Abstract
Background
Brugada syndrome is recognized as being associated with sudden cardiac death; however, the prevalence of non–type 1 Brugada‐type ECG (BrS) or atypical ST‐segment elevation in the right precordial leads (STERP) and the long‐term prognosis for those patients remain unknown.
Methods and Results
We analyzed standard 12‐lead ECGs of 7178 apparently healthy participants (age range 40–64 years) who underwent health checkups from 1982 to 1986 in the Circulatory Risk in Communities Study, a prospective, large, community‐based cohort study in Japan. ECGs with J point amplitude ≥0.2 mV in the right precordial leads were divided into 3 groups: (1) type 1 BrS, (2) type 2 or 3 BrS (non‐type 1 BrS), and (3) STERP. The others served as the non–ST‐segment elevation group. We identified 8 participants (0.1%) with type1 BrS, 84 (1.2%) with non–type 1 BrS, and 228 (3.2%) with STERP. During a median follow‐up of 18.7 years (133 987.0 person‐years), sudden cardiac death was observed in no participants (0.0%) with type 1 BrS, in 1 (1.2%) with non–type 1 BrS, in 7 (3.1%) with STERP, and in 50 (0.7%) with non–ST‐segment elevation. Participants with STERP had a markedly elevated risk of sudden cardiac death (multivariable hazard ratio 3.9, 95% CI 1.7–9.0).
Conclusions
STERP was associated with an elevated risk of sudden cardiac death in a middle‐aged population.
Keywords: Brugada syndrome, electrocardiography, epidemiology, prognosis, sudden cardiac death
Subject Categories: Sudden Cardiac Death
Introduction
Brugada syndrome is a hereditary arrhythmogenic disease characterized by accentuated ST‐segment elevation (type 1) in the right precordial leads (V1 through V3) and increased risk of sudden cardiac death (SCD) without structural heart disease.1, 2, 3 The data from previous studies vary regarding prevalence and prognosis for patients with Brugada‐type ECG (BrS) because of different populations (eg, general population, office workers),4, 5, 6, 7, 8, 9 different ECG definitions,5, 6, 9, 10, 11, 12 relatively short‐term follow‐up,5, 6, 9, 10, 12, 13 or poorly defined SCD.5, 6, 7, 8, 10, 11, 13, 14
The prevalence of non–type 1 BrS and ECGs with a J point amplitude ≥0.2 mV and non–Brugada‐type right precordial ST‐segment (atypical ST‐segment elevation in the right precordial leads [STERP]) and the long‐term prognosis for these patients have not been reported in a prospective community‐based cohort study. Recently, Kamakura et al reported that early repolarization (ER) characterized by notching or slurring in the right precordial leads had a poor prognosis in their hospital‐based study.15 It is difficult, however, to evaluate the J point morphology because the ST‐segment attenuates it; therefore, we also focused on the J point amplitude in STERP. This study aimed to use the data from a study with >20 years of follow‐up of 7178 participants in 3 Japanese populations to investigate the relationship between BrS or ECGs with STERP and the risk of SCD.
Methods
Study Population
The study included community residents aged 40 to 64 years who enrolled in a community‐based cohort of the Circulatory Risk in Community Study (CIRCS). CIRCS is a prospective community‐based study that was launched to examine risk factors of cardiovascular disease from 1963 onward and that is conducted by a research team from Osaka Medical Center for Health Science and Promotion, Osaka University, and the University of Tsukuba. The CIRCS participants lived in Ikawa town, Akita Prefecture (a northeastern rural community); Kyowa town, Ibaraki Prefecture (a central rural community); and Yao City, Osaka Prefecture (a southwestern suburb). Among them, we recruited 10 337 participants (4223 men, 6114 women) who underwent a health checkup from 1982 to 1986. We excluded 3159 participants who met the following exclusion criteria: age <40 or >64 years, no previous ECGs on record, and a past history of myocardial infarction and/or atrial fibrillation at registration (Figure 1). Systematic community surveillance of SCD was done for 7178 people (2886 men, 4292 women) who constituted the participants of this study. The population numbers in each community were 1780 in Ikawa (779 men, 1001 women), 3343 in Kyowa (1431 men, 1912 women), and 2055 in Yao (676 men, 1379 women). Informed consent was not obtained from the participants but was obtained from the community representatives to conduct an epidemiological study based on the guidelines of the Council for International Organizations of Medical Science. The study was approved by the ethics committees of Ibaraki Prefecture, University of Tsukuba, Osaka University, and Osaka Medical Center for Health Science and Promotion.
Figure 1.
