Abstract
Background
Since the release of the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines, significant controversy has surrounded the applicability of the new cholesterol guidelines and the Pooled Cohort Equations. In this present study, we investigated whether eligibility for statin therapy determined by the 2013 ACC/AHA guidelines on the management of blood cholesterol is better aligned with the progression of coronary artery calcification (CAC) detected by coronary computed tomography angiography (CCTA) than the previously recommended 2004 National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III guidelines.
Methods and Results
We enrolled 1246 asymptomatic participants who underwent repeated CAC score measurement during routine health examinations. The CAC score progression was defined as either incident CAC in a population free of CAC at baseline or increase ≥2.5 units between the baseline and final square root of CAC scores participants who had detectable CAC at baseline examination. Application of the ACC/AHA guidelines to the study population increased the proportion of statin‐eligible subjects from 20.5% (according to ATP III) to 54.7%. Statin‐eligible subjects, as defined by ACC/AHA guidelines, showed a higher odds ratio for CAC score progression than those considered statin eligible according to ATP III guidelines (2.73 [95% CI, 2.07–3.61] vs 2.00 [95% CI, 1.49–2.68]).
Conclusions
Compared with the ATP III guidelines, the new ACC/AHA guidelines result in better discrimination of subjects with cardiovascular risk detected by CAC score progression in an Asian population.
Keywords: atherosclerosis, cardiovascular disease, cholesterol guidelines, coronary artery calcification, Pooled Cohort Equation
Subject Categories: Computerized Tomography (CT), Statements and Guidelines, Primary Prevention, Lipids and Cholesterol
In November 2013, with an update in 2014, the American College of Cardiology (ACC) and American Heart Association (AHA) released clinical practice guidelines for the treatment of blood cholesterol to reduce cardiovascular risk.1, 2 These guidelines aimed to replace the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III guidelines. Compared with the 2004 ATP III guidelines, which involved risk‐based low‐density lipoprotein cholesterol (LDL‐C) thresholds for treatment of coronary heart disease (CHD) alone, the ACC/AHA guidelines focus more on the treatment of absolute cardiovascular risk and was broadened to estimate the 10‐year risk of all atherosclerotic cardiovascular diseases (ASCVDs), including CHD and stroke, using Pooled Cohort Equations.1, 2 The application of the ACC/AHA guidelines largely expanded the number of adults endorsed for statin treatment attributed to a lower cut‐off point of 7.5%, and there have been debates about whether the ACC/AHA guidelines have set too low an ASCVD risk threshold for statin therapy eligibility.3, 4
A few studies have compared statin assignment between the ACC/AHA and ATP III guidelines in subjects with coronary atherosclerosis detected by coronary computed tomography angiography (CCTA).5, 6, 7, 8 Two studies performed the comparative analysis between these 2 guidelines in Korean population, and their results showed that the ACC/AHA was more sensitive in identifying subjects with subclinical atherosclerosis as assessed by coronary artery calcification (CAC) score than the previous ATP III guidelines.7, 8 However, these previous studies analyzed a single measurement of CAC score, and the alignment of the 2 guidelines for statin therapy with CAC score progression has not been previously reported. Although baseline CAC score measured by CCTA has been established as a surrogate marker of coronary atherosclerosis, recent studies have shown that CAC score progression is significantly related to an increased risk of future cardiovascular events and all‐cause mortality and a better predictor of cardiovascular events and prognosis than baseline CAC score.9, 10
In light of these findings, we sought to evaluate the accuracy of the ATP III and ACC/AHA guidelines in assigning statin therapy to subjects with CAC score progression, in an asymptomatic, middle‐aged, Korean population without history of CHD.
Methods
Study Population
The study population consisted of 7300 individuals who underwent baseline CCTA using a 64‐slice multidetector computed tomography (MDCT) scanner during routine health evaluations at Asan Medical Center (Seoul, Republic of Korea) between January 2007 and June 2011. Among these 7300 participants, repeated CCTA was performed in 1591 cases through December 2014. This analysis also used data obtained using in‐person follow‐up examinations after the baseline examinations. Each participant completed a questionnaire that covered the history of previous medical and/or surgical diseases, medications, and drinking and smoking habits. Drinking habits were categorized in terms of frequency per week (ie, ≤1 times/week or ≥2 times/week [moderate drinker]), smoking habits as noncurrent or current, and exercise habits as frequency per week (ie, ≤2 times/week or ≥3 times/week [physically active]).
Cardiovascular disease (CVD) history was based on each participant's history of physician‐diagnosed angina, myocardial infarction, and/or cerebrovascular accidents. Participants with diabetes mellitus were defined as those with a fasting plasma glucose (FPG) level of ≥7.0 mmol/L and/or a hemoglobin A1c (HbA1c) level ≥6.5%.11 In addition, participants who reported the use of antidiabetic medications on a self‐report questionnaire were considered to have diabetes mellitus.12 Hypertension was defined as systolic and/or diastolic blood pressure (BP) ≥140/90 mm Hg or the use of hypertensive medications.
We excluded participants with a history of CVD at baseline examinations (n=95), as well as participants receiving statins (n=238). Participants who underwent percutaneous coronary intervention (n=8) or coronary arterial bypass surgery (n=3) after the initial examinations were also excluded. Finally, subjects that were not between the ages of 20 and 79 years were excluded (n=3). Some participants met more than 2 exclusion criteria. After excluding ineligible subjects, 1246 subjects with a mean age of 54.2 years (range, 33–79) were enrolled in our final study population. All participants provided written informed consent. This study was approved by the institutional review board of Asan Medical Center.
