Abstract
Cognitive impairment in age-related neurodegenerative diseases such as Alzheimer's disease may be partly due to long-term exposure and increased susceptibility to inflammatory insults. In the current study, we investigated whether polyphenols in blueberries can reduce the deleterious effects of inflammation induced by central administration of kainic acid by altering the expression of genes associated with inflammation. To this end, 4-month-old male Fischer-344 (F344) rats were fed a control, 0.015% piroxicam (an NSAID) or 2% blueberry diet for 8 weeks before either Ringer's buffer or kainic acid was bilaterally micro-infused into the hippocampus. Two weeks later, following behavioral evaluation, the rats were killed and total RNA from the hippocampus was extracted and used in real-time quantitative RT-PCR (qRT-PCR) to analyze the expression of inflammation-related genes. Kainic acid had deleterious effects on cognitive behavior as kainic acid-injected rats on the control diet exhibited increased latencies to find a hidden platform in the Morris water maze compared to Ringer's buffer-injected rats and utilized non-spatial strategies during probe trials. The blueberry diet, and to a lesser degree the piroxicam diet, was able to improve cognitive performance. Immunohistochemical analyses of OX-6 expression revealed that kainic acid produced an inflammatory response by increasing the OX-6 positive areas in the hippocampus of kainic acid-injected rats. Kainic acid up-regulated the expression of the inflammatory cytokines IL-1β and TNF-α, the neurotrophic factor IGF-1, and the transcription factor NF-κB. Blueberry and piroxicam supplementations were found to attenuate the kainic acid-induced increase in the expression of IL-1β, TNF-α, and NF-κB, while only blueberry was able to augment the increased IGF-1 expression. These results indicate that blueberry polyphenols attenuate learning impairments following neurotoxic insult and exert anti-inflammatory actions, perhaps via alteration of gene expression.
Keywords: Morris water maze, memory, inflammation, aging, oxidative stress, antioxidants, anti-inflammatories, dietary supplementation, blueberries, piroxicam, OX-6, cytokines, flavonoids
Introduction
Inflammation is thought to be a major factor in brain aging and in age-related neurodegenerative diseases such as Alzheimer's disease. Products of inflammatory reactions, such as cytokines, complement proteins, and adhesion molecules are neurotoxic, and may represent extracellular signals that initiate neuronal degeneration through several intracellular pathways.1–5 Interestingly, several inflammatory mediators, produced by multiple endogenous sources including microglia, astrocytes, and brain endothelial cells,6 have been identified in the Alzheimer's disease brain over the last two decades, and the levels of inflammatory cytokines and complement proteins are significantly elevated in the brains of Alzheimer's disease patients.7,8
Cognitive impairment, alone or as part of an age-related neurodegenerative disorder such as Alzheimer's disease, may be due in part to a combination of, or interaction between, long-term exposure and increased susceptibility to inflammatory insults.9–12 As previously reported with respect to oxidative stress,13 we suggest that changes resulting from aging establish environmental conditions in the brain for further inflammatory insult in Alzheimer's disease. Therefore, increasing anti-inflammatory protection in the brain and/or reducing brain susceptibility to inflammatory insults should retard or even reverse the deleterious effects of inflammation.
Previous findings from our research group and others have suggested that this might be accomplished by increasing the dietary intake of fruits and vegetables (see Joseph et al.14 for review). Foods which contain dietary polyphenolics that are high in antioxidant and anti-inflammatory activity,15,16 such as blueberries and strawberries, can prevent and reverse the occurrence of the neurochemical and behavioral changes that characterize the aged organism.17–23 In this regard, our research group has demonstrated in several studies that diets enriched with different varieties of berry-fruit, particularly blueberries, as well as purple grape juice, were able to reverse several parameters of brain aging (e.g. deficits in cell communication), such as dopamine release, as well as age-related motor and cognitive deficits, when fed to aged rats for 2 months.17,19,21,22,24,25
In addition to having antioxidant and anti-inflammatory effects, other possible mechanisms for the berry-fruit's positive effects include: direct effects on signaling to enhance neuronal communication,20 the ability to buffer against excess calcium,26 enhancement of neuroprotective stress shock proteins,27 and reduction of NF-κB.23 Additionally, the anthocyanins contained in blueberries have been shown to enter the brain, and their concentrations were correlated with cognitive performance.28 Measures of hippocampal plasticity, including neurogenesis, were also enhanced in rats on a blueberry-enriched diet.18 Additionally, supplementation with blueberries increased the levels of insulin-like growth factor-1 (IGF-1) and its receptor IGF-1R, and these increases were significantly correlated with improvements in cognitive performance and significantly associated with increases in extracellular signal regulated kinase (ERK) activation.18 Furthermore, the potential anti-inflammatory actions of polyphenolics are supported by a number of studies that have shown them to be able to antagonize arachidonic acid transport29 and suppress the 5-lipoxygenase pathway30 and thus reduce inflammatory responses,31,32 as well as regulate signal transduction processes/transcription factors involved in the regulation of inflammatory genes.33
The current study investigated whether the polyphenols in blueberries can reduce the deleterious effects of inflammation on cognition by altering the expression of inflammatory genes using central administration of kainic acid as a model to induce an inflammatory response. Injections of kainic acid, a neurotoxin and an analog of the excitatory amino acid glutamate, have been shown to induce a characteristic behavioral syndrome and neurodegeneration in several brain areas including the hippocampus, an important neural locus for learning and memory. Kainic acid injections induce selective neuronal loss to hippocampal neurons containing ionotropic kainate glutamatergic receptors.34 We examined the performance of treated young rats in a Morris water maze task and then collected brain tissue for analyzing several markers of inflammatory response in the hippocampus. The hypothesis was that rats on a 2% blueberry diet (equivalent to a human eating approximately 1 cup of blueberries daily) would exhibit less kainic acid-induced impairment in the Morris water maze task and less expression of inflammatory markers compared to rats on a control diet, or a diet containing the non-steroidal anti-inflammatory drug, piroxicam, used as a positive control.
