Abstract
Background
Rehospitalizations following acute myocardial infarction for unplanned coronary revascularization and unstable angina (UA) are often included as parts of composite end points in clinical trials. Although clearly costly, the clinical relevance of these individual components has not been described.
Methods and Results
Patients enrolled in a prospective, 24‐center, US acute myocardial infarction registry were followed for 1 year after an acute myocardial infarction for rehospitalizations, that were independently adjudicated by experienced cardiologists. Patients who did and did not experience UA or revascularization rehospitalization were propensity matched using greedy matching. Among 3283 patients with acute myocardial infarction who were included, mean age was 59 years, 33% were female, and 70% were white. Rehospitalization rates for UA and unplanned revascularization at 1 year were 5.0% and 4.1%, respectively. After propensity matching, we included 2433 patients in the UA rehospitalization group and 2410 in the unplanned revascularization group. Using weighted proportional hazards Cox regression, there was no significant association between a rehospitalization for UA and 5‐year all‐cause mortality (9.6% versus 13.8%; adjusted hazard ratio 0.87, 95% CI 0.60–1.16). Patients rehospitalized for unplanned revascularization had a lower 5‐year mortality risk (7.0% versus 15.1%; hazard ratio 0.68, 95% CI 0.50–0.92) compared with those without such rehospitalizations. Nevertheless, patients with UA and unplanned revascularization had a substantially greater hazard of subsequent rehospitalizations compared with patients without such events (UA: hazard ratio 4.36, 95% CI 3.48–5.47; revascularization: hazard ratio 4.38, 95% CI 3.53–5.44).
Conclusions
Rehospitalizations for UA and unplanned revascularization in the year after an acute myocardial infarction are associated with higher risks of subsequent rehospitalizations but not with mortality.
Keywords: morbidity/mortality, myocardial infarction
Subject Categories: Acute Coronary Syndromes, Coronary Artery Disease, Mortality/Survival
Introduction
Rehospitalizations after acute myocardial infarction (AMI) have attracted major attention by payors and regulators in recent years and now are considered a marker of poor health care quality.1 Although the economic implications of rehospitalizations are indisputable,2 little is known about their clinical impact on patients. In particular, the association between these readmissions and subsequent mortality and morbidity is not known. Given that rehospitalizations for unstable angina (UA) and coronary revascularization are often included as parts of composite end points in clinical trials, better defining their clinical importance in terms of mortality and recurrent events is important.3, 4 Underscoring the heterogeneity of clinical significance for different components of composite clinical end points, a recent study asking clinical trialists and patients to rank individual components of composite end points in terms of their perceived importance found that revascularizations and rehospitalizations were considered less important than the other end points, such as mortality, stroke, and myocardial infarction.3, 5 As such, a better understanding of the clinical importance of rehospitalizations for UA and revascularization could inform the design of composite end points and support the interpretation of studies using these events as part of their primary outcome. To address this gap in knowledge, we sought to examine the association between rehospitalizations for recurrent ischemic coronary events and subsequent mortality and rehospitalizations.
Methods
Study Protocol
The analytic cohort for this study was derived from the TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction patients' Health Status) registry. TRIUMPH is a prospective multicenter observational registry that enrolled 4340 AMI patients from 24 US medical centers between April 11, 2005, and December 31, 2008.6 Eligible patients were aged ≥18 years with a diagnosis of AMI within 24 hours of admission. AMI was defined as elevated cardiac biomarkers and additional clinical evidence supporting the diagnosis of AMI, including electrocardiographic ST changes or clinical signs and symptoms of ischemia. Baseline data were obtained through chart abstraction and a standardized in‐person interview by trained research staff during the index AMI admission. Institutional review board approval was obtained at each participating center, and all patients signed an informed consent for baseline and follow‐up interviews. Patients were also asked to consent to medical record abstractions of hospitalizations over the next year following the index AMI.
Follow‐up and Definition of Exposure
Follow‐up telephone interviews were attempted for all survivors at 1, 6, and 12 months after the index AMI. During these follow‐up interviews, patients were asked to report interval events (eg, procedures, diagnostic tests, hospitalizations, and outpatient visits) since their last study contact. If a patient reported being hospitalized since the previous interview and had consented to medical record review, records of that hospitalization were obtained to classify cardiovascular events. Chart abstractions were sent to 2 cardiologists who independently classified the reason for hospitalization. If there was disagreement between the 2 cardiologists, the record was adjudicated by a third cardiologist; if disagreement persisted, up to 5 cardiologists independently reviewed the charts until consensus was obtained. Patients who did not consent to medical record review or those for whom records were unable to be obtained were excluded from the study to avoid misclassification of rehospitalizations.
Our primary exposure variables were rehospitalizations for UA and unplanned revascularization. UA was defined, based on guidelines, as a hospitalization due to symptoms suggestive of ischemia that was of new onset, that was increasing in severity (ie, more frequent, longer in duration, or lower in threshold), or that occurred at rest.7 Hospitalizations with ischemic symptoms and biomarker positivity or ST‐segment elevations on ECG were excluded. An unplanned revascularization was defined as a revascularization procedure that was not planned at the time of the index AMI admission and that was not performed in the setting of a recurrent AMI (to avoid examining the clinical impact of recurrent AMIs). All staged percutaneous coronary interventions (PCIs) and elective coronary artery bypass grafting (CABG) surgeries performed within 1 month of the index AMI were excluded. Because both cohorts were set up separately, if a patient had admissions for both unplanned revascularization and UA, they were included in both cohorts.
