Abstract
Background
MicroRNAs (miRNAs) are noncoding RNAs that modulate cellular gene expression, primarily at the post-transcriptional level. We sought to examine the functional role of miR-155 in a model of viral-induced neuroinflammation.
Methods
Acute encephalomyelitis and immune-mediated demyelination were induced by intracranial injection with the neurotropic JHM strain of mouse hepatitis virus (JHMV) into C57BL/6 miR-155+/+ wildtype (WT) mice or miR-155−/− mice. Morbidity and mortality, viral load and immune cell accumulation in the CNS, and spinal cord demyelination were assessed at defined points post-infection. T cells harvested from infected mice were used to examine cytolytic activity, cytokine activity, and expression of certain chemokine receptors. To determine the impact of miR-155 on trafficking, T cells from infected WT or miR-155−/− mice were adoptively transferred into RAG1−/− mice, and T cell accumulation into the CNS was assessed using flow cytometry. Statistical significance was determined using the Mantel-Cox log-rank test or Student’s T tests.
Results
Compared to WT mice, JHMV-infected miR-155−/− mice developed exacerbated disease concomitant with increased morbidity/mortality and an inability to control viral replication within the CNS. In corroboration with increased susceptibility to disease, miR-155−/− mice had diminished CD8+ T cell responses in terms of numbers, cytolytic activity, IFN-γ secretion, and homing to the CNS that corresponded with reduced expression of the chemokine receptor CXCR3. Both IFN-γ secretion and trafficking were impaired in miR-155−/−, virus-specific CD4+ T cells; however, expression of the chemokine homing receptors analyzed on CD4+ cells was not affected. Except for very early during infection, there were not significant differences in macrophage infiltration into the CNS between WT and miR-155−/− JHMV-infected mice, and the severity of demyelination was similar at 14 days p.i. between WT and miR-155−/− JHMV-infected mice.
Conclusions
These findings support a novel role for miR-155 in host defense in a model of viral-induced encephalomyelitis. Specifically, miR-155 enhances antiviral T cell responses including cytokine secretion, cytolytic activity, and homing to the CNS in response to viral infection. Further, miR-155 can play either a host-protective or host-damaging role during neuroinflammation depending on the disease trigger.
Keywords: miR-155, Virus, Neuroinflammation, T cells, Demyelination
Background
MicroRNAs (miRNAs) are a new class of evolutionarily conserved gene-regulatory molecules that function to repress key target genes, primarily at the post-transcriptional level through specific mRNA 3′ untranslated region (3′ UTR) interactions [1]. Because miRNAs commonly target critical signaling proteins and transcription factors with potent regulatory impacts on the immune system [2, 3], it is accepted that miRNAs have an important effect on immune system activation and cellular differentiation. Recent work by our group and others has determined that miR-155 is an important regulator of immune cell development and function. Following its original identification as an oncogene in chicken lymphomas [4], miR-155 was discovered to be overexpressed in mammalian hematopoietic cancers and shortly thereafter established as an immunomodulatory noncoding RNA in macrophages and B lymphocytes [5–9]. It is now clear that miR-155 is expressed by and functions within a variety of activated immune cell types that include various T cell populations, NK cells, and dendritic cells [6, 7, 10–12]. In addition, miR-155 represses a variety of immunoregulatory proteins that include signaling molecules such as Ship1 [13] and Socs1 [14], as well as transcriptional regulators such as Jarid2 [15], Ets1 [16, 17], PU.1 [18], and Fosl2 [19].
Consistent with its known roles in regulating immune factors, multiple studies have demonstrated that miR-155 is important in shaping the immune responses that govern viral pathogenesis [20]. Genetic silencing of miR-155 results in increased sensitivity to experimental infection with lymphocytic choriomeningitis virus (LCMV) [21, 22], influenza virus [23], and herpes simplex virus (HSV) [24, 25]. While miR-155 had previously been shown to help tailor CD4+ T cell responses in models of autoimmunity, viral studies have since illustrated the importance of miR-155 in strengthening CD8+ T cell responses. Recent reports showed that miR-155 is required for optimal CD8+ T cell function following experimental infection with LCMV in terms of CTL activity, cytokine secretion, and proliferation [21, 22]. With regard to viral-induced encephalitis, miR-155 is important in controlling neuroinflammation, presumably by regulating T cell responses [24, 26]. These reports have emphasized the importance of miR-155 in augmenting host defense following viral infection; however, there have been few rigorous studies examining how miR-155 influences immune cell responses in a model of viral-induced encephalomyelitis.
Inoculation of the neurotropic JHM strain of mouse hepatitis virus (JHMV) into the CNS of susceptible strains of mice provides an excellent model for examining host response mechanisms responsible for controlling viral replication and modulating neuroinflammation within distinct cell lineages present in the brain [27, 28]. During acute disease, control of viral replication is mediated by infiltrating CD4+ and CD8+ T cells [29–31]; however, clearance of virus is not complete, and animals that survive the acute disease develop an immune-mediated demyelinating disease with both T cells and macrophages amplifying disease severity by contributing to myelin damage [32–38]. Our findings demonstrated that miR-155 was necessary for optimal T cell accumulation, cytolytic activity, cytokine secretion, and trafficking to the CNS after JHMV infection. Macrophage migration and accumulation within the CNS was not impaired in the absence of miR-155 during the time period studied, and there were no differences in the severity of demyelination at 14 days pi, when peak disease severity generally occurs. These results demonstrate that miR-155 has an important role in regulating antiviral T cell responses following viral-induced neuroinflammation.
Methods
Virus and mice
For intracranial (i.c.) injections, age-matched (5–7 weeks) C57BL/6 miR-155+/+ mice (wildtype (WT)) or miR-155−/− mice were anesthetized with an intraperitoneal (i.p.) injection of 200 μl of a mixture of ketamine (Hospira, Lake Forest, IL, USA) and xylazine (Phoenix Pharmaceutical, Saint Joseph, MO, USA) in Hank’s balanced salt solution (HBSS). Mice were injected intracranially (i.c.) with 200 plaque-forming units (PFU) of JHMV (strain V34) suspended in 30 μl HBSS [39]. Clinical severity was assessed using a previously described four-point scoring scale [40]. For analysis of viral titers, mice were sacrificed at indicated time points. One half of each brain was homogenized and used in a plaque assay performed using the DBT mouse astrocytoma cell line [41]. The DM-JHMV (2.5 × 105 PFU) strain [31, 42] was used to immunize experimental mice via i.p. injection to generate virus-specific T cells. This is an established and reliable method to accurately measure T cell responses following JHMV infection [42, 43]. RAG1−/− mice were purchased from Jackson Laboratories. All animal studies were reviewed and approved by the University of Utah Animal Care and Use Committee.
Cell isolation and flow cytometry
Immunophenotyping of immune cells present within brains and spinal cords of JHMV-infected mice at defined times post-infection (p.i.) was accomplished by homogenizing isolated tissue and generating single-cell suspensions for analysis by flow cytometry using previously described procedures [44–46]. In brief, isolated cells were stained with the following antibodies: APC-conjugated rat anti-mouse CD4 and a PE-conjugated tetramer specific for the CD4 immunodominant epitope present within the JHMV matrix (M) glycoprotein spanning amino acids 133-147 (M133-147 tetramer) to determine total and virus-specific CD4+ cells, respectively [47]; APC-conjugated rat anti-mouse CD8a and a PE-conjugated tetramer specific for the CD8 immunodominant epitope present in the spike (S) glycoprotein spanning amino acids 510-518 (S510-518) to identify total and virus-specific CD8+ cells, respectively; and APC-conjugated rat anti-mouse CD45 and FITC-conjugated anti-F4/80 to identify macrophages. Samples were analyzed using a BD LSR Fortessa X-20 flow cytometer and FloJo software.