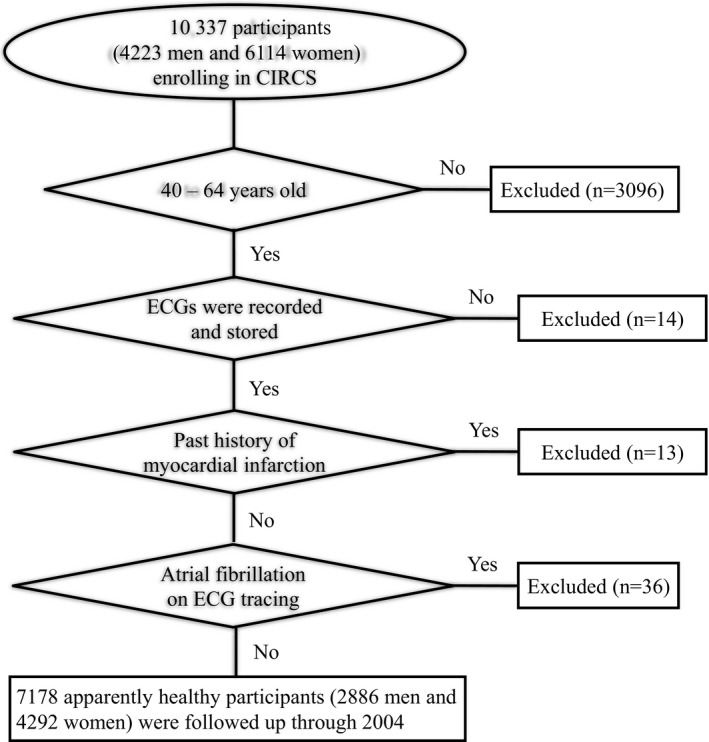
Flowchart of the entry process for the 7178 participants.
Baseline Examinations
Risk factors possibly predisposing to cardiovascular disease were recorded at participant registration. Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg or diastolic BP ≥90 mm Hg or use of antihypertensive medications. Arterial BP was measured by trained observers using standard mercury sphygmomanometers on the right arm of the seated participants after a 5‐minute rest.16 Diabetes mellitus was defined as a fasting glucose level of ≥126 mg/dL, a nonfasting glucose level of ≥200 mg/dL, or use of glucose‐lowering medication. Smoking was defined as only current smoking. Alcohol use was defined as only current use. Height in stocking feet and weight in light clothing were measured. Body mass index was calculated as weight (in kg) divided by the square of height in meters.
ECG Analysis
ECG recordings were performed for all participants every year; however, not all participants regularly underwent a health checkup. To make our study as unbiased as possible, we analyzed only the first ECG that was recorded when participants were aged 40 to 64 years. At baseline, resting standard 12‐lead ECGs were recorded at a paper speed of 25 mm/s in the supine position and were coded according to the Minnesota Code by epidemiologist‐physicians.17 From a panel of 4 cardiologists (Y.Y., N.M., Y.S., H.T.) blinded to the data of risk factor surveys and prognosis, 2 cardiologists independently reviewed the baseline ECGs in paper format without using magnifiers or other tools. In cases of disagreement as to the category, a third cardiologist from the panel determined it.
The types of BrS were classified according to the ECG criteria described in the report of the second consensus conference, which defined type 1 as characterized by a coved‐type J point elevation ≥2 mm (0.2 mV) and non–type 1 as characterized by type 2 or 3 BrS ECG, that is, a saddleback‐type J point elevation ≥2 mm (0.2 mV).3 Patients who had ECGs with J point elevation ≥0.2 mV in the right precordial leads and non‐BrS ECG were categorized as having STERP. Other ECGs were classified as non–ST‐segment elevation (non‐ST). Consequently, ECGs in this study were categorized into 4 groups: type1 BrS (Figure 2A), non–type 1 BrS (Figure 2B and 2C), STERP (Figure 2D and 2E), and non‐ST (Figure 2F).
Figure 2.
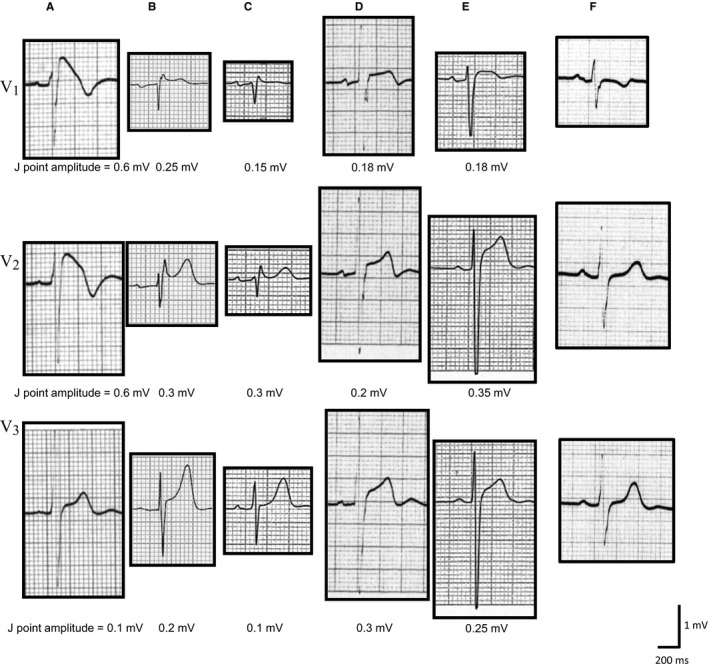
Representative ECG tracings of the type 1 Brugada‐type ECG (BrS), non–type 1 BrS, atypical ST‐segment elevation in the right precordial leads (STERP), and non–ST‐segment elevation (non‐ST) groups. Type 1 BrS (A) is characterized by prominent coved ST‐segment elevation displaying a J point amplitude ≥0.2 mV at its peak, followed by a negative T wave. Non–type 1 BrS (B and C) also has high takeoff ST‐segment elevation, but in this case, the J point amplitude (≥0.2 mV) gives rise to a gradually descending ST‐segment elevation followed by a positive or biphasic T wave that results in a saddleback configuration. STERP (D and E) shows noncoved and nonsaddleback ST‐T morphology with J point elevation ≥0.2 mV in the right precordial leads. F, J point elevation of <0.2 mV in the right precordial leads is included in the non‐ST group.