Outcomes
Our outcomes of interest included all‐cause mortality and rehospitalization. Mortality was assessed over 5 years following the exposure event through a query of the Social Security Death Master File. Rehospitalization was defined as the first rehospitalization after the exposure event (ie, the UA or unplanned revascularization rehospitalization) that occurred within 1 year following the index AMI. These events were also determined through chart abstractions performed by independent cardiologists, as described earlier. In addition, we also examined time to first cardiovascular‐cause rehospitalization, which included admission for AMI, UA, heart failure, stroke, coronary revascularization, or other cardiovascular procedures (eg, implantable cardioverter‐defibrillator implantation, peripheral arterial procedures). A longer follow‐up period for mortality was selected to increase the number of events and thus to improve statistical efficacy. As part of the TRIUMPH registry, however, follow‐up data on rehospitalization was collected for only the 1 year after index AMI; therefore, longer term rehospitalization data were unavailable.
Covariates for Propensity Matching
We included a wide range of variables based on prior literature or clinical judgment to generate propensity‐matched cohorts. Demographic covariates included age; sex; race; and educational, marital, insurance, and work status. Clinical covariates included body mass index, prior AMI (before the AMI at index hospitalization), prior CABG, prior stroke or transient ischemic attack, prior stable angina, cancer, diabetes mellitus, hypertension, hypercholesterolemia, peripheral vascular disease, chronic kidney disease, dialysis, chronic heart failure, chronic lung disease, family history of coronary artery disease, depression, and baseline health status (as measured by Seattle Angina Questionnaire angina frequency and quality of life scores8 and Short Form 12 physical and mental component summary scores9). Index hospitalization clinical covariates included initial systolic blood pressure, initial heart rate, ST‐segment elevations on ECG, left ventricular systolic dysfunction (ejection fraction <40%), Global Registry of Acute Coronary Event discharge risk score,10 and highest serum troponin level. Finally, treatment characteristics during index AMI hospitalization included in‐hospital PCI and CABG and medications at admission and at discharge, including aspirin, beta blockers, statins, angiotensin antagonists, thienopyridines, and insulin.
Statistical Analysis
To account for differences in characteristics of patients with and without UA or unplanned revascularization rehospitalization, we needed to identify patients without UA or revascularization who were similar to those who experienced them. This was accomplished through the creation of propensity scores with UA and unplanned revascularization as the outcomes of interest. To do this, we calculated 2 propensity scores: (1) to be hospitalized for UA and (2) to be hospitalized for unplanned revascularization after AMI. Propensity score models included the covariates described earlier. Any missing data for baseline variables were imputed by sequential regression imputation incorporating all baseline variables. Site of initial hospitalization was included as a fixed effect to account for clustering of patients by site.
We then matched patients with UA rehospitalization to those without an admission for UA using greedy matching on the logit of the propensity score. The caliper width was chosen as 0.2 times the pooled standard deviation of the logit propensity scores for the groups. The same approach was used to develop propensity‐matched cohorts for those with and without unplanned coronary revascularization; therefore, 2 matched cohorts were created: (1) those with and without a UA rehospitalization and (2) those with and without a revascularization rehospitalization. Each patient with an exposure (ie, rehospitalization for UA or unplanned revascularization) was matched to many patients without exposure. The models were conditional on the matched pair, with weights developed from the number of nonexposures that were matched in a pair, so as not to overweight any individual pairing.11 Balance of baseline characteristics between matched cohorts was examined before and after matching using absolute standardized differences, with <10% considered good balance between groups.12
We then used these matched cohorts to examine the association of UA and revascularization hospitalizations with subsequent mortality and rehospitalization. For each of these analyses, time zero for the exposure groups (ie, patients with UA or unplanned revascularization readmissions) was discharge from the first UA or revascularization event. Time zero for the control groups (ie, patients without UA or unplanned revascularization readmission) was also matched such that it was the same date as patients in the exposure group. Subsequently, the patients were tracked forward from that time point for subsequent death or rehospitalization. Cox proportional hazards models were stratified by matched sets and used to examine this association. The proportional hazards assumption was evaluated and found to be valid for all models. Statistical significance was defined by P<0.05, and all analyses were performed with SAS version 9.2 (SAS Institute) and R version 2.11.1 (R Foundation for Statistical Computing).
Results
Patient Population
Between April 2005 and December 2008, 4340 patients with an AMI were enrolled in the TRIUMPH registry. We excluded patients who died within the first month and never had the opportunity for follow‐up (n=71). Of the remaining 4269 patients, 986 patients (23%) were subsequently excluded due to missing data, which was caused by loss to follow‐up (n=637), lack of patient consent for medical chart review (n=77), or hospitals not honoring patients' medical record release forms (n=272). The final analytic cohort consisted of 3283 patients (Figure 1). The mean age of the patients included in our matched cohort was 59 years, 33% were female, and 70% were white. The comorbidity burden was high, with 29% of patients having diabetes mellitus, 20% having prior myocardial infarction, 19% having prior PCI, and 11% having prior CABG. Table 1 shows the baseline characteristics of patients included in the analytic cohort compared with those with missing data. Patients with missing data tended to be younger, were more likely to be female and of nonwhite race, and had more comorbidities compared with patients in the analytic cohort.
Figure 1.
