Abstract
Pulmonary tumour thrombotic microangiopathy (PTTM) resulting in fatal pulmonary hypertension is a rare complication of malignancy. Patients with PTTM generally suffer rapid deterioration of hypoxaemia, and a diagnosis is often only made at autopsy. We report a case of extramammary Paget's disease associated with PTTM. An ante-mortem diagnosis was made based on cytology of blood aspirated from a pulmonary artery catheter in a wedged position. Together with a review of the literature, this case highlights the potential diagnostic value of blood cytology in patients with cancer with rapidly progressing pulmonary hypertension.
Background
Pulmonary tumour thrombotic microangiopathy (PTTM) is a rare complication of cancer. Widespread microscopic tumour emboli in the pulmonary arterioles cause occlusion, and also stimulate activation of localised coagulation and fibrocellular proliferation of endocytes. PTTM usually originates from mucinous adenocarcinomas, predominantly gastric tumours.1 The prognosis is extremely poor, and the diagnosis of PTTM is often only made postmortem. We present the first report of a case of PTTM originating from extramammary Paget's disease (EMPD), diagnosed antemortem. This case highlights the potential value of pulmonary artery blood cytology as a diagnostic procedure for PTTM.
Case presentation
A 70-year-old man was admitted to the hospital with a symptom of shortness of breath. He had a 3-week history of slight right leg oedema and mild fatigue. He had experienced dyspnoea on exertion 4 days prior to admission. His dyspnoea had deteriorated the day before hospitalisation, and he was unable to walk a short distance. He consulted his primary care physician who noted that his percutaneous oxygen saturation was <90% (room air). He was transferred to our emergency department for further tests and treatment. He was a current smoker and drank nearly a litre of sake a day. He had been followed at his primary care clinic for hypertension and chronic liver disease, but he had no known cardiac or pulmonary comorbidities.
Investigations
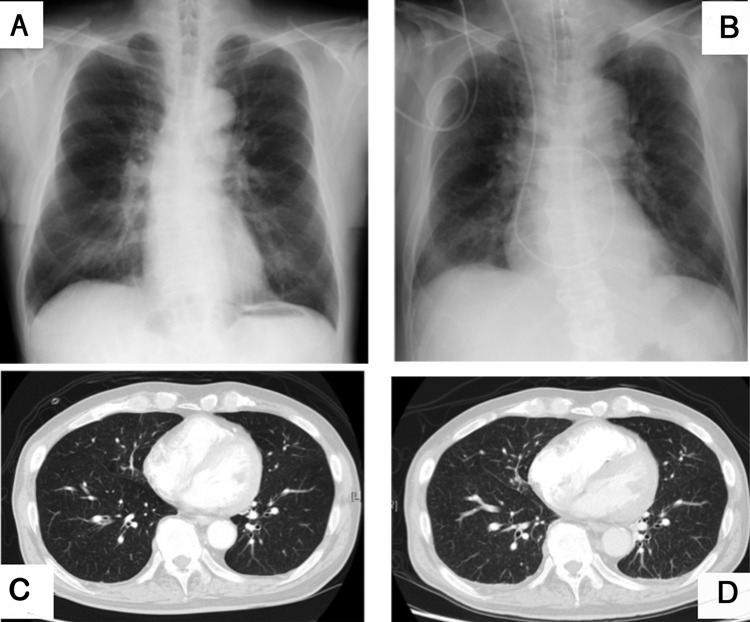
A resting ECG revealed complete right bundle branch block and left axis deviation. A chest radiograph showed no remarkable findings (figure 1). Arterial blood gas was tested in room air and revealed hypoxaemia and respiratory alkalosis (pH 7.47, pO2 58 mm Hg, pCO2 28 mm Hg, HCO3− 20.3 m Eq/L). Other laboratory investigations indicated coagulopathy: antiprothrombin time 54.7 s, prothrombin time–international normalised ratio 1.3, D-dimer 12.3 µg/mL, platelet count 87 000/µL, elevated aspartate aminotransferase (64 IU/L) and lactate dehydrogenase (350 IU/L). The results of other liver function tests were grossly normal, and there were no signs, such as splenomegaly, suggesting cirrhosis or portal hypertension, despite the patient's high alcohol intake. Pulmonary function tests were not indicative of obstructive or restrictive lung disease. CT angiography was performed for presumed acute pulmonary thromboembolic disease, though no embolisms were detected in the pulmonary arteries and the lung fields were clear (figure 1). A transthoracic echocardiogram revealed a structurally normal heart, but the estimated pulmonary artery pressure (PAP) was elevated to 51 mm Hg. A contrast echocardiogram did not show evidence of haemodynamic shunts.