We excluded ECGs with an epsilon wave, which is characteristic of arrhythmogenic right ventricular cardiomyopathy. The epsilon wave is defined as distinct waves of small amplitude that occupy the early phase of the ST‐segment in the right precordial leads and that are distinct from the QRS complex.
To examine the association of J point amplitude with SCD in non‐BrS, we excluded participants with type 1 or non–type 1 BrS and divided ECGs for participants without BrS according to J point amplitude into STERP (group A), ECGs with J point amplitude ≥0.1 to <0.2 mV (group B), and ECGs with J point amplitude <0.1 mV (group C).
Follow‐up
The participants were followed until the end of 2004. Follow‐up was censored at the date the participant moved out of the area or the date of all‐cause death. A total of 1206 participants (16.8%) died during the follow‐up period.
SCD was defined as a sudden unexpected death either within 1 hour of symptom onset (for witnessed events) or within 24 hours of having been observed alive and symptom free. Cases were excluded if death occurred >24 hours following the onset of symptoms or if the apparent cause of death was found to be something other than SCD, such as cerebrovascular disease, cancer, or accidental death. For all residents, SCD was determined from death certificates; national insurance claims; reports by local physicians, public health nurses, and health volunteers; and annual cardiovascular risk surveys. To confirm the diagnosis, all living participants were either telephoned or visited to obtain their medical history in addition to review of their medical records including monitor ECGs or 12‐lead ECGs not only in the hospital but also in the ambulance. For deaths, we obtained histories from families and reviewed medical records to investigate the mode of onset such as physical activity or symptoms. A panel of trained epidemiologist‐physicians who were blinded to the data of cardiovascular risk factors made the final diagnosis of SCD. We also excluded participants with coronary artery disease. The indication of definite myocardial infarction was typical severe chest pain accompanied by new, abnormal, and persistent Q or QS waves or consistent changes in cardiac enzyme levels. Patients who reported typical chest pain but whose ECGs and enzyme levels were nondiagnostic or unattainable were diagnosed as having possible myocardial infarction.18
Statistical Analysis
Continuous variables are expressed as mean±SD, and categorical variables are presented as percentages. ANCOVA or the chi‐square test was used to test differences in age‐adjusted means and proportions of baseline characteristics by the ECG categories. Corrections for multiple comparisons were made according to the Dunnet method. The chi‐square test was used to perform a frequency comparison. Person‐years were calculated as the sum of individual follow‐up times until participants moved out of the communities, the occurrence of all‐cause death, or the end of 2004. Hazard ratios (HRs) and 95% CIs for (1) type 1 BrS, non–type 1 BrS, and STERP groups against the non‐ST group and (2) STERP (J point amplitude >0.2 mV) and the group with J point amplitude >0.1 to <0.2 mV against the group with J point amplitude <0.1 mV for SCD were calculated with a Cox proportional hazards regression model. We adjusted for age (years) and sex and for other potential confounding variables including systolic BP (mm Hg), antihypertensive medication use (yes or no), smoking status (never, former, and current smoker), and diabetes mellitus (yes or no). Two‐tailed testing was performed, and a P<0.05 was considered to indicate significance. All analyses were made with the SAS statistical software package (version 9.2; SAS Institute).
Results
Prevalence and Clinical Characteristics of Type 1 BrS, Non–Type 1 BrS, and STERP
The prevalence of type 1 BrS, non–type 1 BrS, and STERP of the 7178 participants was 0.1% (n=8), 1.2% (n=84), and 3.2% (n=228), respectively. No ECGs with epsilon waves or markedly prolonged QT intervals were observed. Baseline characteristics are listed in Table 1. The median follow‐up period was 18.7 years and was uniform among the 4 groups. Compared with the non‐ST group, participants in the other 3 groups showed male predominance, and those with non–type 1 BrS were older, had lower mean body mass index values, and included a higher proportion of current alcohol use and smoking. Those with STERP were younger; had lower mean values for heart rate, diastolic BP, and serum cholesterol; and included a higher proportion of current alcohol use and smoking. Those with type 1 BrS had a higher proportion of current alcohol use. There were no significant differences in mean systolic BP and triglyceride levels or in the prevalence of hypertension and diabetes mellitus among the 4 groups.
Table 1.
