Abstract
Epstein-Barr virus (EBV) infection is associated with neurological sequellae, but rarely there is acute cerebellar ataxia (ACA) in an adult. We present a novel case of a 26-year-old man, who presented with ACA. He had normal MRI and CSF analysis. Serum testing confirmed active EBV. A course of oral prednisolone 1 mg/kg for 4 weeks, with a subsequent taper was started. He made a full recovery within 3 weeks of presentation.
Background
Epstein-Barr virus (EBV) infection is associated with neurological sequellae, but rarely there is acute cerebellar ataxia (ACA) in an adult.1 We present the case of a 26-year-old man who presented with ACA. The case is novel as his presentation was sudden, without prodromic viral symptoms, and his lumbar puncture and MRI investigations were normal.
Case presentation
A previously well, 26-year-old Caucasian man presented with a 3-day history of imbalance and incoordination, following a 3-day occipital headache with neck pain, which had since resolved. He was an infrequent user of cannabis, but denied any other recreational drug use.
On examination he was, apyrexial, dysarthric and unable to stand or even sit unaided. He had finger-nose dysmetria and an intention tremor. His eye movements had some ocular dysmetria, but there was no nystagmus and the remaining cranial nerves examination was normal. Tone, power and reflexes were also normal in all four limbs, with all modalities of sensation preserved. There was no dysdiadochokinesis.
Investigations
This history and examination clearly pointed towards an ACA. Given the predominant truncal disability and lack of lateralising features, clinically at least, this was likely to be affecting the midline cerebellar structures.
The differential for ACA is wide and includes vascular events (ischaemic, haemorrhagic stroke or dissection), toxic-mediated processes (ie, antiepileptic drugs, chemotherapy, recreational drugs), infection, postinfectious process (typically 1–6 weeks following viral infection, more commonly in children than adult), inflammatory (ie, multiple sclerosis) or neoplastic (local or paraneoplastic). Given the acute history, a vascular event was suspected.
However, a subsequent CT scan, MRI of the brain, including diffusion weighted imaging (DWI) sequence and MR angiogram were normal. Serum blood tests revealed a normal white cell count (WCC) (7.4×109/L), with a slight neutropenia (1.6×109/L) and normal C reactive protein (<5 mg/L). Alanine transaminase (ALT) was slightly elevated (48 μ/L) and platelets were low (113×109/L). HIV and hepatitis serology were negative. A lumbar puncture was performed, which was quiescent; WCC 1, red blood cell count <1, protein 0.26 mg/L and the viral PCRs were also normal (Herpes simplex virus 1/2, EBV, Cytomegalovirus, Varicella zoster virus, Enterovirous and Parechovirus).
In the absence of a relevant drug history, these investigations had ruled out a vascular event and a primary infectious or inflammatory process very unlikely. The aetiology was therefore remaining elusive but then serendipity struck; while performing a serum cell count, a laboratory technician noted atypical cells (figure 1).
Figure 1.
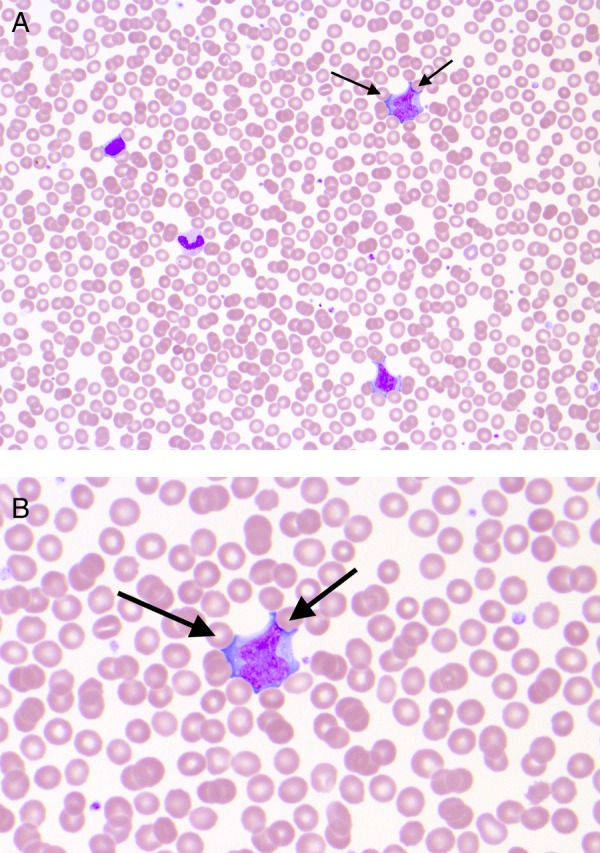
Blood film (×400 magnification). The blood film showing multiple ‘atypical’ lymphocytes, which are pleomorphic, have large amounts of cytoplasm and prominent nucleoli. Indentations of the cytoplasm created by the surrounding red blood cells can be seen (arrows). The changes are a reaction to viral antigenic stimulation. In the presence of Epstein-Barr virus, these cells are sometimes referred to as Downey cells with reference to Hal Downey who contributed to their first characterisation.