Figure 1.
Imaging findings of the chest. (A) Chest X-ray on standing at admission. (B) Last chest X-ray taken in supine position ∼5 hours prior to death. (C) Lung fields were grossly normal on CT scan at admission. (D) CT scan on day 11 revealed diffuse minute granular shadows in all lung fields and increased right ventricle volume.
On examination, there was hepatomegaly and enlarged indolent lymph nodes in his right inguinal region. Skin erosion in his right scrotum and slight oedema of the right leg were also detected (figure 2).
Figure 2.
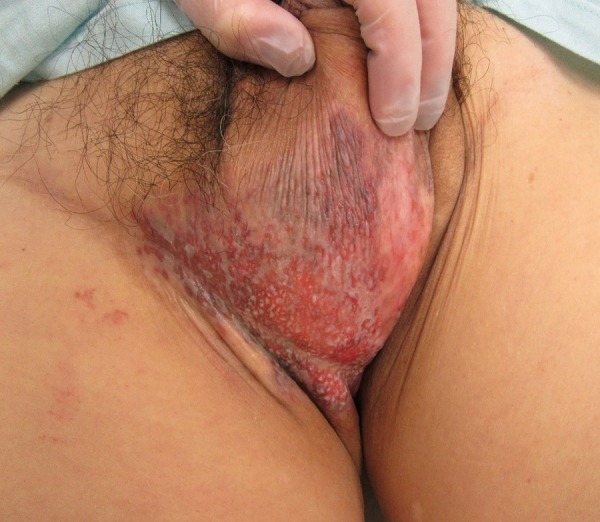
Gross features of the right scrotum. Smooth skin erosion was observed from the scrotum to the perineum. The anus had normal skin and there were no satellite lesions, suggesting benign extramammary Paget's disease. Biopsies were performed in four locations.
EMPD was suspected based on the skin lesion. The patient claimed the lesion had been present for ∼20 years, but he had not consulted a doctor because he was embarrassed by the location. Gross examination of the lesion revealed no patch lesions or nodules. A biopsy later confirmed the diagnosis of EMPD, with no invasion in the basement membrane.
On day 5 after admission, we performed a repeat transthoracic echocardiogram because his respiratory condition had deteriorated. The interventricular septum was remarkably suppressed and flattened during the systolic phase, and the estimated PAP was 75 mm Hg. Rapid progression of his pulmonary hypertension suggested a pathophysiology involving microembolisms in the small pulmonary arteries, and continuous anticoagulation therapy with heparin and sildenafil 60 mg/day was initiated.
On day 9 after admission, lung perfusion scintigraphy revealed diffuse wedge-shaped peripheral perfusion defects. The patient's shortness of breath was worsening, and bosentan 125 mg/day was added to reinforce the treatment for pulmonary hypertension.
The following day, a right inguinal lymph node biopsy was performed, which confirmed metastasis of EMPD. A pulmonary artery catheter was placed for continuous monitoring. Mean values of the PAP and pulmonary capillary wedge pressure were 34 mm Hg and 8 mm Hg, respectively. Cardiac output determined by the repeated thermodilution method was 6.4 L/min and the pulmonary vascular resistance was 324.5 dyn-s/cm5. We also collected blood samples from the catheter wedged in the right inferior and left superior pulmonary artery. Cytology revealed three-dimensional clusters of atypical epithelial cells indicating adenocarcinoma (figure 3). On the basis of the clinical findings, including progressive pulmonary hypertension and coagulopathy, and the imaging and cytological findings, we diagnosed the patient with PTTM originating from invasive EMPD.
Figure 3.
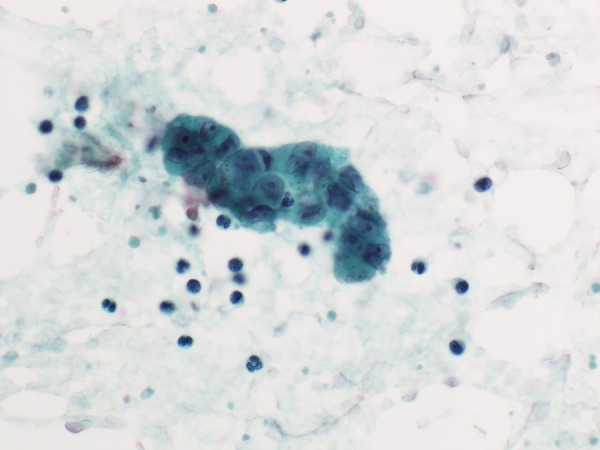
Blood samples aspirated from a wedged pulmonary artery catheter revealed clusters of large epithelial cells. Large irregular nuclei were associated with nucleoli, and the cytoplasm contained many granules. These findings were consistent with metastasis of extramammary Paget's disease (Papanicolaou stain, original magnification ×400).