Baseline Characteristics and Outcomes of the Type 1 BrS, Non–Type 1 BrS, STERP, and Non‐ST Groups
| All Participants | Type 1 BrS | Non–Type 1 BrS | STERP | Non‐ST | P Value | |
|---|---|---|---|---|---|---|
| Number of participants | 7178 | 8 (0.1) | 84 (1.2) | 228 (3.2) | 6858 (95.5) | |
| Follow‐up period, y | 18.7±0.1 | 17.4±5.9 | 17.7±5.4 | 18.4±4.2 | 18.7±4.9 | 0.233 |
| Age, y | 51.8±7.1 | 52.9±7.3 | 54.7±6.9* | 50.3±7.1* | 51.8±7.1 | <0.001 |
| Male | 2886 (40.2) | 7 (87.5) | 74 (88.1) | 216 (94.7) | 2589 (37.8) | <0.001 |
| Height, cm | 152.6±21.3 | 158.4±6.3 | 159.7±7.4* | 161.3±12.6* | 152.2±21.6 | <0.001 |
| Weight, kg | 55.3±11.6 | 56.5±6.6 | 55.6±7.2 | 59.9±8.8* | 55.1±11.7 | <0.001 |
| Body mass index, kg/m2 | 23.4±3.1 | 22.5±1.7 | 21.8±2.7* | 22.9±2.4 | 23.4±3.2 | <0.001 |
| Systolic BP, mm Hg | 132.0±18.5 | 129.3±18.5 | 131.1±21.9 | 134.4±20.0 | 131.9±18.4 | 0.235 |
| Diastolic BP, mm Hg | 80.1±11.4 | 72.5±10.7 | 81.0±12.0 | 81.9±12.7* | 80.1±11.3 | 0.017 |
| Hypertensive | 2552 (35.6) | 2 (25.0) | 33 (39.3) | 97 (42.5) | 2420 (35.3) | 0.113 |
| Serum total cholesterol, mmol/L | 182.7±36.2 | 189.3±39.4 | 184.0±33.7 | 185.7±37.4* | 193.0±36.1 | 0.003 |
| Triglycerides, mg/dL | 140.9±101.6 | 126.4±106.0 | 123.8±74.3 | 143.3±98.4 | 147.1±102.0 | 0.568 |
| Current alcohol use | 2291 (31.9) | 5 (62.5)* | 55 (65.5)* | 172 (75.4)* | 2059 (30.0) | <0.001 |
| Current smoking | 2217 (30.9) | 5 (62.5) | 55 (65.5)* | 155 (68.0)* | 2002 (29.2) | <0.001 |
| Diabetes mellitus | 312 (4.3) | 1 (12.5) | 1 (1.2) | 14 (6.1) | 296 (4.3) | 0.167 |
| ECG findings | ||||||
| Heart rate, bpm | 68.6±11.8 | 64.9±14.4 | 65.9±9.1 | 64.4±8.9* | 68.8±11.9 | <0.001 |
| QRS axis | ||||||
| Left axial deviation | 130 (1.8) | 1 (12.5) | 4 (4.8) | 2 (0.9) | 123 (1.8) | 0.016 |
| Right axial deviation | 3 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.0) | 0.987 |
| LVH by voltage criteria | 249 (3.5) | 0 (0.0) | 3 (3.6) | 13 (5.7) | 233 (3.4) | 0.285 |
Continuous variables are expressed as the mean±SD, and categorical variables are presented as number (percentage). BP indicates blood pressure; BrS, Brugada‐type ECG; LVH, left ventricular hypertrophy; non‐ST, Non–ST‐segment elevation; STERP, atypical ST‐Segment elevation in the right precordial leads.
*P<0.05, test for significance against the non‐ST group.
Prognosis of Type 1 BrS, Non–Type 1 BrS, and STERP
There were 58 SCDs (0.8%) and 1206 all‐cause deaths (16.8%) during the median follow‐up of 18.7 years (133 987.0 person‐years). The number of SCDs in the type 1 BrS, non–type 1 BrS, and STERP groups was 0 (0.0%), 1 (1.2%), and 7 (3.1%), respectively. Participants with STERP had a markedly elevated risk of SCD (age‐ and sex‐adjusted HR 4.1, 95% CI 1.8–9.6; multivariable HR 3.9, 95% CI 1.7–9.0), whereas those with non–type 1 BrS had no excess risk of SCD (age‐ and sex‐adjusted HR 1.2, 95% CI 0.16–8.6; multivariable HR 1.1, 95% CI 0.15–8.3) compared with those with non‐ST (Table 2).
Table 2.
Unadjusted and Age‐ and Sex‐Adjusted and Multivariable HRs of Type 1 BrS, Non–Type 1 BrS, and STERP Groups Against the Non‐ST Group
| Type 1 BrS | Non–Type 1 BrS | STERP | Non‐ST | |
|---|---|---|---|---|
| Person‐years | 202.3 | 2074.4 | 4816.8 | 177472.1 |
| Number at risk | 8 | 84 | 228 | 6858 |
| Number of SCD | 0 (0.0%) | 1 (1.2%) | 7 (3.1%) | 50 (0.7%) |
| Age‐ and sex‐adjusted HR (95% CI) | — | 1.2 (0.16–8.6) | 4.1 (1.8–9.6) | 1 |
| Multivariable HR (95% CI) | 1.1 (0.15–8.3) | 3.9 (1.7–9.0) | 1 |
Multivariable HR adjusted for age, sex, systolic blood pressure, antihypertensive medication use, smoking status, and diabetes mellitus. BrS indicates Brugada‐type ECG; HR, hazard ratio; non‐ST, Non–ST‐segment elevation; SCD, sudden cardiac death; STERP, atypical ST‐segment elevation in the right precordial leads.
SCD in Participants With STERP
Results of further investigation into the ECG findings of the 7 participants with SCD are shown in Table 3. Five of the 7 subjects (71.4%) were aged in their 60s at death, and all were male (100%). P wave duration (52–120 ms), PR interval (140–200 ms), and QRS duration (76–104 ms) were within normal limits in all participants. SV1+RV5 in 3 of the 7 participants (42.9%) was >3.5 mV. Corrected QT interval in 6 of the 7 participants (85.7%) was within normal limits (<440 ms1/2).
Table 3.
