Abstract
Background
Obstructive sleep apnea (OSA) is an important risk factor for the development of cardiovascular diseases including myocardial infarction (MI). The aim of this study was to investigate the effects of OSA on prognosis after MI, and to determine which specific measures of OSA severity best predicted outcomes.
Methods and Results
We performed a prospective study, in which 112 patients without a prior diagnosis of sleep apnea underwent comprehensive polysomnography within a median of 7 days after MI. Patients were followed up at 6‐monthly intervals (±2 weeks) for a total of 48 months. Patients classified with central apnea (n=6) or those using continuous positive airway pressure (n=8) after polysomnography were excluded from analyses. The primary end point was major adverse cardiac events, including death from any cause, recurrent MI, unstable angina, heart failure, stroke, and significant arrhythmic events. Forty of 98 patients (41%) had OSA (apnea‐hypopnea index ≥15 events/h). OSA patients had higher major adverse cardiac event rates when compared to those without OSA (47.5% versus 24.1%; χ2=5.41, P=0.020). In a multivariate model that adjusted for clinically relevant variables including age, left ventricular ejection fraction, diabetes mellitus, oxygen desaturation index, and arousal index, significant hypoxemia, as defined by nocturnal nadir oxygen saturation ≤85%, was an independent risk factor for major adverse cardiac events (hazard ratio=6.05, P=0.004) in follow‐up 15 months after baseline.
Conclusions
Nocturnal hypoxemia in OSA is an important predictor of poor prognosis for patients after MI. These findings suggest that routine use of low‐cost nocturnal oximetry may be an economical and practical approach to stratify risk in post‐MI patients.
Keywords: hypoxemia, major adverse cardiac event, myocardial infarction, obstructive sleep apnea
Subject Categories: Acute Coronary Syndromes, Risk Factors
Introduction
Obstructive sleep apnea (OSA) is an important risk factor for coronary artery disease.1 Although seldom recognized by clinicians, the prevalence of OSA in patients hospitalized for myocardial infarction (MI) is >60% using an apnea‐hypopnea index (AHI) ≥5 events/h,2 and >40% for an AHI ≥15 events/h.2, 3 OSA has been reported as an independent risk factor for the development of MI and for sudden cardiac death.4, 5
OSA may affect cardiovascular risk by several pathophysiological mechanisms, including sleep fragmentation, negative intrathoracic pressure, hypercapnia, and repetitive intermittent hypoxemia. Among these, hypoxemia is thought to be the primary factor in mediating cardiovascular damage.6 The unique oxygen desaturation pattern seen in OSA produces tissue hypoxemia and re‐oxygenation similar to ischemia–reperfusion injury. Low oxygen saturation has been associated with impaired endothelial function,7 sympathetic activation,8 inflammation,9 hypercoagulation,10 oxidative stress,11 and metabolic disorders,12 all of which may increase the risk of MI. Clinically, nocturnal oxygen desaturation is independently associated with prevalence of cardiovascular diseases,13 correlates well with severity of coronary atherosclerosis using invasive coronary angiography,14 and may be an independent predictor of mortality.15 Previous large‐scale studies found that decreases in nocturnal oxygen saturation16 and lowest (nadir) nocturnal oxygen saturation (minSaO2) <78%,5 but not AHI, were independently associated with an increased risk of incident atrial fibrillation and sudden cardiac death, respectively. In a study of MI patients, we reported that minSaO2, but not AHI, was independently associated with impaired endothelial function,7 a marker of cardiovascular risk.17
Currently, severity of OSA is categorized using AHI, which has been used to predict prognosis in coronary artery disease.4, 18, 19 To the best of our knowledge, no prior studies have addressed the effect of nocturnal hypoxemia on prognosis after MI. We tested the hypothesis that post‐MI patients with OSA have worse long‐term prognosis, and examined whether specific measures of OSA severity, such as AHI or hypoxemia, obtained using a fully attended overnight sleep study, would be predictive of risk.
Methods
Study Populations
We conducted a prospective study of patients admitted for newly diagnosed MI at Mayo Clinic, Rochester, Minnesota. The results of baseline assessments have already been published.7, 20, 21 The diagnosis of MI, made by the attending physician, was defined by the Joint European Society of Cardiology/American College of Cardiology Committee (2000) criteria for “acute, evolving or recent MI.”22 A total of 121 eligible patients were enrolled consecutively (Figure 1). Twenty‐three patients were excluded from the final analysis: 1 due to a prior diagnosis of OSA that was managed with continuous positive airway pressure (CPAP), 1 because of a late final diagnosis of pulmonary embolism and not MI before polysomnography (PSG), 7 patients did not complete PSG testing due to technical, logistical, or clinical reasons, 6 had central sleep apnea, and 8 OSA patients used CPAP after PSG. The final study population consisted of 98 patients who completed overnight PSG within a median of 7 days after MI onset. No patients had acute heart failure at baseline, and only 1 was diagnosed with stable chronic obstructive pulmonary disease. This study was approved by the Mayo Clinic Institutional Review Board, and all patients signed informed consent.
