Abstract
Necrotising sialometaplasia is a rare, benign and self-limiting inflammatory lesion that commonly involves minor salivary glands. Its clinical appearance, signs and symptoms very often mimic a carcinomatous lesion, thus creating a diagnostic dilemma for the clinician. Necrotising sialometaplasia being an important differential for an apparent carcinoma should be excluded histologically prior to radical therapy. It commonly occurs on the palatal mucosa following a palatal infiltration anaesthesia. The patient reports to the dentist with a sudden onset of painful ulcerations that rapidly increase in size. This case report describes the occurrence of necrotising sialometaplasia in a 46-year-old man with an unusual clinical presentation at multiple sites in the oral cavity. The importance of history taking, thorough clinical examination and careful histopathological examination in diagnosing necrotising sialometaplasia is highlighted in this paper.
Background
Necrotising sialometaplasia was first described by Abrams et al1 in 1973. It is an acute, self-limiting, necrotising and inflammatory process involving the minor salivary gland tissues in the palatal region. However, there are a very few case reports of this lesion occurring in other intraoral sites, such as the lower lip, tongue, retromolar region, buccal mucosa and a few extraoral sites where salivary gland tissues are present.
Demographically, necrotising sialometaplasia commonly occurs above the age of 40 years with a male predilection (65%).2 Another study reported a female-to-male ratio of 2.7:16 and 1.95:15.3 Unilateral presentation of this lesion is more common than bilateral lesions, which account for only 20%.2
The patient reports of pain, bleeding, dysphagia and increased salivation but rarely present symptoms of anaesthesia or paresthesia. If it occurs in regions where there is no salivary gland tissue, it is called adenometaplasia. The patients will report with a sudden onset of an ulcer that increases in size, accompanied by pain at the site of lesion and referred to the ear and pharynx. This lesion is self-limiting and usually undergoes a complete resolution in about 4–12 weeks.
Case presentation
A male patient aged 46 reported with a symptom of ulcers in the oral cavity associated with pain and dysphagia. History revealed an insidious onset of an ulcer on the inner aspect of the cheek on the right lower molar teeth region 2 months before, which had progressively increased to its present size. The lesion was associated with severe pain (Visual Analogue Scale score: 7), dysphagia and sialorrhoea. The patient could not take normal diet, especially spicy foods due to burning sensation. The patient also reported of generalised tiredness and loss of weight due to decreased food intake since the onset of the ulcer.
Immediately after the onset of ulcer, the patient visited his primary care dentist, who attributed it to be induced by trauma from the grossly decayed 46 and subsequently extracted 46 under local anaesthesia (lignocaine 2% with epinephrine 1:80 000). Even after extraction, the ulcer did not heal and continued to increase in size. One week after extraction as the ulcer was still persistent, his dentist performed an incisional biopsy. The histopathological report just mentioned areas of abundant inflammatory cells with certain areas of the epithelium over the incised specimen showing features suggestive of mild dysplasia, but was inconclusive.
As the problem persisted, he consulted his family physician who treated him with various topical anaesthetics (lignocaine gel) and antiseptic (0.2% topical chlorhexidine gluconate gel).
As there was no improvement in signs and symptoms, the patient was then referred to the oral medicine specialty clinic in our hospital. Medical history revealed episodes of abdominal pain 5 years before for which a complete gastrointestinal tract examination was performed. He was diagnosed with gastritis and was under treatment for 1 year with H2-receptor blocker. Personal history of the patient revealed no tobacco usage in any form. The patient had no other contributing factors.
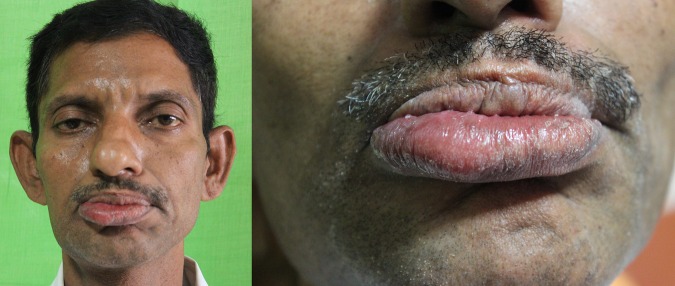
On clinical examination, the patient appeared pale and tired. There was a localised swelling on the right side of his lower lip extending from the angle of the mouth until the midline (figure 1). Mucosa over the enlarged lip showed mild signs of erythema and was soft in consistency.
