pax-7 (abcam ab34360), diluted 1:3000.
Patch Clamp Electrophysiology
Whole-cell patch clamp recordings of the mature myotubes were performed in a recording chamber located on the stage of a Zeiss Axioscope 2FS Plus upright microscope as described previously.13 Patch pipettes were prepared from borosilicate glass (BF150-86-10; Sutter, Novato, CA) with a Sutter P97 pipette puller and filled with intracellular solution (in mM: K-gluconate 140, EGTA 1, MgCl2 2, Na2ATP 2, phosphocreatine 5, phosphocreatine kinase 2.4 mg, Hepes 10; pH = 7.2). The resistance of the electrodes was 6–8 MΩ. Voltage clamp and current clamp experiments were performed with a Multiclamp 700A amplifier (Axon Laboratories, Union City, CA). Signals were filtered at 2 kHz and digitized at 20 kHz with an Axon Digidata 1322A interface. Data recording and analysis were done with pClamp 8 software (Axon Laboratories). Membrane potentials were corrected by subtraction of a 15 mV tip potential, which was calculated using Axon’s pClamp 8 program. Sodium and potassium currents were measured in voltage clamp mode using voltage steps from a −85 mV holding potential. Action potentials were evoked with 1 s depolarizing current injections from a −85 mV holding potential.
Statistical Analysis
30 random fields of view were analyzed across 3 independent experiments. The number of fields of view analyzed was deemed sufficient to provide an accurate representation of the mean as assessed by cumulative frequency analysis (data not shown).
Results and Discussion
DETA surface Modification and Characterization
DETA is a spermidine analog that has been shown to promote the proliferation, maturation and long term survival of an array of cell types.11, 12, 14, 15, 17 Static contact angle and XPS analysis was used for validation of the surface modifications. Contact angles of 46° +/− 2° were shown to be consistent and reproducible across this study. XPS measurements for the ratio of N (1s) to Si (2p) of 1500 +/− 200 indicated that a reaction site limited DETA monolayer was formed on the coverslips.
Development of Serum-free Medium and Plating Techniques
The starting medium formulation, surface modification and plating technique was developed previously by our lab to create a system for the development of myotubes derived from embryonic E18 rat hind-limbs.10 However, this system was insufficient to enable the development of myotubes from satellite cells isolated from the TA of adult rats. The original medium formulation was altered by the addition of bFGF, which is well established as a promoter of muscle proliferation,18 L15 medium, which was previously shown to promote myoblast survival,9 and the addition of calcium, in the form of CaCl2.19 This reformulated medium was sufficient to promote the proliferation of myoblasts. The plating technique was modified by introducing a serial plating protocol whereby the cell suspension was removed after 24 hours and re-plated on a new DETA coverslip.20 After the second serial plating it was determined that the culture had a higher proportion of myoblasts and cell survival in the culture was increased.
After cell proliferation and culture purity were optimized, it was also necessary to alter the technique by which differentiation of myoblasts into myotubes was induced. The previously devised technique was to shock the cultures into differentiation by completely changing the growth medium to differentiation medium after 48 hours of proliferation.10 It was observed that if this technique was employed, it resulted in complete cell death within 12 hours. Instead a gradual decline in growth factors was employed to enable, cell survival and maturation into multinucleated myotubes. 300 uL of growth medium was removed daily and replaced with an equivalent volume of a defined serum-free differentiation medium (Table 2) over the course of 5 days. This gradual reduction in growth factors resulted in robust cell survival and myotube formation. The combination of all these changes resulted in the establishment of a system by which mature myotubes could be routinely formed from adult satellite cells (Figure 1). Functional contractile in vitro myotubes tend to differ slightly from mature in vivo myofibers morphologically; they lack peripheral nuclei and, despite forming well-defined sarcomeric structures, they usually do not promote the formation of extensive and highly organized myofibrils. Therefore, for the purposes of this manuscript, these differentiated myogenic cells are referred to as functional myotubes, rather than myofibers.
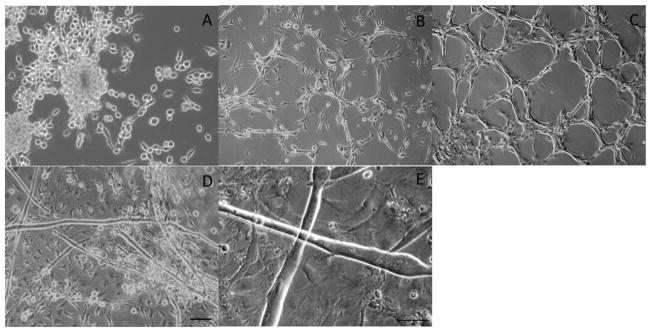
Figure 1.