Seven Cases of Sudden Cardiac Death With STERP
| Case No. | Age at Death (y) | Sex | P Wave Duration (ms) | PR Interval (ms) | QRS Duration (ms) | SV1+RV5 (mm) | QT/QTc (ms/ms1/2) |
|---|---|---|---|---|---|---|---|
| 1 | 61 | Male | 120 | 168 | 104 | 40 | 336/350 |
| 2 | 64 | Male | 52 | 172 | 72 | 32 | 292/326 |
| 3 | 65 | Male | 120 | 180 | 104 | 25 | 400/358 |
| 4 | 68 | Male | 80 | 200 | 80 | 30 | 360/389 |
| 5 | 63 | Male | 84 | 188 | 84 | 47 | 348/362 |
| 6 | 71 | Male | 96 | 140 | 80 | 30 | 480/451 |
| 7 | 80 | Male | 80 | 200 | 76 | 47 | 364/379 |
STERP indicates atypical ST‐segment elevation in the right precordial leads.
Association of J Point Amplitude With SCD in Participants Without BrS
Overall, 615 participants (8.6%) had J point amplitude ≥0.1 to <0.2 mV (group B) and 6243 (87.0%) had J point amplitude <0.1 mV (group C). Compared with the participants in group C, the incidence of SCD in participants in groups A and B was higher (age‐ and sex‐adjusted HR 4.1 [95% CI 1.8–9.6] and 2.4 [95% CI 1.1–5.1], respectively; multivariable HR 3.9 [95% CI 1.7–9.0] and 2.5 [95% CI 1.2–5.3], respectively) (Table 4).
Table 4.
Unadjusted, Age‐ and Sex‐Adjusted, and Multivariable HRs for SCD of STERP
| Group A | Group B | Group C | |
|---|---|---|---|
| Person‐years | 4816.8 | 8951.1 | 117210.7 |
| Number at risk | 228 | 615 | 6243 |
| Number with SCD | 7 (3.1%) | 11 (1.8%) | 39 (0.1%) |
| Age‐ and sex‐adjusted HR (95% CI) | 4.1 (1.8–9.6) | 2.4 (1.1–5.1) | 1 |
| Multivariable HR (95% CI) | 3.9 (1.7–9.0) | 2.5 (1.2–5.3) | 1 |
HRs are for group A (J point amplitude >0.2 mV) and group B (J point amplitude >0.1 to <0.2 mV) versus group C (J point amplitude <0.1 mV). The multivariable HR is adjusted for age, sex, systolic blood pressure, antihypertensive medication use, smoking status, and diabetes mellitus. HR indicates hazard ratio; SCD, sudden cardiac death; STERP, atypical ST‐segment elevation in the right precordial leads.
Discussion
Major Findings
We revealed an interesting finding in our large‐scale and long‐term observational study: Participants with STERP had an excess risk of SCD compared with those in the non‐ST group. Our study is the first to find that STERP is a distinct clinical entity with a high risk of SCD in the middle‐aged Japanese general population.
Prevalence of BrS and Prognosis for Participants With BrS
The prevalence of type1 BrS (0.1%) and non–type 1 BrS (1.2%) in this cohort was compatible with that in previous studies: 0.0% to 0.2%5, 6, 7, 8, 9, 10, 12, 13, 14, 19 for type 1 BrS and 0.0% to 6.0% for non–type 1 BrS.4, 5, 7, 9, 10, 13, 14, 20 In keeping with previous reports,4, 7, 9, 10, 21, 22 there was a male predominance among participants with BrS. In the present study, no SCD among participants with type 1 BrS and only 1 SCD among those with non–type 1 BrS was observed during the follow‐up period, which was consistent with previous community‐based studies,6, 8, 9, 12, 14 except for the Atomic Heart Study.11 In that study, the definition of type 1 BrS, which was characterized by the coexistence of coved and saddleback morphology with J point elevation ≥0.1 mV in V1 through V3, was different from that in the second consensus report.3
Prognosis and Characteristics of Participants With STERP
The arrhythmogenic potential of ER in the inferior leads was highlighted recently by Haïssaguerre et al.22 Nevertheless, in the cohort studies examining early repolarization syndrome (ERS), the right precordial leads were not included in the analysis to avoid including BrS or arrhythmogenic right ventricular cardiomyopathy. Kamakura et al extended the definition of non–type 1 BrS and concluded that the long‐term prognosis of probands with non–type 1 BrS was similar to that of probands with type 1 BrS in a hospital‐based multicenter study.23 Moreover, Kamakura et al investigated the significance of non–type 1 anterior ER in patients with idiopathic ventricular fibrillation and inferolateral ER in their hospital‐based study.15 They concluded that the coexistence of non–type 1 anterior ER was a predictor of poor outcome in patients with inferolateral ER and ventricular fibrillation; however, these studies included patients with non–type 1 BrS. Little is known about what ST‐T morphology, except for BrS in the right precordial leads, is associated with malignant arrhythmia. Consequently, we investigated ECGs with STERP without BrS and analyzed the clinical characteristics and long‐term prognosis of participants with STERP. These participants had a markedly elevated risk of SCD compared with those with non‐ST. To the best of our knowledge, this report is the first to show a significantly higher risk of SCD in participants with STERP.