Atypical reactive CD8 lymphocytes, although not pathognomonic for EBV, are common during acute infection. The neutropenia and thrombocytopenia might also support viral infection.
On further serum testing, EBV viral capsid antigen IgG and IgM antibodies were detected and antibodies to EBV nuclear antigen (EBNA) were absent. As antibodies to EBNA appear later, the serology was consistent with recent, primary EBV infection. EBV DNA was detected by real-time PCR in serum, confirming the presence of the virus in peripheral blood. EBV DNA was not detected in cerebrospinal fluid (CSF) by real-time PCR.
Outcome and follow-up
Our patient was started on oral prednisolone 1 mg/kg for 4 weeks, with a subsequent taper. He made a full recovery within 3 weeks of presentation.
Discussion
EBV infection is estimated to manifest neurological sequallae in 2–5% of infected patients.1 Of these associations, in adults, ACA is particularly uncommon. A systematic search of MEDLINE showed 25 case reports for EBV-associated ACA.
Among these cases, normal CSF and MRI are reported. The absence of any prodromal illness or clinical symptoms typical of EBV infection is unusual. With the benefit of the ‘retrospectoscope’, the slight derangement in platelets, neutrophils and liver enzymes, would be in keeping with viral infection.
The exact mechanism of EBV-mediated ACA is unknown, but positive antibodies in some cases point towards a parainfectious molecular mimicry, rather than direct viral effect.2 Negative CSF EBV PCRs have been reported in a similar cases and this may reflect the non-infectious aetiology of the disease.1 The literature suggests the prognosis is generally good, with the majority making a full recovery. However, there are descriptions of associated fatal cerebellitis, through tonsillar herniation or hydrocephalus.3 Reported management is variable, with some opting for supportive measures alone and others for steroids, intravenous immunoglobulin (IVIG) and/or antivirals uniquely or in combination. In our case, in the absence of active CSF or CSF EBV, antiviral therapy was not given but steroids were started.
Learning points.
Epstein-Barr virus (EBV) is a rare cause of acute cerebellar ataxia.
It can occur in the absence of infective symptoms (clearly an Epstein-Barr humbug).
A normal CSF and MRI of the brain is common.
On this basis, it is always worth considering EBV infection screening in acute cerebellar ataxia by serum testing.
Footnotes
Contributors: All authors were involved in the care of the patient in question. BD was the lead author involved in drafting the article. Authors NM, TL, MAH reviewed, critically appraised and amended the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.McCarthy CL, McColgan P, Martin P. Acute cerebellar ataxia due to Epstein-Barr virus. Pract Neurol 2012;12:238–40. 10.1136/practneurol-2011-000115 [DOI] [PubMed] [Google Scholar]
- 2.Uchibori A, Sakuta M, Kusunoki S et al. Autoantibodies in postinfectious acute cerebellar ataxia. Neurology 2005;65:1114–6. 10.1212/01.wnl.0000178802.38268.1e [DOI] [PubMed] [Google Scholar]
- 3.Wise FM, Olver J, Infeld B. A 19-year-old male with cerebellar ataxia and cognitive impairment following glandular fever. J Clin Neurosci 2013;20:749–50. 10.1016/j.jocn.2012.03.052 [DOI] [PubMed] [Google Scholar]