Phase images of myoblasts and myotubes derived from the Tibalias anterior of adult Sprague Dawley rats. Phase images of proliferating myoblasts at (A) 2 DIV, (B) 4 DIV, (C) 8 DIV. Phase images of fused myoblasts at 12 DIV (D,E). Scale Bar 50 μm, the scale bar in panel D pertains to panels A–D.
Characterization of the Satellite Cells and Resultant Myotubes
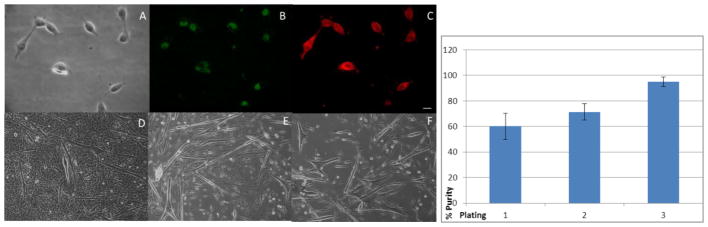
Satellite cells express the transcription factor Pax-7. Cells of a myogenic lineage will also express the muscle specific marker Myo-D. There is some contention as to whether Pax-7 is expressed in quiescent versus activated satellite cells, nevertheless it is ubiquitously expressed in satellite cells as a whole.21–23 As such, these markers were used to quantify the number and purity of muscle satellite cells isolated from the TA. Figure 2a shows the co-localization of Pax-7 and Myo-D in myoblasts expanding in proliferation medium after 2 days in vitro (DIV). Multiple coverslips were imaged, using cumulative frequency analysis, and the cultures were determined to be 60.15% +/− 10.39% positive for both Pax-7 and MyoD in the first plating, 71.36% +/− 6.3% for the second plating, and 94.96% +/− 3.85% after the final serial plating (Figure 2b). This establishes that serial plating was needed to provide a relatively pure culture of proliferating myoblasts that promotes myotube formation in the serum-free medium. Figure 2a, panels D-F, also shows phase images of the myotubes from 12 DIV, indicating the continued purity of the culture.
Figure 2.
Figure 2a. Pax-7 and Myo-D immunostaing of 2 DIV myoblasts derived from plate three of serial plating. (A) Phase image of myoblasts, (B) Pax-7 transcription factor staining, (C) Myo-D muscle marker staining. Phase images indicating the relative purity of myotube cultures from serial plating at 12 DIV. (E) Plate 1, (D) Plate 2, (F) Plate 3. Scale bar 10 μm. Figure 2b: Indicates the relative purity of the culture starting at 60% purity at plate 1 going to 94% purity at plate 3.
Immunocytochemical Characterization of the Myotubes
After induction of myoblast fusion into myotubes the culture was characterized immunocytochemically. DIV 14 myotubes were stained with anti-MyHC antibody A4.1025 from the DSHB. MyHC is an essential element in the contractile apparatus of mature myotubes. Figure 3 shows highly striated muscle indicating mature myotubes with a highly developed contractile apparatus.
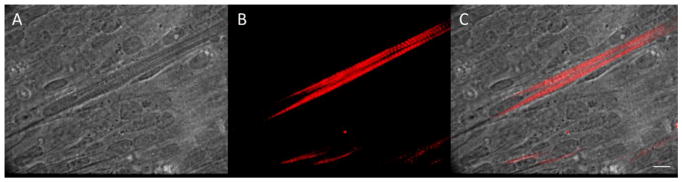
Figure 3.
(A) Phase image (B) MYHC immunostaining and (C) overlay of 14 DIV myotubes. A4.1025 MyHC antibody staining showing highly striated myotubes. Striations are an indication of a highly organized contractile apparatus and mature myotubes. Scale Bar 50 μm.