Figure 1.
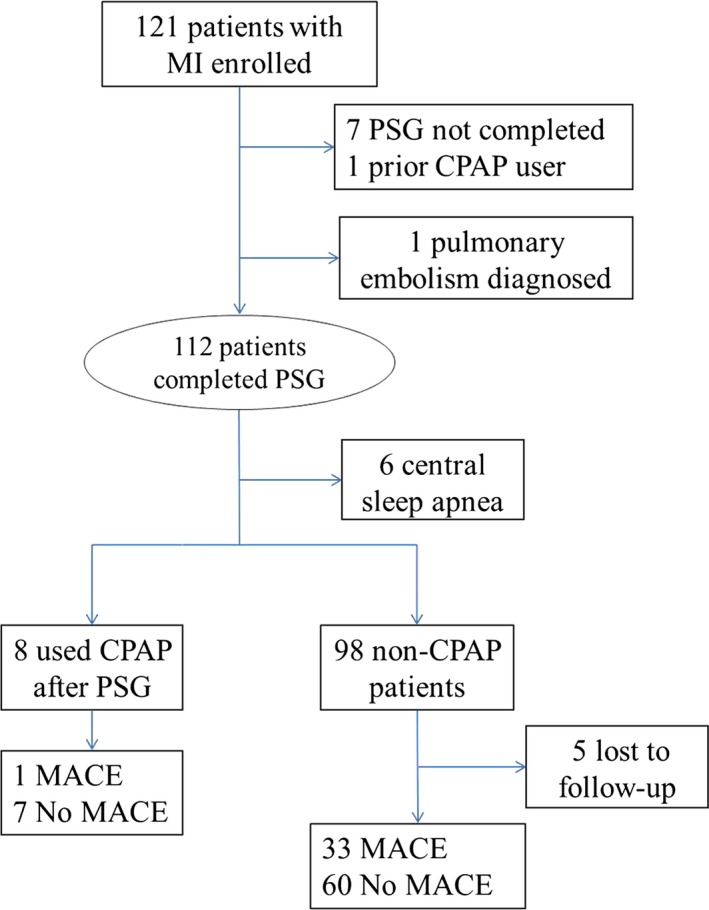
Flow chart of study progress. CPAP indicates continuous positive airway pressure; MACE, major adverse cardiac events; MI, myocardial infarction; PSG, polysomnography.
Management of MI
All patients received standard post‐MI care at the discretion of the attending clinician at Mayo Clinic. Eight patients did not undergo revascularization (percutaneous coronary intervention or coronary artery bypass grafting) or thrombolysis.
Polysomnography
Detailed PSG methods have been described previously.7, 20, 21 All patients underwent full‐night PSG between 10:00 pm and 6:00 am. PSG was recorded on an E‐Series Comprehensive Networked‐Linked Amplifier System for Sleep/EEG (Compumedics Ltd, Abbotsford, Victoria, Australia) or Siesta 802 wireless amplifier/recorder for ambulatory PSG and long‐term electroencephalography (Compumedics Ltd). All PSGs were recorded through PSG Online 2 recording software and scored online during data acquisition using Profusion PSG 2 software (Compumedics Ltd). Sleep stages, breathing events, oxygen desaturation, and arousals were scored by an experienced registered PSG technologist. Apneas were defined as a ≥90% decrease of airflow for at least 10 s (as viewed on the thermal airflow channel), and hypopneas were defined by a ≥30% decline in airflow for at least 10 s (as viewed on the nasal pressure channel) accompanied by an oxygen desaturation of ≥4%. Apneas without evidence of respiratory effort were scored as central, while those with respiratory effort were categorized as obstructive. After classification, frequency of disordered breathing events was determined by AHI (mean number of breathing events per hour), and patients with AHI ≥15 events/h were considered to have OSA. However, if >50% of these AHI events were central, then they were classified as central sleep apnea.23 Severity of hypoxemia was quantified by minSaO2, and hypoxemia duration was calculated as the percentage of total sleep time associated with saturation lower than 90% (T90SaO2).7 The oxygen desaturation index (ODI) was calculated as the number of times the oxygen saturation dropped by ≥4% from baseline, per hour of sleep.
Follow‐Up and End Point Definitions
Follow‐up contact was made via mail or telephone every 6 months (±2 weeks) to ascertain vital status and incidence of the outcome variables. In addition, the subjects’ Social Security and (for those over 65 years) Medicare numbers were obtained to assist with determining vital status of subjects who were lost to follow‐up using the state health department, National Death Index, and Social Security Administration records.