Figure 1.
Prominent enlargement of lower lip on the right side.
Intraorally, on the labial mucosa, there was a diffuse irregular ulcer with numerous lobulations having a typical cobblestone appearance extending from 41 to 44 region (figure 2). Edges of the ulcer were irregular. Margins of the ulcer on the inferior aspect was slightly everted and raised. The floor of the ulcer was covered by greyish-white slough with pinpoint haemorrhagic spots uniformly dispersed over it. Mild induration was felt on palpation.
Figure 2.
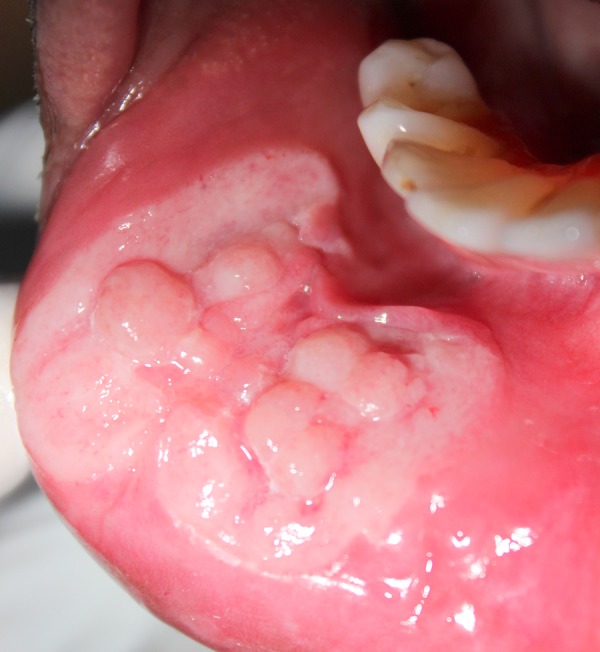
Irregular ulcer with lobulations on the lower labial mucosa.
A well-circumscribed, solitary ulcer measuring about 0.5 cm with a mild fissure in the centre surrounded by a white keratotic margin was present on the buccal vestibule in 46 region (figure 3).
Figure 3.
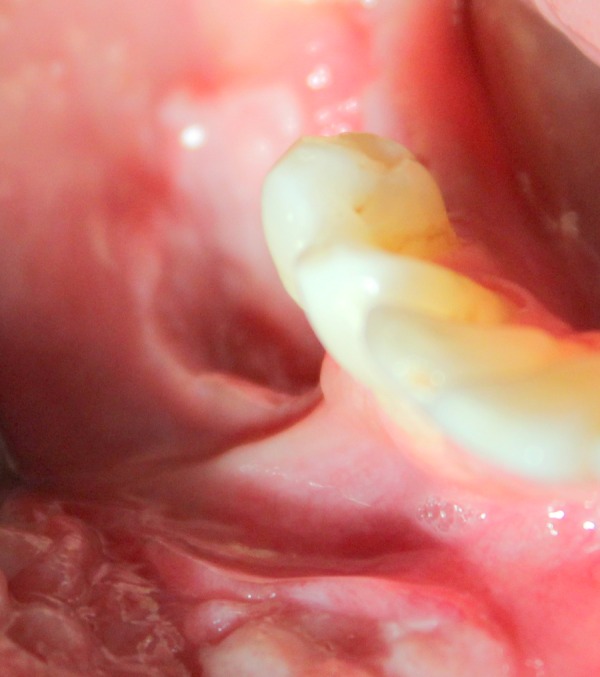
Solitary ulcer with fissuring in buccal vestibule of 46 region.
Another well-circumscribed ovoid ulcer measuring about 0.5 cm with an erythematous halo, mild corrugated appearance was present on the inner aspect of labial mucosa in relation to 31 and 32 regions (figure 4). The floor of the ulcer was covered by a greyish-white slough. There was mild bleeding on probing from the ulcer margin. There was no trauma from teeth along the areas where the ulcer was present.
Figure 4.