Characterizing Mature MyHC expression
One of the limiting factors in the use of embryonic derived cultures is the limitation for those cultures to express more advanced isoforms of MyHC such as neonatal and/or adult.24, 25 Figure 4 shows positive staining for both the mature MyHC slow isoform as well as the mature MyHC fast isoform. It was determined that approximately 73.89 ± 4.6% of myotubes at 12 DIV expressed the MyHC fast twitch isoform and that 10.67 ± 1.1% of those myotubes also expressed the mature slow twitch isoform. This is in contrast to previous results indicating approximately 25% neonatal/adult conversion after 45 DIV for embryonic derived tissue.17
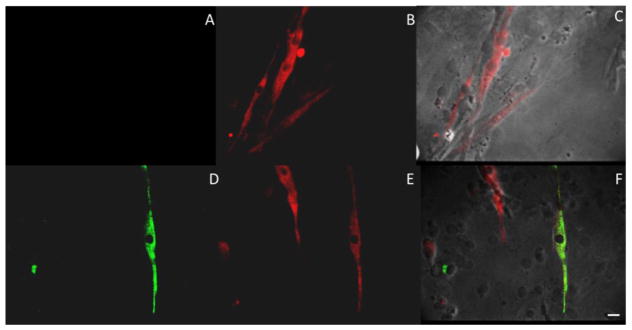
Figure 4.
Immunostaining of 12 DIV myotubes for adult MyHC isoforms. Panels (A and D) show staining for adult MyHC type I isoform for two separate coverslips. Panels (B and E) show staining for adult MyHC type IIb isoforms for those same myotubes. Panels (C and F) are the composite images of those cultures. Cultures indicate at 12 DIV 73.89 ± 4.6% of myotubes stain for type IIb and approximately 10.67 ± 1.1% of those also stain for type I isoform. Scale Bar 20 μm.
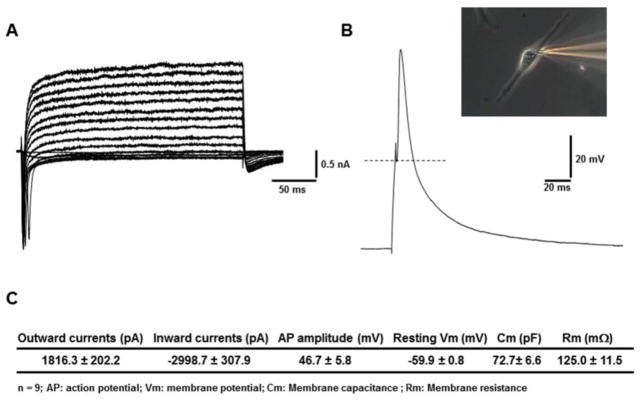
Electrophysiological Characterization
Muscle specific myoblasts were shown to fuse into MyHC positive myotubes. These myotubes were then tested to determine if the ion channels were functional utilizing whole cell patch clamp electrophysiology. DIV 14 myotubes were patched according to the described protocol and as can be seen in Figure 5 fired action potentials (APs) when stimulated. The myotubes expressed a mean inward current of −2998.7 +/− 307.9 pA with a mean outward current of 1816.3 +/− 202.2 pA indicating functional sodium and potassium channels and the APs had a mean amplitude of 46.7 +/− 5.8 mV (errors are expressed as +/− the SEM, with n = 9). The resting membrane potential of −59.9 +/− .8 mV measured in our system more closely mimics ideal in vivo potentials than previously reported membrane potentials of comparably aged in vitro rat myotubes in serum-containing medium.26
Figure 5.
Patch clamp electrophysiology of adult tissue derived myotubes. (A) Representative voltage clamp trace obtained from a 14 DIV myotube. (B) Representative current clamp trace of same myotube. Inset is a phase image of the myotube. (C) Statistics showing mean parameters derived from n = 9 myotubes.
The results described above document the development of a new serum free adult skeletal muscle culture system. This system furthers the evolution of physiologically relevant in vitro myotube models. Specifically, this manuscript documents the development of a medium formulation, surface composition and culture technique that results in myotubes that better recapitulates in vivo functionality and maturation by more rapid expression of adult myosin isoforms compared to embryonic tissue-derived myotube cultures.
Current in vitro muscle cultures derived from adult tissue primarily rely on animal sera for the division and growth of myoblasts.27 The limitations of this approach are the variability of serum production and the lack of definition of the contents. A serum-free medium was developed, supplemented with a cocktail of defined growth factors supporting adult-derived myoblast proliferation and fusion as well as myotube maturation, both structurally and functionally. The completely defined nature of this medium formulation also makes it advantageous for high-content drug screening and in vitro/in vivo correlative studies. It could also be utilized in tissue engineering and regenerative medicine investigations by demonstrating new techniques for isolation and expansion of adult satellite cells that are capable of functional maturation.