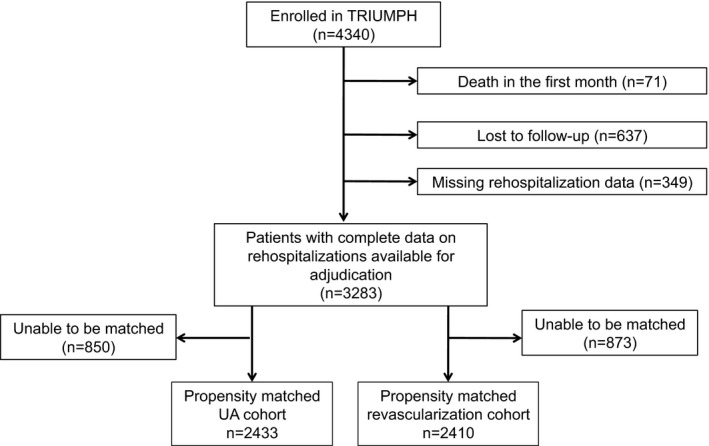
Study population. UA indicates unstable angina.
Table 1.
Baseline Characteristics of Study Cohort Compared With Excluded Patients
| Characteristic | Analytic Cohort (n=3283) | Missing Data (n=986) | P Value |
|---|---|---|---|
| Age, y (mean±SD) | 59.4±12.0 | 57.5±13.2 | <0.001 |
| Female sex | 32.7 | 35.4 | 0.11 |
| Race | <0.001 | ||
| White | 69.9 | 59.2 | |
| Black | 23.5 | 33.2 | |
| Other | 6.5 | 7.6 | |
| Married | 53.4 | 44.8 | <0.001 |
| High school education | 79.9 | 77.8 | 0.16 |
| Lack of insurance | 19.3 | 25.0 | <0.001 |
| Currently employed | 50.6 | 45.1 | 0.003 |
| Dyslipidemia | 49.4 | 48.0 | 0.43 |
| Hypertension | 66.1 | 67.7 | 0.34 |
| Prior CVA/TIA | 6.7 | 7.7 | 0.28 |
| Peripheral vascular disease | 4.7 | 4.6 | 0.90 |
| Diabetes mellitus | 29.0 | 35.8 | <0.001 |
| Prior myocardial infarction | 19.6 | 24.8 | <0.001 |
| Prior angina | 14.3 | 17.0 | 0.03 |
| Prior CABG | 10.6 | 13.6 | 0.01 |
| Prior PCI | 19.1 | 21.4 | 0.12 |
| Chronic kidney disease | 6.3 | 10.4 | <0.001 |
| Chronic lung disease | 7.0 | 7.8 | 0.40 |
| Chronic heart failure | 6.9 | 13.6 | <0.001 |
| History of malignancy | 7.3 | 6.7 | 0.53 |
| Current smoker | 38.4 | 43.2 | 0.01 |
| Depression | 7.5 | 8.6 | 0.26 |
| BMI, mean±SD | 29.6±6.5 | 29.5±6.5 | 0.64 |
| Family history of CAD | 74.7 | 71.3 | 0.04 |
| Initial systolic blood pressure, mm Hg (mean±SD) | 143±30 | 144±31 | 0.52 |
| Initial heart rate, bpm (mean±SD) | 82±22 | 85±23 | <0.001 |
| STEMI | 44.6 | 37.6 | <0.001 |
| In‐hospital CABG | 10.1 | 6.8 | 0.002 |
| In‐hospital PCI | 67.0 | 59.8 | <0.001 |
| GRACE 6‐month score, mean±SD | 99.9±28.9 | 100.9±33.1 | 0.36 |
| Highest serum troponin level, mean±SD | 29.3±75.6 | 25.7±61.4 | 0.17 |
| Aspirin at discharge | 95.0 | 92.3 | 0.001 |
| Beta blocker at discharge | 90.7 | 90.2 | 0.61 |
| Statin at discharge | 88.5 | 87.1 | 0.26 |
| ACEI/ARB at discharge | 74.6 | 74.7 | 0.91 |
Data are shown as percentages except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CVA, cerebrovascular accident; GRACE, Global Registry of Acute Coronary Event; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction; TIA, transient ischemic attack.
Among 3283 patients who were followed for 1 year after AMI, 140 patients (Kaplan–Meier estimate of 5.0%) were rehospitalized due to UA, with a median time to event of 4.1 months (interquartile range 1.2–7.3 months), and 113 patients (Kaplan–Meier estimate of 4.1%) were rehospitalized for unplanned coronary revascularizations, with a median time to event of 3.6 months (interquartile range 1.5–7.4 months). There were 56 patients who were admitted with both of these events and thus were included in both cohorts. The propensity‐matched cohorts for UA and unplanned coronary revascularization included 2433 and 2410 patients, respectively. There were no significant differences between the matched cohorts for either UA or revascularization, as suggested by small standardized differences between the groups (Table 2). Figure 2A shows the balance between patients with and without UA rehospitalization, and Figure 2B shows the balance between patients with and without unplanned coronary revascularization before and after propensity score matching.
Table 2.