CD8+ T cell cytotoxicity assay
WT and miR-155−/− mice were infected i.p. with the DM strain of JHMV (DM-JHMV, 2.5 × 105 PFU), and a cytolytic T cell (CTL) assay was performed as previously described [31]. In brief, RMA-S target cells were seeded at a density of 10,000 cells/well in a flat-bottom 96-well tissue culture plate (Corning Life Sciences) and pulsed overnight with 50 μM OVA peptide or the immunodominant CD8 peptide specific for MHV spike (S) glycoprotein spanning amino acids 510-518 (S510-518, Bio-Synthesis). CD8+ T cells were isolated from splenocytes of infected mice at day 8 p.i. using MACS® Separation Columns and CD8+ T cell Isolation kit (Miltenyi). Equivalent numbers of virus-specific CD8+ T cells from WT and miR-155−/− mice were then incubated with RMA-S cells at different effector-to-target (E:T) ratios. Co-cultures were incubated for 4 h at 37 °C in 5 % CO2 at a final volume of 200 μL/well. The levels of lactate dehydrogenase released from lysed cells were determined using a LDH Cytotoxicity Assay Kit (Pierce). The percentage of CTL-mediated lysis was determined as specified by the manufacturer’s specifications as previously described [31].
IFN-γ production
CD4+ and CD8+ T cells were isolated from the spleens of WT and miR-155−/− mice infected i.p. with DM-JHMV (2.5 × 105 PFU) and used to assess cytokine secretion in response to defined viral epitopes [39]. CD4+ and CD8+ T cells were isolated as described above using an isolation kit according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA). Enriched T cell subsets (1 × 106 cells) were incubated with antigen-presenting cells in flat-bottom 96-well plates and incubated for 24 h at 37 °C in 5 % CO2 in the presence of 5 μM of either OVA, M133, or S510 peptides. Supernatants were collected and IFN-γ levels were measured using ELISA (R & D Systems, Minneapolis, MN, USA).
Histology
Spinal cords were isolated at defined time points and fixed overnight with 4 % paraformaldehyde at 4 °C. Sections were subsequently cryoprotected in 30 % sucrose for 5–7 days, separated into 12 coronal sections, and embedded in optimum cutting temperature (OCT) formulation (VWR, Radnor, PA, USA) [48]. Coronal sections (8 μm thick) were cut, and sections were stained with luxol fast blue (LFB) in combination with hematoxylin and eosin (H & E). Areas of total white matter and demyelinated white matter were determined with Image J Software. The percent demyelination was calculated by dividing the area of demyelinated white matter by the total white matter area using established methods previously described [47, 49].
Adoptive transfer
Adoptive transfer experiments were performed using previously described protocols [42]. In brief, WT and miR-155−/− mice were injected i.p. with JHMV-DM (2.5 × 105 PFU) and spleens removed at day 7 p.i.. CD4+ and CD8+ T cells were enriched via magnetic separation (Miltenyi) and equal numbers of virus-specific T cells (determined by tetramer staining) were adoptively transferred via intravenous (i.v.) injection into the retro-orbital sinus of RAG1−/− mice 3 days following i.c. infection with 200 PFU of JHMV. Mice were sacrificed 9 days post-transfer (12 days p.i.), and brains and spinal cords were removed. One half of the brains were used for flow cytometry analysis, and the remaining halves were used to determine viral titers. Control animals included JHMV-infected RAG-1−/− mice.
Results
Increased disease severity in JHMV-infected miR-155−/− mice
Age-matched WT or miR-155−/− mice were i.c. inoculated with JHMV (200 PFU), and the severity of clinical disease and survival were monitored. JHMV-infected miR-155−/− mice demonstrated delayed onset of disease compared to WT mice, yet clinical disease was sustained in miR-155−/− animals compared to WT mice (Fig. 1a). By day 30 p.i., 85 % of WT and 54 % of miR-155−/− mice had survived (Fig. 1b). Assessment of viral titers within the brains of infected mice revealed similar titers 5 days p.i.; however, by day 7 p.i., WT mice had dramatically reduced viral titers, and by day 14 p.i., titers were below the level of detection (~100 PFU/g) (Fig. 1c). In contrast, JHMV-infected miR-155−/− mice were unable to control viral replication and demonstrated high viral titers out to 21 p.i. (Fig. 1c). Collectively, these data indicate that miR-155 expression enhances immune-mediated control of viral replication within the CNS.
Fig. 1.
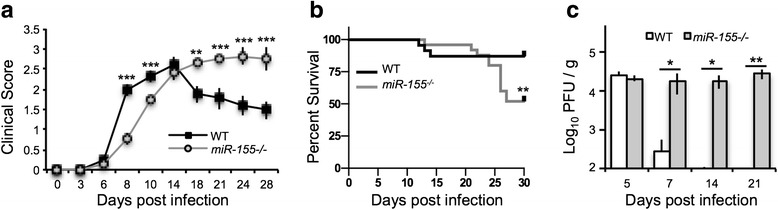
Increased morbidity/mortality in JHMV-infected miR-155 −/− mice was associated with elevated viral titers within the brain. WT (n = 12) and miR-155 −/− mice (n = 12) were infected via i.c. injection with 200 PFU of JHMV. Clinical scores (a) and survival (b) were assessed throughout infection. The increase in both clinical disease and mortality correlated with an impaired ability to control viral replication within the brains at the indicated times p.i. c Statistical significance was determined using Mantel-Cox log-rank test or one-tailed, unpaired, Student’s T tests. Data are representative of at least two independent experiments; * p < 0.05; ** p < 0.01; *** p < 0.001
Impaired T cell response in JHMV-infected miR-155−/− mice
T cell responses are critical for controlling JHMV replication within the CNS [27, 29, 50–57]. Therefore, we next wished to determine whether increased morbidity/mortality and inability to control viral replication correlated with impaired T cell accumulation within the brains of JHMV-infected miR-155−/− mice. Brains were harvested from JHMV-infected WT or miR-155−/− mice at 5, 7, 14, and 21 days p.i., and immune cells were isolated and immunophenotyped using flow cytometry [47, 58, 59]. Both total CD4+ and virus-specific CD4+ cells were decreased in brains from miR-155−/− mice compared to WT mice at 5, 7, and 14 days p.i. (Fig. 2a, b); however, by 21 days p.i., no differences were detected. In addition, levels of total and virus-specific CD8+ cells were dramatically decreased in brains from miR-155−/− mice compared to WT mice at 5, 7, and 14 days p.i. yet not at day 21 p.i. (Fig. 2c, d). There were decreased levels of macrophages at 5 days p.i. in brains of miR-155−/− mice compared to WT mice; however, there were no significant differences in CNS macrophage accumulation at later times (Fig. 2e). The degree of demyelination at day 14 p.i. was similar between JHMV-infected WT (35.1 ± 4.9 %, n = 4) and miR-155−/− mice (36.7 % ± 4.3 %, n = 4) (Fig. 2f, g). These results demonstrate that miR-155 is necessary for optimal T cell accumulation in the CNS in the context of JHMV infection, and is consistent with previous studies.
Fig. 2.