Previously, studies conducted in our lab resulted in the development of a defined skeletal muscle myotube system derived from the hind-limbs of embryonic day 18 rat fetuses.10 This culture system provided a starting point for studying myotube physiology in a serum-free environment, but was limited in its ability to reproduce in vivo mature muscle characteristics. This previous work has shown, and is supported by observations from our experiments, that MyHC class switching from an embryonic isoform to a more adult like phenotype occurs over a 45–50 day culture period, after which the culture will express ~25% neonatal/adult MyHC isoforms.10 While the use of DETA provides for long term muscle cultures, up to a maximum of 6 months in our hands (data not shown), it would be more time efficient and cost effective to have myotube cultures that express adult MyHC isoforms at earlier time points.
We have shown here that skeletal muscle myofibers derived from the TA of adult rats undergo a more accelerated MyHC isoform switching than do their embryonic muscle counterparts under these serum-free media conditions and using a defined organosilane surface. Figure 4 shows both mature MyHC fast-twitch fiber expression and MyHC slow-twitch fiber type expression by day 12 in culture. This equates to a 75% decrease in time to maturity over embryonic derived muscle in serum-free conditions. We have also shown that 73.89+/−4.6% of myotubes in culture at day 12 expressed the mature MyHC fast twitch isoform, and 10.67 +/−1.1% of those also expressing the adult type slow twitch isoform; a 3-fold increase in mature MyHC expression over an embryonic-derived culture system.10, 17, 28 The ratio of fast to slow twitch isoform expression in myotubes maintained for 12 DIV in serum-free conditions mirrors data attained using serum-containing media at equivalent time-points.25
It was observed that 31.0% +/− 19.1% of the 12 DIV myotubes contracted spontaneously at any one time, as well as approximately 70% of myotubes contracted under broad field stimulation.29 Further studies have shown that this new culture technique and medium formulation allows for the interrogation of myotube contractile force and fatigue.29 This system will allow for ease of isolation of tissue, reduction in culture cost for media, reduction in time to analysis, and increased relevance to mature muscle, making it a more representative culture system to study adult myopathies. This formulation is also compatible with neuronal culture, so it should facilitate multi cellular construct formation in vivo. Furthermore, the adult nature of the myoblasts used in this system facilitates the accurate investigation of adult myopathies, muscle regeneration and neuromuscular junction physiology. This opens new possibilities of directly culturing cells from transgenic animals after they began exhibiting their deficits or changes due to genetic manipulation.
Conclusion
In conclusion, this serum-free skeletal myotube culture system provides a model by which to investigate the role specific growth factors and drugs might play on adult physiological processes such as myotube maturation as well as disease processes associated with muscle regeneration including the muscular dystrophies.
Acknowledgments
We would like to acknowledge NIH grant number(s) R01NS050452, R01EB005459 and R01EB009429 for supporting this work.
Footnotes
Author Disclosure Statement
“No competing financial interests exist.”
Contributor Information
Christopher W. McAleer, NanoScience Technology Center, University of Central Florida, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA
John W. Rumsey, NanoScience Technology Center, University of Central Florida, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA.
Maria Stancescu, NanoScience Technology Center, University of Central Florida, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA.
James J. Hickman, NanoScience Technology Center, University of Central Florida, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA.
