Abstract
Ramucirumab, a monoclonal antibody targeting the vascular endothelial growth factor (VEGF) pathway, in combination with paclitaxel is becoming part of standard second-line systemic therapy for advanced oesophagogastric cancer, based on the results of the REGARD and RAINBOW trials. Common well-known side effects of VEGF pathway inhibitors are hypertension and infusion-related reactions. Here, we describe hypertension as the predominant feature of an infusion-related reaction in 2 patients with metastasised oesophagogastric carcinoma treated with ramucirumab and paclitaxel as second-line treatment and propose possible explanations of this side effect previously undescribed for ramucirumab.
Background
Despite advances in treatment options, oesophagogastric cancer has a dismal prognosis with an overall survival rate of ∼26%.1 2 In the majority of cases, the disease is advanced at the time of diagnosis, precluding surgical options. Median survival in this group of patients is less than a year, even with extensive systemic or combined systemic and radiotherapy treatment.1 2 After progression on first-line treatment, second-line treatment may improve overall survival and quality of life.3–5 Recently, the benefit of ramucirumab in this setting was established.6–8 Ramucirumab is a human IgG1 monoclonal antibody (MOAB) inhibiting the vascular endothelial growth factor (VEGF) pathway targeting angiogenesis, by binding to the VEGF receptor 2 (VEGFR2) (figure 1).
Figure 1.
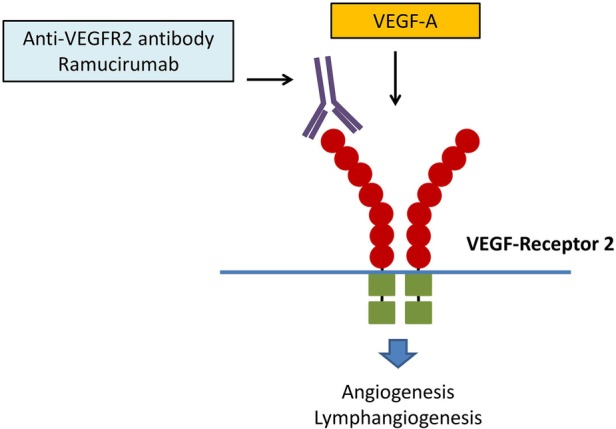
VEGF receptor 2 (based on Lankhorst et al16). VEGF-A binds to the thyrosine kinase receptor VEGF receptor 2 and by activating this pathway promotes angiogenesis and lymphangiogenesis. Ramucirumab inhibits this pathway by binding to the VEGF receptor 2. VEGF, vascular endothelial growth factor.
In the REGARD trial, monotherapy ramucirumab was compared to placebo as second-line palliative treatment in patients with gastric or gastro-oesophageal junction adenocarcinoma. Median overall survival was 5.2 months in the ramucirumab group vs 3.8 months in the supportive care group (HR 0.776; 95% CI 0.603 to 0.998; p=0.047).7 The RAINBOW trial compared ramucirumab in combination with paclitaxel versus placebo in combination with paclitaxel. Overall survival in the ramucirumab plus paclitaxel group was significantly longer (median 9.6 vs 7.4 months; HR 0.807; 95% CI 0.678 to 0.926; p=0.017).8
Hypertension is a well-known side effect of inhibitors of the VEGF pathway, while infusion-related reactions (IRRs) are a common side effect in treatment with MOABs. However, to the best of our knowledge, IRRs predominated by hypertension have not previously been reported for ramucirumab. While awaiting local marketing authorisation and approval by the regulatory authorities for the use of ramucirumab in combination with paclitaxel for second-line systemic treatment in advanced or metastasised oesophagogastric cancer in the Netherlands, patients in our hospital were able to participate in the ramucirumab compassionate use programme. Patients who fulfilled the eligibility criteria—identical to the inclusion criteria of the RAINBOW trial—could receive ramucirumab 8 mg/kg on days 1 and 15 in combination with paclitaxel 80 mg/m2 on days 1, 8 and 15 of a 28-day cycle, until progression or unacceptable toxicity. Standard premedication with ranitidine (H2 receptor antagonist), clemastine (H1 receptor antagonist) and dexamethasone was given. Here, we present two patients from this programme who experienced acute hypertension during infusion with ramucirumab.
Case presentation: case 1
A 65-year-old man with a history of well-regulated hypertension treated with amlodipine and ibesartan was referred to the department of medical oncology for palliative systemic treatment after an exploratory laparoscopy had revealed peritoneal metastasis of gastric adenocarcinoma. First-line systemic treatment consisted of three-weekly cycles with capecitabine (1000 mg/m2 two times per day on days 1–14) in combination with oxaliplatin (130 mg/m2 on day 1). This was given for three cycles, followed by three-weekly cycles of capecitabine monotherapy (1000 mg/m2 on days 1–14) after a 2-week delay because of thrombocytopenia. After three cycles of capecitabine monotherapy, progression was found on the CT scan. At this time, the patient had little symptoms, except for mild peripheral oedema and light headiness, attributed to the use of amlodipine and this was withheld 6 weeks prior to the start of ramucirumab. Before start of treatment, the patient had a blood pressure of 128/88 mm Hg. On day 1 of cycle 1 blood pressure was 130/77 mm Hg. The first cycle of second-line treatment was complicated by febrile neutropenia and day 15 treatment was withheld. Blood pressure was 134/82 mm Hg before start of day 1 of the second treatment cycle. Directly after infusion of ramucirumab, the patient experienced chills and was found to be hypertensive with a blood pressure of 171/119 mm Hg, while his pulse and temperature were normal (pulse 81/min, temperature 37.4°C).