The primary composite end points were major adverse cardiac events (MACE),24 including all‐cause mortality, re‐admission for recurrent nonfatal MI, hospitalized unstable angina leading to revascularization or not, hospitalized heart failure, stroke, and significant arrhythmic events (sustained ventricular tachycardia, ventricular fibrillation, or asystole). The diagnosis of unstable angina was based on new onset, worsening, or resting angina associated with evidence of ST‐segment elevation or depression without evidence of myocardial necrosis. Significant arrhythmias were detected and documented by emergency medical services, in the hospital, or by internal cardioverter defibrillators.
Patients with an AHI ≥5 events/h were advised to seek formal clinical assessment. When CPAP therapy was subsequently initiated by the treating physicians, these patients were followed up including obtaining basic usage data from devices.
Statistical Analysis
All 98 patients included in the final analysis were divided into OSA and non‐OSA groups. Shapiro–Wilk test was used to assess continuous variables for normality. Because normal distribution was not seen in every subgroup, except for age, Wilcoxon tests were used for comparison for all continuous variables, while Pearson's χ2 tests were used for all dichotomous variables. Continuous variables were described as medians with interquartile range, whereas dichotomous variables were described as frequency and percentage.
To minimize the influence of potential benefit of CPAP for post‐MI patients, CPAP users after PSG testing were not included in the follow‐up analysis. Survival was calculated by the Kaplan–Meier product limit method as the time from the initial sleep study to MACE, with data censored at the time of the last available follow‐up. The log‐rank test was used to assess difference. Multivariable Cox proportional hazards models were applied for further investigation of independent risk factors of end points, adjusting for age, left ventricular ejection fraction, diabetes mellitus, ODI, and arousal index. The appropriateness of the proportional hazards assumption was examined using graphical methods and tested by the method of Grambsch and Therneau25 using STATA 12 (Stata Corp, College Station, TX). Proportional hazard assumptions were violated in 3 of the 4 models. Covariates that failed the assumption were modeled with a hazard for the first 15 months and ≥15 months, with 2 separate hazard functions to reflect their relationship with the outcome of interest. Analyses were performed using JMP, version 10 (SAS Institute, Cary, NC), and a P<0.05 was considered statistically significant.
Results
Forty patients (41%) of the 98 included in the follow‐up analysis had OSA. Baseline characteristics based on the presence or absence of OSA are shown in Table 1. Patients with OSA were older, had a higher incidence of diabetes mellitus, and lower left ventricular ejection fraction. As expected, patients with OSA exhibited lower minSaO2, higher T90SaO2, greater ODI, and more frequent arousals during sleep, compared to non‐OSA patients.
Table 1.
Clinical Characteristics of Patients With MI Based on the Presence or Absence of OSA (n=98)
| Clinical Characteristics | OSA (n=40) | Non‐OSA (n=58) | P Value |
|---|---|---|---|
| MinSaO2, % | 83 (79, 85) | 88 (85, 90) | <0.001 |
| T90SaO2, % | 8.8 (2.4, 32.6) | 0.4 (0, 1.5) | <0.001 |
| ODI, events/h | 24.2 (3.6, 42.3) | 3.4 (0, 14.8) | <0.001 |
| AI, events/h | 42.5 (33.1, 52.6) | 30.0 (20.5, 41.6) | <0.001 |
| Age, y | 68 (53, 80) | 56 (52, 66) | 0.008 |
| Male | 32 (80.0) | 44 (75.9) | 0.629 |
| Body mass index, kg/m2 | 29 (26, 33) | 28 (26, 32) | 0.334 |
| Fasting glucose, mmol/L | 6.3 (5.7, 7.5) | 6.1 (5.6, 7.1) | 0.425 |
| Peak creatine kinase MB isoenzyme, ng/mL | 92 (36, 157) | 100 (25, 202) | 0.980 |
| Total cholesterol, mmol/L | 4.5 (3.6, 5.0) | 4.3 (3.7, 5.2) | 0.592 |
| Triglyceride, mmol/L | 1.4 (0.9, 2.1) | 1.3 (0.8, 1.9) | 0.629 |
| High‐density cholesterol, mmol/L | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.903 |
| Low‐density cholesterol, mmol/L | 2.6 (1.9, 3.3) | 2.5 (2.0, 3.4) | 0.759 |
| LVEF, % | 50 (40, 56) | 58 (46, 63) | 0.027 |
| Diabetes mellitus | 12 (30.0) | 7 (12.1) | 0.027 |
| Hypertension | 24 (60.0) | 31 (53.4) | 0.521 |
| Prior myocardial infarction | 8 (20.0) | 8 (13.8) | 0.414 |
| Chronic heart failure | 2 (5) | 2 (3.4) | 0.703 |
| ST‐segment elevated myocardial infarction | 28 (70.0) | 43 (74.1) | 0.652 |
| Aspirin | 38 (95.0) | 54 (93.1) | 0.700 |
| Adenosine diphosphate receptor inhibitor | 31 (77.5) | 51 (87.9) | 0.170 |
| Statins | 38 (95.0) | 55 (94.8) | 0.970 |
| β‐Blockade | 39 (97.5) | 57 (98.3) | 0.790 |
| Angiotensin‐converting‐enzyme inhibitor/Angiotensin receptor blocker | 33 (82.5) | 45 (77.6) | 0.553 |
| Implantable cardioverter defibrillator | 2 (5.0) | 0 (0) | 0.085 |
Values are median (25th, 75th percentile) or n (%). AI indicates arousal index; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MinSaO2, minimum oxygen saturation during sleep; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; T90SaO2, percentage of total sleep time with saturation <90%.
