Abstract
A 33-year-old woman with no premorbidities presented to us with chest pain and worsening dyspnoea since 1 week. Systemic examination was suggestive of acute pulmonary oedema and preliminary investigations revealed evolved anterior wall myocardial infarction (MI). The patient was stabilised and taken up for angiography which revealed spontaneous coronary artery dissection (SCAD) of the left anterior descending (LAD) artery. She underwent percutaneous coronary intervention (PCI) for the same. Further investigation into the cause for the SCAD came strongly positive for systemic lupus erythematosus (SLE). She had no prior symptoms suggestive of SLE and the SCAD was its very first clinical manifestation.
Background
Spontaneous coronary artery dissection (SCAD) is a rare cause for MI in young. Earlier reports suggested the incidence to be upto 1.1% among patients referred for angiography but was likely to be under-reported due to high risk of associated sudden cardiac death and difficulty in identifying the lesion on angiography.1 With advancements in intracardiac imaging more recent studies suggest the incidence to be about 1–4%.2 It has a striking predilection for women who account for over 80% of the cases.3 We report a case of MI secondary to SCAD in a young female with no risk factors. Further evaluation for possible aetiology of SCAD helped clinch the diagnosis of systemic lupus erythematosus (SLE). SLE causing SCAD is rare, moreover SCAD as the presenting feature of SLE with no prior symptoms has never been described before. MI in young females is rare and while atherosclerotic coronary artery disease is the most common cause for ACS in young, non-atherosclerotic aetiology must be considered especially in the absence of risk factors. Moreover, in a case of SCAD, the patient must be evaluated for secondary causes.
Case presentation
A 33-year-old woman presented to us with a 1 week history of left side chest pain and breathlessness. The chest pain was dull with no radiation to the shoulder, jaw or back and did not worsen on deep inspiration. The patient reported of associated dyspnoea on exertion since 1 week, which had progressed over the last 24 hours and had brought the patient to the hospital. There was no history of trauma, cough, fever, syncope or palpitations. The patient denied any history of smoking or substance abuse and was not on any medications. She had no history of abortions.There was no history of sudden cardiac death or connective tissue disorder in the family to the best of her knowledge. On examination, the patient was afebrile with a regular pulse rate of 100bpm, peripheral pulses were well felt, blood pressure (BP) was 110/70 mm Hg and saturation was 97% on room air. She was tachypnoeic with a respiratory rate of 22 breaths/min and jugular venous pressure was elevated. Systemic examination revealed normal first and second heart sounds and an audible third heart sound. Bilateral air entry into the lungs was equal and bibasal crepitations were audible on auscultation. The remaining systemic examination was normal. Examination findings were highly suggestive of acute pulmonary oedema. The history of chest pain was suggestive of a cardiac cause for the pulmonary oedema.
Investigations
ECG showed qRBBB pattern in leads V1 and V2 suggestive of an evolved anterior wall MI. Troponin T was positive. Echocardiogram showed anterior wall hypokinesia with moderate left ventricular dysfunction. Chest X-ray was suggestive of pulmonary oedema with no cardiomegaly and D-dimer was normal.
Differential diagnosis
All preliminary investigations pointed towards an evolved anterior wall MI and acute pulmonary oedema. Since atherosclerotic disease is the most common cause of MI in both old and young, it was considered the most likely possibility. However, in view of the age and the absence of risk factors a possibility of arterial emboli, muscle bridge, SCAD and congenital anomalies were also kept in mind.
Treatment
The patient was treated for the acute pulmonary oedema with intravenous diuretics and oxygen by mask. She received loading doses of aspirin 325 mg and clopidogrel 300 mg. Once stable, she underwent a coronary angiogram that unveiled an unexpected finding. The coronary angiogram was suggestive of a coronary artery dissection in the proximal LAD artery with 80–90% occlusion (video 1). No other abnormalities were noted on angiography. The patient underwent percutaneous transluminal coronary angioplasty for the same. The lesion was crossed with 0.014 galeo floppy wire and stented with 2.75×37 mm metafor. Postprocedure thrombolysis in myocardial infarction (TIMI) flow III was noted (figure 1). The patient tolerated the procedure well and postprocedure hospital stay was uneventful. Postprocedure ECG revealed qRBBB.
Figure 1.