Baseline Characteristics of Propensity‐Matched Cohorts
| Unstable Angina Cohort | Unplanned Revascularization Cohort | |||||
|---|---|---|---|---|---|---|
| UA (n=140) | No UA (n=2293) | Standardized Difference | Revasc (n=113) | No Revasc (n=2297) | Standardized Difference | |
| Age, y (mean±SD) | 58.1±11.6 | 59.3±11.9 | 3.44 | 59.4±10.7 | 59.6±11.9 | 3.11 |
| Female sex | 46.4 | 34.2 | 3.60 | 35.4 | 33.1 | 6.02 |
| Race | ||||||
| White | 65.7 | 71.9 | 2.98 | 78.8 | 72.8 | 2.5 |
| Black | 23.6 | 22.3 | 4.76 | 16.8 | 21.5 | 1.5 |
| Other | 10.7 | 5.8 | 12.99 | 4.4 | 5.7 | 4.9 |
| Married | 43.6 | 53.9 | 0.56 | 54.0 | 54.6 | 0.91 |
| High School Education | 85.0 | 82.6 | 6.30 | 87.6 | 82.2 | 2.29 |
| Lack of insurance | 24.3 | 17.7 | 2.46 | 17.7 | 16.9 | 4.13 |
| Currently employed | 42.9 | 51.6 | 3.01 | 46.0 | 51.9 | 0.54 |
| Dyslipidemia | 51.4 | 49.8 | 1.45 | 48.7 | 50.4 | 0.03 |
| Hypertension | 72.1 | 65.3 | 1.11 | 64.6 | 65.9 | 2.03 |
| Prior CVA/TIA | 7.9 | 6.4 | 5.3 | 5.3 | 6.4 | 4.52 |
| Peripheral vascular disease | 8.6 | 4.3 | 2.47 | 6.2 | 4.7 | 1.46 |
| Diabetes mellitus | 37.1 | 28.5 | 5.40 | 30.1 | 27.7 | 3.97 |
| Prior myocardial infarction | 22.9 | 18.8 | 1.17 | 17.7 | 18.7 | 0.63 |
| Prior CABG | 20.7 | 10.9 | 2.07 | 15.9 | 10.7 | 2.30 |
| Prior PCI | 29.3 | 19.2 | 3.33 | 23.0 | 19.1 | 1.30 |
| Chronic kidney disease | 7.1 | 6.0 | 0.11 | 4.4 | 5.1 | 0.66 |
| Chronic lung disease | 7.1 | 6.0 | 1.95 | 7.1 | 7.0 | 3.03 |
| Chronic heart failure | 7.1 | 6.3 | 4.17 | 4.4 | 5.6 | 1.50 |
| Current smoker | 42.1 | 37.6 | 2.49 | 29.2 | 36.7 | 3.77 |
| Depression | 10.7 | 8.2 | 0.34 | 5.3 | 7.5 | 0.91 |
| BMI, mean±SD | 29.5±7.5 | 29.7±6.4 | 2.21 | 29.0±6.2 | 29.6±6.5 | 0.14 |
| Systolic blood pressure, mm Hg (mean±SD) | 142±30 | 143±30 | 1.87 | 141±30 | 143±30 | 0.71 |
| Heart rate, bpm (mean±SD) | 82±21 | 81±22 | 1.34 | 79±19 | 81±22 | 0.24 |
| STEMI | 42.9 | 46.5 | 2.22 | 52.2 | 46.6 | 1.05 |
| In‐hospital CABG | 3.6 | 6.4 | 0.23 | 2.7 | 8.4 | 5.35 |
| In‐hospital PCI | 78.6 | 72.4 | 2.12 | 80.5 | 69.7 | 4.81 |
| GRACE score, mean±SD | 95.7±28.2 | 98.6±28.2 | 0.79 | 97.5±25.6 | 99.4±28.2 | 2.87 |
| Peak troponin, ng/dL | 40.6±138.2 | 31.0±74.1 | 4.45 | 39.1±140.9 | 29.8±69.8 | 1.36 |
| Aspirin at discharge | 94.3 | 94.7 | 1.94 | 95.6 | 95.5 | 1.06 |
| Beta blocker at discharge | 85.7 | 91.1 | 0.81 | 89.4 | 90.8 | 0.002 |
| Statin at discharge | 90.0 | 88.1 | 1.14 | 89.4 | 88.6 | 1.15 |
| ACEI/ARB at discharge | 82.9 | 76.8 | 2.82 | 83.2 | 75.4 | 6.61 |
Data are shown as percentages except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minutes; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; GRACE, Global Registry of Acute Coronary Event; PCI, percutaneous coronary intervention; Revasc, revascularization; STEMI, ST‐segment elevation myocardial infarction; TIA, transient ischemic attack.
Figure 2.