Thirty‐three patients had MACE, including 1 death from esophageal cancer and 1 death from leukemia. Reinfarction was the most frequent MACE, occurring in 12 patients (12.2%), followed by hospitalization for heart failure (n=9 [9.2%]) and hospitalization for unstable angina (n=7 [7.1%]). Five (5.1%) patients had sudden cardiac arrest, with 4 resuscitated by implantable cardioverter defibrillator and/or manual defibrillation. Strokes occurred in 3 patients (3.1%). Seven (7.1%) patients died of cardiac diseases, including 1 death from sudden cardiac arrest. Fifteen patients (45.5%) had MACE within 15 months of follow‐up, and 18 (54.5%) patients had MACE after 15 months.
As shown in Figure 2A, patients with OSA had higher MACE rates than patients without OSA (47.5% versus 24.1%; χ2=5.41, P=0.020). To investigate the prognostic value of oxygen desaturation, patients were also divided into subgroups by median values of minSaO2 (85%) and T90SaO2 (1.3%), respectively. Patients with minSaO2 ≤85% and T90SaO2 ≥1.3% had higher MACE rates than patients with minSaO2 >85% (50% versus 16.7%; χ2=11.21, P<0.001) and T90SaO2 <1.3% (44.9% versus 22.5%; χ2=4.77, P=0.029) (Figure 2B and 2C, respectively).
Figure 2.
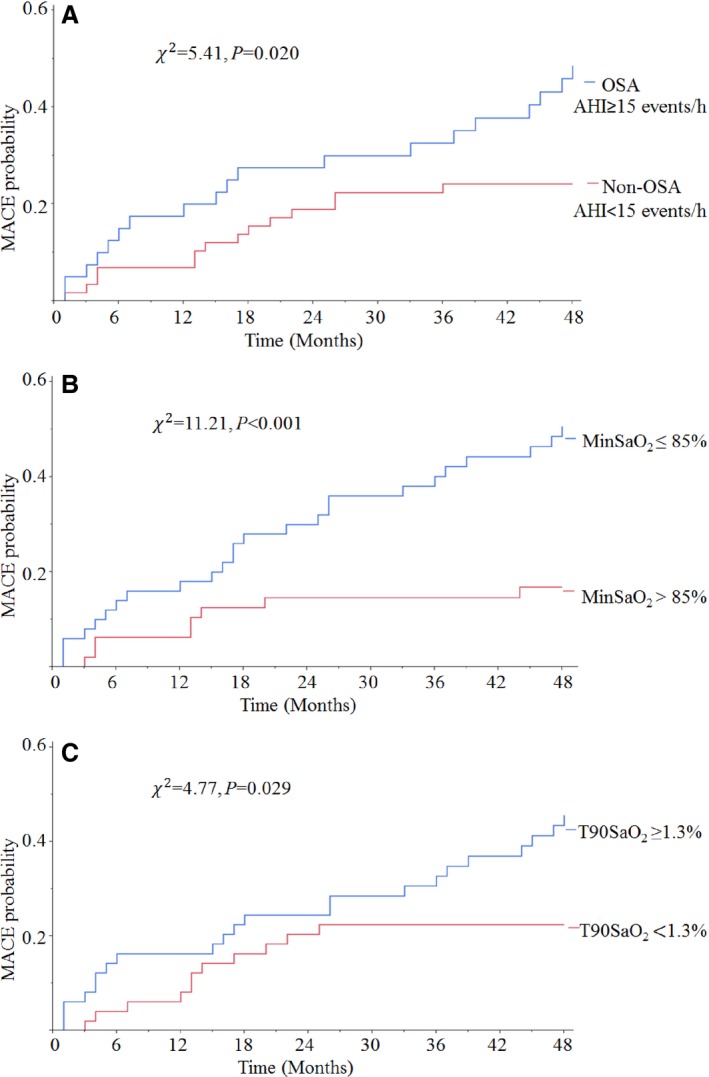
Kaplan–Meier curves of all MACE estimates after MI for non‐CPAP users included in follow‐up analysis (n=98). Cumulative incidence curves for MACE, according to the presence of OSA (A), level of minSaO2 (B) and T90SaO2 (C). χ2 and P‐values were calculated from log‐rank tests. AHI indicates apnea‐hypopnea index; CPAP, continuous positive airway pressure; MACE, major adverse cardiac events; MinSaO2, minimum oxygen saturation during sleep; OSA, obstructive sleep apnea; T90SaO2, percentage of total sleep time with saturation <90%.