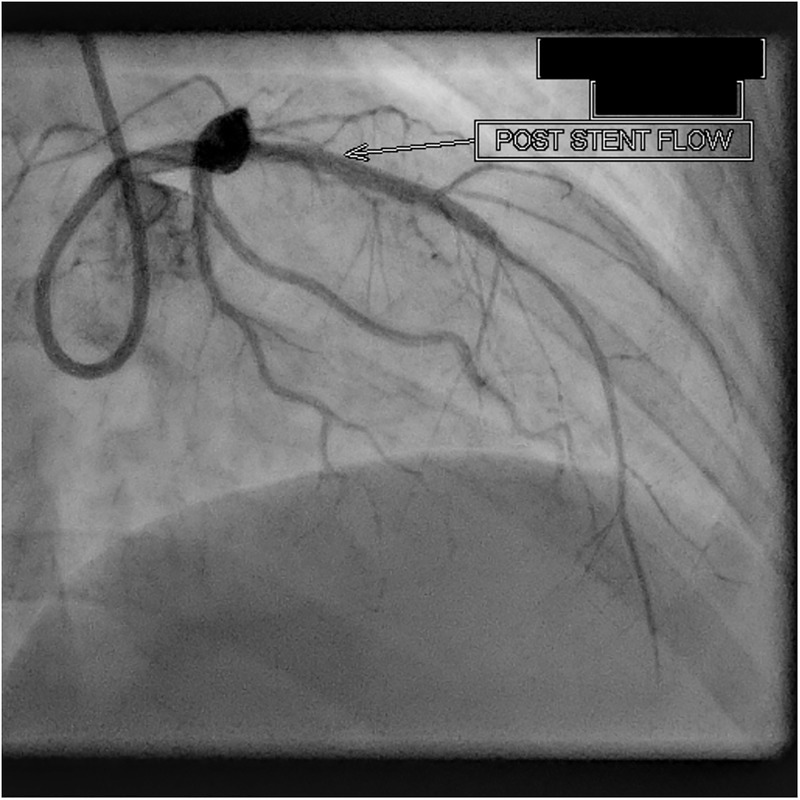
Poststent flow in left anterior descending artery.
Video 1.
Coronary angiogram showing dissection of the left anterior descending artery.
Echocardiogram was suggestive of apical and anterior wall hypokinesia, mild mitral regurgitation (MR) moderate left ventricular dysfunction with an ejection fraction of 42%.
Outcome and follow-up
The young age, absence of atherosclerosis or an on-going or recent pregnancy in our patient prompted us to look for other possible aetiologies for the SCAD. We repeated a detailed history and examination, which revealed no evidence of features suggestive of Marfan's syndrome, SLE, scleroderma, arthritis or other markers of connective tissue disorder. The suspicion of fibromuscular dysplasia (FMD) was low on our list in view of normal BP and low prevalence of the disease in our population. A drug screen was not performed as there was no history or examination finding suggestive of the possibility of drug abuse or of any other high-risk behaviour. We revisited the laboratory investigations which revealed an erythrocyte sedimentation rate) (ESR) of 67 mm/hour and a platelet count of 104 000/µL. Serum ferritin was 1406 µg/L Other than thrombocytopenia and elevated ESR and ferritin, routine investigations; including blood counts, biochemical parameters, urine microscopy and protein were normal. Work-up was sent for connective tissue disorders and vasculitis and was found to be strongly positive for anti-nuclear antibody (ANA) and anti-sm antibodies. Direct Coombs test was positive. Antineutrophil cytoplasmic antibodies (ANCA) and anticardiolipin antibody were negative. Anti-double standard DNA titres were not carried out. The patient was diagnosed to have SLE as per the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria and had presented with SCAD as the primary presentation of her SLE.
The patient was started on hydroxychloroquine 200 mg two times a day. While systemic vasculitis secondary to SLE is treated with steroids and cytotoxic therapy, due to the low incidence, no clear guidelines exist regarding the treatment of SCAD in association with SLE. We initially considered starting steroids in our patient; however, as she was showing significant improvement and the postprocedure period was free of complications we decided to withhold steroids and keep her under a close observation. Also, steroids are associated with poor tissue healing in MI and worsening of the traditional risk factors of coronary artery disease such as atherosclerosis and diabetes mellitus which were avoided.
The patient has been on regular follow-up, and at 1 month and 6 months follow-up she has been doing well with β- blockers, ACE inhibitors, oral diuretic, dual antiplatelet therapy and hydroxychloroquine.