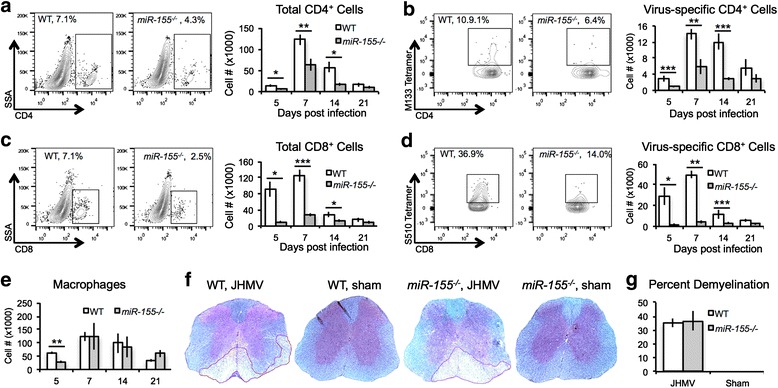
JHMV-infected miR-155 −/− mice demonstrated reduced CNS T cell infiltration. WT and miR-155 −/− mice were infected i.c. with JHMV (200 PFU). Mice from each group were sacrificed 5, 7, 14, and 21 days p.i., and brains were collected. Flow analysis indicated reduced infiltration of total CD4+ T cells (a) and CD8+ T cells (c), as well as reduced virus-specific CD4+ T cells (b) and CD8+ T cells (d), as determined by tetramer staining [95, 96]. In contrast, while macrophage (CD45 + F4/80hi) infiltration into the CNS was lower in miR-155 −/− mice at 5 days p.i. (e), the levels were similar at later time points. Representative spinal cords from JHMV-infected and sham-infected mice stained with LFB at day 14 p.i. showed similar levels of demyelination between infected WT mice (35.1 + 4.9 %, n = 4) and miR-155 −/− mice (36.7 + 4.3, n = 4) whereas no demyelination is observed in sham-infected animals (f, g). Data presented are derived from two independent experiments with a minimum of four mice/experimental group. Data are presented as average ± SEM. Statistical significance was measured using unpaired, one-tailed Student’s T tests; * p < 0.05; ** p < 0.01; *** p < 0.001
These findings suggest that the absence of miR-155 during acute viral-induced encephalomyelitis affects either the ability of virus-specific T cells to expand and/or traffic to the CNS [45, 46]. To test whether miR-155 affects expansion in the context of JHMV infection, we immunized WT and miR-155−/− mice by intraperitoneal (i.p.) injection with 2.5 × 105 PFU of DM-JHMV [31, 42] and isolated splenocytes at day 8 p.i. to determine the frequency and number of virus-specific T cells by tetramer staining. Similar numbers of M133-147 virus-specific CD4+ T cells were present in miR-155−/− mice compared to WT (Fig. 3a). In contrast, there was a significant (p < 0.001) decrease in S510-518-specific CD8+ T cells in splenocytes from miR-155−/− mice compared to those from WT mice (Fig. 3b), indicating that miR-155 is necessary for optimal CD8+ T cell expansion.
Fig. 3.
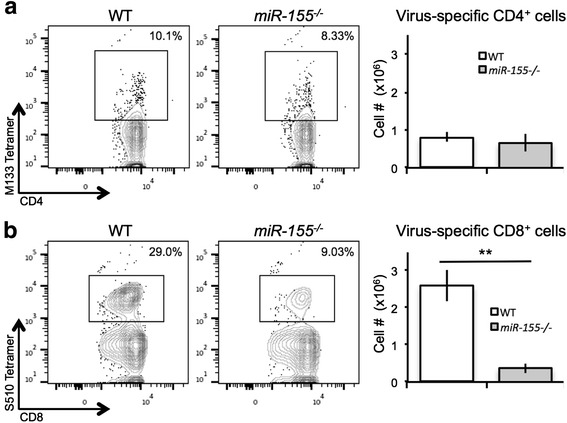
miR-155enhanced expansion of virus-specific CD8+ T cells. WT and miR-155 −/− mice were i.p. infected with DM-JHMV. Spleens were removed at day 8 p.i., and virus-specific T cells were identified by tetramer staining. Representative dot blots indicated that while similar frequencies and numbers of virus-specific CD4+ T cells (a) were present in WT and miR-155 −/− mice, there was a significant (**p < 0.001) decrease in both the frequency and numbers of virus-specific CD8+ T cells (b) in miR-155 −/− mice compared to WT mice. Histograms are presented as average ± SEM; statistical significance was measured using unpaired, one-tailed Student’s T tests. Data presented are derived from two independent experiments with a minimum of four mice/experimental group. * p < 0.05; ** p < 0.01; *** p < 0.001
Antiviral T cell activity is muted in miR-155−/− mice
We next examined whether T cell antiviral effector responses were altered in the absence of miR-155 expression. Both cytolytic activity by CD8+ T cells [27, 50, 57, 60], as well as secretion of IFN-γ by virus-specific CD4+ and CD8+ T cells are important for controlling JHMV replication within the CNS [31, 53, 54, 61, 62]. WT and miR-155−/− mice were infected i.p. with DM-JHMV. Splenocytes were removed 8 days p.i., and the antiviral activity of virus-specific T cells was determined. As shown in Fig. 4a, virus-specific, miR-155−/− CD8+ T cells showed reduced (p < 0.05) cytolytic activity compared to WT CD8+ T cells. In addition, secretion of IFN-γ by CD4+ and CD8+ T cells from immunized miR-155−/− mice was reduced (p < 0.001) compared to WT mice (Fig. 4b, c). These findings argue that in the absence of miR-155, virus-specific T cell functions are blunted, consistent with previous reports [21, 22, 24, 25].
Fig. 4.
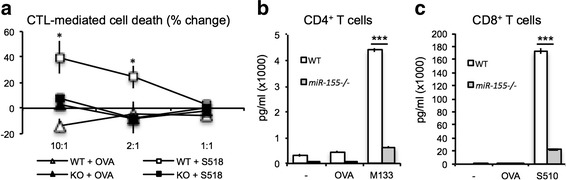
T cells from miR-155 −/− mice exhibited impaired antiviral effector function. WT and miR-155 −/− mice were immunized with DM-MHV via i.p. injection. a Animals were sacrificed 8 days p.i., and CD4+ and CD8+ T cells were isolated and pooled. The frequencies of total and virus-specific CD8+ T cells were determined by tetramer staining [96]. Equivalent numbers of virus-specific CTLs were added to target cells pulsed with either the immunodominant CD8+ T cell epitope within the spike (S) glycoprotein spanning residues 510-518 (S510-518, 50 μM) or control ovalbumin peptide (50 μM) at the indicated ratios, and lytic activity was determined as previously described [31, 96]. In addition, antigen recall responses to the immunodominant CD4 T cell epitope (M133-147) (b) or CD8 T cell epitope S510-518 (c) was performed, and IFN-γ levels were determined by ELISA as previously described [96]. Data are representative of at least two independent experiments, with at least five mice from each group. * p < 0.05; *** p < 0.001
miR-155 ablation impairs T cell migration to the CNS of JHMV-infected RAG-1−/− mice
Our findings indicate that in the absence of miR-155, antiviral T cell responses are dampened following JHMV infection of the CNS. In addition, the inability to control viral replication within the CNS was associated with fewer numbers of T cells within the CNS of JHMV-infected miR-155−/− mice compared to infected WT mice, raising the possibility of a deficiency in T cell homing in the absence of miR-155. We have previously shown that T cell expression of the chemokine receptor CXCR3, the signaling receptor for the chemokine CXCL10, is important in enhancing the ability of these cells to migrate and accumulate within the CNS of JHMV-infected mice [44, 45, 63–65]. We therefore tested whether expression of CXCR3 was decreased on T cells from JHMV-infected miR-155−/− mice. There were no differences in expression of CXCR3 on M133-147-specific CD4+ T cells (Fig. 5a, b). In contrast, there was an overall reduction (p < 0.05) in the frequency of CXCR3-positive S510-518-positive CD8+ T cells (Fig. 5c), as well as a reduction (p < 0.01) of CXCR3 on a per-cell level (Fig. 5d). These findings indicate that miR-155 regulates expression of CXCR3 on CD8+ T cells, and this corresponds with impaired trafficking of these cells to the CNS following JHMV infection. The paucity in CD4+ T cell trafficking to the CNS of JHMV-infected miR-155−/− mice suggests the possibility that other T cell homing receptors such as CCR5 may be affected by miR-155 deficiency and account for impaired CNS migration [66]; however, analysis of CCR5 on virus-specific CD4+ (Fig. 5e, f) and CD8+ T cells (Fig. 5g, h) indicated no differences in surface expression of this homing receptor between WT and miR-155−/− T cells.