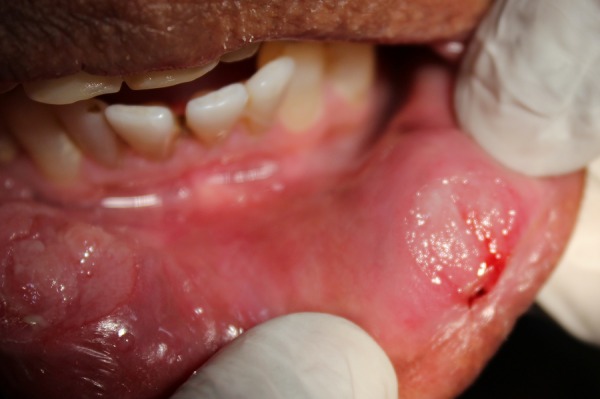
Circumscribed ovoid ulcer (0.5 cm) on the labial mucosa in relation to 31 and 32.
On the basis of history and clinical findings, a provisional diagnosis of malignant ulcer involving the lower labial and buccal mucosa and a possible aphthous-like ulcer on the left labial mucosa was made.
Investigations
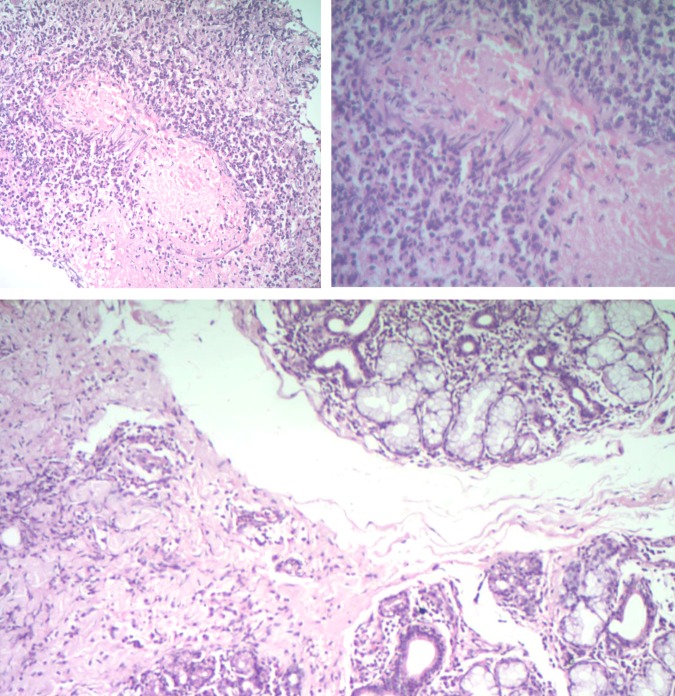
A full blood count test, liver function test and renal function test were performed, but they did not reveal any abnormalities. A second repeat incisional biopsy was carried out at two sites. Site A was over the right labial mucosa, which had a lobulated cobblestone appearance. Site B was over the solitary ulcer on the left labial mucosa. The hisotological findings in both sections showed squamous epithelium with extensive oedema and diffuse neutrophil infiltrate. Submucosa showed abscess with necrosis and dense neutrophil infiltrate. The deeper tissue showed small islands of salivary gland with a normal lobular architecture and a sparse-to-moderate lymphocytic infiltrate, intralobular and extralobular. The complete block was studied. The deeper section showed medium arteriole vasculitis, obliteration of its lumen and an area of necrosis around it. Both sections did not have any dysplastic cells nor was there was any granuloma. These findings were suggestive of necrotising sialometaplasia with submucosal abscess, sialadenitis and vasculitis (figure 5).
Figure 5.
Histological sections reveal islands of salivary gland tissue with intralobular and extralobular lymphocytic infiltrate. Areas of extensive oedema around the squamous epithelium with neutrophilic infiltration. Submucosa revealing medium arteriole vasculitis, obliteration of lumen with necrosis.
Differential diagnosis
Malignant ulcer: The clinical picture of our case would definitely favour a possible diagnosis of an oral squamous cell carcinoma (OSCC). OSCC arises from diffuse ‘field change’ brought about by tobacco and/or alcohol abuse. The patient did not have any of these risk factors. However, carcinoma can arise spontaneously without any pre-existing lesion, which can be confirmed by histopathology. In our case, histopathology of both sections did not show any dysplastic cells and the basement membrane was intact, and it is quite unusual for carcinoma to occur at multiple sites within the oral cavity.
Crohn's disease: Clinical presentation of mucosal tags, cobblestone appearance of buccal mucosa, swelling of lower lip and a medical history of gastritis could suggest an oral Crohn's disease as a possible differential. However, biochemical liver function test, gastrointestinal examination and the histological absence of non-caseating granuloma and multinucleated giant cells rule out a possible Crohn's disease.