Assignment to Statin Therapy According to the 2004 ATP III Guidelines
Because we excluded subjects with known CVD, no subject had CHD or stroke at the time of enrollment. Diabetes mellitus was regarded as a CHD risk equivalent.13 CHD risk factors included smoking, hypertension, low high‐density lipoprotein cholesterol (HDL‐C; <1.0 mmol/L), family history of premature CHD (ie, CHD in a male first‐degree relative <55 years of age; CHD in a female first‐degree relative <65 years of age), and age (men≥45 years; women≥55 years).13 The 10‐year risk for CHD was calculated using the modified Framingham risk scoring (FRS) system.14 Subjects assigned to statin therapy according to the ATP III guidelines included those categorized as: (1) high risk (CHD risk equivalents or CHD risk factors ≥2 and 10‐year risk for CHD >20%) with an LDL‐C level of ≥2.6 mmol/L; (2) moderately high risk (CHD risk factors ≥2 and 10‐year risk for CHD 10–20%) with an LDL‐C level of ≥3.4 mmol/L; (3) moderate risk (CHD risk factors ≥2 and 10‐year risk for CHD <10%) with an LDL‐C level of ≥4.1 mmol/L; and (4) lower risk (0–1 CHD risk factors) with an LDL‐C level of ≥4.9 mmol/L.13
Assignment to Statin Therapy According to the 2013 ACC/AHA Guidelines
Based on the exclusion criteria, no subject included in this study had CVD at the time of enrollment. Subjects who were recommended statin therapy for primary prevention included (1) adults ≥21 years of age with primary elevations in LDL‐C ≥4.9 mmol/L, (2) patients with diabetes mellitus aged 40 to 75 years with an LDL‐C level of 1.8 to 4.9 mmol/L, (3) individuals aged 40 to 75 years with no clinical ASCVD or diabetes with an LDL–C of 1.8 to 4.9 mmol/L and estimated 10‐year ASCVD risk of ≥7.5%, and (4) nondiabetic individuals with 5% to <7.5% 10‐year ASCVD risk among 40 to 75 years of age with LDL‐C 1.8 to 4.9 mmol/L.1 The 10‐year ASCVD risk was estimated using the Pooled Cohort Equations for non‐Hispanic white developed by the Risk Assessment Work Group.1 Parameters required for Pooled Cohort Equations included sex, age, race, total cholesterol, HDL‐C, systolic BP, use of antihypertensive medication, diabetes mellitus, and smoking.1 The Pooled Cohort Equations were not applied to subjects younger than 40 years or older than 75 years.1
Clinical and Laboratory Measurements
Height and weight were obtained while the participants wore light clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Waist circumference (WC; in cm) was measured midway between the costal margin and the iliac crest at the end of a normal expiration. BP was measured on the right arm after ≥5‐minute rests using an automatic manometer with an appropriate cuff size. After overnight fasting, early‐morning blood samples were drawn from the antecubital vein into vacuum tubes and subsequently analyzed by the central certified laboratory at Asan Medical Center. Measurements included the concentrations of fasting glucose, insulin, high‐sensitivity C‐reactive protein (hsCRP), several lipid parameters, and liver enzymes. The methods used were detailed previously.15
Use of MDCT to Assess the CAC Score
MDCT examinations were performed by using either 64‐slice, single‐source, computed tomography (CT; LightSpeed VCT; GE, Milwaukee, WI) or dual‐source CT (Somatom Definition or Somatom Definition Flash; Siemens, Erlangen, Germany), as previously described.16, 17 The CAC score was calculated using an automated software program using the Agatston scoring method,18 and participants were categorized according to the cut‐off points used by Greenland et al.19 (ie, none, 0; mild, 1–100; moderate, 101–300; severe, >300).
Estimating Changes in the CAC Score
Progression of CAC was defined as (1) incident CAC, which indicates baseline Agatston score of zero converting to detectable CAC at the follow‐up examination in a population free of CAC at baseline,20, 21 or (2) increase of ≥2.5 units between the baseline and final square root of CAC scores participants who had detectable CAC at baseline examination.9, 22, 23 To eliminate the dependence of residual interscan variability on the baseline CAC score, square root transformation of the CAC score was performed before the estimation of CAC progression. Using the data published by Hokanson et al., progressors were defined as individuals with a difference of ≥2.5 units between the baseline and final square root of their CAC scores (ie, the “SQRT method” [the square root‐transformed difference]).9, 22, 23 To put it differently, a change of <2.5 units between the baseline and final square root of the CAC score was considered to be within the margin of error for the estimation of the CAC score using MDCT and thus was attributed to interscan variability; such participants were classified as nonprogressors.9, 22, 23
Statistical Analysis
Continuous variables with normal distributions are expressed as the mean±SD, whereas continuous variables with skewed distributions are expressed as the median (and interquartile range). Categorical variables are expressed as the percentage. After we categorized participants according to the CAC score progression, the demographic and biochemical characteristics of subgroups were compared using the Student t test or Mann–Whitney U test for continuous variables or the chi‐squared test for categorical variables.
Logistic regression analysis was performed to calculate the odds ratios (ORs) and 95% CIs of the 2 guidelines on statin eligibility for the prediction of CAC progression. C statistics, which are analogous to the area under the receiver operating characteristic (ROC) curve, were calculated to compare the ability of different logistic models to discriminate between the 2 sets of criteria for the prediction of CAC score progression.24 Comparison between the 2 C statistics was performed by MedCalc software (version 11.6.1.0 for Windows; MedCalc Software, Mariakerke, Belgium) according to the method described by DeLong et al.10
Next, to compare the 2 risk assessment methods (ie, FRS system and the estimated 10‐year ASCVD risk) for the prediction of CAC score progression, we plotted ROC curves and calculated the areas under the curve (AUCs). The Youden index was used to identify the best cut‐off point. Comparisons of ROC curves were performed using the method of DeLong et al.,10 as mentioned above. All statistical analyses, except ROC curves, were performed using SPSS software (version 20.0 for Windows; SPSS, Inc., Chicago, IL). In our current analyses, P < 0.05 was considered statistically significant.