Discussion
Classically, the aetiology of SCAD is classified into three categories; peripartum, atherosclerotic and idiopathic.4 However, more recently, it has also been described in relation with Marfan’s syndrome,5 Ehler-Danlos,6 FMD,7 cocaine abuse,8 strenuous exercise9 and oral contraceptive pill (OCP) use.10 An extensive literature search yielded only five cases of SCAD in association with SLE hence, this case report emphasises the need to consider the possibility of connective tissue disorders, especially SLE when investigating a case of SCAD.
The exact pathogenesis of SCAD remains unknown. It is, however, believed to be complex and multifactorial. In case of SLE coronary artery disease is thought to result from vasculitis, chronic vessel wall inflammation and increased risk of atherosclerosis.11 Oestrogen is believed to cause hypertrophy of the smooth muscles in the vessel wall along with increase in mucopolysaccharides and reduction in collagen production resulting in loosening of the intercellular matrix, leaving them more prone to dissection.12 This may explain the increased propensity of vessels to dissect in pregnancy and peripartum period, and among women in general.
SCAD often presents as MI but is most commonly picked up on autopsy. It has a female predilection and most commonly involves the LAD artery.3 Three of five cases reported previously in association with SLE were women and the dissection involved LAD in two,13 14 and left circumflex,15 left main coronary artery,16 obtuse marginal in one case each.17 The patient with left main coronary artery (LCMA) involvement had succumbed to the MI.16
There are no specific guidelines for the treatment of SCAD and the primary aim is to achieve patency of the affected vessel. Thrombolysis should be avoided as it may cause propagation of the dissection due to progression of the intramural haematoma and lead to further narrowing of the arterial lumen.18 PCI is the treatment of choice when there is significant narrowing of the lumen or the disease affects the epicardial vessels; however, occasionally wiring the true lumen can be challenging and any error may lead to extension of the dissection and further damage. In a study by Tweet et al,19 procedural failure rates were as high as 53% among those treated by PCI while conservative treatment resulted in uneventful hospital course in 90% cases with 10% eventually needing revascularisation. In case normal flow is noted on angiogram and the patient is haemodynamically stable with no further worsening of symptoms, PCI may be deferred and conservative treatment justified. Hence, treatment must be individualised based on hemodynamic stability, location of the dissection and TIMI flow. In our patient, as the duration of chest pain was 7 days, attempting thrombolysis before a PCI was ruled out and incidentally helped us avoid thrombolysis even before the diagnosis of SCAD was determined. The presence of 80–90% lumen narrowing on angiography warranted immediate PCI.
Three of the five patients mentioned above had been diagnosed with SLE prior to the coronary event. Patients with SLE have a 50-fold increase in prevalence of coronary artery disease as compared to an age-matched control.11 In the remaining two, while the patients were diagnosed to have SLE only following the presentation of SCAD both patients had subtle symptoms of SLE prior to the event that had not been further investigated. In a case reported by Nisar et al,15 a male patient had reported subtle symptoms such as oral ulcers, arthralgia and fatigue before the cardiac event. Subtle non-specific symptoms and the female predilection of the disease may delay diagnosis in men. In another case report, by Aldoboni et al,13 a 39-year-old woman had symptoms of malar rash, photosensitivity and skeletal pains for which she did not seek medical attention. At the time of presentation, the patient had pancytopenia and raised inflammatory markers that prompted them to look for evidence of SLE. Even on detailed history taking our patient reported no prior symptoms suggestive of SLE making SCAD truly the first presentation of her underlying disease. Signs, symptoms and investigation findings suggestive of an underlying connective tissue disorder may be subtle and may be overlooked unless its possibility is kept in mind. The possibility of a connective tissue disorder should be considered in patients presenting with SCAD especially when pregnancy and atherosclerosis is ruled out.
Learning points.
Spontaneous coronary artery dissection (SCAD) is a rare cause of myocardial infarction (MI) and must be considered especially in young individuals who present with MI in the absence of other risk factors.
Initially thought to be upto 1.1%, the incidence of SCAD is now reported to be about 1–4% due to higher rate of detection with the advancement in intracardiac imaging.
In patients presenting with SCAD, the possibility of underlying connective tissue disorders should be considered especially when pregnancy and atherosclerosis are ruled out.
Treatment must be individualised based on haemodynamic stability, location of the dissection and TIMI flow.