Materials and methods
Animals
Seventy male F344 rats (Harlan Sprague Dawley, Indianapolis, IN, USA), weighing 269–332 g (mean ± SEM, 294.11 ± 1.49 g) and 3 months of age at the start of the experiment, were used in this study. They were individually housed in stainless steel mesh suspended cages, provided food and water ad libitum, and maintained on a 12-h light/dark cycle. Following at least a 2-week acclimatization period to the facility, the rats were weight-matched and then randomly assigned to one of three diet groups – control, 2% blueberry diet, or 0.015% piroxicam diet. All animals were observed daily for clinical signs of disease. These animals were utilized in compliance with all applicable laws and regulations as well as principles expressed in the National Institutes of Health, USPHS, Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Use and Care Committee of the USDA, HNRCA at Tufts University.
Diets
The blueberry diet was prepared by homogenizing blueberries (1:1 w/v) for 3 min and then centrifuging the recovered homogenate at 13,000 g for 15 min at 4°C. The supernatant was then frozen, crushed, and lyophilized, and the freeze-dried extracts were shipped to Harlan Teklad (Madison, WI, USA) where they were combined with the control diet, which was a modification of the NIH-31 diet (20 g/kg diet, 2% w/w). This diet is the same one used in previous studies in which beneficial effects on aging were found.18,20,22,23 The piroxicam diet was prepared by adding piroxicam powder (P0847; Sigma-Aldrich, St Louis, MO, USA) to the NIH-31 diet (0.15 g/kg diet, 0.015% w/w). For both diets, the amount of corn in the control diet was adjusted to compensate for the added volume. The rats were maintained on either the control, blueberry, or piroxicam diet for 8 weeks prior to kainic acid micro-infusion.
Kainic acid micro-infusions
Kainic acid (K0250, Sigma-Aldrich) or Ringer's buffer was bilaterally micro-infused stereotaxically into the CA3 region of the hippocampus (kainic acid dose = 300 ng in 0.5 μl Ringer's buffer each side for a total of 600 ng) during a 3-min infusion. Rats were anesthetized with pentobarbital (50 mg/kg) and restrained in a stereotaxic instrument. A 1-μl Hamilton microsyringe was mounted onto the stereotaxic instrument and lowered to coordinates A/P −3.6 mm; M/L ± 2.4 mm; D/V −3.0 mm. Seven of the original 70 rats did not survive the micro-infusion procedure. The rats were subsequently divided into 6 groups: control diet-Ringer's (control diet-R, n = 10), control diet-kainic acid (control diet-KA (n = 12), blueberry diet-Ringer's, (BB-R, n = 9), blueberry–kainic acid (BB-KA, n = 12), piroxicam diet-Ringer's (PX-R, n = 9), piroxicam–kainic acid (PX-KA, n = 11), although afterwards two rats in the PX-KA group were unable to perform the behavioral tasks. Animals were allowed to recover in polycarbonate cages with contact bedding for 1 week before being transferred back to the suspended cages for behavioral testing.
Morris water maze testing
The Morris water maze is a commonly used age35–37 and diet19,22,38 sensitive spatial learning and memory paradigm. This paradigm requires that rats to find the location of a hidden platform (10 cm in diameter) just below the surface (2 cm) of a circular pool of water (134 cm in diameter × 50 cm in height, maintained at 23°C) based on distal cues that remain constant from trial to trial while placement into the maze on given trials varies.39 Accurate navigation to the platform is rewarded by escape from the water. The maze is placed in a room with the lights dimmed, and there are numerous extra-maze cues placed on the walls that the rat can use to navigate the maze.
Rats were given four consecutive days of training in the Morris water maze (6 trials/day) 10 days following Ringer's buffer or kainic acid micro-infusion. At the beginning of each trial, the rat was gently immersed in the water, facing the wall, at one of three randomized start positions (located in the center of each quadrant not containing the platform). Each rat was allowed 60 s to escape onto the platform; if the rat failed to escape within this time, it was guided to the platform. Once the rat reached the platform, it remained there for 10 s. At the end of each trial, the rat was towel-dried, returned to its home cage for approximately 15–20 min (during which the remaining rats were tested) before being returned to the maze for its next trial. On days 2 and 3, trial 6 was a probe trial (60 s swim), where the platform was removed and the rat's swim path was monitored to assess the development of a spatially guided learning strategy. On day 4, a reversal test (platform moved to the quadrant diagonally opposite to the training quadrant) was performed for 5 trials, followed by a probe trial, in order to assess the ability of the rats to relearn a new platform location. Performances were videotaped and analyzed with image tracking software (HVS Image, UK), which allows measurements of latency to find the platform (s), path length (cm), and swimming speed (cm/s), as well as information on spatial strategies during the probe trials, such as number of crossings and time spent in the area where the platform had been previously located. Detailed descriptions of the maze and the paradigm can be found in a previous publication.40
OX-6 determination
One day following behavioral testing, one-half of the rats in each group were perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, and the brains prepared for immunohistochemical measurement of inflammatory markers using primary antibodies against OX-6, a monomorphic determinant for rat class II MHC molecules on macrophage-like cells and reactive microglia. The sections were incubated at room temperature overnight with purified anti-rat RT1B (OX-6; BD PharMingen, San Diego, CA, USA) diluted 1:400. The sections were incubated with biotinylated secondary antibody followed by incubation with avidin and biotinylated peroxidaseconjugated tertiary antibody complex (ABC Elite; Vector Laboratories, Burlingame, CA, USA). Immunostaining was visualized enzymatically with chromogen 3,3′-diaminobenzidine (DAB substrate kit, Vector Laboratories). OX-6 immunoreactivity was quantified by two independent raters, on a scale of 1–8 (1 being no activation and 8 being high activation).
Total RNA isolation and real-time qRT-PCR analysis
One day following behavioral testing, one-half of the rats in each group were decapitated, hippocampi were dissected out and immediately frozen in liquid nitrogen, then stored at −80°C for later analysis. Total RNA was extracted from the hippocampal tissues, and residual genomic DNA was digested by RNase-free DNase I with the RNeasy kit from Qiagen (Valencia, CA, USA). RNA concentration was assessed by RiboGreen RNA Assay kit from Invitrogen (Carlsbad, CA, USA). One microgram of total RNA was used to synthesize cDNA in 20 μl total volume with the ImProm-II reverse transcription system from Promega Corp. (Madison, WI, USA). At the conclusion of reverse transcription, the total volume of the reaction mix was increased to 125 μl by adding DNase/RNase-free water and 4 μl of the resulting cDNA template was subsequently used for real-time PCR. Real-time PCR was carried out for each sample in duplicates on an ABI PRISM 7700 Sequence Detection System in 25 μl total volume with the SYBR Green PCR Master Mix (Foster City, CA, USA). Relative mRNA expression was measured in several markers: the neurotrophic factor IGF-1, inflammatory cytokines IL-1β and TNF-α, and the transcription factor NF-κB. The primers (sequences available upon request) for PCR amplification were designed with Primer3 software.41 A comparative threshold cycle (CT) method was used to analyze the real-time PCR data where the amount of target, normalized to an endogenous reference (β-actin) and relative to a calibrator (untreated sample), was calculated by the equation 2−ΔΔCT (for derivation of the equation see Livak and Schmittgen42).