Minor salivary gland neoplasm: As the oral mucosa has numerous minor salivary glands, either a mucoepidermoid carcinoma or an acinic cell carcinoma is a possibility. In our case, the histopathological section does not show the presence of mucous-secreting cells surrounded by epidermoid cells. Furthermore, features of cystic changes and cellular atypia were absent, which are pathognomic in mucoepidermoid or acinic cell carcinomas.
Treatment
Our patient was prescribed topical application of triamcinolone ointment 0.1% (kenalog orabase) three times a day, along with topical 0.2% chlorhexidine gluconate gel and a paracetamol tablet three times a day for 7 days. The first follow-up that was carried out 7 days later did not show any improvement. The patient's symptoms of pain, dysphagia and burning sensation increased. Subsequently, the patient was put on 5 mg methyl prednisolone (Medrol) once daily for 7 days along with pantoprazole 40 mg to prevent gastric irritation. The second review showed significant improvement in signs and symptoms. The patient was further asked to continue 5 mg methyl prednisolone for another 7 days, and then it was tapered to 2.5 mg for the next 7 days. Along with tapering dose of methyl prednisolone, the patient was advised to continue topical 0.1% triamcinolone acetonide ointment. The lesion showed gradual resolution over a period of 3 weeks. Since he was on steroids for 3 weeks, the patient's full blood count was analysed, and all parameters were normal.
Outcome and follow-up
There was resolution of the ulcer (figure 6). The patient's symptoms of pain and burning sensation were completely relieved. Routine follow-up was performed for 3 months, and there was no recurrence.
Figure 6.
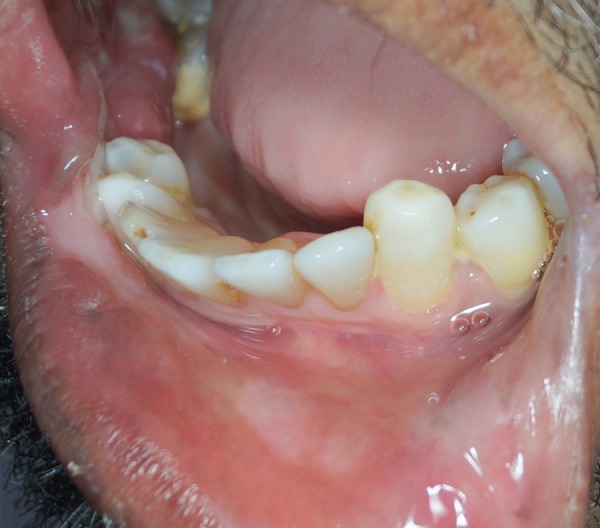
Three weeks postoperative showing complete resolution of the ulcer.
Discussion
Necrotising sialometaplasia is a benign self-limiting inflammatory lesion occurring commonly in the oral cavity in the sites where the minor salivary gland tissue is present mainly on the palate, labial mucosa and floor of the mouth. This lesion is rare, and it is well evident from that literature that just about 200 cases have been reported since 1973.1
This lesion has a male predilection, occurring predominantly in the fourth decade of life. Recent case reports have also shown necrotising sialometaplasia occurring in childhood.4 This lesion occurs as a result of an injury to the blood vessel that produces ischaemic changes, and this leads to infarction of salivary gland causing necrosis and inflammation, following which there is an attempt to repair the areas of ischaemia and necrosis. This attempt to repair induces metaplasia and changes in ductal architecture and further cicatrisation.5
This lesion is induced as a sequel to local infiltration anaesthesia over the palatial mucosa, surgical procedures, self-induced vomiting in patients with anorexia nervosa and bulimia nervosa. Rarely, tobacco users and cocaine addicts develop this, which is due to local ischaemia caused by constriction effects of tobacco and cocaine.6
The pathogenesis of necrotising sialometaplasia is histologically categorised into five stages: infarction, sequestration, ulceration, repair and healing phase.7
There is also evidence during the early stages that there is squamous metaplasia of salivary gland ducts and coagulation necrosis of acini, whereas the late stages show a reactive fibrosis.5