Conservative management is preferred when the flow is normal, no further worsening of symptoms occurs and the patient is haemodynamically stable. Percutaneous coronary intervention must be considered in case of significant narrowing of lumen or a haemodynamically unstable patient.
Footnotes
Contributors: SR contributed to the summary, background and video. TV contributed to the history, examination and discussion. NCGS was involved in the treatment and discussion. RKS was involved in ideas for discussion, editing and approval of the final version.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rosengarten JA, Dana A. Recurrent spontaneous coronary artery dissection: acute management and literature review. Eur Heart J Acute Cardiovasc Care 2012;1:53–6. 10.1177/2048872612442404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiguchi T, Tanaka A, Ozaki Y et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2016;5:263–70. 10.1177/2048872613504310 [DOI] [PubMed] [Google Scholar]
- 3.Garcia NA, Khan AN, Boppana RC et al. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect. 2014;4:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Maio SJ Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 1989;64:471–4. [DOI] [PubMed] [Google Scholar]
- 5.Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366:1965–76. 10.1016/S0140-6736(05)67789-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henkin S, Negrotto SM, Tweet MS et al. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders . Heart 2016;102:876–81. 10.1136/heartjnl-2015-308645 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed Z, Bajwa A, Bhardwaj B et al. Spontaneous coronary artery dissection: the management dilemma continues. BMJ Case Rep 2015;2015:pii: bcr2015211061 10.1136/bcr-2015-211061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhauer JR, Caulfield JB. Spontaneous coronary artery dissection associated with cocaine abuse: case report and brief review. Cardiovasc Pathol 2001;10:141–5. [DOI] [PubMed] [Google Scholar]
- 9.Choi JW, Davidson CJ. Spontaneous multivessel coronary dissection in a long distance runner successfully treated with oral antiplatelet therapy a case report and review of the literature. J Invasive Cardiol 2002;14:675–8. [PubMed] [Google Scholar]
- 10.Evangelou D, Letsas KP, Korantzopoulos P et al. Spontaneous coronary artery dissection associated with oral contraceptive use: a case report and review of the literature . Int J Cardiol 2006;112:380–2. 10.1016/j.ijcard.2005.07.069 [DOI] [PubMed] [Google Scholar]
- 11.Karrar A, Sequeira W, Block JA. Coronary artery disease in systemic lupus erythematosus: a review of the literature. Semin Arthritis Rheum 2001;30:436–43. 10.1053/sarh.2001.23498 [DOI] [PubMed] [Google Scholar]
- 12.Asuncion CM, Hyun J. Dissecting intramural hematoma of the coronary artery in pregnancy and the puerperium. Obstet Gynecol 1972;40:202–10. [PubMed] [Google Scholar]
- 13.Aldoboni AH, Hamza EA, Majdi K et al. Spontaneous dissection of coronary artery treated by primary stenting as the first presentation of systemic lupus erythematosus . J Invasive Cardiol 2002;14:694–6. [PubMed] [Google Scholar]
- 14.Rekik S, Lanfranchi P, Jacq L et al. Spontaneous coronary artery dissection in a 35 year-old woman with systemic lupus erythematosus successfully treated by angioplasty. Heart Lung Circ 2013;22:955–8. 10.1016/j.hlc.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 15.Nisar MK, Mya T. Spontaneous coronary artery dissection in the context of positive anticardiolipin antibodies and clinically undiagnosed systemic lupus erythematosus. Lupus 2011;20:1436–8. 10.1177/0961203311406765 [DOI] [PubMed] [Google Scholar]
- 16.Sharma AK, Farb A, Maniar P et al. Spontaneous coronary artery dissection in a patient with systemic lupus erythematosis. Hawaii Med J 2003;62:248–53. [PubMed] [Google Scholar]
- 17.Kothari D, Ruygrok P, Gentles T et al. Spontaneous coronary artery dissection in an adolescent man with systemic lupus erythematosus. Intern Med J 2007;37:342–3. 10.1111/j.1445-5994.2007.01345.x [DOI] [PubMed] [Google Scholar]
- 18.Buys EM, Suttorp MJ, Morshuis WJ et al. Extension of a spontaneous coronary artery dissection due to thrombolytic therapy. Cathet Cardiovasc Diagn 1994;33:157–60. [DOI] [PubMed] [Google Scholar]
- 19.Tweet MS, Eleid MF, Best PJ et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777–86. 10.1161/CIRCINTERVENTIONS.114.001659 [DOI] [PubMed] [Google Scholar]