Statistical analyses
For each measure, analysis of variance (ANOVA) models using group as a between-subjects factor were performed using Systat (SPSS, Inc., Chicago, IL, USA) to test for statistical significance at the P < 0.05 level (overall ANOVA results for group are presented in the figure captions). Post-hoc comparisons, to determine differences among the groups, were performed using Fisher's LSD post-hoc analysis. Three specific comparisons included: the kainic acid-injected versus the matched diet Ringer's buffer group; differences from the control diet-Ringer's buffer group; and differences from the control diet–kainic acid group. For the Morris water maze, trials over each day were averaged, and each day was analyzed separately. Escape latency was one dependent measure in the Morris water maze, while on the probe trials, number of crossings of and latency to the region of the pool marking the exact position and surface area of the previous location of the hidden platform were analyzed.
Results
Body weights
An analysis of body weights indicated no significant differences between the groups initially (because they were weight-matched) or at the time of surgery. However, 3 days following surgery, the control diet–KA rats weighed significantly less (P < 0.05) than the control diet-R group, and this difference persisted until the day of euthanization 10 days later. Although these decreases in body weight were noted in the BB-KA and PX-KA groups post-surgery, the decreases were attenuated by the blueberry and piroxicam diets by the time the rats were euthanized (P > 0.05).
Cognitive performance
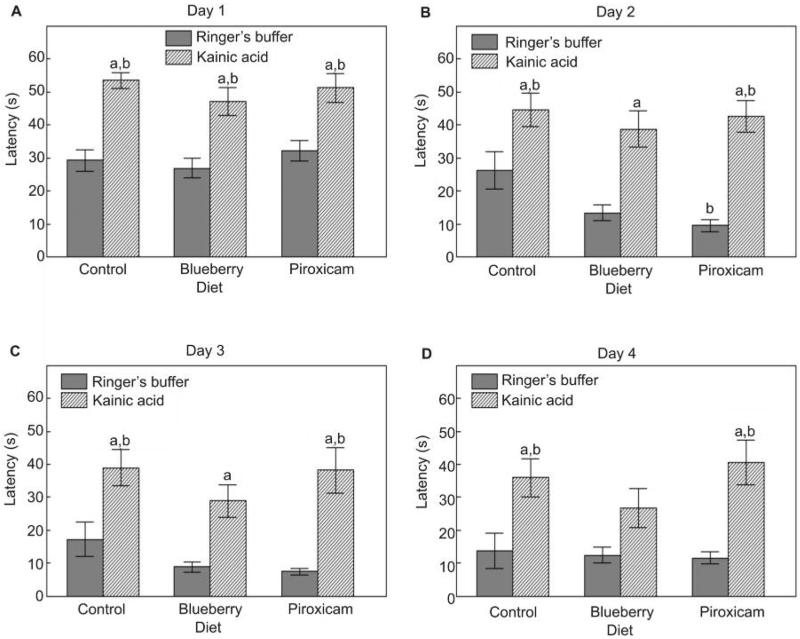
Kainic acid micro-infusions had deleterious effects on all measures of cognitive behavior, including latency to find the platform on days 1–3 and the reversal day, as well as performance on the probe trials. These deficits were improved by the blueberry diet and, to a lesser degree, the piroxicam diet. Interestingly, both the blueberry and piroxicam diets were able to improve performance in the rats administered Ringer's buffer on some measures. Specifically, rats on the control diet given kainic acid had increased latencies to find the hidden platform in the Morris water maze on days 1–3, and on day 4 during reversal learning, compared to the Ringer's buffer group (P < 0.05; Fig. 1). These deficits were improved by the blueberry diet. Specifically, latencies to find the platform on days 2–4 in the BB-KA group were not different than the control diet-R group (P > 0.05), and, on day 4, latencies for the BB-KA group were also not different than the BB-R group (Fig. 1B–D). On day 2, the piroxicam diet improved performance compared to the control diet (P < 0.05; Fig. 1B).
Figure 1.
Mean escape latencies (s; mean ± SEM) to find the hidden platform in the Morris water maze for (A) day 1 (trials 1–6), (B,C) days 2–3 (trials 1–5), and (D) for reversal testing on day 4 (trials 2–5) for Ringer's buffer or kainic acid injected rats fed the control, blueberry or piroxicam diet for 8 weeks prior to micro-infusion. Significant group effects were seen for day 1 [F(5,55) = 11.79, P < 0.001], day 2 [F(5,55) = 9.93, P < 0.001], day 3 [F(5,55) = 8.00, P < 0.001], and day 4 [F(5,55) = 5.57, P < 0.001]. Post-hoc testing revealed a significant difference (aP < 0.05) between the kainic acid injected and the matched diet Ringer's group, and a significant difference (bP < 0.05) from the control diet-Ringer's group
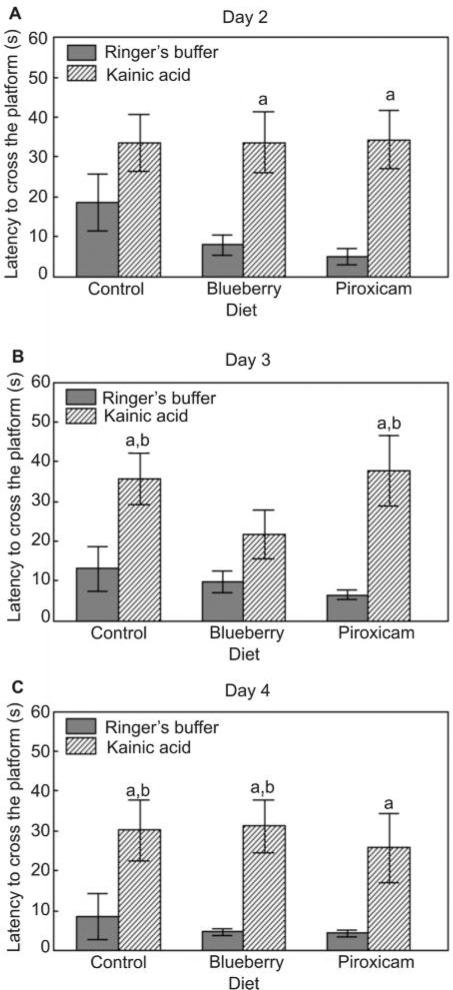
Rats on the control diet given kainic acid had increased latencies to cross the previous platform location during the probe trials in the Morris water maze on days 3 and 4 (Fig. 2B,C), but not day 2 (Fig. 2A), compared to the Ringer's buffer group (P < 0.05). These deficits were improved by the blueberry diet on day 3 as latencies to cross the platform in the BB-KA group were not different from either the BB-R or the control diet-R group (Fig. 2B). On day 4, the piroxicam diet improved performance, since latencies for the PX-KA group were not different than the control diet-R group (Fig. 2C).