Eight patients with OSA (median AHI=40.6 events/h and minSaO2=83%) received CPAP therapy. The end point of MACE was rare in CPAP users (1 patient out of 8), and no CPAP user died within the 48 months of follow‐up.
Variables that predicted MACE in Single Predictor Hazard Models are shown in Table 2. OSA patients had 2.22 times higher crude risk than patients without OSA (95% CI 1.12–4.51, P=0.023). Similarly, patients with minSaO2 ≤85% and T90SaO2 ≥1.3% had a higher risk (hazard ratio [HR]=3.57, 95% CI 1.68–8.46, P<0.001 and HR=2.19, 95% CI 1.08–4.69, P=0.029). After adjusting for clinically relevant variables including age, left ventricular ejection fraction, diabetes mellitus, ODI, and arousal index (Table 3), patients with significant hypoxemia (ie, those in the minSaO2 ≤85% group) had a greater than 6‐fold higher risk of MACE in the ≥15‐month period (minSaO2 model 1: HR=6.05, 95% CI 1.79–20.46, P=0.004). The prognostic value of OSA and T90SaO2 was not significant after adjusting for covariates (AHI model: HR=2.43, 95% CI 0.91–6.48, P=0.077; T90SaO2 model: HR=1.25, 95% CI 0.50–3.16, P=0.630, respectively). Of the covariates, age, ODI, and arousal index were not statistically significant risk factors (Table 2), while diabetes mellitus was significant in an unadjusted model, and left ventricular ejection fraction was found to be a significant independent risk factor in all models. The independent prognostic value of minSaO2 in the ≥15‐month period alone remains consistently strong even if AHI and the aforementioned variables were adjusted for (minSaO2 model 2: HR=6.05, 95% CI 1.80–20.36, P=0.004).
Table 2.
Risk of MACE for Non‐CPAP Users, Single Predictor Hazard Models (n=98)
| Covariates | HR | Unadjusted 95% CI | P Value |
|---|---|---|---|
| Age, per year | 1.02 | 0.99 to 1.05 | 0.126 |
| LVEF (per 1%) | 0.96 | 0.94 to 0.99 | 0.005 |
| Diabetes mellitus | 2.88 | 1.34 to 5.83 | 0.008 |
| ODI, per events/h | 1.01 | 0.99 to 1.03 | 0.148 |
| AI, per events/h | 1.02 | 1.00 to 1.04 | 0.07 |
| AHI, per events/h | 1.03 | 1.01 to 1.04 | 0.015 |
| OSA | 2.22 | 1.12 to 4.51 | 0.023 |
| MinSaO2 ≤85% | 3.57 | 1.68 to 8.46 | <0.001 |
| T90SaO2 ≥1.3% | 2.19 | 1.08 to 4.69 | 0.029 |
AHI indicates apnea‐hypopnea index; AI, arousal index; CPAP, continuous positive airway pressure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MinSaO2, minimum oxygen saturation during sleep; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; T90SaO2, percentage of total sleep time with saturation <90%.
Table 3.
Multivariate Analysis Addressing Variables Associated With MACE for Non‐CPAP Users (n=98)
| Covariates | HR | Adjusted a95% CI | P Value | |
|---|---|---|---|---|
| AHI model | ||||
| Diabetes mellitus | 1.96 | 0.77 to 4.99 | 0.160 | |
| LVEF (per 1%) | 0.96 | 0.93 to 0.99 | 0.014 | |
| ODI, per events/h | 1.01 | 0.99 to 1.03 | 0.395 | |
| AI, per events/h | 1.00 | 0.98 to 1.03 | 0.771 | |
| OSAb | <15 months | 0.12 | 0.01 to 1.23 | 0.075 |
| Age, per yearb | <15 months | 1.08 | 0.99 to 1.18 | 0.099 |
| OSAb | ≥15 months | 2.43 | 0.91 to 6.48 | 0.077 |
| Age, per yearb | ≥15 months | 0.97 | 0.93 to 1.01 | 0.133 |
| MinSaO2 model 1 | ||||
| Diabetes mellitus | 1.46 | 0.58 to 3.67 | 0.419 | |
| LVEF (per 1%) | 0.96 | 0.93 to 0.99 | 0.004 | |
| ODI, per events/h | 0.99 | 0.97 to 1.02 | 0.512 | |
| MinSaO2 ≤85%b | <15 months | 0.12 | 0.01 to 1.13 | 0.063 |
| Age, per yearb | <15 months | 1.08 | 0.99 to 1.18 | 0.073 |
| AI, per events/hb | <15 months | 0.98 | 0.92 to 1.05 | 0.574 |
| MinSaO2 ≤85%b | ≥15 months | 6.05 | 1.79 to 20.46 | 0.004 |
| Age, per yearb | ≥15 months | 0.96 | 0.92 to 1.00 | 0.034 |
| AI, per events/hb | ≥15 months | 1.02 | 0.99 to 1.05 | 0.221 |