Clinical characteristics were as follows. First, participants with STERP were predominantly male (94.7%) and were significantly younger than participants in the non‐ST group, which is the same as that with Brugada syndrome and ERS.18, 24, 25 This suggests a particular young and male background that relates to heredity, hormonal factors, or autonomic nervous function. Haruta et al proposed a hypothesis that testosterone may modulate cardiac mortality in ERS.26 The plasma concentration of testosterone is higher in men with BrS than in other age‐matched men15 and was reported to increase net outward current of the epicardium, to aggravate the transmural voltage gradient between the epicardium and endocardium, and to lead to the J point seen in ERS and BrS.27 In our study, there was no significant difference in body mass index that would indicate an influence by testosterone between the STERP and non‐ST groups, indicating that testosterone may not play a leading role in the prognosis of participants with STERP even if it could influence the J point amplitude. Junttila et al28 reported that testosterone levels were closely associated with not only lateral J point elevation but also with a rapidly ascending ST‐segment after J point elevation, which Tikkanen et al29 reported as being benign in 3 types of ST‐segments (ascending, horizontal, or descending.) The mechanism of the J point in the right precordial leads associated with testosterone would be the same as that in the inferior leads.
Second, in the large number of participants without BrS, the higher amplitude of the J point in the right precordial leads was significantly associated with SCD incidence. A hospital‐based study with a small number of participants (n=85) reported that the incidence of SCD of probands with non–type 1 BrS including ECGs with a J point amplitude ≥0.1 to <0.2 mV was similarly as high as those with type 1 BrS.15 In terms of J point amplitude, our result is identical to that of a previous large‐scale community‐based study that investigated the inferior leads and showed that J point elevation of at least 0.1 mV in the inferior leads was associated with a high risk of cardiac death, and J point elevation of >0.2 mV in the inferior leads had a higher risk of arrhythmia events and cardiac death.25 Similarly, we focused on the right precordial leads in this middle‐aged Japanese general population without BrS and revealed that the amplitude of the J point elevation in the right precordial leads had some prognostic value, and there was a higher risk of SCD among participants with a markedly elevated J point (>0.2 mV) than among those with more moderate elevation (≥0.1 mV).
Study Limitations
First, we did not systematically obtain data on the history of syncope or the family history of SCD in our population‐based cohort study, which indicates that our cases of SCD could be sporadic. For Brugada syndrome, however, if a substantial number of SCD cases had not been sporadic, the benign feature of BrS in our study would be strengthened.
Second, the statistical power was insufficient for persons aged <40 years, and generalization would be questionable because almost no SCD was observed in this age group. It is possible that ER in our participants had an identity different from that in younger persons. Moreover, to exclude lethal conditions other than arrhythmias that can be present in participants aged >65 years, we limited participant age to 40 to 65 years. The mean age (51.8 years) at registration in the present study was older than that in most of the previous hospital‐ or community‐based studies that analyzed participants diagnosed as having ERS or Brugada syndrome.15, 25, 27 Nevertheless, ≈90% of the SCDs were observed in participants aged >40 years.30 With participant age at registration (40–64 years) and the >18‐year follow‐up period, we could cover almost all possible age ranges during which a malignant arrhythmia would occur.
Third, to exclude structural heart disease from this study, we excluded only participants who were diagnosed as having myocardial infarction at registration and/or whose ECG displayed atrial fibrillation without performing echocardiography, cardiac catheterization, and electrophysiological study. The study population might include patients with structural heart disease, which also features ERS or BrS. Miyazaki et al reported that J point elevation is typically observed in patients with hypothermia, hypercalcemia, myocardial ischemia, and brain injury31; however, these conditions were not commonly found among the participants in routine health checkups. Even a self‐reporting system without evaluation by ECG or echocardiography can be useful to exclude cardiac disease from the baseline population.32
Fourth, we examined only standard ECGs with precordial positions recorded from the fourth intercostal space in a single examination and did not perform drug provocation tests. A higher precordial position might have increased the prevalence of type 1 BrS33; therefore, the prevalence of spontaneous and drug‐induced type 1 BrS may be underestimated.
Fifth, the J amplitude of ER and the type of BrS can fluctuate over time, especially immediately before ventricular fibrillation,27 which might cause us to underestimate the prevalence of ER and BrS, respectively. Repetitive ECG recordings are necessary to investigate the mode of fluctuation in ECGs with atypical J point elevation.
Sixth, the prevalence of type 1 BrS in this study is as rare as that in previous studies, which revealed that almost no SCD occurred in participants with type 1 BrS. In this study, only 8 participants with type 1 BrS were found, and none of them died. To increase statistical power, a much greater number of participants would be needed.
Conclusion
STERP rather than BrS was associated with an elevated risk of SCD in a middle‐aged Japanese general population.
Appendix
CIRCS Investigators
The CIRCS is a collaborative study managed by the Osaka Medical Center for Health Science and Promotion, University of Tsukuba, Osaka University, and Ehime University. The CIRCS investigators who contributed to this study are as follows: Tomoko Sankai, Kazumasa Yamagishi, Mitsumasa Umesawa, Choy‐Lye Chei, Kimiko Yokota, and Minako Tabata, University of Tsukuba, Tsukuba; Hiroyasu Iso, Tetsuya Ohira, Hironori Imano, Renzhe Cui, Ai Ikeda, Hiroyuki Noda, Satoyo Ikehara, Isao Muraki, and Yuji Shimizu, Osaka University, Suita; Yoshinori Ishikawa, Akihiko Kitamura, Masahiko Kiyama, Masakazu Nakamura, MD, Takeo Okada, Kenji Maeda, Masatoshi Ido, Masakazu Nakamura, PhD, Masamitsu Konishi, Takashi Shimamoto, Minoru Iida, and Yoshio Komachi, Osaka Medical Center for Health Science and Promotion, Osaka; Shinichi Sato, Chiba Prefectural Institute of Public Health, Chiba; Yoshihiko Naito, Mukogawa Women's University, Nishinomiya; Hideki Ozawa, Senri‐Kinran University, Suita; Takeshi Tanigawa, Isao Saito, Susumu Sakurai, and Shinichi Hitsumoto, Ehime University, Tōon; and Masayuki Yao, Ranryoen Hospital, Ibaraki.