Fig. 5.
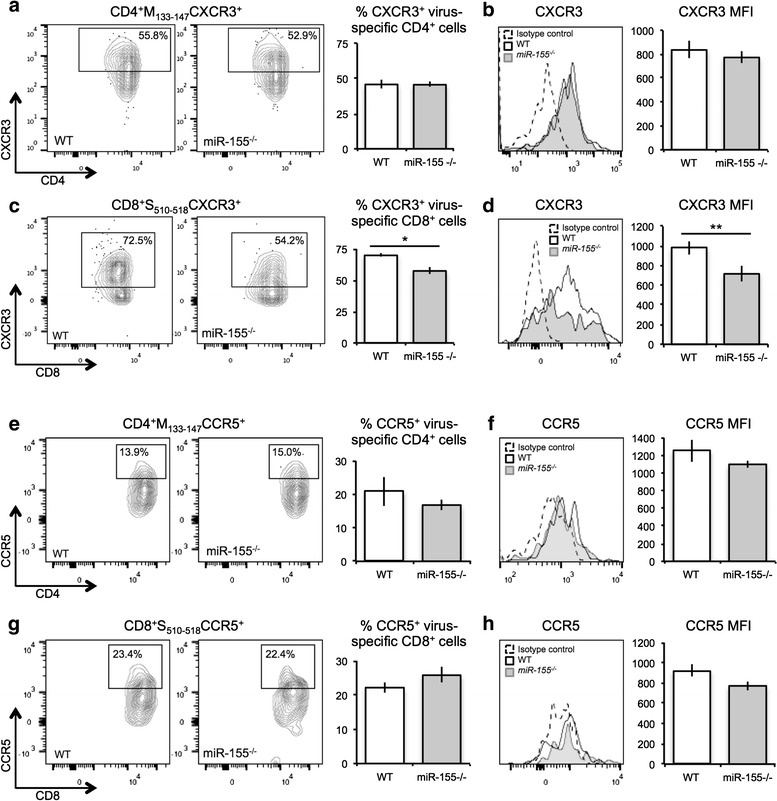
Chemokine receptor expression on virus-specific WT and miR-155 −/− mice. WT and miR-155 −/− mice were infected i.p. with DM-MHV and spleens were isolated at day 8 p.i.. Flow cytometric analysis revealed similar levels of CXCR3 on virus-specific CD4+ T cells isolated from WT and miR-155 −/− mice (a, b). However, expression of CXCR3 was decreased (p < 0.05) on virus-specific CD8+ T cells isolated from miR-155 −/− mice compared to WT mice (c, d). Flow cytometric analysis revealed similar levels of CCR5 on both virus-specific CD4+ T cells (e, f) and virus-specific CD8+ T cells (g, h) isolated from WT and miR-155 −/− mice. Plots represent average ± SEM; statistical significance was measured using unpaired, one-tailed Student’s T tests. Data are representative of two independent experiments, with a minimum of four mice per group per experiment. * p < 0.05; ** p < 0.01
As an additional test to determine if the absence of miR-155 affected T cell migration into the CNS, we performed adoptive transfer experiments. WT and miR-155−/− mice were injected i.p. with DM-MHV. Eight days p.i., spleens were isolated and equal numbers of virus-specific CD4+ or CD8+ T cells from WT or miR-155−/− mice were injected i.v. into RAG-1−/− mice (deficient in functional T and B lymphocytes) that had been infected i.c. with JHMV 3 days prior. As shown in Fig. 6a, JHMV-infected RAG-1−/− recipients of either virus-specific CD4+ or CD8+ T cells from WT mice showed increased (p < 0.05) clinical disease severity compared to recipients of miR-155−/− T cells. Animals were sacrificed at day 9 post-transfer (day 12 p.i.), and viral titers and T cell infiltration into the CNS were assessed. Our findings indicate that viral titers within the brain were higher in JHMV-infected RAG1−/− mice that received either virus-specific miR-155−/− CD4+ or CD8+ T cells compared to recipients of WT virus-specific T cell subsets (Fig. 6b). Importantly, CNS accumulation of both virus-specific CD4+ (Fig. 6c) and CD8+ (Fig. 6d) T cells was significantly (p < 0.01) reduced in mice that received miR-155−/− T cells compared to recipients of WT T cells. These results provide further evidence that miR-155 is important for T cell trafficking.
Fig. 6.