Results
Clinical and Biochemical Characteristics of the Study Subjects
Baseline characteristics of the study population according to CAC score progression are listed in Table 1. Overall, mean age was 54.2±7.4 years and males predominated (81.8%). Compared with nonprogressors, progressors were significantly older and had a higher BMI, WC, and systolic and diastolic BP. In addition, progressors were more likely to be male, current smokers, and frequent drinkers. Progressors also had a less‐favorable risk profile, which included higher prevalences of hypertension and diabetes mellitus and higher levels of FPG, HbA1c, triglycerides (TG), uric acid, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma‐glutamyltransferase (GGT), and hsCRP. The 10‐year FRS and 10‐year ASCVD risk score were significantly higher in progressors than nonprogressors (Table 1).
Table 1.
Clinical and Biochemical Characteristics of the Study Participants According to CAC Score Progression
| Total | Nonprogressor | Progressor | P Value | |
|---|---|---|---|---|
| N (%) | 1,246 | 933 (74.9) | 313 (25.1) | – |
| Age, y | 54.2±7.4 | 53.5±7.1 | 56.3±7.9 | <0.001 |
| Sex (% male) | 81.8 | 78.3 | 92.0 | <0.001 |
| BMI, kg/m2 | 25.0±3.0 | 24.8±3.1 | 25.4±2.6 | 0.002 |
| WC, cm | 87.0±8.2 | 86.3±8.3 | 89.0±7.4 | <0.001 |
| Systolic BP, mm Hg | 119.6±12.8 | 118.7±12.4 | 122.4±13.6 | <0.001 |
| Diastolic BP, mm Hg | 76.8±10.5 | 76.2±10.4 | 78.4±10.8 | 0.002 |
| Current smoker (%) | 27.3 | 25.0 | 36.2 | 0.002 |
| Moderate drinker (%) | 52.8 | 51.0 | 58.1 | 0.031 |
| Physically active (%) | 43.6 | 42.1 | 47.6 | 0.100 |
| Family history of diabetes mellitus (%) | 24.4 | 23.8 | 26.2 | 0.403 |
| Diabetes mellitus, n (%) | 168 (13.5) | 105 (11.3) | 63 (20.1) | <0.001 |
| Hypertension, n (%) | 420 (33.7) | 277 (29.7) | 143 (45.7) | <0.001 |
| FPG, mmol/L | 5.8±1.0 | 5.8±1.0 | 6.0±1.1 | <0.001 |
| HbA1c (%) | 5.5 (5.3–5.9) | 5.5 (5.3–5.8) | 5.6 (5.3–6.0) | 0.005 |
| Total cholesterol, mmol/L | 5.1±0.8 | 5.1±0.8 | 5.1±0.9 | 0.565 |
| TG, mmol/L | 1.3 (0.9–1.8) | 1.3 (0.9–1.8) | 1.4 (1.0–1.8) | 0.027 |
| LDL‐C, mmol/L | 3.3±0.7 | 3.2±0.7 | 3.3±0.7 | 0.750 |
| HDL‐C, mmol/L | 1.3±0.3 | 1.6±0.3 | 1.3±0.3 | 0.006 |
| Uric acid, μmol/L | 344.9±82.6 | 340.7±83.2 | 357.6±79.4 | 0.002 |
| AST, U/L | 25 (22–31) | 25 (21–31) | 27 (22–33) | 0.001 |
| ALT, U/L | 23 (17–31) | 22 (17–31) | 24 (19–34) | <0.001 |
| GGT, U/L | 25 (16–40) | 24 (16–38) | 30 (20–44) | <0.001 |
| hsCRP, mg/L | 0.6 (0.3–1.3) | 0.6 (0.3–1.3) | 0.7 (0.4–1.5) | 0.049 |
| 10‐year FRS (%) | 6.0 (3.0–10.0) | 6.0 (2.0–10.0) | 10.0 (6.0–12.0) | <0.001 |
| 10‐year ASCVD risk score (%) | 5.6 (2.7–9.8) | 4.8 (2.3–8.7) | 8.1 (4.9–13.4) | <0.001 |
| Baseline CAC score | 0 (0–23) | 0 (0–11) | 12 (0–98) | <0.001 |
| Last follow‐up CAC score | 1 (0–50) | 0 (0–16) | 64 (11–234) | <0.001 |
| Baseline CAC score category | ||||
| 0, n (%) | 714 (57.3) | 602 (64.5) | 112 (35.8) | <0.001 |
| 1 to 100, n (%) | 385 (30.9) | 258 (27.7) | 127 (40.6) | |
| 101 to 300, n (%) | 93 (7.5) | 49 (5.3) | 44 (14.1) | |
| >300, n (%) | 54 (4.3) | 24 (2.6) | 30 (9.6) | |
| Follow‐up interval (years) | 3.0 (2.1–3.8) | 2.9 (2.0–3.6) | 3.1 (2.5–4.0) | <0.001 |
ALT indicates alanine aminotransferase; ASCVD, atherosclerotic cardiovascular disease; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CAC, coronary artery calcification; FPG, fasting plasma glucose; FRS, Framingham Risk Score; GGT, gamma‐glutamyltransferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; TG, triglyceride; WC, waist circumference.
*Continuous variables with normal distributions are expressed as the mean±SD, whereas continuous variables with skewed distributions are expressed as the median (and interquartile range). Categorical variables are expressed as the percentage (%).
Statin Eligibility According to the 2004 ATP III and 2013 ACC/AHA Guidelines
When the ATP III and ACC/AHA guidelines were each applied separately to the study population, 256 (20.5%) and 681 (54.7%) of subjects were eligible for statin therapy, respectively (Figures 1 and 2). In subjects who were older than 60 years, 84.5% of the subjects were eligible for statins according to ACC/AHA guidelines compared with 26.1% recommendation by ATP III guidelines (Figure 1B). The increase in statin eligibility was mainly observed in subjects eligible for statins attributed to an increase in the risk predicted by the risk calculator (Figure 1).
Figure 1.