Figure 2.
Mean latency to cross (s; mean ± SEM) the previous location of the hidden platform for the first time during the probe trials on testing days 2–4 (A, B, and C, respectively) in the Morris water maze for Ringer's solution or kainic acid injected rats fed the control, blueberry or piroxicam diet for 8 weeks prior to micro-infusion. Significant group effects were seen for day 2 [F(5,55) = 4.21, P < 0.01], day 3 [F(5,55) = 5.04, P < 0.01], and day 4 [F(5,55) = 4.25, P < 0.01]. Post-hoc testing revealed a significant difference (aP < 0.05) between the kainic acid injected and the matched diet Ringer's group, and a significant difference (bP < 0.05) from the control diet-Ringer's group
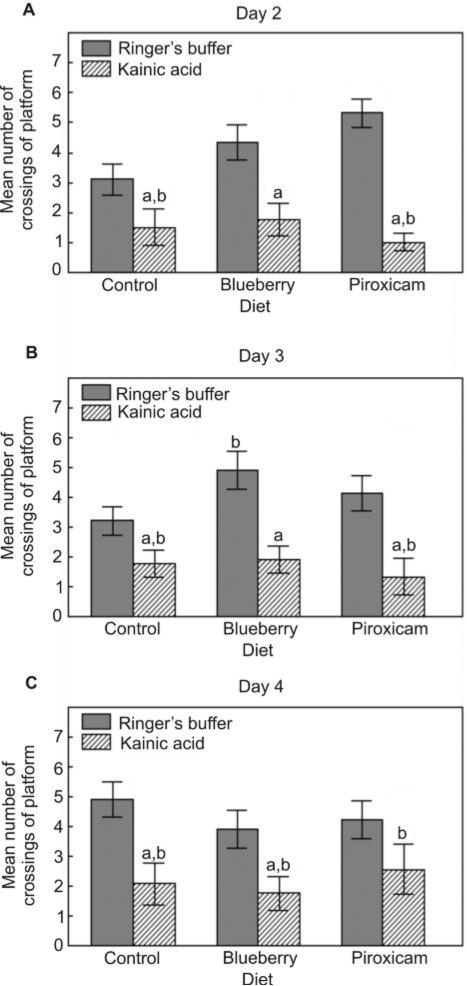
Control diet-KA rats had fewer crossings of the previous platform location during the probe trials in the Morris water maze on days 2–4, compared to the Ringer's buffer group (P < 0.05; Fig. 3). These deficits were improved by the blueberry diet on days 2 and 3 as number of crossings in the BB-KA group were not different from the control diet-R group (Fig. 3A,B), and by the piroxicam diet on day 4 as number of crossings in the PX-KA group were not different from the PX-R group (Fig. 3C). The blueberry and piroxicam diets improved performance on days 2 (Fig. 3A) and 3 (Fig. 3B), respectively, since number of crossings were higher than those of the control diet-R group (P < 0.05).
Figure 3.
Mean number of crossings of the previous location (mean ± SEM) of the hidden platform during the probe trials on testing days 2–4 (A, B, and C, respectively) in the Morris water maze for Ringer's or kainic acid injected rats fed the control, blueberry or piroxicam diet for 8 weeks prior to micro-infusion. Significant group effects were seen for day 2 [F(5,55) = 9.52, P < 0.001], day 3 [F(5,55) = 6.90, P < 0.001], and day 4 [F(5,55) = 3.81, P < 0.01]. Post-hoc testing revealed a significant difference (aP < 0.05) between the kainic acid injected and the matched diet Ringer's group, and a significant difference (bP < 0.05) from the control diet-Ringer's group
The control diet-KA rats had decreased swim speeds compared to the control diet-R group on days 1, 2 and 4 (P < 0.05), and these decreases in speed were attenuated by the blueberry and piroxicam diets on days 2 and 4 (data not shown). However, there were no differences in speed between the control diet-R group and the kainic acid-treated diet groups (control diet-KA, BB-KA, or PX-KA; P > 0.05) on the probe trials on days 2–4.
Inflammatory responses
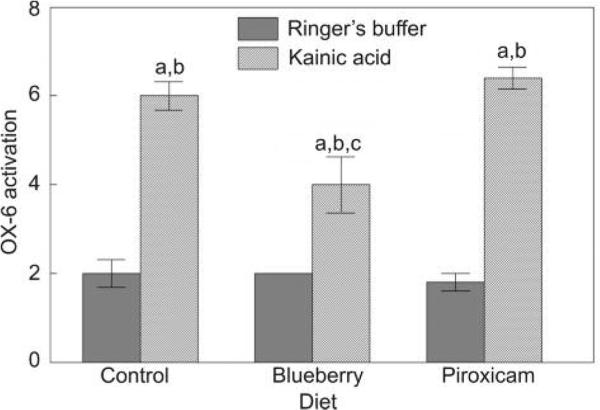
Kainic acid produced an inflammatory response as shown by increased OX-6 activation in the hippocampus in the control diet-KA group compared to the control diet-R group (P < 0.05; Fig. 4). The blueberry diet reduced this inflammatory response (P < 0.05 from the control diet-KA group); however, the response was not returned to control levels (without kainic acid). The piroxicam diet had no effect on OX-6 activation.