Sources of Funding
This work was supported by a Grant‐in‐Aid from the Ministry of Health, Labour and Welfare, and Health and Labour Sciences Research Grants, Japan (Research on Health Services: Intractable Diseases Conquest Research: H21‐Nanchi‐Ippan‐059, Intractable Diseases Conquest Research: H22‐Nanchi‐Ippan‐144, and Intractable Diseases Conquest Research: H23‐Nanchi‐Ippan‐144).
Disclosures
None.
Acknowledgments
The authors would like to thank the staff and participants of CIRCS for their important contributions.
(J Am Heart Assoc. 2016;5:e002899 doi: 10.1161/JAHA.115.002899)
References
- 1. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiolographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. [DOI] [PubMed] [Google Scholar]
- 2. Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA; Study Group on the Molecular Basis of Arrhythmias of the European Society of Cardiology . Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. [DOI] [PubMed] [Google Scholar]
- 3. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademancee K, Perez Riera AR, Shimizu W, Schulze‐Bahr E, Tan H, Wilde A. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. [DOI] [PubMed] [Google Scholar]
- 4. Furuhashi M, Uno K, Tsuchihashi K, Nagahara D, Myakukoku M, Ohtomo T, Satoh S, Nishimiya T, Shimamoto K. Prevalence of asymptomatic ST segment elevation in right bundle branch block (Brugada‐type ST shift) among the general Japanese population. Heart. 2001;86:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atarashi H, Ogawa S, Harumi K, Sugimoto T, Inoue H, Murayama M, Toyama J, Hyakawa H; Idiopathic Ventricular Fibrillation Investigators . Three‐year follow‐up of patients with right bundle branch block and ST segment elevation in the right precordial leads. J Am Coll Cardiol. 2001;37:1916–1920. [DOI] [PubMed] [Google Scholar]
- 6. Hermida J, Lemoine J, Aoun F, Jarry G, Rey J, Quiret J. Prevalence of the Brugada syndrome in an apparantly healthy population. Am J Cardiol. 2000;86:91–94. [DOI] [PubMed] [Google Scholar]
- 7. Junttila M, Raatikainen M, Karjalainen J, Kauma H, Kesaniemi Y, Huikuri H. Prevalence and prognosis of subjects with Brugada‐type ECG pattern in a young and middle‐aged Finnish population. Eur Heart J. 2004;25:874–878. [DOI] [PubMed] [Google Scholar]
- 8. Lee C, Soni A, Tate R, Cuddy T. The incidence and prognosis of Brugada electrocardiographic pattern in the Manitoba Follow‐Up Study. Can J Cardiol. 2005;21:1286–1290. [PubMed] [Google Scholar]
- 9. Gallagher M, Forleo G, Behr E, Magliano G, Luca L, Morgia V, Lieberato F, Romeo F. Prevalence and significance of Brugada‐type ECG in 12,012 apparently healthy European subjects. Int J Cardiol. 2008;130:44–48. [DOI] [PubMed] [Google Scholar]
- 10. Tohyo Y, Nakazawa K, Takenobu H, Akagi T, Miyake H, Murayama M. A survey in the incidence of right bundle branch block with ST elevation among normal population. Jpn J Electrocardiol. 1995;15:223–226. [Google Scholar]
- 11. Matsuo K, Akahoshi M, Nakashima E, Suyama A, Seto S, Hayano M, Yano K. The prevalence, incidence and prognostic value of the Brugada‐type electrocardiogram: a population‐based study of four decades. J Am Coll Cardiol. 2001;38:765–770. [DOI] [PubMed] [Google Scholar]
- 12. Tsuji H, Sato T, Morisaki K, Iwasaka T. Prognosis of subjects with Brugada‐type electrocardiogram in a population of middle‐aged Japanese diagnosed during a health examination. Am J Cardiol. 2008;102:584–587. [DOI] [PubMed] [Google Scholar]
- 13. Sakabe M, Fujiki A, Tani M, Nishida K, Mizumaki K, Inoue H. Proportion and prognosis of healthy people with coved or saddle‐back type ST segment elevation in the right precordial leads during 10 years follow‐up. Eur Heart J. 2003;24:1488–1493. [DOI] [PubMed] [Google Scholar]
- 14. Ito H, Yano K, Chen R, He Q, Curb J. Brugada‐type electrocardiogram in a population of middle‐aged Japanese‐American men with follow‐up of three decades. Am J Med Sci. 2006;331:25–29. [DOI] [PubMed] [Google Scholar]
- 15. Kamakura T, Kawata H, Nakajima I, Yamada Y, Miyamoto K, Okamura H, Noda T, Satomi K, Aiba T, Takaki H, Aihara N, Kamakura S, Kimura T, Shimizu W. Significance of non‐type 1 anterior early repolarization in patients with inferolateral early repolarization syndrome. J Am Coll Cardiol. 2013;62:1610–1618. [DOI] [PubMed] [Google Scholar]
- 16. Kirkendall W, Feinleb M, Freis E, Mark A. Recommendations for human blood pressure determination by sphygmomanometers. Subcommittee of the AHA Postgraduate Eduation Committee. Circulation. 1980;62:1146A–1155A. [PubMed] [Google Scholar]