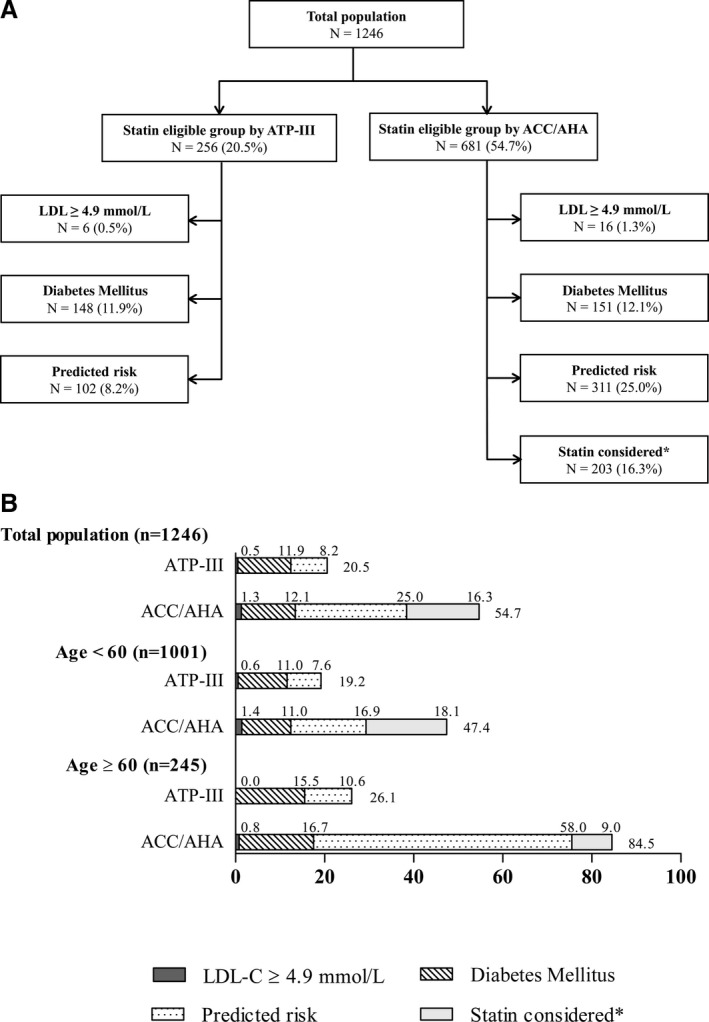
A, Flow diagram of participants who would be eligible for statin therapy according to each set of guidelines and age group. B, Proportion of participants who would be eligible for statin therapy according to each set of guidelines and age group. Proportions were calculated within the total population (N=1289). *“Statin considered” indicates statin candidates who are nondiabetic individuals with 5% to <7.5% 10‐year ASCVD risk among 40 to 75 years of age with LDL‐C 1.8 to 4.9 mmol/L. ACC/AHA indicates American College of Cardiology/American Heart Association; ASCVD, atherosclerotic cardiovascular disease; ATP III, Adult Treatment Panel III; LDL‐C, low‐density lipoprotein cholesterol.
Figure 2.
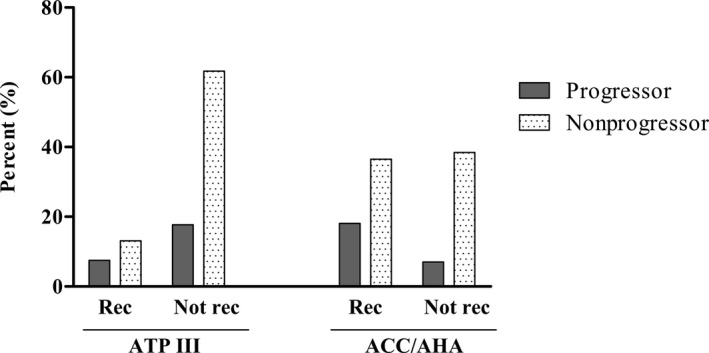
Comparison of the proportion of nonprogressor and progressor in statin‐eligible population according to each set of guideline. Proportions were calculated within the total population (N=1289). ACC/AHA indicates American College of Cardiology/American Heart Association; ATP III, Adult Treatment Panel III; Not Rec, not recommended for statin; Rec, Recommended for statin.
Subjects who were eligible for statin under ACC/AHA guideline tended to be older. On the other hand, subjects who were eligible for statin under ATP III guidelines are more likely to be current smokers and less physically active than statin‐eligible subjects according to ACC/AHA guidelines. Statin eligible subjects under ATP III guidelines also have higher prevalence of hypertension and diabetes mellitus, more frequent family history of diabetes mellitus, and worse lipid profiles (Table 2). Statin‐eligible subjects by ACC/AHA guidelines tended to have higher baseline and last follow‐up CAC score (Table 3).
Table 2.