Figure 4.
Level of OX-6 activation in the hippocampus (mean ± SEM) for Ringer's or kainic acid injected rats fed the control, blueberry or piroxicam diet for 8 weeks prior to micro-infusion. A significant group effect was seen [F(5,24) = 37.82, P < 0.001] and post-hoc testing showed a significant difference (aP < 0.05) between the kainic acid injected and the matched diet Ringer's group, a significant difference (bP < 0.05) from the control diet-Ringer's group, and a significant difference (cP < 0.05) from the control diet-kainic acid group
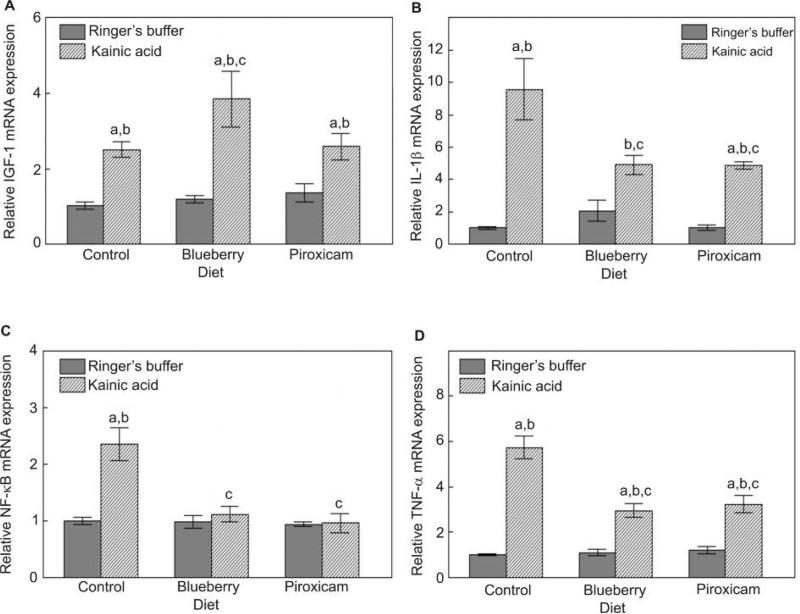
Kainic acid also significantly increased hippocampal IGF-1, IL-1β, NF-κB, and TNF-α mRNA expression compared to rats on the control diet not administered kainic acid (P-values < 0.05; Fig. 5). Although the blueberry diet did not have a significant effect on IGF-1 in the group given Ringer's buffer, this diet further increased IGF-1 expression in the kainic acid group compared to the control diet-KA group (P < 0.05; Fig. 5A), while the piroxicam diet had no effect on IGF-1 mRNA level. Both blueberry and piroxicam diets reduced IL-1β (Fig. 5B), NF-κB (Fig. 5C), and TNF-α (Fig. 5D) mRNA expression in the rats given kainic acid (P < 0.05 from the control diet-KA group).
Figure 5.
Level of (A) IGF-1, (B) IL-1β, (C) NF-κB and (D) TNF-α activation in the hippocampus (mean ± SEM) for Ringer's or kainic acid injected rats fed the control, blueberry or piroxicam diet for 8 weeks prior to micro-infusion. A significant group effect was seen for IGF-1 [F(5,21) = 9.57, P < 0.001], IL-1β [F(5,21) = 13.24, P < 0.001], NF-κB [F(5,21) = 11.60, P < 0.001], and TNF-α [F(5,21) = 34.81, P < 0.001]. Post-hoc testing showed a significant difference (aP < 0.05) between the kainic acid injected and the matched diet Ringer's group, a significant difference (bP < 0.05) from the control diet-Ringer's group, and a significant difference (cP < 0.05) from the control diet-kainic acid group
Discussion
In this study, administration of an inflammatory stimulus disrupted spatial learning and memory in rats injected with kainic acid as observed in: (i) increased latencies to find a hidden platform in the Morris water maze, particularly on the probe trials; and (ii) a deficit in the ability to learn a new platform location (and forget the old location), compared to Ringer's buffer injected rats. These decrements are very similar to those observed in aged animals, including an initial deficit in the acquisition of the task,43 difficulty in learning a new platform location during reversal training,44 and lack of a spatial preference compared to young animals when tested in a probe trial (i.e. less time spent in the training quadrant and less platform crossings).43,45 One reason for the similarity in behavior deficits between aged and kainic acid-injected rats, as mentioned above, might be an increase in inflammation or susceptibility to inflammatory insults in aging, which may be responsible for the induction of cognitive deficits.9–12
Furthermore, these results indicate that blueberry polyphenols have anti-inflammatory actions, comparable to or greater than piroxicam, a known anti-inflammatory drug, since young rats fed a diet supplemented with a 2% blueberry extract for 2 months, prior to injection of an inflammatory stimulus into hippocampus, were significantly less impaired in their spatial learning and memory ability. That is, the cognitive performance of rats in the BB-KA group was not different from control diet-R rats; however, the blueberry effect was not robust enough for performance to also be different from the control diet-KA rats, perhaps because of the extensive damage caused by kainic acid. Additionally, rats fed the blueberry diet prior to kainic acid injection had less activation of the inflammatory marker OX-6 and increased expression in the neuroprotective trophic factor IGF-1. Both blueberry and piroxicam were able to reduce expression of the inflammatory cytokines IL-1β and TNF-α, and the transcription factor NF-κB. Therefore, one possible mechanism of action of the blueberry polyphenols could be reduction of the deleterious effects of an inflam matory stimulus by altering the expression of genes associated with inflammation, protecting the brain and/or reducing its susceptibility to insults. Of course, there could be other mechanisms not tested in this study which could be responsible for the behavioral effects observed.
In this respect, it has been known for a number of years that dietary polyphenols can inhibit several inflammatory stress markers including arachidonic acid peroxidation,46 cyclooxygenase (COX) activity,47–49 peroxis ome prolifer ator activated receptors (PPARs), nitric oxide, and NF-κB in a variety of models (see Yoon and Baek50 for review). Studies also indicate that polyphenols, in a similar manner to that seen with NSAIDs, can increase NSAID activated gene-1 (NAG-1).50
Additionally, flavonoids have been found to reduce TNF-α in several in vivo and in vitro experiments including T-cells (grape-seed extract),51 keratinocytes (grape-seed extract),52 blood mononuclear cells (quercetin),53 and lung tissue (perforatum extract).54 Similar findings have been observed when TNF-α gene expression was assessed.53,55 As observed in the present study, similar down-regulation effects have been observed for TNF-α in the kainic acid animals supplemented with blueberries or given piroxicam.