Literature Cited
- 1.Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 2.Hayward LJ, Schwartz RJ. Sequential expression of chicken actin genes during myogenesis. J Cell Biol. 1986;102:1485–1493. doi: 10.1083/jcb.102.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced differentiation potential of primary myod −/− myogenic cells derived from adult skeletal muscle. J Cell Biol. 1999;144:631–643. doi: 10.1083/jcb.144.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, Valentini R, Crawford G. A drug screening platform based on the contractility of tissue engineered muscle. Muscle Nerve. 2008;37:438–447. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 5.Springer ML, Rando TA, Blau HM. Current protocols in human genetics. John Wiley & Sons, Inc; 2001. Gene delivery to muscle. [DOI] [PubMed] [Google Scholar]
- 6.Bjare U. Serum-free cell culture. Pharmacol Ther. 1992;53:355–374. doi: 10.1016/0163-7258(92)90056-6. [DOI] [PubMed] [Google Scholar]
- 7.van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, Thalen M, Baumans V. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol in Vitro. 2004;18:1–12. doi: 10.1016/j.tiv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Allen R, Dodson M, Luiten L, Boxhorn L. A serum-free medium that supports the growth of cultured skeletal muscle satellite cells. In Vitro Cell Dev Biol. 1985;21:636–640. doi: 10.1007/BF02623296. [DOI] [PubMed] [Google Scholar]
- 9.Das M, Gregory CA, Molnar P, Riedel LM, Wilson K, Hickman JJ. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27:4374–4380. doi: 10.1016/j.biomaterials.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Das M, Rumsey JW, Bhargava N, Gregory C, Riedel L, Kang JF, Hickman JJ. Developing a novel serum-free cell culture model of skeletal muscle differentiation by systematically studying the role of different growth factors in myotube formation. In Vitro Cell Dev Biol Animal. 2009;45:378–387. doi: 10.1007/s11626-009-9192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg T, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 12.Kaeberein M. Spermidine surprise for a long life. Nat Cell Biol. 2009;11:1277–1278. doi: 10.1038/ncb1109-1277. [DOI] [PubMed] [Google Scholar]
- 13.Das M, Rumsey JW, Gregory CA, Bhargava N, Kang JF, Molnar P, Riedel L, Guo X, Hickman JJ. Embryonic motor neuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007;146:481–488. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Gonzalez M, Stancescu M, Vandenburgh H, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32:9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X, Das M, Rumsey JW, Gonzalez M, Stancescu M, Hickman JJ. Neuromuscular junction formation between human stem cell derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng Part C Methods. 2010;16:1347–1355. doi: 10.1089/ten.tec.2010.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukazawa T, Matsumoto M, Imura T, Khalesi E, Kajiume T, Kawahara Y, Tanimoto K, Yuge L. Electrical stimulation accelerates neuromuscular junction formation through adam19/neuregulin/erbb signaling in vitro. Neurosci Lett. 2013;545:29–34. doi: 10.1016/j.neulet.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31:4880–4888. doi: 10.1016/j.biomaterials.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannon K, Kudla AJ, McAvoy MJ, Clase KL, Olwin BB. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J Cell Biol. 1996;132:1151–1159. doi: 10.1083/jcb.132.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przybylski R, MacBride R, Kirby A. Calcium regulation of skeletal myogenesis. I. Cell content critical to myotube formation. In Vitro Cell Dev Biol Plant. 1989;25:830–838. doi: 10.1007/BF02623667. [DOI] [PubMed] [Google Scholar]
- 20.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 22.Tapscott SJ, Weintraub H. Myod and the regulation of myogenesis by helix-loop-helix proteins. J Clin Invest. 1991;87:1133–1138. doi: 10.1172/JCI115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yablonka-Reuveni Z, Day K, Vine A, Shefer G. Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci. 2008;86:E207–E216. doi: 10.2527/jas.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. Skeletal muscle tissue engineering: A maturation model promoting long-term survival of myotubes, structural development of the excitation-contraction coupling apparatus and neonatal myosin heavy chain expression. Biomaterials. 2009;30:5392–5402. doi: 10.1016/j.biomaterials.2009.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torgan CE, Daniels MP. Regulation of myosin heavy chain expression during rat skeletal muscle development in vitro. Mol Biol Cell. 2001;12:1499–1508. doi: 10.1091/mbc.12.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie AK, Fambrough DM. Electrophysiological properties of the membrane and acetylcholine receptor in developing rat and chick myotubes. J Gen Physiol. 1975;66:327–355. doi: 10.1085/jgp.66.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniels MP, Lowe BT, Shah S, Ma JX, Samuelson SJ, Lugo B, Parakh T, Uhm CS. Rodent nerve-muscle cell culture system for studies of neuromuscular junction development: Refinements and applications. Microsc Res Tech. 2000;49:26–37. doi: 10.1002/(SICI)1097-0029(20000401)49:1<26::AID-JEMT4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka Y, Inoue A. Controlled differentiation of myoblast cells into fast and slow muscle fibers. Cell Tissue Res. 2008;332:123–132. doi: 10.1007/s00441-008-0582-z. [DOI] [PubMed] [Google Scholar]
- 29.McAleer CW, Smith AST, Najjar S, Pirozzi K, Long CJ, Hickman JJ. Mechanistic investigation of adult myotube response to exercise and drug treatment in vitro using a multiplexed functional assay system. J Appl Physiol. 2014 doi: 10.1152/japplphysiol.00612.2014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]