Clinical and Biochemical Characteristics of the Study Participants According to the 2004 ATP III and 2013 ACC/AHA Guidelines
| Statin‐Eligible by ATP‐III Guidelines | Statin‐Eligible by ACC/AHA Guidelines | |
|---|---|---|
| N (%) | 256 (20.5) | 681 (54.7) |
| Age, y | 55.8±7.1 | 57.0±7.1 |
| Sex (% male) | 92.2 | 94.6 |
| BMI, kg/m2 | 25.5±2.6 | 25.4±3.0 |
| WC, cm | 89.1±6.9 | 89.2±7.4 |
| Systolic BP, mm Hg | 123.5±13.7 | 122.4±12.7 |
| Diastolic BP, mm Hg | 79.7±11.2 | 79.0±10.4 |
| Current smoker (%) | 48.8 | 41.9 |
| Moderate drinker (%) | 55.1 | 57.7 |
| Physically active (%) | 40.6 | 43.9 |
| Family history of diabetes mellitus (%) | 34.8 | 22.8 |
| Diabetes mellitus, n (%) | 127 (49.6) | 155 (22.8) |
| Hypertension, n (%) | 132 (51.6) | 305 (44.8) |
| FPG, mmol/L | 6.7±1.6 | 6.1±1.3 |
| HbA1c (%) | 6.0 (5.6–6.8) | 5.7 (5.4–6.1) |
| Total cholesterol, mmol/L | 5.7±0.9 | 5.2±0.8 |
| TG, mmol/L | 1.6 (1.2–2.2) | 1.4 (1.1–2.0) |
| LDL‐C, mmol/L | 3.8±0.7 | 3.4±0.7 |
| HDL‐C, mmol/L | 1.2±0.3 | 1.2±0.3 |
| Uric acid, μmol/L | 356.5±80.2 | 358.2±78.6 |
| AST, U/L | 27 (22–34) | 26 (22–32) |
| ALT, U/L | 27 (20–35) | 24 (22–32) |
| GGT, U/L | 32 (22–47) | 29 (20–43) |
| hsCRP, mg/L | 0.8 (0.4–1.8) | 0.8 (0.4–1.6) |
| 10‐year FRS (%) | 12.0 (8.0–16.0) | 10.0 (8.0–12.0) |
| 10‐year ASCVD risk score (%) | 11.7 (8.2–17.3) | 9.0 (6.6–13.3) |
ACC/AHA indicates American College of Cardiology/American Heart Association; ALT, alanine aminotransferase; ASCVD, atherosclerotic cardiovascular disease; AST, aspartate aminotransferase; ATP III, Adult Treatment Panel III; BMI, body mass index; BP, blood pressure; CAC, coronary artery calcification; FPG, fasting plasma glucose; FRS, Framingham Risk Score; GGT, gamma‐glutamyltransferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; TG, triglyceride; WC, waist circumference.
*Continuous variables with normal distributions are expressed as the mean±SD, whereas continuous variables with skewed distributions are expressed as the median (and interquartile range). Categorical variables are expressed as the percentage (%).
Table 3.
Baseline CAC Score and CAC Score Change of the Study Participants According to the 2004 ATP III and 2013 ACC/AHA Guidelines
| Statin‐Eligible by ATP‐III Guidelines | Statin‐Eligible by ACC/AHA Guidelines | |
|---|---|---|
| N (%) | 256 (20.5) | 681 (54.7) |
| Baseline CAC score | 3 (0–49) | 6 (0–70) |
| Last follow‐up CAC score | 15 (0–104) | 21 (0–131) |
| Baseline CAC score category | ||
| 0, n (%) | 110 (43.7) | 308 (45.2) |
| 1–100, n (%) | 106 (42.1) | 263 (38.6) |
| 101–300, n (%) | 25 (9.9) | 65 (9.5) |
| >300, n (%) | 11 (4.4) | 45 (6.6) |
| Follow‐up interval (years) | 2.9 (2.1–3.5) | 2.9 (2.0–3.5) |
ACC/AHA indicates American College of Cardiology/American Heart Association; ATP III, Adult Treatment Panel III; CAC, coronary artery calcification.
*Continuous variables with skewed distributions are expressed as the median (and interquartile range). Categorical variables are expressed as the percentage (%).
As shown in Figure 2, subjects who were recommended for statin under the ACC/AHA guidelines were more likely to be progressors compared to those who were recommended for statin under ATP III guidelines (18.1 vs 7.5%). In the case of subjects who were not recommended for statin under each guideline, those who were judged based on ACC/AHA guidelines were less likely to be progressors compared to those who were assorted based on ATP III guidelines (7.0 vs 17.7%; Figure 2). Statistical analysis could not be performed because the 2 groups are not independent groups and some of the subjects could overlap.
Comparison of the 2 Risk Scoring Systems for Predicting CAC Score Progression
When the ORs for the presence of CAC progression were analyzed in statin‐eligible subjects by the 2 guidelines, subjects considered statin eligible by ACC/AHA guidelines showed a higher OR than those considered statin eligible according to ATP III guidelines: 2.73 (95% CI, 2.07–3.61) versus 2.00 (95% CI, 1.49–2.68; Table 4). The C statistic for the ACC/AHA guidelines was 0.62 (95% CI, 0.59–0.64), and use of the ATP III risk prediction model resulted in a C statistic of 0.56 (95% CI, 0.53–0.59). A significant difference was observed between the 2 guidelines in their ability to predict CAC progression (P = 0.006; Table 4).
Table 4.
Comparisons of the Odds Ratio of the 2 Statin Eligibility Guidelines for the Prediction of CAC Score Progression
| Guidelines Applied | C Statistic (95% CI) | OR (95% CI) | P Value | P Valuea |
|---|---|---|---|---|
| Statin eligible by ATP III guidelines | 0.56 (0.53–0.59) | 2.00 (1.49–2.68) | <0.001 | 0.0006 |
| Statin eligible by ACC/AHA guidelines | 0.62 (0.59–0.64) | 2.73 (2.07–3.61) | <0.001 |
ACC/AHA indicates American College of Cardiology/American Heart association; ATP III, Adult Treatment Panel III; CAC, coronary artery calcification; OR, odds ratio.
P value between ATP III guidelines and ACC/AHA guidelines. P value is calculated by comparison of C statistic using the method of DeLong et al.10
To compare the efficacy of the 10‐year FRS and 10‐year ASCVD risk scoring systems for assigning subjects to statin therapy, we further excluded subjects who were not between the ages of 40 and 75 years and those with an LDL‐C level of ≥4.9 mmol/L and/or diabetes mellitus, in whom the calculation of the 10‐year ASCVD risk is not indicated.1 After these exclusions, we conducted ROC curve analysis and compared the 2 risk scoring systems for their ability to accurately detect CAC progression in the remaining 1225 subjects (Table 5). The optimal cut‐off values for detecting CAC score progression were 5% and 4.19% in the 10‐year ASCVD scoring system and the 10‐year FRS system, respectively. The AUCs of the 10‐year ASCVD risk scores for detecting CAC progression were slightly higher than those of the 10‐year FRS system: 0.67 (95% CI 0.64–0.70) versus 0.66 (95% CI, 0.62–0.69). However, no statistically significant differences were observed between the 2 guidelines (P = 0.056; Table 5).