The results in the present study, as well as numerous others, demonstrate that polyphenols derived from a number of sources decreased IL-1β activation in oxidative and inflammatory stress-mediated studies (e.g. muscadine grape extracts in blood mononuclear cells,56 ellagic acid in activated pancreatic stellate cells,57 and epicatechin conjugates in whole blood.58 However, there do not appear to be, at the writing of this paper, studies of IL-1β gene expression in similar paradigms with various fruit extracts. Interestingly, the piroxicam-induced decreases in IL-1β in the PX-KA group were similar to those seen in the BB-KA group.
Kainic acid administration also increased IGF-1 mRNA; however, it appeared that in the blueberry-supple mented rats given kainic acid there was a synergistic effect such that there were significantly greater increases compared to those observed in the kainic acid group maintained on the control diet. This synergistic effect was not seen in the rats administered kainic acid and treated with piroxicam. This finding is important since numerous previous experiments have indicated that there are decreases in IGF-1 in inflammation. It is known, for example, that patients with rheumatoid arthritis have low serum concentrations of IGF-159 and rats given adjuvant-induced arthritis showed significant decreases in circulating IGF-1.60 Conversely, IGF-1 has been shown to protect the CNS against oxidative stressors.61–64 Moreover, it is also known that levels of IGF-1 mRNA decrease in hippocampal neurons of aged rats65 and that levels of IGF-1 and IGF-1 receptor proteins decrease in the cerebral cortex of old rats.66 Importantly, the reduced IGF-1 appears to contribute to age-related memory disorders.67
Research also indicates that Ames dwarf mice showed increased IGF-1 and growth hormone as compared to controls, which led to activation of downstream signals such as Akt and CREB (cAMP responsive element-binding protein), both of which are important in learning and memory.68 Thus, in the present study, the improved cognitive performance in the BB-KA group may reflect both direct reductions in kainate-induced oxidative/inflammatory stressors as well as synergistic increases in IGF-1, thereby enhancing IGF-1 protection against inflammation and possibly directly affecting cognitive function through the increases in IGF-1 immunoreactivity (see Sonntag et al.69 and Casadesus et al.18). Note that these synergistic effects were not seen in the PX-KA group and there were differential effects of the blueberry diet and piroxicam diet on cognitive function. However, it is also possible that there were differences in sites of action of blueberry and piroxicam.
In this regard, the positive behavioral effects of the blueberry diet were notable during the probe trials primarily on days 2 and 3, while the piroxicam diet showed improved performance only on day 4, the reversal day. The kainic acid injections caused impairments in probe trial performance that were similar to those seen with aged animals, in that kainic acid-injected rats were unable to develop appropriate search strategies due to a decline in the ability to process or retain place information.70 While the blueberry diet seemed to improve overall spatial preference and search strategies in the kainic acid-injected rats, the piroxicam only had beneficial effects on spatial learning on the reversal day. It is known that aged rats have difficulty in learning a new platform location during reversal training.44 Because blueberries and piroxicam had differential effects on cognition, it is possible that they are acting in different brain regions or through different mechanisms to produce their beneficial effects. Rats with damage to the dorsal striatum preferentially learn that the relationships among the cues in the room predict the location of the platform, and therefore are subsequently impaired in learning a new platform location, while rats with damage to the hippocampus fail to learn about the platform's location in space and are impaired in learning the location of the platform over time.71 It is thought that the hippocampus mediates allocentric spatial navigation (i.e. place learning) while the dorsomedial striatum mediates egocentric spatial orientation (i.e. response and cue learning) and plays an important role in the acquisition of procedural aspects of both place and cue learning.72 In this regard, we have shown that whole-body irradiation with 1.5 Gy of 1 GeV/n high-energy 56Fe-particles impaired performance in the Morris water maze, and measures of dopamine release, 1 month following radiation, and that these deficits were protected by blueberry- and strawberry-supplemented diets.73 Interestingly, the strawberry diet offered better protection against spatial deficits in the maze because strawberry-fed animals were better able to retain place information (a hippocampally-mediated behavior) compared to controls. The blueberry diet, on the other hand, seemed to improve reversal learning, a behavior more dependent on intact striatal function. These data suggest polyphenols from different fruits might be acting in different brain regions or via different mechanisms of action.73 Therefore, if both blueberries and piroxicam had been fed to the rats in the same diet, they may have had a synergistic effect to improve memory better than either compound alone, particularly hippocampally-mediated behaviors, especially since, as indicated above, polyphenols (e.g. resveratrol, EGCG genistein) and NSAIDs can activate NAG-1 and reduce cytokines.74–76
Interestingly, the blueberry and piroxicam diets were also able to improve performance in rats administered Ringer's solution, where presumably vulnerability to oxidative stress and inflammation are not enhanced, as it is in the rats given kainic acid. We have seen this enhanced effect of the blueberry diet in previous studies.77 We believe that blueberries may also have direct effects (i.e. not mediated through oxidative stress or inflammatory pathways) on the brain by directly increasing signaling20 as well as neurogenesis,18 and by enhancing neuroprotective stress shock proteins,27 while reducing NF-κB.23
Conclusions
It appears that the significant effects of blueberries on cognitive behavior are due to a multiplicity of direct and indirect actions, the former involving effects on neuronal communication and the latter involving antioxidant and anti-inflammatory activity. We found that blueberries can reduce the deleterious effects of kainic acid by altering the expression of genes associated with inflammation, thus protecting the brain and/or reducing its susceptibility to inflammatory insults. Considering that many of the diseases of aging, including Alzheimer's disease, have an inflammatory component, this research suggests that it is possible to mitigate the effects of inflammation via dietary means. The next steps in this research might be focused on what it is about foods such as blueberries that provided the protection that we observed in this study. More extensive research will be required to characterize and isolate the chemical composition of the blueberry extract and further assess the beneficial effects on function in animals and humans before any conclusions can be made. However, based upon the evidence provided in the present paper, as well as that of previous research, it might be speculated that the differences observed between blueberry and piroxicam treatments in their protective effects against kainic acid on behavior may involve differential increases in IGF-1 and reductions in OX-6. The selective reductions in OX-6 activation by blueberry-supplementation could have protected against over-activation of the microglia and maintained their ability to protect against kainate-induced hippocampal cell loss. It has been shown, for example, that activated microglia over-expressing IL-1 are readily detectable in the brains of Alzheimer's disease patients.78 Furthermore, highly activated microglia, not astrocytes, have been found in the substantia nigra of Parkinson's disease brains.79 Additionally, Duffy and colleagues80 observed, via stereological analyses, that rats receiving excitotoxic kainic acid injections exhibited loss of CA1 neurons, and this cell loss was attenuated by a blueberry-enriched diet. Finally, it is possible that although piroxicam and blueberry-supplementation had similar effects on cytokines and NF-κB, there may have been differential modification of additional downstream inflammatory markers (e.g. NAG-1, COX-1). In this regard, we are presently investigating the effects of blueberry and other fruit supplementations on these additional inflammatory markers in both cell and animal experiments.