| MinSaO2 model 2 | ||||
| Diabetes mellitus | 1.65 | 0.64 to 4.22 | 0.299 | |
| LVEF (per 1%) | 0.95 | 0.92 to 0.98 | 0.002 | |
| ODI, per events/h | 0.99 | 0.97 to 1.02 | 0.685 | |
| AHI, per events/h | 0.97 | 0.94 to 1.01 | 0.201 | |
| MinSaO2 ≤85%b | <15 months | 0.16 | 0.02 to 1.62 | 0.121 |
| Age, per yearb | <15 months | 1.09 | 0.99 to 1.19 | 0.066 |
| AI, per events/hb | <15 months | 0.99 | 0.93 to 1.06 | 0.848 |
| MinSaO2 ≤85%b | ≥15 months | 6.05 | 1.80 to 20.36 | 0.004 |
| Age, per yearb | ≥15 months | 0.96 | 0.92 to 1.00 | 0.035 |
| AI, per events/hb | ≥15 months | 1.02 | 0.99 to 1.05 | 0.239 |
| T90SaO2 model | ||||
| T90SaO2 ≥1.3% | 1.25 | 0.50 to 3.16 | 0.630 | |
| Diabetes mellitus | 1.89 | 0.69 to 4.69 | 0.203 | |
| Age, per year | 1.01 | 0.98 to 1.04 | 0.585 | |
| LVEF (per 1%) | 0.96 | 0.93 to 0.99 | 0.016 | |
| ODI, per events/h | 1.00 | 0.98 to 1.02 | 0.741 | |
| AI, per events/h | 1.00 | 0.98 to 1.03 | 0.867 | |
AHI indicates apnea‐hypopnea index; AI, arousal index; CPAP, continuous positive airway pressure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MinSaO2, minimum oxygen saturation during sleep; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; T90SaO2, percentage of total sleep time with saturation <90%.
Adjusted for age, LVEF, diabetes mellitus, ODI, and AI for AHI model, MinSaO2 model 1, and T90SaO2 model; adjusted for age, LVEF, diabetes mellitus, ODI, AI, and AHI for MinSaO2 model 2.
Variables deviated from the proportional hazards assumption and had HR reported for the follow‐up 15 months after baseline.
Discussion
This prospective study shows that the presence of OSA predicts the incidence of MACE in post‐MI patients in univariate analysis and that nocturnal hypoxemia is an independent prognostic marker in this patient population. To the best of our knowledge, this is the first prospective study investigating the prognostic value of the severity of OSA, as measured by indices of hypoxemia, in post‐MI patients. Our data are consistent with previous findings that OSA may increase the risk of adverse events after MI.4, 19 As the conventionally accepted standard to diagnose OSA, AHI is commonly used to predict outcomes related to the diagnosis.4, 18, 19, 26 Because hypoxemia may be the physiological consequence triggering most of the adverse effects caused by breathing events,6 indices of hypoxemia, such as minSaO2 and T90SaO2, could provide a better prognostic marker in this cohort.
As to the prognostic implications of hypoxemia, our results are consistent with previous studies examining the relationship between sleep measures and cardiac prognosis in non‐MI patients. In a retrospective study of 3542 subjects, the degree of nocturnal oxygen desaturation was independently associated with onset of atrial fibrillation,16 and in a study of 10 701 adults, nadir nocturnal oxygen saturation (<78%) was an independent risk factor for sudden cardiac death,5 while AHI was not identified as an independent predictor risk for incident atrial fibrillation or sudden cardiac death. Punjabi and colleagues showed that desaturation in the range of 4.0% to 4.9% was associated with prevalent cardiovascular disease, but the association between prevalent cardiovascular disease and oxygen desaturation <4% was not significant.13
Despite the high prevalence of OSA in patients after MI, it may not be economical or logistically feasible to obtain sleep studies in all these patients. Due to the prognostic value of nocturnal hypoxemia, overnight oximetry would potentially be a more cost‐effective widely applicable screening tool, with repeated oximetry during follow‐up easily measured to observe progression of OSA.