Table 5.
Comparison of the Sensitivity and Specificity of the Framingham and 10‐year ASCVD Risk Scoring Systems for the Alignment of Statin Therapy in Subjects According to CAC Score Progression
| Framingham Risk Scoring | 2013 ASCVD Risk Scoring | P Valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | Sensitivity | Specificity | AUC (95% CI) | Cutoff | Sensitivity | Specificity | AUC (95% CI) | ||
| Progression | 5 | 76.2 | 48.6 | 0.66 (0.63–0.68) | 4.19 | 79.5 | 49.3 | 0.67 (0.64–0.70) | 0.056 |
ASCVD indicates atherosclerotic cardiovascular disease; AUC, area under the curve.
P value between Framingham and 10‐year ASCVD risk scoring system. P value is calculated by comparison of AUCs using the method of DeLong et al.10
Discussion
In the present study, we observed that a higher proportion of participants were eligible for statin therapy according to ACC/AHA cholesterol treatment guidelines (54.7%) than according to ATP III guidelines (20.5%; Figure 1). Using CAC score progression as a surrogate marker of CHD risk, we examined the performance of the ACC/AHA guidelines and compared it with that of the ATP III guidelines to calibrate the risk of CHD. Overall, under the ACC/AHA guidelines, the probability of being assigned statin therapy better tracks CAC score progression, which has predictive value for cardiovascular events (Table 4).
The ACC/AHA guidelines released in 2013 have been shown to broaden statin recommendations in Western populations.25, 26 National Health and Nutrition Examination Survey data and several Korean studies reported that the ACC/AHA guidelines increased the statin‐eligible population compared with the ATP III guidelines, and the increase was mainly observed in subjects eligible for statin therapy attributed to an increased number of adults who would be classified on the basis of their 10‐year risk of a cardiovascular event calculated by the new Pooled Cohort Equations.7, 8, 25, 27 Similar to previous studies, the ACC/AHA guidelines would substantially expand the number of adults who would be eligible for statin therapy in our population (Figure 1), and this increase seemed to mostly occur among those who would be considered eligible according to their predicted 10‐year ASCVD risk by the new calculator, especially subjects older than 60 years (Figure 1).
There have been general concerns that the new guidelines overestimate risk and cause too many patients to be eligible for statins.3, 4, 28 However, although the ACC/AHA guidelines recommend statin to considerable population, it is less likely to miss patients who could be beneficiary of statins. Our results demonstrated that subjects who were recommended for statin under the ACC/AHA guidelines were more likely to be progressors compared to those who were recommended for statin under ATP III guidelines (18.1 vs 7.5%; Fig. 2). Furthermore, a review of randomized statin trials demonstrated that statin therapy is associated with a slightly increased risk of transaminase elevations, but not of myalgias, creatine kinase elevations, rhabdomyolysis, or withdrawal of therapy compared with placebo.29 Last, ASCVD is one of the most important global causes of morbidity and mortality, and the most important and clinically treatable risk factor is LDL‐C.30 Therefore, given the favorable safety profile of statins and disastrous outcomes of CHD, the benefits of statin therapy clearly outweigh the risks of overtreatment in an era when CHD remains a world‐wide public health issue.29, 30, 31
In addition, some studies reported that the ACC/AHA guidelines improved the alignment of statin eligibility.5, 32, 33 Recently, Ko et al.27 reported that, in the Korean population, the ACC/AHA guidelines would substantially increase the number of adults who would potentially be eligible for statin therapy and would recommend statin therapy for more adults who would have higher ASCVD events. Furthermore, a retrospective cohort study in an Asian population showed that the apparent overprediction of the pooled risk score could be a result of increased number of patients receiving statin therapy, which result would lead to a reduction of ASCVD events. The researchers also asserted that the pooled cohort risk score would be possibly appropriate for use in a primary care setting in the absence of validation of the pooled cohort risk score in an untreated population.34
Regarding coronary imaging studies comparing the ACC/AHA and ATP III guidelines, the new guidelines showed better discrimination of coronary atherosclerosis than the ATP III guidelines, which suggests that the new guidelines will better predict future coronary events.5, 6, 7, 8 Pursnani et al.5 showed the superiority of the ACC/AHA guidelines in predicting CHD detected by CCTA in patients who presented to the emergency department with acute chest pain but who were not diagnosed with acute coronary syndrome. Johnson et al.6 also reported that the ACC/AHA guidelines better matched total plaque burden than the ATP III guidelines with only a modest increase in the number of patients who were assigned statins. More recently, 2 studies in Korea demonstrated that the ACC/AHA guidelines were more sensitive in identifying subjects with subclinical CHD and could better identify subjects with high‐risk individuals who could benefit from statin therapy.7, 8 In our study, the ACC/AHA guidelines showed better ability to detect CAC score progression than the ATP III guidelines (Table 4), which is concordant with the findings of previous studies. Furthermore, whereas previous studies mostly had a cross‐sectional design and used a single measurement of coronary atherosclerosis, precluding analysis of the temporal relationship between exposure and outcomes, we followed the change in the CAC score and used the CAC score progression as a surrogate marker of future CHD risk. The CAC score progression is predictive of an increase in all‐cause mortality, as well as hard and total CHD events.9, 10 In addition, considering that atherosclerosis progression is a dynamic and continuous process, monitoring of CAC progression by serial CAC scanning may be a more useful predictor of a patient's risk of future events than the baseline CAC score. To the best of our knowledge, this is the first report to compare the ability of the ACC/AHA guidelines and ATP III guidelines to discriminate CAC score progression in an asymptomatic Asian population.