Acknowledgements
This research was supported, in part, by the USDA and the Alzheimer's Association. The authors would like to thank Laura Simon and Donna Bielinski for assisting with data collection.
Footnotes
This article was written and prepared by officers/employees of the US Government as part of their official duties and is not copyrightable.
References
- 1.Rosenman SJ, Shrikant P, Dubb L, Benveniste EN, Ransohoff RM. Cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and astrocytoma cell lines. J Immunol. 1995;154:1888–1899. [PubMed] [Google Scholar]
- 2.Schipper HM. Astrocytes, brain aging, and neurodegeneration. Neurobiol Aging. 1996;17:467–480. doi: 10.1016/0197-4580(96)00014-0. [DOI] [PubMed] [Google Scholar]
- 3.Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148:1819–1838. [PMC free article] [PubMed] [Google Scholar]
- 4.Stella N, Estelles A, Siciliano J, et al. Interleukin-1 enhances the ATP-evoked release of arachidonic acid from mouse astrocytes. J Neurosci. 1997;17:2939–2946. doi: 10.1523/JNEUROSCI.17-09-02939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodroofe MN. Cytokine production in the central nervous system. Neurology. 1995;45:S6–S10. doi: 10.1212/wnl.45.6_suppl_6.s6. [DOI] [PubMed] [Google Scholar]
- 6.Rogers J, Webster S, Lue LF, et al. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996;17:681–686. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- 7.Hauss-Wegrzyniak B, Willard LB, Del Soldato P, Pepeu G, Wenk GL. Peripheral administration of novel anti-inflammatories can attenuate the effects of chronic inflammation within the CNS. Brain Res. 1999;815:36–43. doi: 10.1016/s0006-8993(98)01081-6. [DOI] [PubMed] [Google Scholar]
- 8.Mrak RE, Griffin WST. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harman D. Role of free radicals in aging and disease. Ann NY Acad Sci. 1992;673:126–141. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 11.Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL. Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Res. 2000;859:157–166. doi: 10.1016/s0006-8993(00)01999-5. [DOI] [PubMed] [Google Scholar]
- 12.Olanow CW. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- 13.Joseph JA, Villalobos-Molina R, Denisova NA, Erat S, Jimenez N, Strain J. Increased sensitivity to oxidative stress and the loss of muscarinic receptor responsiveness in senescence. Ann NY Acad Sci. 1996;786:112–119. doi: 10.1111/j.1749-6632.1996.tb39056.x. [DOI] [PubMed] [Google Scholar]
- 14.Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81:313S–316S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- 15.Olszanecki R, Gebska A, Kozlovski VI, Gryglewski RJ. Flavonoids and nitric oxide synthase. J Physiol Pharmacol. 2002;53:571–584. [PubMed] [Google Scholar]
- 16.Rice-Evans CA, Miller MJ, Bolwell PG, Branley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolics flavonoids. Free Radic Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 17.Bickford P, Gould T, Briederick L, et al. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 18.Casadesus G, Shukitt-Hale B, Stellwagen HM, et al. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 19.Joseph JA, Shukitt-Hale B, Denisova NA, et al. Reversals of age-related declines in neuronal signal transduction cognitive and motor behavioral deficits with blueberry spinach or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph JA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- 21.Shukitt-Hale B, Galli RL, Meterko V, et al. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age. 2005;27:49–57. doi: 10.1007/s11357-005-4004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youdim KA, Shukitt-Hale B, Martin A, et al. Short term dietary supplementation of blueberry polyphenolics: beneficial effects on aging brain performance and peripheral tissue function. Nutr Neurosci. 2000;3:383–397. [Google Scholar]
- 23.Goyarzu P, Malin DH, Lau FC, et al. Blueberry supplemented diet: Effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci. 2004;7:75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- 24.Bickford PC, Shukitt-Hale B, Joseph J. Effects of aging on cerebellar noradrenergic function and motor learning: nutritional interventions. Mech Ageing Dev. 1999;111:141–154. doi: 10.1016/s0047-6374(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 25.Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. The effects of grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Joseph JA, Fisher DR, Carey AN. Fruit extracts antagonize AB- or dopamine-induced deficits in Ca2+ flux in M1-transfected COS-7 cells. J Alz Dis. 2004;6:403–411. doi: 10.3233/jad-2004-6408. [DOI] [PubMed] [Google Scholar]
- 27.Galli RL, Bielinski DF, Szprengiel A, Shukitt-Hale B, Joseph JA. Blueberry supplemented diet reverses age-related decline in hippocampal HSP70 neuroprotection. Neurobiol Aging. 2006;27:344–350. doi: 10.1016/j.neurobiolaging.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 29.Krischer SM, Eisenmann M, Bock A, Mueller MJ. Protein-facilitated export of arachidonic acid from pig neutrophils. J Biol Chem. 1997;272:10601–10607. doi: 10.1074/jbc.272.16.10601. [DOI] [PubMed] [Google Scholar]
- 30.Mirzoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukoc Essent Fatty Acids. 1996;55:441–449. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 31.Bors W, Heller W, Michel C, Stettmaier K. Flavonoids and polyphenols: chemistry and biology. In: Cadenas E, Packer L, editors. Handbook of Antioxidants. Marcel Dekker; New York: 1996. pp. 409–466. [Google Scholar]
- 32.Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 33.Gerritsen ME. Flavonoids: inhibitors of cytokine induced. gene expression. In: Manthey JA, Buslig BS, editors. Flavonoids in the Living System. Vol. 439. Plenum; New York: 1998. pp. 183–190. [DOI] [PubMed] [Google Scholar]
- 34.Gobbo OL, O'Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behav Brain Res. 2005;159:21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 35.Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 36.Ingram DK, Jucker M, Spangler E. Behavioral manifestations of aging. In: Mohr U, Cungworth DL, Capen CC, editors. Pathobiology of the Aging Rat. Vol. 2. ILSI Press; Washington: 1994. pp. 49–70. [Google Scholar]