Investigations
Upon physical examination, no abnormalities of the heart, lungs and abdomen were found. No signs of infection were observed after laboratory examination nor did an X-ray of the chest indicate an infection.
Treatment
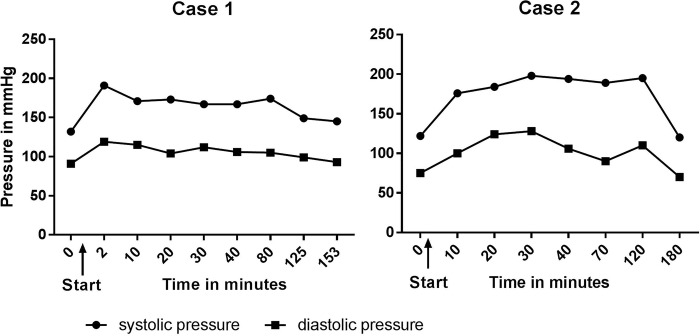
Under suspicion of an allergic reaction, additional dexamethasone 8 mg intravenous was administered. However, blood pressure remained elevated (figure 2), and 40 min after onset of the symptoms, patient developed a rise in temperature. Dexamethasone 8 mg was repeated, and 2 hours after start of the symptoms, blood pressure normalised without further medical intervention. Paclitaxel was withheld that day.
Figure 2.
Blood pressure during IRR. Both patients experience a rise in blood pressure shortly after starting infusion with ramucirumab. IRR, infusion-related reaction.
Outcome and follow-up
The infusion rate for the next dose of ramucirumab (day 15) was halved, and premedication remained unchanged. Before start of infusion, blood pressure was 149/88 mm Hg. Nevertheless, at the end of the infusion, blood pressure rose to 164/111 mm Hg, while no other symptoms were noticed. Amlodipine was restarted and no further hypertension was reported. Owing to progression, treatment had to be stopped after two cycles. No further cytotoxic treatment was started and the patient died at home 19 weeks after the last chemotherapy.
Case presentation: case 2
A 71-year-old man was referred to the department of medical oncology in our hospital for second-line palliative treatment in the ramucirumab compassionate use programme after being diagnosed with a recurrence of his gastric adenocarcinoma, carcinomatous peritonitis and lymphogenic metastasis 3 months after completing adjuvant chemotherapy. He had received six cycles of chemotherapy with epirubicin (50 mg/m2), cisplatinum (60 mg/m2) and capecitabine (1000 mg/m2 on days 2–14) in three-weekly cycles perioperatively. Therefore, he did not receive additional first-line palliative therapy with platinum and fluoropyrimidines. The first course was complicated with thrombosis, and during the final cycle, epirubicin and cisplatin were withheld due to symptoms of peripheral neuropathy. During the preoperative treatment, the patient also suffered from a transient ischaemic attack. Symptoms of the patient at the time of the recurrence were a loss of appetite, nausea and abdominal discomfort with concurrent weight loss. He had no history of hypertension, and upon the last visit to the outpatient clinic before the start of treatment, blood pressure was 110/65 mm Hg. The patient also suffered from melancholy.
On day 1 of the first cycle of second-line treatment, the patient developed a reaction 20 min after start of infusion with ramucirumab, with facial redness and hypertension (blood pressure 176/100 mm Hg). There were no other signs of an anaphylactic reaction.
Treatment
Additional clemastine 2 mg and dexamethasone 8 mg were given. Blood pressure remained high (195/110 mm Hg) after 2 hours (figure 2). Two hours after start of the symptoms, patient received 10 mg of nifedipine after which the blood pressure quickly normalised to 125/70 mm Hg. Ramucirumab infusion was not restarted and paclitaxel was withheld.
Outcome and follow-up
Owing to rapid clinical deterioration, the patient chose to stop palliative chemotherapy and no further chemotherapy was given. He died a few weeks later.
Discussion
IRRs to MOABs have frequently been described.9 Hypertension can occur as a symptom of an IRR, as has been described for the anti-CD20 antibodies of atumumab and obinutuzumab, for example.10 11 For bevacizumab, a monoclonal anti-VEGF antibody, hypertension as a feature of IRRs has been reported in the manufacturer’s prescribing information.12 To the best of our knowledge, there are no case reports on acute onset hypertension after infusion with ramucirumab nor is it described as symptom of IRRs in the prescribing information.13
In the REGARD trial, <1% of the reported adverse events in the ramucirumab group were IRRs. In the RAINBOW trial, 5.8% IRRs were reported. The exact pathophysiological mechanism of hypertension as part of an IRR is still unclear. Most likely, it is induced by an antibody–antigen-mediated reaction resulting in cytokine release.9 The majority of reactions occur during the first courses of treatment; however, IRRs can occur anytime during treatment.14 Although the rapid onset of hypertension in our cases suggests an IRR, the limited response to additional dexamethasone administration and the need for antihypertensive medication could indicate another mechanism of action.