Treatment
We administered docetaxel 100 mg for invasive EMPD on day 13 of admission.
Outcome and follow-up
The patient's mean PAP continued to rise to 39 mm Hg on day 16, and 46 mm Hg on day 18. Despite aggressive haemodynamic and ventilator support, the patient suffered severe respiratory failure and died on day 19 of hospitalisation.
An autopsy was performed within 4 hours of death. The lungs were grossly congested, but there were no signs of solid neoplasms. Microscopic examination revealed widespread tumour emboli associated with thromboemboli and fibrocellular intimal proliferation within the pulmonary arterioles, consistent with PTTM (figure 4). The tumour cells were identical to EMPD histologically, and stained strongly for gross cystic disease fluid protein 15, which is specific to apocrine sweat-secreting cells. Metastasis was also detected in the medulla of the thoracic and lumbar vertebra and in the para-aorta lymph nodes.
Figure 4.
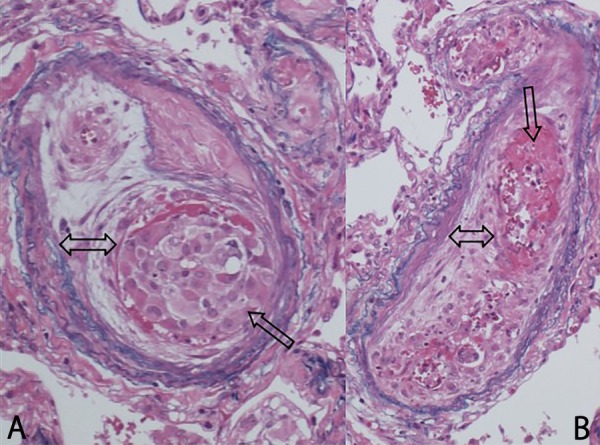
(A) Arteriole with tumour cells adhering to the vessel wall and evidence of fibrointimal proliferation. (B) Blood clot with thickening of the endothelium (H&E stain, original magnification ×40).
Local EMPD lesions were reviewed carefully and few invasions were found in the basement membrane of the primary skin lesion.
Discussion
PTTM is a rare complication of cancer, first proposed in 1990 by von Herbay et al.1 The incidence of PTTM was 1.4–3.3% in an autopsy series of patients who died of cancer.1 2 Widespread microscopic tumour emboli in the pulmonary arterioles cause occlusion and also activate localised coagulation and fibrocellular proliferation of the endocytes. PTTM should be clearly distinguished from pulmonary tumour embolism (PTE) as a cause of pulmonary hypertension, because the main pathophysiology of PTTM involves increased pulmonary vascular resistance caused by fibrocellular proliferation and luminal stenosis.3 Furthermore, PTE is classified as class 4 in the clinical classification of pulmonary hypertension (2015 European Society of Cardiology/European Respiratory Society Guidelines for Diagnosis and Treatment of Pulmonary Hypertension), while PTTM is class 5.4
PTTM usually originates from mucinous adenocarcinomas, particularly gastric tumours, followed by lung or breast neoplasms.1–3 Based on a review of cases in PubMed-indexed journals, the current case appears to be the first report of EMPD or Paget's disease as a cause of PTTM.
Patients with PTTM commonly seek medical assistance because of progressive dyspnoea. However, the prognosis is extremely poor, and most patients die within hours or days of experiencing breathlessness. Patients typically present with normal findings on chest radiography. Laboratory findings are consistent with consumptive coagulopathy, which may progress to disseminated intravascular coagulopathy (DIC), including thrombocytopenia, prolonged antiprothrombin time and prothrombin time, elevated D-dimer or fibrinogen degradation products and elevated lactate dehydrogenase.5 Chest CT scan may show non-specific features such as ground-glass opacities in a tree-in-bud appearance in the lung fields. Contrast-enhanced CT scan or a pulmonary angiography is usually unremarkable. If performed in a timely fashion, lung perfusion scintigraphy or fluorodeoxyglucose (18F)–positron emission tomography–CT scan may be helpful in assessing PTTM. However, both tests are costly and lack evidence to support their true diagnostic values.