Although nocturnal hypoxemia from OSA predicts poor outcome, it is unknown whether patients with MI benefit from oxygen therapy. Breathing events and sleep fragmentation still occur in spite of the elevated oxygen saturation.27 Concerns regarding lengthened apnea duration and risk of hypercapnia have been raised.28, 29 Gottlieb and colleagues report that CPAP, rather than oxygen therapy, may reduce blood pressure in OSA patients, although patients with AHI >50 events/h or long duration of severe nocturnal hypoxemia (SaO2 <85% for >10% of sleep) were excluded from the study.30 On the other hand, it has been shown that sleep‐disordered breathing is common in patients with precapillary pulmonary hypertension,31 and nocturnal oxygen therapy seems to have a positive effect on prognostic markers and may improve outcomes in this patient population.32 Therefore, considering that CPAP may be intolerable for some patients with OSA, oxygen therapy may be an alternative therapeutic strategy to reduce severe hypoxemia and consequent target organ damage, although it may be prudent to consider oxygenating patients with persistently low oxygen saturation (<88%) after CPAP,33 or hypoxemia without apneic events. Despite our limited sample of CPAP users, our findings are consistent with a previous study showing that CPAP therapy may reduce the risk of recurrent MI and revascularization in OSA patients after MI.4
A potential cardioprotective role of intermittent ischemia from recurrent episodes of apneas during MI via preconditioning has been described.34 Although reduced myocardial injury verified by cardiac enzyme levels after MI in patients with OSA has been reported, untreated OSA may increase the risk of MI and recurrence of MI.4 Although survival advantage from even moderately elevated AHI has been seen in several cohorts, especially in older patients,35 this may be due to ascertainment bias. However, given the paucity of data on this subject, further investigation testing the hypothesis that severe desaturation and reoxygenation can be protective for post‐MI patients is needed.
Strengths of the current study include first, its prospective design. Second is the use of comprehensive, overnight PSG to detect the presence and severity of OSA and hypoxemia by technologists blinded to the follow‐up. Nevertheless, this study has limitations. First, because of the small sample size, we could only address a limited number of clinically relevant variables, which may decrease the generalizability of the prognostic value of nocturnal hypoxemia and OSA. Second, CPAP users were not included in survival analysis and multivariable Cox proportional hazards model because of the limited number of cases. Therefore, prognostic indices such as AHI, nocturnal hypoxemia, or other clinical factors could not be determined in this subgroup. Our data suggest a marked reduction in MACE in those patients receiving CPAP. However, whether CPAP therapy can improve long‐term prognosis in post‐MI patients with OSA is still to be determined. Third, it is possible that the prevalence and severity of OSA may change in the months to years following an MI.36 Hence, data acquired 7 days after a MI may not be representative of the severity of OSA for all patients over the subsequent 48 months. However, this does not contradict our finding that OSA‐induced hypoxemia, measured early after MI, is an important predictor of MACE over 4 years of follow‐up. Furthermore, it is possible that serial observation by oximetry may provide further information on cardiovascular risk.
Conclusions
In stable patients after MI, untreated OSA is associated with increased risk of adverse events, and nocturnal hypoxemia is an independent predictor of MACE. Despite the high prevalence of OSA in patients with MI, it may not be feasible to conduct full‐attended PSGs routinely. Overnight oximetry may be a more practical cost‐effective alternative approach to stratify risk of MACE after MI, and to identify those who warrant a full sleep study and who may potentially benefit from intervention.
Sources of Funding
This study was supported by funding from National Institutes of Health (NIH) Grants HL65176 and M01‐RR00585. This study was also supported by a gift to the Mayo Foundation by the Phillip Respironics Foundation.
Disclosures
Dr Somers has served as a consultant for ResMed, Phillips, GlaxoSmithKline, Respicardia, Rhonda Gray, Price Waterhouse Coopers, and U‐Health. He has spoken at meetings sponsored by Phillips and ResMed. He works with Mayo Health Solutions and their industry partners on intellectual property related to sleep and to obesity. Dr Kuniyoshi became a full‐time employee of Philips Respironics after the collection of the data provided in this article. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Acknowledgments
We would like to thank Debra L. Pfeifer and Ann B. Peterson for their superb secretarial and administrative assistance and Diane E. Davison, RN, MA, for her expertise in coordinating the studies.