- 37.Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33:615–624. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 38.Joseph JA, Shukitt-Hale B, Denisova NA, et al. Long term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 40.Shukitt-Hale B, Casadesus G, McEwen JJ, Rabin BM, Joseph JA. Spatial learning and memory deficits induced by exposure to iron-56-particle radiation. Radiat Res. 2000;154:28–33. doi: 10.1667/0033-7587(2000)154[0028:slamdi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana; Totowa: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- 44.Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- 45.Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 46.Baumann J, von Bruchhausen F, Wurm G. Flavonoids and related compounds as inhibition of arachidonic acid peroxidation. Prostaglandins. 1980;20:627–639. doi: 10.1016/0090-6980(80)90103-3. [DOI] [PubMed] [Google Scholar]
- 47.Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure–activity relations. Biochem Pharmacol. 1984;33:1525–1530. doi: 10.1016/0006-2952(84)90423-4. [DOI] [PubMed] [Google Scholar]
- 48.Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85:1010–1017. doi: 10.1002/jnr.21205. [DOI] [PubMed] [Google Scholar]
- 49.Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyaniding glycosides in cherries and berries. Phytomedicine. 2001;5:362–369. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- 50.Yoon J, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46:585–596. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagchi D, Bagchi M, Stohs S, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann NY Acad Sci. 2002;957:260–270. doi: 10.1111/j.1749-6632.2002.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 52.Roy S, Khanna S, Alessio HM, et al. Anti-angiogenic property of edible berries. Free Radic Res. 2002;36:1023–1031. doi: 10.1080/1071576021000006662. [DOI] [PubMed] [Google Scholar]
- 53.Nair MP, Mahajan S, Reynolds JL, et al. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappabeta system. Clin Vaccine Immunol. 2006;13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menegazzi M, Di Paola R, Mazzon E, et al. Hypericum perforatum attenuates the development of carrageenan-induced lung injury in mice. Free Radic Biol Med. 2006;40:740–753. doi: 10.1016/j.freeradbiomed.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 55.Sueoka N, Suganuma M, Sueoka E, et al. A new function of green tea: prevention of lifestyle-related diseases. Ann NY Acad Sci. 2001;928:274–280. doi: 10.1111/j.1749-6632.2001.tb05656.x. [DOI] [PubMed] [Google Scholar]
- 56.Greenspan P, Bauer JD, Pollock SH, et al. Antiinflammatory properties of the muscadine grape (Vitis rotundifolia). J Agric Food Chem. 2005;53:8481–8484. doi: 10.1021/jf058015+. [DOI] [PubMed] [Google Scholar]
- 57.Masamune A, Satoh M, Kikuta K, Suzuki N, Satoh K, Shimosegawa T. Ellagic acid blocks activation of pancreatic stellate cells. Biochem Pharmacol. 2005;70:869–878. doi: 10.1016/j.bcp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Mitgans M, del Campo J, Abajo C, et al. Immunomodulatory activity of a new family of antioxidants obtained from grape polyphenols. J Agric Food Chem. 2004;52:7294–7299. doi: 10.1021/jf049403z. [DOI] [PubMed] [Google Scholar]
- 59.Johansson AG, Baylink DJ, Ekenstam E, Lindh E, Mohan S, Ljunghall S. Circulating levels of insulin-like growth factor-I and -II, and IGF-binding protein-3 in inflammation and after parathyroid hormone infusion. Bone Miner. 1994;24:25–31. doi: 10.1016/s0169-6009(08)80128-6. [DOI] [PubMed] [Google Scholar]
- 60.Lopez-Calderon A, Soto L, Martin AI. Chronic inflammation inhibits GH secretion and alters the serum insulin-like growth factor system in rats. Life Sci. 1999;65:2049–2060. doi: 10.1016/s0024-3205(99)00472-5. [DOI] [PubMed] [Google Scholar]
- 61.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 62.Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- 63.Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Lai M, Hibberd CJ, Gluckman PD, Seckl JR. Reduced expression of insulin-like growth factor 1 messenger RNA in the hippocampus of aged rats. Neurosci Lett. 2000;288:66–70. doi: 10.1016/s0304-3940(00)01170-8. [DOI] [PubMed] [Google Scholar]
- 66.Sonntag WE, Lynch CD, Bennett SA, et al. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269–279. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- 67.Zhao WQ, Cheng H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 68.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Gallagher M, Pelleymounter MA. Spatial learning deficits in old rats: a model for memory decline in the aged. Neurobiol Aging. 1988;9:549–556. doi: 10.1016/s0197-4580(88)80112-x. [DOI] [PubMed] [Google Scholar]
- 71.McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 72.Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- 73.Shukitt-Hale B, Carey AN, Jenkins D, Rabin BM, Joseph JA. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol Aging. 2007;28:1187–1194. doi: 10.1016/j.neurobiolaging.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 74.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25:2425–2432. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- 75.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–434. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 76.Wilson LC, Baek SJ, Call A, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene (NAG-1) is induced by genistein through the expression of p53 in colorectal cancer cells. Int J Cancer. 2003;105:747–753. doi: 10.1002/ijc.11173. [DOI] [PubMed] [Google Scholar]
- 77.Shukitt-Hale B, Carey A, Casadesus G, Galli RL, Joseph JA. Mechanisms involved in blueberry enhancements of motor and cognitive function in young and old rats. Soc Neurosci Abs. 2003;29:633.14. [Google Scholar]
- 78.Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- 80.Duffy KB, Spangler EL, Devan BD, et al. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.002. e-pub. [DOI] [PubMed] [Google Scholar]