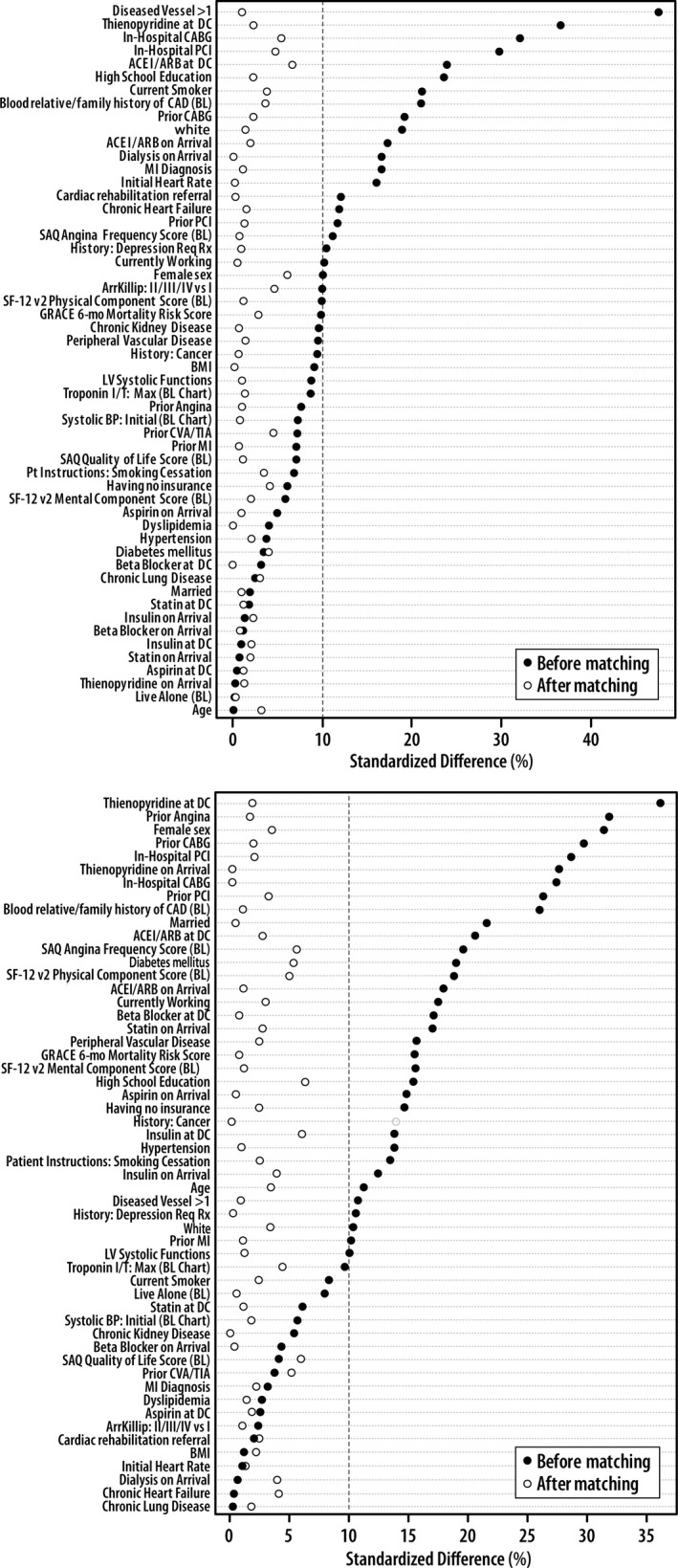
Assessment of balance before and after propensity matching between patients with and without unstable angina rehospitalizations (A) and unplanned coronary revascularization rehospitalizations (B). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ArrKillip, Killip class on arrival; BL, baseline; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CVA, cerebrovascular accident; DC, discharge; GRACE, Global Registry of Acute Coronary Event; LV, left ventricular; max, maximum; MI, myocardial infarction; Mo, month; PCI, percutaneous coronary intervention; Pt, patient; ReqRx, requiring treatment; SAQ, Seattle Angina Questionnaire; SF‐12, Short Form 12; TIA, transient ischemic attack.
All‐Cause Mortality
Among patients in the entire cohort, the Kaplan–Meier estimated 5‐year mortality rates were 13.7% among patients with UA rehospitalization compared with 14.6% among patients without UA rehospitalizations (unadjusted hazard ratio [HR] 0.81, 95% CI 0.62–1.07). The 5‐year mortality rates among patients with unplanned revascularization readmissions were 9.7% compared with 15.2% in those without such readmissions (unadjusted HR 0.61, 95% CI 0.45–0.82).
Among patients in the propensity‐matched cohorts, the Kaplan–Meier estimated 5‐year mortality rates (after the index rehospitalization event) were 9.6% versus 13.8% in patients with versus without a UA rehospitalization, respectively, and 7.0% versus 15.1% in patients with versus without unplanned revascularization rehospitalizations, respectively (log‐rank P=0.25 for UA rehospitalizations and P=0.003 for unplanned coronary revascularization) (Figure 3A and 3B). The hazard for all‐cause mortality did not differ between patients with and without UA rehospitalizations (HR 0.84, 95% CI 0.63–1.13). Patients with unplanned revascularization, however, were less likely to die over the following 5 years compared with those without revascularization (HR 0.62, 95% CI 0.45–0.84) (Table 3).
Figure 3.
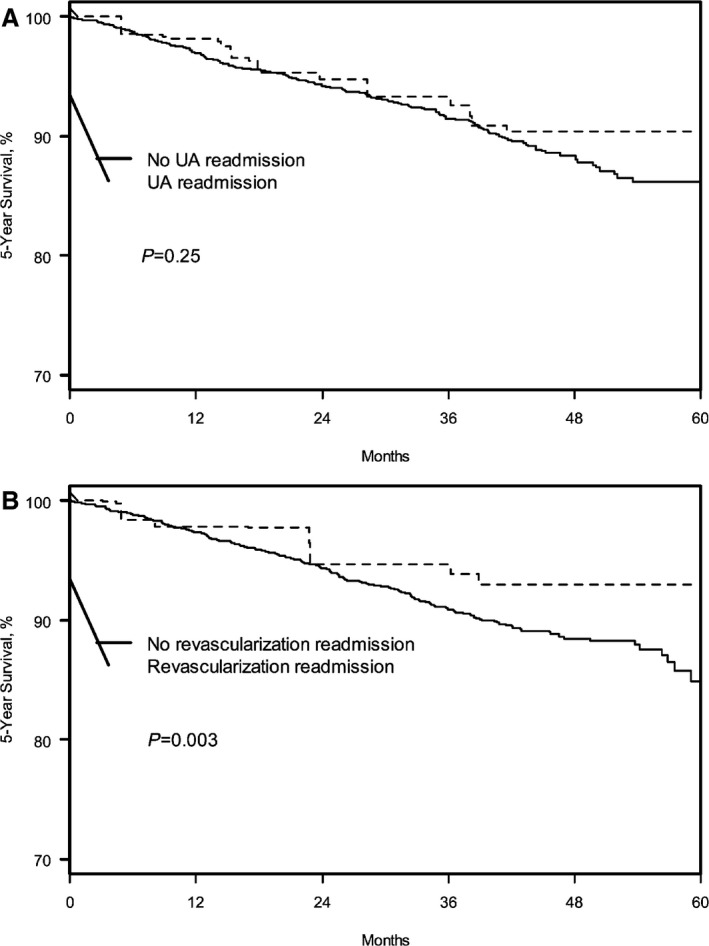
Kaplan–Meier curves for probability of 5‐year mortality in the propensity‐matched cohorts for UA rehospitalizations (A) and unplanned coronary revascularization (B). UA indicates unstable angina.