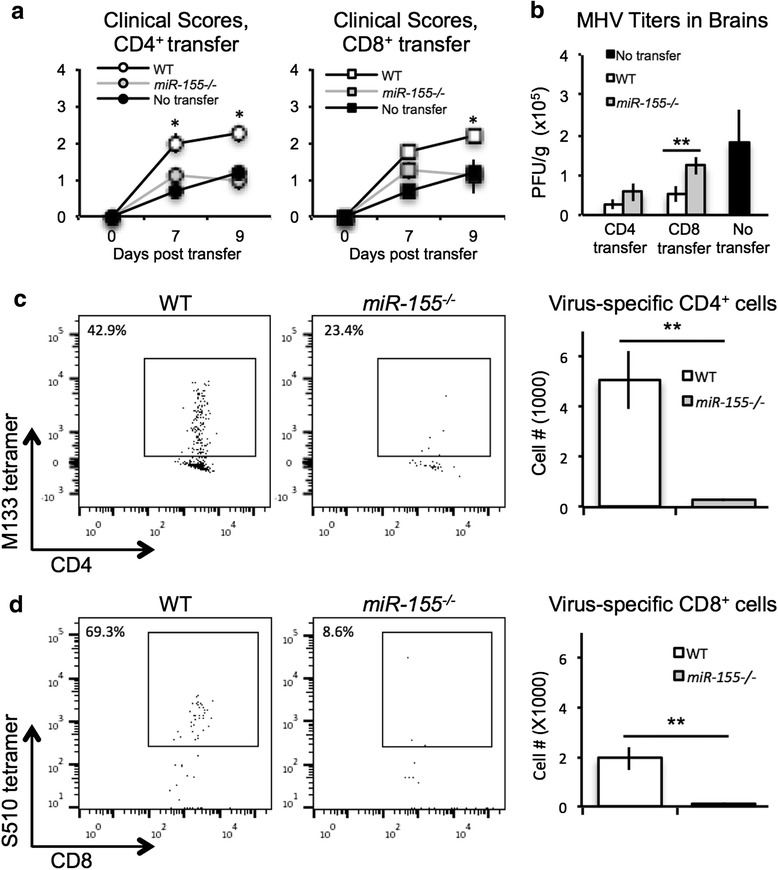
Silencing of miR-155 dampened the accumulation of adoptively transferred virus-specific T cells within the CNS of JHMV-infected RAG-1 −/− mice. JHMV-infected RAG1 −/− mice received equal numbers of either WT or miR-155 −/− virus-specific CD4+ or CD8+ T cells via i.v. injection on the day following i.c. instillation of virus. a Clinical disease in mice that received either miR-155 −/− CD4+ (n = 3) or CD8+ T cells (n = 3) was reduced (p < 0.05) when compared to recipients of WT CD4+ (n = 3) or CD8+ (n = 3) cells. Increased disease severity was associated with an inability to control viral replication within the CNS (b) and a dramatic reduction (p < 0.01) in migration of CD4+ (c) and CD8+ (d) T cells into the brains compared to WT cells at day 9 post-transfer. a, b Data are presented as average ± SEM. c, d Representative dot blots are shown and histograms are presented as average ± SEM. Statistical significance was measured using unpaired, one-tailed Student’s T tests. * p < 0.05; ** p < 0.01
Discussion
In this report, we have examined the mechanisms by which miR-155 affects both host defense and disease progression following JHMV infection of the CNS. Our findings revealed that miR-155 expression is associated with susceptibility to JHMV-induced neurologic disease. Expression of miR-155 is necessary for effective antiviral T cell responses as ablation of miR-155 resulted in increased morbidity/mortality that was associated with elevated viral titers within the CNS. Increased disease severity most likely reflects dampened CD8+ T cell responses, as reflected by reduced CNS accumulation of virus-specific CD8+ T cells. Furthermore, cytolytic activity by CD8+ T cells, as well as secretion of IFN-γ, was reduced in miR-155−/− CD8+ T cells, highlighting a role for this molecule in configuring effective responses by virus-specific CD8+ T cells. While expansion of virus-specific CD4+ T cells was not affected in the absence of miR-155, IFN-γ secretion by CD4+ T cells was diminished. Importantly, the ability of T cells to migrate to the CNS was dramatically reduced in the absence of miR-155 expression, and this was associated with increased susceptibility to JHMV-induced neurologic disease. Whether increased susceptibility to JHMV-induced neurologic disease reflects a T cell intrinsic problem or whether our findings reflect an extrinsic effect via other immune cells, e.g., dendritic cells, is not known at this time. While this is an important question, we believe that muted antiviral T cell responses in miR-155−/− mice following JHMV infection reflects an intrinsic problem in that (i) adoptive transfer of miR-155−/− virus-specific T cells into JHMV-infected mice was unable to effectively reduce CNS viral titers and (ii) recent studies employing experimental infection of miR-155−/− mice with lymphocytic choriomeningitis virus (LCMV) indicate that impaired T cell responses are due to specific deficiencies in T cells [21].
Within the context of neuroinflammatory diseases, miR-155 was initially shown to be critical in the induction of myelin-reactive Th17 cells in EAE, the prototypic model of the human demyelinating disease multiple sclerosis (MS) [67, 68]. In addition, miR-155 expression by endothelial cells of the blood-brain barrier (BBB) has been shown to regulate BBB function and affect neuroinflammation during EAE [69]. More recently, a role for miR-155 has been implicated in contributing to neuroinflammation in models of Parkinson’s disease [70], Alzheimer’s disease [71], alcohol-induced neuroinflammation [72], and amyotrophic lateral sclerosis (ALS) [73]. Although the mechanisms by which miR-155 affects neuroinflammation have not been firmly established, an emerging concept is that expression of miR-155 by microglia is important in regulating expression of proinflammatory genes that subsequently influence neuroinflammation [74–77]. We are currently further investigating the mechanisms by which miR-155 affects host defense and disease progression in models of viral encephalitis. Results from the current study are congruent with recent reports by Rouse and colleagues [24] demonstrating that miR-155 affects susceptibility to HSV-1-induced encephalitis as a result of impaired antiviral T cell responses as well as homing to the CNS. Similarly, the severity of neuroinflammation is reduced following experimental infection with Japanese encephalitis virus (JEV) in the absence of miR-155, and this is associated with dampened expression of proinflammatory cytokines [26]. These findings emphasize an important role for miR-155 in augmenting host defense in response to CNS infection by neurotropic viruses through different mechanisms, including regulating gene expression by resident glia and tailoring T cell responses. With regard to the former, negative regulation of Ship1 by miR-155 was found to affect expression of proinflammatory cytokines and modulate neuroinflammation during JEV infection [26]. A number of different mechanisms by which miR-155 controls T cell responses following viral infection have been proposed. In the absence of miR-155, virus-specific CD8+ T cells have enhanced type-I interferon signaling, leading to increased susceptibility to interferon’s anti-proliferative effect [23]. Impaired antiviral CD8+ T cell responses have also been associated with reduced activation of the prosurvival Akt pathway, arguing that miR-155 promotes T cell survival/function in response to viral infection [21]. Targeting of Socs1 by miR-155 has also been shown to disrupt T cell function in response to viral infection, and these studies emphasized the importance of both cell type and context in determining how miR-155 affects lymphocyte function [22]. Whether these miR-155-related pathways and/or targets are affected in response to JHMV infection of the CNS remains to be determined and is the focus of ongoing studies by our group.
Previous work from our lab and others has implicated chemokines as important in regulating lymphocyte migration to the CNS in response to viral infection [78]. Specifically, we have shown that expression of both CXCR3 and CCR5 promote migration of virus-specific T cells into the CNS of JHMV-infected mice [42, 43, 45, 79]. Our findings that impaired migration of miR-155-deficient, virus-specific CD8+ T cells to the CNS of JHMV-infected mice correlated with reduced expression of CXCR3, but not CCR5, are interesting and argue that expression of chemokine homing receptors may be modulated by miR-155. In our hands, this effect was restricted to CD8+ T cells, as neither CXCR3 nor CCR5 expression was affected in miR-155-deficient CD4+ T cells. Nonetheless, homing to the CNS by CD4+ cells was reduced, arguing that the absence of miR-155 may affect the ability of these cells to efficiently migrate to sites of infection. This theory was further supported by adoptive transfer experiments demonstrating that in RAG-1−/− mice that received miR-155-deficient CD4+ or CD8+ cells, there was a dramatic deficiency in CNS accumulation of CD4+ or CD8+ T cells, respectively. In addition, there was an impaired ability to control viral replication compared to recipients of WT cells. Recent work has demonstrated that miRNAs, including miR-155, may influence chemokine receptor expression on circulating lymphocytes [80–82], suggesting that sufficient expression of these homing receptors is intrinsically influenced by miRNAs.