The diagnosis of PTTM is predominantly made postmortem, but an ante-mortem diagnosis is essential to enable the initiation of therapy for PTTM. Imatinib has been reported to alleviate pulmonary hypertension, with increased survival of 54 days to 1 year.6–9 Chemotherapy is an alternative option, and has shown some success in two cases.10 11 A search of full-text case reports written in English identified 19 patients who were diagnosed antemortem, including seven who underwent transbronchial lung biopsy (TBLB),2 7 9 11 12 five who received video-assisted thoracoscopic surgery or thoracotomy (including 1 patient who was not diagnosed by TBLB),2 8 10 13 14 one patient who underwent CT-guided biopsy,2 four diagnosed by cytology,2 6 15 16 and two cases who were diagnosed based on clinical decisions without specific pathological testing.17 18 The treatment and survival of these cases are shown in table 1.
Table 1.
Methods of ante-mortem diagnosis and treatment of cases with PTTM
| Year | Author | Primary site | Method of diagnosis | Treatment after diagnosis | Survival* |
|---|---|---|---|---|---|
| 2003 | Roberts15 | Bladder | Cytology | NA | NA |
| 2007 | Miyano14. | Stomach | VATS† | Chemotherapy, dexamethasone | Recovered |
| 2011 | Ueda12 | Oesophagus | TBLB | Chemotherapy | 9 days |
| 2012 | Kim HJ17 | Breast | Clinical-based decision | Anticoagulation, dexamethasone | 2 days |
| 2012 | Kayatani10 | Unknown (signet ring) | VATS | Chemotherapy | 15 months |
| 2012 | Kitamura11 | Breast | TBLB | Chemotherapy | 3 months |
| 2013 | Demirag13 | Epithelioid angiosarcoma | Lung resection | Refusal of treatment | 5 weeks |
| 2013 | Higo7 | Colon | TBLB | Imatinib, chemotherapy, bosentan, tadalafil | 12 months |
| 2013 | Ogawa9 | Stomach and duodenum | TBLB | Imatinib, chemotherapy, bosentan, epoprostenol | 9 months |
| 2013 | Uruga2 | Lung | CT-guided lung biopsy | Chemotherapy | 7 months |
| 2013 | Uruga2 | Lung | Cytology | NA | 69 days |
| 2013 | Uruga2 | Stomach | TBLB | NA | 3 days |
| 2013 | Uruga2 | Stomach | TBLB | NA | 10 days |
| 2013 | Uruga2 | Stomach | TBLB | NA | 10 days |
| 2013 | Uruga2 | Stomach | VATS | NA | 5 days |
| 2014 | Mandaliya18 | Stomach | Clinical-based decision | Anticoagulation, corticosteroids | Uncertain |
| 2015 | Fukada6 | Breast | Cytology | Imatinib, chemotherapy | 54 days |
| 2015 | Minatsuki8 | Stomach | VATS | Imatinib, chemotherapy, sildenafil, ambrisentan | 12 months |
| 2015 | Yamakawa16 | Bladder | Cytology | Chemotherapy | 12 days |
*Survival indicates time from initial admission in most cases.
†Unsuccessful diagnosis with TBLB.
NA, not applicable; PTTM, pulmonary tumour thrombotic microangiopathy; TBLB, transbronchial lung biopsy; VATS, video-assisted thoracoscopic surgery.
Although tissue histology remains the diagnostic golden standard for PTTM, lung biopsy (TBLB, video-assisted thoracoscopic surgery or CT guided) is limited to patients with stable respiratory status. Among these techniques, blood aspiration using a wedge catheter is less invasive and has little impact on the patient's breathing. However, PTE cannot be excluded by cytology alone, and distinguishing tumour cells from pulmonary megakaryocytes is technically challenging.19 If a tissue biopsy is not available, the diagnosis of PTTM should be supported by a combination of cytology and clinical features of progressive acute pulmonary hypertension and DIC, after excluding pulmonary thromboembolism by imaging studies.
Learning points.
Pulmonary tumour thrombotic microangiopathy (PTTM) is rare, but should be considered as a possible diagnosis in patients with cancer.
A full physical examination, including the genital organs, is essential to reach the correct diagnosis, and should not be overlooked.
Evaluation of blood cytology from the pulmonary artery may be useful for diagnosing PTTM.
Progressive acute pulmonary hypertension and DIC may be clues for diagnosing PTTM.