Table 3.
Association Between Rehospitalizations for Unstable Angina and Unplanned Coronary Revascularizations With Outcomes
| Unstable Angina Adjusted HR (95% CI) (n=2433) | Unplanned Revascularization Adjusted HR (95% CI) (n=2410) | |
|---|---|---|
| All‐cause mortality | 0.81 (0.62–1.07) | 0.61 (0.45–0.82) |
| Rehospitalization (all‐cause) | 3.99 (2.97–5.35) | 4.27 (3.23–5.66) |
| Rehospitalization (cardiac) | 6.40 (5.00–11.75) | 3.87 (2.56–5.86) |
HR, hazard ratio.
Rehospitalizations
In the year following index AMI, 53.2% of patients with UA readmissions had at least 1 subsequent rehospitalization compared with 15.3% of patients without UA readmissions (log‐rank P<0.001) (Figure 4A). In the adjusted analyses, we observed a 3.99 fold increased hazard of subsequent rehospitalization (95% CI 2.97–5.35). Similarly, there was a significant association between UA rehospitalization and subsequent first cardiovascular rehospitalization (36.8% versus 6.4%; HR 7.66, 95% CI 5.00–11.75). The most common reasons for rehospitalizations among patients who had a prior UA rehospitalization were repeated UA, noncardiac chest pain, and heart failure (Table 4).
Figure 4.
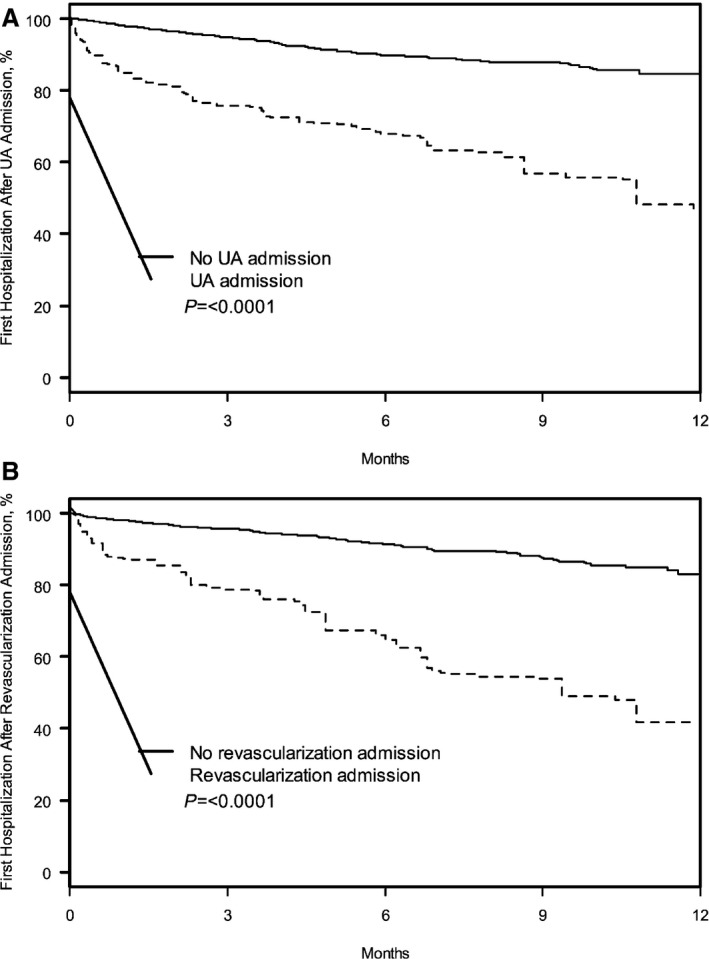
Kaplan–Meier curves for probability of all‐cause rehospitalizations in the propensity‐matched cohorts for UA rehospitalizations (A) and unplanned coronary revascularization (B). UA indicates unstable angina.
Table 4.
One‐Year Kaplan–Meier Estimates for Readmission Etiologies
| Readmission Cause | UAa | Revasca | ||
|---|---|---|---|---|
| Number of Subsequent Readmissions | UA n=66 | No UA n=229 | Revasc n=45 | No Revasc n=235 |
| Emergent | 84.8 | 77.7 | 88.9 | 86.8 |
| Noncardiac | 34.9 | 57.6 | 42.2 | 54.5 |
| Cardiac | 65.1 | 42.4 | 57.8 | 45.5 |
| Demand ischemia with positive enzymes | 0 | 1.3 | 0 | 0.8 |
| UA with ischemia | 4.5 | 0 | 2.2 | 0.4 |
| UA or chest pain without ischemia | 21.2 | 0 | 20.0 | 11.5 |
| Stable angina | 1.5 | 3.5 | 2.2 | 0.8 |
| Noncardiac chest pain | 15.8 | 17.5 | 20.0 | 22.4 |
| Subacute stent thrombosis | 0 | 0 | 0 | 0.8 |
| Heart failure | 15.8 | 14.9 | 6.7 | 12.3 |
| Arrhythmia | 3.0 | 4.4 | 2.2 | 2.1 |
| Bleeding | 1.5 | 0.4 | 2.2 | 3.4 |
| Elective cardiac catheterization | 3.0 | 8.3 | 2.2 | 1.7 |
| Syncope | 1.5 | 2.2 | 0 | 2.1 |
| Other cardiac reason | 3.0 | 2.6 | 2.2 | 4.3 |
Data are shown as percentages. Revasc indicates revascularization; UA, unstable angina.