Even though the literature says the lesion is self-limiting and undergoes complete resolution in 4–6 weeks, our patient had pain and dysphagia, which was treated with topical steroids and antiseptic gel, but did not heal satisfactorily. As the ulcers were present at multiple sites, increasing in size, associated with dysphagia, weight loss, not resolving spontaneously or with topical medications, systemic therapy was initiated for our case. We administered a 3-week course of systemic steroid, after which there was healing. Previous reports of similar cases had a spontaneous regression, and the literature also does not mention the need for an active therapy. Necrotising sialometaplasia if it is present in multiple sites and does not regress spontaneously definitely requires medical intervention. The majority of the cases of necrotising sialometaplasia reported in the literature have unilateral presentation either in palate, lip or buccal mucosa. Our case is different with the lesion clinically being present in three different sites, namely left lower lip, right labial mucosa and right buccal vestibule. A similar bilateral presentation following bilateral greater palatine anaesthesia has also been reported.8 Whether mandibular block anaesthesia could have aggregated the ischaemic process needs to be considered. Dentists should be well updated and aware of this rare inflammatory condition of minor salivary gland, which requires careful clinical and histopathological diagnosis. Histologically, necrotising sialometaplasia shows ischaemic lobar necrosis of sero mucinous glands, coagulative necrosis of mucinous acini but with an intact lobular architecture. The outlines of the acini are pale. The nuclei are hypochromatic. Squamous metaplasia of the ducts and acini are common features. These metaplastic cells exhibit minimal hyperchromatism and few mitotic figures. It is the overlying epithelium which is hyperplastic with thick, elongated, complex, rete processes, pseudoepitheliomatous hyperplasia and extensive ductal metaplasia which makes them histologically resemble a squamous cell carcinoma.8 This histological resemblance of necrotising sialometaplasia to OSCC can possibly lead to a misdiagnosis, which can change the treatment modality entirely.
Learning points.
Necrotising sialometaplasia is a localised self-regressing inflammatory lesion occurring at sites where salivary gland tissue minor is present.
Routine common dental procedures such as palatal infiltration and local anaesthesia can trigger this condition.
Clinically, it mimics a carcinomatous lesion posing an alarm to the dental surgeon, which should be carefully excluded histologically.
Careful history taking, clinical examination and thorough histopathological observation could help in the right diagnosis, and a very conservative management approach is needed for this curable lesion which a dentist could encounter in practice.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Abrams AM, Melrose RJ, Howell FV. Necrotizing sialometaplasia. A disease simulating malignancy. Cancer 1973;32:130–5. [DOI] [PubMed] [Google Scholar]
- 2.Brannon RB, Fowler CB, Hartman KS. Necrotizing sialometaplasia: a clinicopathological study of sixty-nine cases and review of the literature. Oral Surg 1991;72:317–25. 10.1016/0030-4220(91)90225-2 [DOI] [PubMed] [Google Scholar]
- 3.Lynch DP, Crago CA, Martinez MG Jr. Necrotising sialometaplasia. A review of the literature and report of two additional cases. Oral Surg Oral Med Oral Pathol 1979;47:63–9. 10.1016/0030-4220(79)90103-8 [DOI] [PubMed] [Google Scholar]
- 4.Ylikontiola L, Siponen M, Salo T et al. . Sialometaplasia of the soft palate in a 2-year-old girl. J Can Dent Assoc 2007;73(4):333–6. [PubMed] [Google Scholar]
- 5.Imbery TA, Edwards PA. Necrotizing sialometaplasia: literature review and case reports. J Am Dent Assoc 1996;127:1087–92. 10.14219/jada.archive.1996.0334 [DOI] [PubMed] [Google Scholar]
- 6.Femopase L, Hernández SL, Gendelman H et al. . Necrotizing sialometaplasia: report of five cases. Med Oral 2004;9:304–8. [PubMed] [Google Scholar]
- 7.Anneroth G, Hansen LS. Necrotizing sialometaplasia: the relationship of its pathogenesis to its clinical characteristics. Int J Oral Surg 1982;11:283–91. 10.1016/S0300-9785(82)80027-6 [DOI] [PubMed] [Google Scholar]
- 8.Keogh PV, O'Regan E, Toner M et al. . Necrotizing sialometaplasia: an unusual bilateral presentation associated with antecedent anaesthesia and lack of response to intralesional steroids. Case report and review of the literature. Br Dent J 2004;196:79–81. 10.1038/sj.bdj.4810892 [DOI] [PubMed] [Google Scholar]