Mice persistently infected with JHMV develop an immune-mediated demyelinating disease in which chronic infiltration of virus-specific T cells and macrophages amplifies the severity of demyelination. The profile of clinical symptoms and accompanying histopathology associated with JHMV persistence has been employed as a pre-clinical animal model of the human demyelinating disease multiple sclerosis (MS) [28, 83, 84]. Previous studies have demonstrated that genetic silencing of miR-155 ameliorates the severity of EAE and this was associated with a reduction in the severity of neuroinflammation and demyelination, highlighting that miR-155 has a functional role in pre-clinical MS models [67, 68]. Clinical studies in MS patients have suggested that microRNAs may be used as novel diagnostic and predictive biomarkers, as well as affect disease progression [85–87]. Evidence demonstrating a potentially important role for miR-155 in MS includes demonstration that miR-155 expression is increased in peripheral blood mononuclear cells [88] as well as in brain lesions [89] of MS patients. In addition, glatiramer acetate treatment resulted in normalization of deregulated miRNAs, including miR-155, in peripheral blood mononuclear cells in patients with relapsing-remitting MS, arguing that miR-155 has a role in the regulation of immune responses in MS patients [90]. Other potential roles for miR-155 in controlling disease progression include regulation of proinflammatory responses in blood-derived and CNS-resident myeloid cells [91]. Furthermore, microRNAs may represent novel regulators of oligodendrocyte differentiation via control of transcriptional networks that influence myelin gene expression and cell cycle transitions [92, 93]. Our findings indicate that in the JHMV model, miR-155 does not affect demyelination per se, as there were similar levels of myelin damage in JHMV-infected WT and miR-155−/− mice at the peak of disease. Whether these results reflect the use of the JHM strain of MHV is not known at this time. The A59 strain of MHV has been shown to induce demyelination in the absence of the adaptive immune suggesting that macrophage/microglia may be sufficient to initiate white matter damage [94]. We are currently investigating whether miR-155 influences processes governing demyelination and/or remyelination at later stages of JHMV through control of oligodendrocyte progenitor maturation. In addition, we are examining whether the absence of miR-155 affects proinflammatory gene expression by resident glia, e.g., astrocytes and microglia.
Conclusions
This study demonstrates that miR-155 contributes to antiviral T cell responses in a model of viral-induced encephalomyelitis. Our findings illustrate that the absence of miR-155 increases susceptibility to death in response to viral infection of the CNS and that this correlates with increased viral replication within the CNS, limited T cell trafficking to the CNS, muted secretion of IFN-γ, and reduced cytolytic activity. However, macrophage trafficking and the severity of demyelination were not significantly affected in virally infected miR-155−/− mice, indicating increased disease severity reflected impaired T cell responses. Importantly, because miR-155 plays a host-protective role during JHMV-mediated neuroinflammation, yet plays a pathogenic role in autoimmune models of neuroinflammation and demyelination following immunization with encephalitogenic peptides, its therapeutic targeting in the clinic should be carefully considered.
Acknowledgements
The authors gratefully acknowledge Dr. Timothy Hanley for his scientific and editorial suggestions and Joshua Daugherty for his technical assistance.
Funding
This work was supported by NIH grant R01 NS041249 to TEL and R01 AG047956 to RMO. LLD is supported by Postdoctoral Fellowship FG20105A1 from the National Multiple Sclerosis Society.
Availability of data and materials
The authors will make available all relevant raw data used in generating conclusions put forth within this manuscript via Open Science Framework (osf.io).
Authors’ contributions
LLD designed and conducted experiments, analyzed and interpreted the data, and wrote the manuscript. CLW and JLG assisted in the experiments and collecting the data. TEL and ROC designed the research, analyzed and interpreted the data, and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
All authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All animal studies were reviewed and approved by the University of Utah Animal Care and Use Committee.
Abbreviations
- CNS
Central nervous system
- CTL
Cytotoxic T lymphocyte
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- H & E
Hematoxylin and eosin
- i.c.
Intracranial
- i.p.
Intraperitoneal
- IFNγ
Interferon-gamma
- LDH
Lactate dehydrogenase
- LFB
Luxol fast blue
- miR-155
microRNA 155
- MRI
Magnetic resonance imaging
- p.i.
Post-infection
- PFU
Plaque-forming units
Contributor Information
Laura L. Dickey, Email: laura.dickey@path.utah.edu
Colleen L. Worne, Email: colleen.worne@path.utah.edu
Jessica L. Glover, Email: jessica.glover@utah.edu
Thomas E. Lane, Phone: (801) 585-5554, Email: tom.lane@utah.path.edu
Ryan M. O’Connell, Phone: (801) 213-4153, Email: ryan.oconnell@path.utah.edu
References
- 1.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell RM, Baltimore D. MicroRNAs and hematopoietic cell development. Curr Top Dev Biol. 2012;99:145–174. doi: 10.1016/B978-0-12-387038-4.00006-9. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 4.Tam W, Ben-Yehuda D, Hayward WS. bic, a novel gene activated by proviral insertions in avian leukosis virus-induced lymphomas, is likely to function through its noncoding RNA. Mol Cell Biol. 1997;17:1490–1502. doi: 10.1128/MCB.17.3.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 8.Dahlke C, Maul K, Christalla T, Walz N, Schult P, Stocking C, Grundhoff A. A microRNA encoded by Kaposi sarcoma-associated herpes virus promotes B-cell expansion in vivo. PLoS One. 2012;7:e49435. doi: 10.1371/journal.pone.0049435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205:585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haasch D, Chen YW, Reilly RM, Chiou XG, Koterski S, Smith ML, Kroeger P, McWeeny K, Halbert DN, Mollison KW, Djuric SW, Trevillyan JM. T cell activation induces a noncoding RNA transcript sensitive to inhibition by immunosuppressant drugs and encoded by the proto-oncogene, BIC. Cell Immunol. 2002;217:78–86. doi: 10.1016/S0008-8749(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 11.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa R, Leyland R, Meyer-Hermann M, Lu D, Turner M, Arbore G, Phan TG, Brink R, Vigorito E. MicroRNA-155 controls affinity-based selection by protecting c-MYC+ B cells from apoptosis. J Clin Invest. 2016;126:377–388. doi: 10.1172/JCI82914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Hu R, Huffaker TB, Kagele DA, Runtsch MC, Bake E, Chaudhuri AA, Round JL, O’Connell RM. MicroRNA-155 confers encephalogenic potential to Th17 cells by promoting effector gene expression. J Immunol. 2013;190:5972–5980. doi: 10.4049/jimmunol.1300351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KG, Rada C, Enright AJ, Toellner KM, Maclennan IC, Turner M. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu R, Kagele DA, Huffaker TB, Runtsch MC, Alexander M, Liu J, Bake E, Su W, Williams MA, Rao DS, Moller T, Garden GA, Round JL, O’Connell RM. miR-155 promotes T follicular helper cell accumulation during chronic, low-grade inflammation. Immunity. 2014;41:605–619. doi: 10.1016/j.immuni.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng FR, Tang LJ, He Y, Garcia RC. An update on the role of miRNA-155 in pathogenic microbial infections. Microbes Infect. 2015;17:613–621. doi: 10.1016/j.micinf.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Lind EF, Elford AR, Ohashi PS. Micro-RNA 155 is required for optimal CD8+ T cell responses to acute viral and intracellular bacterial challenges. J Immunol. 2013;190:1210–1216. doi: 10.4049/jimmunol.1202700. [DOI] [PubMed] [Google Scholar]