Footnotes
Contributors: AB drafted the article. KC and HK revised and edited for important intellectual content. KN helped with finalising the manuscript and gave the final approval of the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.von Herbay A, Illes A, Waldherr R et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 1990;66:587–92. [DOI] [PubMed] [Google Scholar]
- 2.Uruga H, Fujii T, Kurosaki A et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med 2013;52:1317–23. 10.2169/internalmedicine.52.9472 [DOI] [PubMed] [Google Scholar]
- 3.Patrignani A, Purcaro A, Calcagnoli F et al. Pulmonary tumor thrombotic microangiopathy: the challenge of the antemortem diagnosis. J Cardiovasc Med (Hagerstown) 2014;15:828–33. 10.2459/JCM.0b013e328354e473 [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903–75. 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 5.Gavin MC, Morse D, Partridge AH et al. Clinical problem-solving. Breathless. N Engl J Med 2012;366:75–81. 10.1056/NEJMcps1011918 [DOI] [PubMed] [Google Scholar]
- 6.Fukada I, Araki K, Minatsuki S et al. Imatinib alleviated pulmonary hypertension caused by pulmonary tumor thrombotic microangiopathy in a patient with metastatic breast cancer. Clin Breast Cancer 2015;15:e167–70. 10.1016/j.clbc.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Higo K, Kubota K, Takeda A et al. Successful antemortem diagnosis and treatment of pulmonary tumor thrombotic microangiopathy. Intern Med 2014;53:2595–9. 10.2169/internalmedicine.53.2379 [DOI] [PubMed] [Google Scholar]
- 8.Minatsuki S, Miura I, Yao A et al. Platelet-derived growth factor receptor-tyrosine kinase inhibitor, imatinib, is effective for treating pulmonary hypertension induced by pulmonary tumor thrombotic microangiopathy. Int Heart J 2015;56:245–8. 10.1536/ihj.14-220 [DOI] [PubMed] [Google Scholar]
- 9.Ogawa A, Yamadori I, Matsubara O et al. Pulmonary tumor thrombotic microangiopathy with circulatory failure treated with imatinib. Intern Med 2013;52:1927–30. 10.2169/internalmedicine.52.0718 [DOI] [PubMed] [Google Scholar]
- 10.Kayatani H, Matsuo K, Ueda Y et al. Pulmonary tumor thrombotic microangiopathy diagnosed antemortem and treated with combination chemotherapy. Intern Med 2012;51:2767–70. 10.2169/internalmedicine.51.7682 [DOI] [PubMed] [Google Scholar]
- 11.Kitamura A, Nishimura N, Jinta T et al. A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy and treated with chemotherapy and long-term oxygen and anticoagulation therapies. Case Rep Pulmonol 2013;2013:259080 10.1155/2013/259080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda A, Fuse N, Fujii S et al. Pulmonary tumor thrombotic microangiopathy associated with esophageal squamous cell carcinoma. Intern Med 2011;50:2807–10. 10.2169/internalmedicine.50.6113 [DOI] [PubMed] [Google Scholar]
- 13.Demirag F, Cakir E, Yazici U et al. Pulmonary tumor thrombotic microangiopathy from metastatic epithelioid angiosarcoma. J Thorac Dis 2013;5:E107–11. 10.3978/j.issn.2072-1439.2012.10.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyano S, Izumi S, Takeda Y et al. Pulmonary tumor thrombotic microangiopathy. J Clin Oncol 2007;25:597–9. 10.1200/JCO.2006.09.0670 [DOI] [PubMed] [Google Scholar]
- 15.Roberts KE, Hamele-Bena D, Saqi A et al. Pulmonary tumor embolism: a review of the literature. Am J Med 2003;115:228–32. 10.1016/S0002-9343(03)00305-X [DOI] [PubMed] [Google Scholar]
- 16.Yamakawa H, Yoshida M, Yamada M et al. Pulmonary tumor thrombotic microangiopathy associated with urothelial carcinoma of the urinary bladder: antemortem diagnosis by pulmonary microvascular cytology. Clin Case Rep 2015;3:735–9. 10.1002/ccr3.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Kwak MH, Kong SY et al. A case of locally advanced breast cancer complicated by pulmonary tumor thrombotic microangiopathy. Cancer Res Treat 2012;44:267–70. 10.4143/crt.2012.44.4.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandaliya R, Farhat S, Uprety D et al. Occult gastric cancer presenting as hypoxia from pulmonary tumor thrombotic microangiopathy. J Gastric Cancer 2014;14:142–6. 10.5230/jgc.2014.14.2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abati A, Landucci D, Danner RL et al. Diagnosis of pulmonary microvascular metastases by cytologic evaluation of pulmonary artery catheter-derived blood specimens. Hum Pathol 1994;25:257–62. 10.1016/0046-8177(94)90197-X [DOI] [PubMed] [Google Scholar]