Data were missing for 4.2% of patients in the UA cohort and 3.6% of patients in the unplanned revascularization cohort.
Among patients with unplanned coronary revascularization admission, 58.2% of patients had at least 1 subsequent all‐cause rehospitalization in the year following index AMI (after the unplanned revascularization) compared with 17.1% of patients without unplanned revascularization readmissions (log‐rank P<0.001) (Figure 4B). In adjusted analyses, we observed a 4.27‐fold increased hazard of subsequent rehospitalization (95% CI 3.23–5.66). In addition, there was also a significant association between unplanned coronary revascularization and subsequent first cardiovascular rehospitalization (27.6% versus 6.1%; HR 3.87, 95% CI 2.56–5.86). The most common reasons for rehospitalizations among patients who had a prior revascularization rehospitalization were UA and noncardiac chest pain (Table 4).
Discussion
In a large multicenter registry, we found that rehospitalizations for UA and unplanned coronary revascularization within the first year after an AMI were not associated with a higher risk of mortality. Nevertheless, these events were associated with a higher hazard for subsequent rehospitalizations, both all‐cause and cardiovascular. These findings suggest that a rehospitalization for UA or unplanned revascularization after an AMI is a marker for patients at very high risk of repeated hospitalizations. Further work is needed to illuminate strategies that can mitigate this risk to minimize the economic impact of these rehospitalizations,2 even if they are not associated with increased mortality.
Given the lack of proven clinical impact of UA and revascularization rehospitalizations, prior studies have raised concerns regarding the use of rehospitalizations both as a quality metric and as an outcome within composite end points for clinical trials.3, 4 Part of this concern stems from the knowledge that these events are, in part, determined by the actions of clinicians and patients rather than by the disease process alone and may introduce substantial bias when used as an outcome in clinical trials.13 In an analysis from the Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) trial, for example, CABG patients who required repeated revascularization had much worse angina and health status compared with PCI patients who had a repeat procedure, suggesting that there was a different threshold for reintervention depending on the patient's prior revascularization procedure.14 In contrast, outcomes such as death or myocardial infarctions, which are objective and quantifiable, are not subject to these potential biases. Further challenging the importance of these events, a recent study that asked patients and trialists to rank the importance of the components of composite end points used in cardiovascular clinical trials, rehospitalizations and revascularizations were ranked as least important by both patients and trialists.3, 5 Our study confirms that these events are likely not as clinically relevant as events such as myocardial infarctions and strokes, given that we did not find UA and revascularization events to be associated with an increased risk of mortality. Our study, however, provides new insights into the clinical implications of such rehospitalizations, particularly in identifying a group of patients at high risk for recurrent rehospitalizations. Given our prior work showing that UA and coronary revascularization rehospitalizations are also associated with impaired quality of life,15 interventions to prevent these rehospitalizations or to at least break the cycle of recurrent rehospitalizations are needed.
Understanding how to prevent recurrent rehospitalizations is of key importance to hospitals, which are being held increasingly accountable for these rehospitalizations. Our previous work has shown that the predictors of UA and revascularization rehospitalizations after an AMI fall into 1 of 2 categories: disease burden or psychosocial factors.16 It is not surprising that patients with a higher burden of cardiovascular disease (eg, prior CABG, prior PCI, peripheral artery disease) are more likely to be rehospitalized for recurrent cardiac events; however, knowing how to differentially affect disease progression in these high‐risk patients is difficult because aggressive secondary prevention efforts are indicated in all post‐AMI patients. The second group of factors that are associated with rehospitalizations (eg, younger age, female sex, uninsured, nonworking status) may help identify a group of patients for whom multidisciplinary efforts to reduce rehospitalizations could be useful. Interventions other than secondary prevention (eg, exercise, stress reduction, social work visits) might be useful in preventing the cycle of rehospitalizations, could have important economic and health status implications, and should be prospectively tested.
Our results also have an important implication for interpreting clinical trial results that frequently use composite clinical end points. Such composite end points frequently incorporate rehospitalizations due to UA and unplanned repeat coronary revascularizations. Composite end points help increase statistical efficiency of trials and decrease duration of follow‐up needed for such trials by increasing the number of clinical events.17 This has become increasingly more important as hard events such as mortality after AMI continue to decline, with the result that several trials reach statistical significance on their composite simply secondary to decreases in rehospitalization or revascularization rates.13 Consequently, interpreting such trial results is challenging because the clinical impact of these events has not been established, making the risk–benefit balance difficult to be appropriately weighed.17 Accordingly, our study results help establish the lack of clinical impact of these end points and, we believe, will help in interpreting clinical trial results.