- 22.Lu LF, Gasteiger G, Yu IS, Chaudhry A, Hsin JP, Lu Y, Bos PD, Lin LL, Zawislak CL, Cho S, Sun JC, Leslie CS, Lin SW, Rudensky AY. A single miRNA-mRNA interaction affects the immune response in a context- and cell-type-specific manner. Immunity. 2015;43:52–64. doi: 10.1016/j.immuni.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, Katsikis PD. The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat Immunol. 2013;14:593–602. doi: 10.1038/ni.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhela S, Mulik S, Reddy PB, Richardson RL, Gimenez F, Rajasagi NK, Veiga-Parga T, Osmand AP, Rouse BT. Critical role of microRNA-155 in herpes simplex encephalitis. J Immunol. 2014;192:2734–2743. doi: 10.4049/jimmunol.1302326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhela S, Mulik S, Gimenez F, Reddy PB, Richardson RL, Varanasi SK, Jaggi U, Xu J, Lu PY, Rouse BT. Role of miR-155 in the pathogenesis of herpetic stromal keratitis. Am J Pathol. 2015;185:1073–1084. doi: 10.1016/j.ajpath.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thounaojam MC, Kundu K, Kaushik DK, Swaroop S, Mahadevan A, Shankar SK, Basu A. MicroRNA 155 regulates Japanese encephalitis virus-induced inflammatory response by targeting Src homology 2-containing inositol phosphatase 1. J Virol. 2014;88:4798–4810. doi: 10.1128/JVI.02979-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane TE, Hosking MP. The pathogenesis of murine coronavirus infection of the central nervous system. Crit Rev Immunol. 2010;30:119–130. doi: 10.1615/CritRevImmunol.v30.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marten NW, Stohlman SA, Bergmann CC. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 2001;14:1–18. doi: 10.1089/08828240151061329. [DOI] [PubMed] [Google Scholar]
- 30.Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J Virol. 2012;86:2416–2427. doi: 10.1128/JVI.06797-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaisted WC, Weinger JG, Walsh CM, Lane TE. T cell mediated suppression of neurotropic coronavirus replication in neural precursor cells. Virology. 2014;449:235–243. doi: 10.1016/j.virol.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheever FS, Daniels JB, Pappenheimer AM, Bailey OT. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. J Exerimental Med. 1949;90:181–194. doi: 10.1084/jem.90.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlman SR, Lane TE, Buchmeier MJ. Coronaviruses: hepatitis, peritonitis, and central nervous system disease. In: Cunningham MW, Fujinami RS, editors. Effects of microbes on the immune system. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 331–348. [Google Scholar]
- 34.Stohlman SA, Ramakrishna C, Tschen SI, Hinton DR, Bergmann CC. The art of survival during viral persistence. J Neurovirol. 2002;8(Suppl 2):53–58. doi: 10.1080/13550280290167884. [DOI] [PubMed] [Google Scholar]
- 35.Wang FI, Stohlman SA, Fleming JO. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J Neuroimmunol. 1990;30:31–41. doi: 10.1016/0165-5728(90)90050-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen O, Perry D, Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980;37:478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- 37.Houtman JJ, Hinze HC, Fleming JO. Demyelination induced by murine coronavirus JHM infection of congenitally immunodeficient mice. Adv Exp Med Biol. 1995;380:159–163. doi: 10.1007/978-1-4615-1899-0_26. [DOI] [PubMed] [Google Scholar]
- 38.Houtman JJ, Fleming JO. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J Neurovirol. 1996;2:101–110. doi: 10.3109/13550289609146543. [DOI] [PubMed] [Google Scholar]
- 39.Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/JVI.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane TE, Asensio VC, Yu N, Paoletti AD, Campbell IL, Buchmeier MJ. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol. 1998;160:970–978. [PubMed] [Google Scholar]
- 42.Glass WG, Lane TE. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology. 2003;312:407–414. doi: 10.1016/S0042-6822(03)00237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glass WG, Lane TE. Functional expression of chemokine receptor CCR5 on CD4(+) T cells during virus-induced central nervous system disease. J Virol. 2003;77:191–198. doi: 10.1128/JVI.77.1.191-198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stiles LN, Hardison JL, Schaumburg CS, Whitman LM, Lane TE. T cell antiviral effector function is not dependent on CXCL10 following murine coronavirus infection. J Immunol. 2006;177:8372–8380. doi: 10.4049/jimmunol.177.12.8372. [DOI] [PubMed] [Google Scholar]
- 45.Stiles LN, Hosking MP, Edwards RA, Strieter RM, Lane TE. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]
- 46.Stiles LN, Liu MT, Kane JA, Lane TE. CXCL10 and trafficking of virus-specific T cells during coronavirus-induced demyelination. Autoimmunity. 2009;42:484–491. doi: 10.1080/08916930902810708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Coleman R, Leang R, Tran H, Kopf A, Walsh CM, Sears-Kraxberger I, Steward O, Macklin WB, Loring JF, Lane TE. Human neural precursor cells promote neurologic recovery in a viral model of multiple sclerosis. Stem Cell Rep. 2014;2:825–837. doi: 10.1016/j.stemcr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Totoiu MO, Nistor GI, Lane TE, Keirstead HS. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol. 2004;187:254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plaisted WC, Zavala A, Hingco E, Tran H, Coleman R, Lane TE, Loring JF, Walsh CM. Remyelination is correlated with regulatory t cell induction following human embryoid body-derived neural precursor cell transplantation in a viral model of multiple sclerosis. PLoS One. 2016;11:e0157620. doi: 10.1371/journal.pone.0157620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergmann CC, Parra B, Hinton DR, Chandran R, Morrison M, Stohlman SA. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J Immunol. 2003;170:3204–3213. doi: 10.4049/jimmunol.170.6.3204. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez JM, Bergmann CC, Ramakrishna C, Hinton DR, Atkinson R, Hoskin J, Macklin WB, Stohlman SA. Inhibition of interferon-gamma signaling in oligodendroglia delays coronavirus clearance without altering demyelination. Am J Pathol. 2006;168:796–804. doi: 10.2353/ajpath.2006.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parra B, Bergmann CC, Hinton DR, Atkinson R, Stohlman SA. IFN-gamma secreted by virus-specific CD8+ T cells contribute to CNS viral clearance. Adv Exp Med Biol. 2001;494:335–340. doi: 10.1007/978-1-4615-1325-4_50. [DOI] [PubMed] [Google Scholar]
- 53.Parra B, Hinton DR, Marten NW, Bergmann CC, Lin MT, Yang CS, Stohlman SA. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol. 1999;162:1641–1647. [PubMed] [Google Scholar]