Overall, 714 (57.3%) of subjects had no detectable CAC at baseline, of which 112 subjects (15.7%) developed incident CAC at follow‐up examination. Of 532 subjects (42.7%) with baseline CAC >0, 201 subjects (37.8%) were categorized to progressor based upon their CAC root change. When we performed additional analysis in these subgroups according to baseline CAC, the ACC/AHA guidelines demonstrated better alignment with the incident CAC than the ATP III guidelines (P = 0.0044; Table S1). In participants with baseline CAC >0, statin eligibility by both guidelines was associated with CAC progression; however, a statistical difference for the prediction of CAC progression was not observed between 2 statin guidelines (P = 0.7918; Table S2). These findings suggested that the ACC/AHA guidelines performed better especially in the prediction of incident CAC compared to ATP III guidelines.
The new ACC/AHA guidelines substantially lowered the cutoff for treatment to an evidence‐based threshold of 7.5%.35, 36 According to the ACC/AHA guidelines, statin treatment was regarded to be beneficial for reducing CVD events in subjects with a 10‐year ASCVD risk ranging between 5% and 7.5%.1 However, different populations may decide on very different thresholds because populations vary in their genetic backgrounds, lifestyles, and environmental factors, as well as their resources. We previously reported that, in our Korean cohort, the optimal cut‐off values for the 10‐year ASCVD scoring system for detecting significant coronary stenosis, CAC score >100, and any plaque were 5.85%, 4.86%, and 4.26%, respectively.8 Similarly, in the current study, we found that the calculated optimal cut‐off value for detecting CAC score progression was 4.19%, which was lower than the recommended value of 5%.1 Although it has been suggested that the pooled cohort risk score seemed to overestimate CVD risk because of the lower cut‐off point, the relatively low cut‐off values in our results support the view that a cut‐off value of 5% for statin eligibility might not be too low in Asian populations. In addition, whereas the ATP‐III guidelines were only based on the 10‐year risk of CHD, the ACC/AHA guidelines broaden to comprise risk of all hard ASCVD, including CHD and stroke, using the Pooled Cohort Equations, which could contribute to the lower cut‐off value of the 10‐year ASCVD scoring system.1 However, our analysis demonstrated no statistically significant differences between the 2 risk scoring systems in the prediction of CAC score progression (Table 5). Therefore, the predictive value of 2 guidelines might not be derived solely from the difference of cut‐off values of the scoring system. Ideally, a comprehensive cost‐benefit analysis would help to estimate the clinical benefit of the treatment and set a reasonable cutoff in terms of cost‐effectiveness.
There are several limitations to this study. First, the retrospective nature of our study limits the establishment of causality. Second, we used the CAC score progression detected by MDCT, not actual coronary events, as the outcome measure to evaluate the accuracy of the statin guidelines. Therefore, although our results showed the superiority of the ACC/AHA guidelines over the ATP III guidelines for detecting CAC score progression, the absolute benefit of statin treatment according to these guidelines remains unclear because we did not investigate the actual occurrence of ASCVD. Third, although the definition of “clinical ASCVD” according to the ACC/AHA guidelines included peripheral artery disease and “CHD risk equivalents” in the high‐risk category included subjects with peripheral artery disease, abdominal aortic aneurysm, or carotid artery disease according to the ATP III guidelines, we could not obtain participant histories regarding peripheral artery disease, abdominal aortic aneurysm, and carotid artery disease. Fourth, the prescriptions for statin after study enrollment of the participants were not considered in the analyses, which might have resulted in promoted calcification of coronary plaque.37 Fifth, we could not determine whether subjects were representative of the general Korean population because participants were voluntarily recruited during routine health examinations, which might have introduced a selection bias. There remains a possibility that more high‐risk participants would be more likely to repeat their CAC measurement. Finally, the definition of CAC progression that we used might be problematic, given that there is still no consensus regarding this definition.31 However, it has been demonstrated that the best CAC progression model that can predict mortality is the SQRT method, which we used in our present study, and a SQRT difference of 2.5 provides the best fit for the data.9
Despite these limitations, to the best of our knowledge, our study is the first to compare these 2 guidelines in terms of cardiovascular risk predicted by CAC score progression in an asymptomatic Korean population.
In conclusion, our results suggest that the new ACC/AHA guidelines provide better alignment of statin eligibility with CAC score progression than the ATP III guidelines. Further prospective, randomized, control trials that compare the accurate allocation of statins and the actual risk reduction by 2 guidelines are needed in the Asian population.
Disclosures
None.