(J Am Heart Assoc. 2016;5:e003162 doi: 10.1161/JAHA.115.003162)
References
- 1. Basner RC. Cardiovascular morbidity and obstructive sleep apnea. N Engl J Med. 2014;370:2339–2341. [DOI] [PubMed] [Google Scholar]
- 2. Konecny T, Kuniyoshi FH, Orban M, Pressman GS, Kara T, Gami A, Caples SM, Lopez‐Jimenez F, Somers VK. Under‐diagnosis of sleep apnea in patients after acute myocardial infarction. J Am Coll Cardiol. 2010;56:742–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee CH, Khoo SM, Tai BC, Chong EY, Lau C, Than Y, Shi DX, Lee LC, Kailasam A, Low AF, Teo SG, Tan HC. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–1495. [DOI] [PubMed] [Google Scholar]
- 4. Garcia‐Rio F, Alonso‐Fernández A, Armada E, Mediano O, Lores V, Rojo B, Fernández‐Lahera J, Fernández‐Navarro I, Carpio C, Ramírez T. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168:1328–1335. [DOI] [PubMed] [Google Scholar]
- 5. Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, Herges RM, Howard DE, Somers VK. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sert Kuniyoshi FH, Singh P, Gami AS, Garcia‐Touchard A, van der Walt C, Pusalavidyasagar S, Wright RS, Vasquez EC, Lopez‐Jimenez F, Somers VK. Patients with obstructive sleep apnea exhibit impaired endothelial function after myocardial infarction. Chest. 2011;140:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985). 1989; 67:2101–2106. [DOI] [PubMed] [Google Scholar]
- 9. Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, Le Jemtel TH. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips CL, McEwen BJ, Morel‐Kopp MC, Yee BJ, Sullivan DR, Ward CM, Tofler GH, Grunstein RR. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo‐controlled crossover study. Thorax. 2012;67:639–644. [DOI] [PubMed] [Google Scholar]
- 11. Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–1484. [DOI] [PubMed] [Google Scholar]
- 12. Levy P, Bonsignore MR, Eckel J. Sleep, sleep‐disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–260. [DOI] [PubMed] [Google Scholar]
- 13. Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep‐disordered breathing and cardiovascular disease: an outcome‐based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayashi M, Fujimoto K, Urushibata K, Uchikawa S, Imamura H, Kubo K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest. 2003;124:936–941. [DOI] [PubMed] [Google Scholar]
- 15. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep‐disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 17. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buchner S, Satzl A, Debl K, Hetzenecker A, Luchner A, Husser O, Hamer OW, Poschenrieder F, Fellner C, Zeman F, Riegger GA, Pfeifer M, Arzt M. Impact of sleep‐disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J. 2014;35:192–199. [DOI] [PubMed] [Google Scholar]
- 19. Lee CH, Khoo SM, Chan MY, Wong HB, Low AF, Phua QH, Richards AM, Tan HC, Yeo TC. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sert Kuniyoshi FH, Zellmer MR, Calvin AD, Lopez‐Jimenez F, Albuquerque FN, van der Walt C, Trombetta IC, Caples SM, Shamsuzzaman AS, Bukartyk J, Konecny T, Gami AS, Kara T, Somers VK. Diagnostic accuracy of the Berlin Questionnaire in detecting sleep‐disordered breathing in patients with a recent myocardial infarction. Chest. 2011;140:1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuniyoshi FH, Garcia‐Touchard A, Gami AS, Romero‐Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez‐Jimenez F, Somers VK. Day‐night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. [DOI] [PubMed] [Google Scholar]
- 23. Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. [DOI] [PubMed] [Google Scholar]
- 24. Ng LL, Sandhu JK, Narayan H, Quinn PA, Squire IB, Davies JE, Bergmann A, Maisel A, Jones DJ. Proenkephalin and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2014;63:280–289. [DOI] [PubMed] [Google Scholar]
- 25. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 26. Nakashima H, Katayama T, Takagi C, Amenomori K, Ishizaki M, Honda Y, Suzuki S. Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J. 2006;27:2317–2322. [DOI] [PubMed] [Google Scholar]
- 27. Loredo JS, Ancoli‐Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo‐CPAP‐controlled study. Sleep. 2006;29:564–571. [DOI] [PubMed] [Google Scholar]
- 28. Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta‐analysis. J Clin Sleep Med. 2013;9:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alford N, Fletcher E, Nickeson D. Acute oxygen in patients with sleep apnea and COPD. Chest. 1986;89:30–38. [DOI] [PubMed] [Google Scholar]
- 30. Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, Tracy RP, Rueschman M, Blumenthal RS, Lewis EF, Bhatt DL, Redline S. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jilwan FN, Escourrou P, Garcia G, Jaïs X, Humbert M, Roisman G. High occurrence of hypoxemic sleep respiratory disorders in precapillary pulmonary hypertension and mechanisms. Chest. 2013;143:47–55. [DOI] [PubMed] [Google Scholar]
- 32. Schumacher DS, Müller‐Mottet S, Hasler ED, Hildenbrand FF, Keusch S, Speich R, Bloch KE, Ulrich S. Effect of oxygen and acetazolamide on nocturnal cardiac conduction, repolarization, and arrhythmias in precapillary pulmonary hypertension and sleep‐disturbed breathing. Chest. 2014;146:1226–1236. [DOI] [PubMed] [Google Scholar]
- 33. Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, Parthasarathy S, Quan SF, Rowley JA; for the Positive Airway Pressure Titration Task Force, American Academy of Sleep Medicine . Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–171. [PMC free article] [PubMed] [Google Scholar]
- 34. Shah N, Redline S, Yaggi HK, Wu R, Zhao CG, Ostfeld R, Menegus M, Tracy D, Brush E, Appel WD, Kaplan RC. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2013;17:819–826. [DOI] [PubMed] [Google Scholar]
- 35. Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18:397–403. [DOI] [PubMed] [Google Scholar]
- 36. Buchner S, Greimel T, Hetzenecker A, Luchner A, Hamer OW, Debl K, Poschenrieder F, Fellner C, Riegger GA, Pfeifer M, Arzt M. Natural course of sleep‐disordered breathing after acute myocardial infarction. Eur Respir J. 2012;40:1173–1179. [DOI] [PubMed] [Google Scholar]