Indeed, a common and well-known side effect of angiogenesis inhibitors is hypertension.15 In the REGARD trial, the reported rate of hypertension was 16%, whereas in the RAINBOW trial, the reported rate of hypertension was 24%.8 Several possible mechanisms that could explain the development of hypertension have been proposed: rarefaction (hypertension due to the reduction of microvasculature leading to an increased peripheral resistance), impairment in renal function with increased salt sensitivity and alteration in the nitric oxide (NO) pathway leading to increased vascular resistance.16
Reduced NO availability has been described as the first step in the onset of hypertension due to VEGF pathway inhibition.17 Interaction of the VEGFR2 with VEGF-A causes activation of NO synthetase resulting in the production of NO. Inhibition of this pathway leads to a reduced bioavailability of NO.16 A reduced bioavailability of NO will rapidly increase vascular resistance leading to a rise in blood pressure. Rapid onset of hypertension, directly after start of treatment with VEGFR2 inhibitors, has been described in experimental animal studies. In these studies, a reduced expression of endothelial and neuronal NO synthases in the kidney was found and inhibition of NO activity led to a similar rise in blood pressure as VEGF pathway inhibition.17 Contrarily, infusion of VEGF leads to acute vasodilation and it is suggested that this is caused by NO synthesis.18 Furthermore, it is postulated that patients with hypertension due to angiogenesis inhibition might benefit from treatment with NO donors (eg, isosorbide dinitrate).19
Learning points.
Hypertension may be part of an infusion-related reaction, but VEGFR2 pathway inhibition may be another plausible explanation.
Independent from the underlying mechanism, the cases we presented stress the importance of close monitoring of blood pressure during treatment with ramucirumab.
Starting with monitoring already during the very first infusion.
Acknowledgments
The authors thank the patients and their families for permitting them to publish their cases.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cunningham D, Starling N, Rao S et al. . Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36–46. 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM et al. . Global cancer statistics. CA Cancer J Clin 2011;61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3.Thuss-Patience PC, Kretzschmar A, Bichev D et al. . Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306–14. 10.1016/j.ejca.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Lee SI, Lim do H et al. . Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–18. 10.1200/JCO.2011.39.4585 [DOI] [PubMed] [Google Scholar]
- 5.Ford HER, Marshall A, Bridgewater JA et al. . Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. 10.1016/S1470-2045(13)70549-7 [DOI] [PubMed] [Google Scholar]
- 6.Boere IA, Hamberg P, Sleijfer S. It takes two to tango: combinations of conventional cytotoxics with compounds targeting the vascular endothelial growth factor-vascular endothelial growth factor receptor pathway in patients with solid malignancies. Cancer Sci 2010;101:7–15. 10.1111/j.1349-7006.2009.01369.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs CS, Tomasek J, Yong CJ et al. . Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 8.Wilke H, Muro K, Van Cutsem E et al. . Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 9.Dillman RO, Hendrix CS. Unique aspects of supportive care using monoclonal antibodies in cancer treatment. Support Cancer Ther 2003;1:38–48. 10.3816/SCT.2003.n.003 [DOI] [PubMed] [Google Scholar]
- 10.AZERRA® (ofatumumab) injection, for intravenous use [prescribing information]. East Hanover: Novartis Pharmaceuticals Corporation, 2016.
- 11.GAZYVARO® (obinutuzumab) concentrate for solution for infusion [prescribing information]. Welwyn Garden City: Roche, 2015.
- 12.AVASTIN® (bevacizumab) injection, Solution for intravenous infusion [prescribing information]. South San Francisco: Genentech Inc. 2015.
- 13.CYRAMZA (ramucirumab) injection, for intravenous use [prescribing information].Indianapolis: Eli Lilly and Company, 2015.
- 14.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007;12:601–9. 10.1634/theoncologist.12-5-601 [DOI] [PubMed] [Google Scholar]
- 15.Verheul HMW, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer 2007;7:475–85. 10.1038/nrc2152 [DOI] [PubMed] [Google Scholar]
- 16.Lankhorst S, Saleh L, Danser AHJ et al. . Etiology of angiogenesis inhibition-related hypertension. Curr Opin Pharmacol 2015;21:7–13. 10.1016/j.coph.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Facemire CS, Nixon AB, Griffiths R et al. . Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 2009;54:652–8. 10.1161/HYPERTENSIONAHA.109.129973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry TD, Annex BH, McKendall GR et al. . The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 2003;107:1359–65. 10.1161/01.CIR.0000061911.47710.8A [DOI] [PubMed] [Google Scholar]
- 19.Kruzliak P. VEGF pathway inhibitors–induced hypertension: next step in therapy. J Clin Hypertens (Greenwich) 2014;16:617 10.1111/jch.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]