- 17. Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, MA: John Wright‐PSG, Inc.; 1982. [Google Scholar]
- 18. Ohira T, Maruyama M, Imano H, Kitamura A, Kiyama M, Okada T, Maeda K, Yamagishi K, Noda H, Cui R, Masuda S, Kimura H, Tachikawa K, Ishikawa Y, Iso H. Risk factors for sudden cardiac death among Japanese: the Circulatory Risk in Communities Study. J Hypertens. 2012;30:1137–1143. [DOI] [PubMed] [Google Scholar]
- 19. Gervacio‐Domingo G, Isidro J, Tirona J, Gabriel E, David G, Amarillo M, Morales D, Dans A. The Brugada type 1 electrocardiographic pattern is common Filipinos. J Clin Epidemiol. 2008;61:1067–1072. [DOI] [PubMed] [Google Scholar]
- 20. Sinner M, Pfeufer A, Perz S, Schulze‐Bahr E, Mönnig G, Eckardt L, Beckmann B, Wichmann H, Greithardt G, Steinbeck G, Fabritz L, Kääb S, Kirchhof P. Spontaneous Brugada electrocardiogram pattern are rare in the German general population: results from the KORA study. Europace. 2009;11:1338–1344. [DOI] [PubMed] [Google Scholar]
- 21. Shimizu W, Matsuo K, Kokubo Y, Satomi K, Kurita T, Noda T, Nagaya N, Suyama K, Aihara N, Kamakura S, Inamoto N, Akahoshi M, Tomoike H. Sex hormone and gender difference–role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:415–421. [DOI] [PubMed] [Google Scholar]
- 22. Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquié J, Nogami A, Babuty D, Yli‐Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Larcoix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O'Neill M, Hocini M, Lim K, Knecht S, Veenhuzen G, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein G, Clémenty J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. [DOI] [PubMed] [Google Scholar]
- 23. Kamakura S, Ohe T, Nakazawa K, Aizawa Y, Shimizu A, Horie M, Ogawa S, Okumura K, Tsuchihashi K, Sugi K, Makita N, Hagiwara N, Inoue H, Atarashi H, Aihara N, Shimizu W, Kurita T, Suyama K, Noda T, Satomi K, Okamura H, Tomoike H; Japan BSIi . Long‐term prognosis of probands with Brugada‐pattern ST‐elevation in leads V1‐V3. Circ Arrhythm Electrophysiol. 2009;2:495–503. [DOI] [PubMed] [Google Scholar]
- 24. Tikkanen J, Anttonen O, Junttila J, Aro A, Kerola T, Rissanen H, Reunanen A, Huikuri H. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. [DOI] [PubMed] [Google Scholar]
- 25. Nam G, Ko K, Kim J, Park K, Rhee K, Choi K, Kim Y, Antzelevitch C. Mode of onset of ventrctricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haruta D, Matsuo K, Tsuneto A, Ichimaru S, Hida A, Sera N, Imaizumi M, Nakashima E, Maemura K, Akahoshi M. Incidence and prognostic value of early repolarization pattern in the 12‐lead electrocardiogram. Circulation. 2011;123:2931–2937. [DOI] [PubMed] [Google Scholar]
- 27. Sekiguchi Y, Aonuma K, Takagi M, Aihara N, Yokoyama Y, Hiraoka M. New clinical and electrocardiographic classification in patients with idiopathic ventricular fibrillation. J Cardiovasc Electrophysiol. 2013;24:902–908. [DOI] [PubMed] [Google Scholar]
- 28. Junttila MJ, Tikkanen JT, Porthan K, Oikarinen L, Jula A, Kenttä T, Salomaa V, Huikuri HV. Relationship between testosterone level and early repolarization on 12‐lead electrocardiograms in men. J Am Coll Cardiol. 2013;62:1633–1634. [DOI] [PubMed] [Google Scholar]
- 29. Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, Sager SJ, Rissanen HA, Myerburg RJ, Reunanen A, Huikuri HV. Early repolarization: electrocardiographic phenotypes associated with favorable long‐term outcome. Circulation. 2011;123:2666–2673. [DOI] [PubMed] [Google Scholar]
- 30. Tokashiki T, Muratani A, Kimura Y, Muratani H, Fukiyama K. Sudden death in the general population in Okinawa: incidence and cause of death. Jpn Circ J. 1999;63:37–42. [DOI] [PubMed] [Google Scholar]
- 31. Miyazaki S, Shah A, Haïssagurerre M. Early repolarization syndrome—a new electrical disorder associated wtih sudden cardiac death. Circ J. 2010;74:2039–2044. [DOI] [PubMed] [Google Scholar]
- 32. Yamagishi K, Ikeda A, Iso H, Inoue M, Tsugane S; JPHC Study Group . Self‐reported stroke and myocardial infarction had adequate sensitivity in a population‐based prospective study JPHC (Japan Public Health Center)‐based Prospective Study. J Clin Epidemiol. 2009;62:667–673. [DOI] [PubMed] [Google Scholar]
- 33. Shimeno K, Takagi M, Maeda K, Tatsumi H, Doi A, Yoshiyama M. Usefulness of multichannel Holter ECG recording in the third intercostal space for detecting type 1 Brugada ECG: comparison with repeated 12‐lead ECGs. J Cardiovasc Electrophysiol. 2009;20:1026–1031. [DOI] [PubMed] [Google Scholar]