The results of our study should be interpreted in the context of several potential limitations. First, we included only patients for whom all rehospitalizations were available for adjudication, and that limited our sample size and may have limited the generalizability of our results. Because the abstraction process was triggered by self‐reporting of rehospitalizations, this likely resulted in underreporting of events. Nevertheless, the adjudication process for identifying the UA and revascularization rehospitalizations increased the specificity of the defined hospitalizations, ensuring that we were examining the intended associations. Second, we were unable to provide longer term data on rehospitalizations beyond 1 year of follow‐up because they were not collected as part of the registry. Nonetheless, we provided 5‐year data on mortality, and that is comparable to the standard follow‐up period for most clinical trials. Finally, as with all other observational studies, we could not prove a causal association between UA and revascularization rehospitalizations and subsequent rehospitalizations, and the issue of unmeasured confounding remains. We believe that such rehospitalizations may be markers, rather than mediators, of subsequent events.
In conclusion, we found that patients who were rehospitalized for UA or unplanned coronary revascularization after an AMI were not at increased risk for subsequent mortality compared with patients without these rehospitalizations. These patients, however, were much more likely to be subsequently rehospitalized at some point over the same 12‐month period. These findings highlight that rehospitalizations for UA and unplanned coronary revascularizations are economically important and may support the use of rehospitalizations as an end point in clinical trials. More importantly they suggest that such rehospitalizations in the first year after AMI are markers of a cohort of patients at high risk for recurrent rehospitalizations. Future work is needed to understand how to break these cycles of rehospitalization, whether through aggressive secondary prevention efforts or potential psychosocial interventions that address noncardiac factors.
Sources of Funding
TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, and Blood Institute): Washington University School of Medicine SCCOR Grant #P50HL077113‐01. This project was funded through a research grant from Eli Lilly. The funding organizations and sponsors of this study had no impact on the design and conduct of the study; as well as collection, management, analysis, and interpretation of the data. Drs Shore and Arnold had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
Dr Zhao is a minor stock holder and employee of Eli Lilly. Dr Smolderen received research grant support from Eli Lilly. Dr Wang received research grant support to the Duke Clinical Research Institute from Astra Zeneca, Boston Scientific, Bristol Myers Squib, Daiichi Sankyo, Eli Lilly, Gilead Sciences, Glaxo Smith Kline and Regeron Pharmaceuticals with consulting fees from Astra Zeneca, Eli Lilly and Premier Inc. Dr Spertus received research grant support from Eli Lilly and owns the copyright to the Seattle Angina Questionnaire. The other authors have no relevant disclosures.
(J Am Heart Assoc. 2016;5:e003129 doi: 10.1161/JAHA.115.003129)
References
- 1. Centers for Medicare & Medicaid Services . Readmissions reduction program. Available at: http://www.Cms.Gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.Html. Accessed October 9, 2013.
- 2. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 3. Ferreira‐Gonzalez I, Busse JW, Heels‐Ansdell D, Montori VM, Akl EA, Bryant DM, Alonso‐Coello P, Alonso J, Worster A, Upadhye S, Jaeschke R, Schunemann HJ, Permanyer‐Miralda G, Pacheco‐Huergo V, Domingo‐Salvany A, Wu P, Mills EJ, Guyatt GH. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ. 2007;334:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montori VM, Permanyer‐Miralda G, Ferreira‐Gonzalez I, Busse JW, Pacheco‐Huergo V, Bryant D, Alonso J, Akl EA, Domingo‐Salvany A, Mills E, Wu P, Schunemann HJ, Jaeschke R, Guyatt GH. Validity of composite end points in clinical trials. BMJ. 2005;330:594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stolker JM, Spertus JA, Cohen DJ, Jones PG, Jain KK, Bamberger E, Lonergan BB, Chan PS. Rethinking composite end points in clinical trials: insights from patients and trialists. Circulation. 2014;130:1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA; Cardiovascular Outcomes Research C . Translational research investigating underlying disparities in acute myocardial infarction patients' health status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. [DOI] [PubMed] [Google Scholar]
- 8. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 9. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 10. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA; Investigators G . A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6‐month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. [DOI] [PubMed] [Google Scholar]
- 11. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One‐to‐many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69–80. [DOI] [PubMed] [Google Scholar]
- 12. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 13. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289:2554–2559. [DOI] [PubMed] [Google Scholar]
- 14. Arnold SV, Magnuson EA, Wang K, Serruys PW, Kappetein AP, Mohr FW, Cohen DJ. Do differences in repeat revascularization explain the antianginal benefits of bypass surgery versus percutaneous coronary intervention? Implications for future treatment comparisons. Circ Cardiovasc Qual Outcomes. 2012;5:267–275. [DOI] [PubMed] [Google Scholar]
- 15. Shore S, Li Y, Smolderen K, Jones PG, Arnold SV, Cohen DJ, Zhenxiang J, Wang TY, Ho PM, Spertus JA. Health status outcomes in acute myocardial infarction patients following rehospitalization for unplanned revascularization or unstable angina. Circ Cardiovasc Qual Outcomes. 2014;7:A283. [Google Scholar]
- 16. Arnold SV, Smolderen KG, Kennedy KF, Li Y, Shore S, Stolker JM, Wang TY, Jones PG, Zhao Z, Spertus JA. Risk factors for rehospitalization for acute coronary syndromes and unplanned revascularization following acute myocardial infarction. J Am Heart Assoc. 2015;4:e001352 doi: 10.1161/JAHA.114.001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomlinson G, Detsky AS. Composite end points in randomized trials: there is no free lunch. JAMA. 2010;303:267–268. [DOI] [PubMed] [Google Scholar]