- 54.Stohlman SA, Hinton DR, Parra B, Atkinson R, Bergmann CC. CD4 T cells contribute to virus control and pathology following central nervous system infection with neurotropic mouse hepatitis virus. J Virol. 2008;82:2130–2139. doi: 10.1128/JVI.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phares TW, Stohlman SA, Hinton DR, Bergmann CC. Enhanced CD8 T-cell anti-viral function and clinical disease in B7-H1-deficient mice requires CD4 T cells during encephalomyelitis. J Neuroinflammation. 2012;9:269. doi: 10.1186/1742-2094-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savarin C, Bergmann CC, Hinton DR, Ransohoff RM, Stohlman SA. Memory CD4+ T-cell-mediated protection from lethal coronavirus encephalomyelitis. J Virol. 2008;82:12432–12440. doi: 10.1128/JVI.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stohlman SA, Bergmann CC, Lin MT, Cua DJ, Hinton DR. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 58.Hosking MP, Tirotta E, Ransohoff RM, Lane TE. CXCR2 signaling protects oligodendrocytes and restricts demyelination in a mouse model of viral-induced demyelination. PLoS One. 2010;5:e11340. doi: 10.1371/journal.pone.0011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tirotta E, Duncker P, Oak J, Klaus S, Tsukamoto MR, Gov L, Lane TE. Epstein-Barr virus-induced gene 3 negatively regulates neuroinflammation and T cell activation following coronavirus-induced encephalomyelitis. J Neuroimmunol. 2013;254:110–116. doi: 10.1016/j.jneuroim.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergmann CC, Parra B, Hinton DR, Ramakrishna C, Dowdell KC, Stohlman SA. Perforin and gamma interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J Virol. 2004;78:1739–1750. doi: 10.1128/JVI.78.4.1739-1750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Templeton SP, Perlman S. Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM. Immunol Res. 2007;39:160–172. doi: 10.1007/s12026-007-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitman L, Zhou H, Perlman S, Lane TE. IFN-gamma-mediated suppression of coronavirus replication in glial-committed progenitor cells. Virology. 2009;384:209–215. doi: 10.1016/j.virol.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu MT, Chen BP, Oertel P, Buchmeier MJ, Armstrong D, Hamilton TA, Lane TE. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- 64.Liu MT, Keirstead HS, Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- 65.Stiles LN, Liu MT, Kane JAC, Lane TE. CXCL10 and trafficking of virus-specific T cells during coronavirus demyelination. Autoimmunity In Press;2009. [DOI] [PMC free article] [PubMed]
- 66.Glass WG, Hickey MJ, Hardison JL, Liu MT, Manning JE, Lane TE. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol. 2004;172:4018–4025. doi: 10.4049/jimmunol.172.7.4018. [DOI] [PubMed] [Google Scholar]
- 67.Murugaiyan G, Beynon V, Mittal A, Joller N, Weiner HL. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2213–2221. doi: 10.4049/jimmunol.1003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. 2014;28:2551–2565. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 70.Thome AD, Harms AS, Volpicelli-Daley LA, Standaert DG. microRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J Neurosci. 2016;36:2383–2390. doi: 10.1523/JNEUROSCI.3900-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guedes JR, Custodia CM, Silva RJ, de Almeida LP, Pedroso de Lima MC, Cardoso AL. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum Mol Genet. 2014;23:6286–6301. doi: 10.1093/hmg/ddu348. [DOI] [PubMed] [Google Scholar]
- 72.Lippai D, Bala S, Csak T, Kurt-Jones EA, Szabo G. Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLoS One. 2013;8:e70945. doi: 10.1371/journal.pone.0070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parisi C, Arisi I, D’Ambrosi N, Storti AE, Brandi R, D’Onofrio M, Volonte C. Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis. 2013;4:e959. doi: 10.1038/cddis.2013.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cardoso AL, Guedes JR, de Lima MC. Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr Opin Pharmacol. 2016;26:1–9. doi: 10.1016/j.coph.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS One. 2013;8:e79416. doi: 10.1371/journal.pone.0079416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61:91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su W, Aloi MS, Garden GA. MicroRNAs mediating CNS inflammation: small regulators with powerful potential. Brain Behav Immun. 2016;52:1–8. doi: 10.1016/j.bbi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosking MP, Lane TE. The role of chemokines during viral infection of the CNS. PLoS Pathog. 2010;6:e1000937. doi: 10.1371/journal.ppat.1000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glass WG, Liu MT, Kuziel WA, Lane TE. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology. 2001;288:8–17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dyskova T, Fillerova R, Novosad T, Kudelka M, Zurkova M, Gajdos P, Kolek V, Kriegova E. Correlation network analysis reveals relationships between micrornas, transcription factor T-bet, and deregulated cytokine/chemokine-receptor network in pulmonary sarcoidosis. Mediators Inflamm. 2015;2015:121378. doi: 10.1155/2015/121378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei Y, Nazari-Jahantigh M, Chan L, Zhu M, Heyll K, Corbalan-Campos J, Hartmann P, Thiemann A, Weber C, Schober A. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–1619. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 82.Yang J, Zhang P, Krishna S, Wang J, Lin X, Huang H, Xie D, Gorentla B, Huang R, Gao J, Li QJ, Zhong XP. 2016. Unexpected positive control of NFkappaB and miR-155 by DGKalpha and zeta ensures effector and memory CD8+ T Cell differentiation. Oncotarget doi:10.18632/oncotarget.8164. [DOI] [PMC free article] [PubMed]
- 83.Hosking MP, Lane TE. The biology of persistent infection: inflammation and demyelination following murine coronavirus infection of the central nervous system. Curr Immunol Rev. 2009;5:267–276. doi: 10.2174/157339509789504005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lane TE, Buchmeier MJ. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kucukali CI, Kurtuncu M, Coban A, Cebi M, Tuzun E. Epigenetics of multiple sclerosis: an updated review. Neuromolecular Med. 2015;17:83–96. doi: 10.1007/s12017-014-8298-6. [DOI] [PubMed] [Google Scholar]
- 86.Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li Y, Singh N, Nagarkatti M, Nagarkatti P. Expression, regulation and function of microRNAs in multiple sclerosis. Int J Med Sci. 2014;11:810–818. doi: 10.7150/ijms.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thamilarasan M, Koczan D, Hecker M, Paap B, Zettl UK. MicroRNAs in multiple sclerosis and experimental autoimmune encephalomyelitis. Autoimmun Rev. 2012;11:174–179. doi: 10.1016/j.autrev.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Paraboschi EM, Solda G, Gemmati D, Orioli E, Zeri G, Benedetti MD, Salviati A, Barizzone N, Leone M, Duga S, Asselta R. Genetic association and altered gene expression of mir-155 in multiple sclerosis patients. Int J Mol Sci. 2011;12:8695–8712. doi: 10.3390/ijms12128695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R, Meinl E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;132:3342–3352. doi: 10.1093/brain/awp300. [DOI] [PubMed] [Google Scholar]
- 90.Waschbisch A, Atiya M, Linker RA, Potapov S, Schwab S, Derfuss T. Glatiramer acetate treatment normalizes deregulated microRNA expression in relapsing remitting multiple sclerosis. PLoS One. 2011;6:e24604. doi: 10.1371/journal.pone.0024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moore CS, Rao VT, Durafourt BA, Bedell BJ, Ludwin SK, Bar-Or A, Antel JP. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurol. 2013;74:709–720. doi: 10.1002/ana.23967. [DOI] [PubMed] [Google Scholar]
- 92.Li JS, Yao ZX. MicroRNAs: novel regulators of oligodendrocyte differentiation and potential therapeutic targets in demyelination-related diseases. Mol Neurobiol. 2012;45:200–212. doi: 10.1007/s12035-011-8231-z. [DOI] [PubMed] [Google Scholar]
- 93.Svaren J. MicroRNA and transcriptional crosstalk in myelinating glia. Neurochem Int. 2014;77:50–57. doi: 10.1016/j.neuint.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matthews AE, Lavi E, Weiss SR, Paterson Y. Neither B cells nor T cells are required for CNS demyelination in mice persistently infected with MHV-A59. J Neurovirol. 2002;8:257–264. doi: 10.1080/13550280290049697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blanc CA, Grist JJ, Rosen H, Sears-Kraxberger I, Steward O, Lane TE. Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination. Am J Pathol. 2015;185:2819–2832. doi: 10.1016/j.ajpath.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blanc CA, Rosen H, Lane TE. FTY720 (fingolimod) modulates the severity of viral-induced encephalomyelitis and demyelination. J Neuroinflammation. 2014;11:138. doi: 10.1186/s12974-014-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will make available all relevant raw data used in generating conclusions put forth within this manuscript via Open Science Framework (osf.io).