Supporting information
Table S1. Comparisons of the Odds Ratio of the 2 Statin Eligibility Guidelines for the Prediction of CAC Score Progression in Subjects With Baseline CAC Score=0
Table S2. Comparisons of the Odds Ratio of the 2 Statin Eligibility Guidelines for the Prediction of CAC Score Progression in Subjects With Baseline CAC Score>0
(J Am Heart Assoc. 2016;5:e003410 doi: 10.1161/JAHA.116.003410)
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 2. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 3. Kavousi M, Leening MJ, Nanchen D, Greenland P, Graham IM, Steyerberg EW, Ikram MA, Stricker BH, Hofman A, Franco OH. Comparison of application of the ACC/AHA guidelines, adult treatment panel III guidelines, and European society of cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311:1416–1423. [DOI] [PubMed] [Google Scholar]
- 4. Muntner P, Colantonio LD, Cushman M, Goff DC Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd‐Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pursnani A, Mayrhofer T, Ferencik M, Hoffmann U. The 2013 ACC/AHA cardiovascular prevention guidelines improve alignment of statin therapy with coronary atherosclerosis as detected by coronary computed tomography angiography. Atherosclerosis. 2014;237:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson KM, Dowe DA. Accuracy of statin assignment using the 2013 AHA/ACC cholesterol guideline versus the 2001 NCEP ATP III guideline: correlation with atherosclerotic plaque imaging. J Am Coll Cardiol. 2014;64:910–919. [DOI] [PubMed] [Google Scholar]
- 7. Rhee EJ, Park SE, Oh HG, Park CY, Oh KW, Park SW, Blankstein R, Plutzky J, Lee WY. Statin eligibility and cardiovascular risk burden assessed by coronary artery calcium score: comparing the two guidelines in a large Korean cohort. Atherosclerosis. 2015;240:242–249. [DOI] [PubMed] [Google Scholar]
- 8. Jung CH, Lee MJ, Kang YM, Yang DH, Kang JW, Kim EH, Park DW, Park JY, Kim HK, Lee WJ. 2013 ACC/AHA versus 2004 NECP ATP III guidelines in the assignment of statin treatment in a Korean population with subclinical coronary atherosclerosis. PLoS ONE. 2015;10:e0137478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all‐cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. [DOI] [PubMed] [Google Scholar]
- 10. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 11. American Diabetes Association . Standards of medical care in diabetes‐2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, Schaefer EJ, Hayashi J. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa population study (KOPS). Diabetologia. 2011;54:3028–3036. [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 14. National Cholesterol Education Program Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults . Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15. Cho YK, Kang YM, Hwang JY, Kim EH, Yang DH, Kang JW, Park JY, Lee WJ, Kim HK, Jung CH. Association between serum gamma‐glutamyltransferase and the progression of coronary artery calcification. Atherosclerosis. 2015;243:300–306. [DOI] [PubMed] [Google Scholar]
- 16. Jung CH, Lee MJ, Hwang JY, Jang JE, Leem J, Yang DH, Kang JW, Kim EH, Park JY, Kim HK, Lee WJ. Association of metabolically healthy obesity with subclinical coronary atherosclerosis in a Korean population. Obesity (Silver Spring). 2014;22:2613–2620. [DOI] [PubMed] [Google Scholar]
- 17. Moon JS, Yoon JS, Won KC, Cho IH, Lee HW. Diagnostic accuracy of 64‐slice MDCT coronary angiography for the assessment of coronary artery disease in Korean patients with type 2 diabetes. Diabetes Metab J. 2013;37:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 19. Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. [DOI] [PubMed] [Google Scholar]
- 20. DeFilippis AP, Blaha MJ, Ndumele CE, Budoff MJ, Lloyd‐Jones DM, McClelland RL, Lakoski SG, Cushman M, Wong ND, Blumenthal RS, Lima J, Nasir K. The association of Framingham and Reynolds risk scores with incidence and progression of coronary artery calcification in MESA (multi‐ethnic study of atherosclerosis). J Am Coll Cardiol. 2011;58:2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carson AP, Steffes MW, Carr JJ, Kim Y, Gross MD, Carnethon MR, Reis JP, Loria CM, Jacobs DR Jr, Lewis CE. Hemoglobin a1c and the progression of coronary artery calcification among adults without diabetes. Diabetes Care. 2015;38:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the multi‐ethnic study of atherosclerosis (MESA). Circulation. 2007;115:2722–2730. [DOI] [PubMed] [Google Scholar]
- 23. Hokanson JE, MacKenzie T, Kinney G, Snell‐Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. Am J Roentgenol. 2004;182:1327–1332. [DOI] [PubMed] [Google Scholar]
- 24. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford: Oxford University Press; 2003. [Google Scholar]
- 25. Pencina MJ, Navar‐Boggan AM, D'Agostino RB Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population‐based sample. N Engl J Med. 2014;370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 26. Vaucher J, Marques‐Vidal P, Preisig M, Waeber G, Vollenweider P. Population and economic impact of the 2013 ACC/AHA guidelines compared with European guidelines to prevent cardiovascular disease. Eur Heart J. 2014;35:958–959. [DOI] [PubMed] [Google Scholar]
- 27. Ko MJ, Kim YJ, Park CM, Lee SM, Lee WJ, Pencina MJ, Navar‐Boggan AM, Park DW. Applicability and potential clinical effects of 2013 cholesterol guidelines on major cardiovascular events. Am Heart J. 2015;170:598–605.e597. [DOI] [PubMed] [Google Scholar]
- 28. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, Krumholz HM. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. [DOI] [PubMed] [Google Scholar]
- 30. Kim NH, So MS, Kang JG, Cho DS, Byrne CD, Lee SJ, Sung KC. Application of new guidelines for the primary prevention of atherosclerotic cardiovascular disease in a Korean population. J Atheroscler Thromb. 2015;22:293–303. [DOI] [PubMed] [Google Scholar]
- 31. Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, Shea S, Sidney S, O'Leary DH, Chan C, Lloyd‐Jones DM. Prevalence and progression of subclinical atherosclerosis in younger adults with low short‐term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi‐ethnic study of atherosclerosis. Circulation. 2009;119:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chia YC, Lim HM, Ching SM. Does use of pooled cohort risk score overestimate the use of statin? A retrospective cohort study in a primary care setting. BMC Fam Pract. 2014;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johansen ME, Green LA, Sen A, Kircher S, Richardson CR. Cardiovascular risk and statin use in the United States. Ann Fam Med. 2014;12:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chia YC, Lim HM, Ching SM. Validation of the pooled cohort risk score in an Asian population: a retrospective cohort study. BMC Cardiovasc Disord. 2014;14:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinto S, Costa J, Vaz Carneiro A, Fernandes R. Analysis of the Cochrane review: antibiotics for acute otitis media in children. Cochrane database syst rev. 2013; 1: Cd000219.. Acta Med Port. 2013;26:633–636. [PubMed] [Google Scholar]
- 37. Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons of the Odds Ratio of the 2 Statin Eligibility Guidelines for the Prediction of CAC Score Progression in Subjects With Baseline CAC Score=0
Table S2. Comparisons of the Odds Ratio of the 2 Statin Eligibility Guidelines for the Prediction of CAC Score Progression in Subjects With Baseline CAC Score>0


