Abstract
Background
The prognostic value of the change in heart rate from the supine to upright position (∆HR) in patients with chronic heart failure (HF) is unknown.
Methods and Results
∆HR was measured in patients enrolled in the Trial of Intensified Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME‐CHF) who were in sinus rhythm and had no pacemaker throughout the trial (n=321). The impact of ∆HR on 18‐month outcome (HF hospitalization‐free survival) was assessed. In addition, the prognostic effect of changes in ∆HR between baseline and month 6 on outcomes in the following 12 months was determined. A lower ∆HR was associated with a higher risk of death or HF hospitalization (hazard ratio 1.79 [95% confidence interval {95% CI} 1.19‐2.75] if ∆HR ≤3 beats/min [bpm], P=0.004). In the multivariate analysis, lower ∆HR remained an independent predictor of death or HF hospitalization (hazard ratio 1.75 [95% CI, 1.18‐2.61] if ∆HR ≤3 bpm, P=0.004) along with ischemic HF etiology, lower estimated glomerular filtration rate, presence and extent of rales, and no baseline β‐blocker use. In patients without event during the first 6 months, the change in ∆HR from baseline to month 6 predicted death or HF hospitalization during the following 12 months (hazard ratio=2.13 [95% CI 1.12–5.00] if rise in ∆HR <2 bpm; P=0.027).
Conclusions
∆HR as a simple bedside test is an independent prognostic predictor in patients with chronic HF. ∆HR is modifiable, and changes in ∆HR also provide prognostic information, which raises the possibility that ∆HR may help to guide treatment.
Clinical Trial Registration Information
URL: www.isrctn.org. Unique identifier: ISRCTN43596477.
Keywords: autonomic nervous system, heart failure, heart rate/heart rate variability, prognosis, TIME‐CHF
Subject Categories: Heart Failure
Introduction
Autonomic dysfunction is a typical phenomenon in patients with chronic heart failure (HF).1 Measures of more advanced autonomic dysfunction are indicators of the severity of HF and adverse prognosis.2, 3, 4, 5, 6 They may include heart rate variability,4, 7 heart rate recovery,3 baroreflex sensitivity,5 measurement of norepinephrine spillover,2, 7 and muscle nerve sympathetic activity.8 However, the techniques needed to identify these are complex, and their application requires considerable infrastructure and time and are, therefore, not well suited for serial testing in clinical practice. Less sophisticated tests of autonomic function are not available in HF patients yet. However, an easy and quick test for assessing autonomic function that provides prognostic information in HF patients would be highly desirable.
In contrast to the situation in HF, a battery of simple tests based on the heart rate (HR) and blood pressure response to a variety of maneuvers has been in clinical use for decades for the assessment of diabetic autonomic neuropathy.9 One of these tests assesses the HR response to standing using continuous ECG monitoring of HR and RR intervals, respectively. The normal response to standing is characterized by a rapid baroreceptor‐mediated rise in HR, which peaks around the 15th beat (shortest RR interval), followed by a relative bradycardia with a maximum RR interval around the 30th beat after getting up. The ratio of the longest (~30th beat) and shortest (~15th beat) RR intervals (“30/15 ratio”) is used to describe this HR response. However, after the 30th beat, the HR remains elevated compared to the supine baseline in the presence of an intact sympathicovagal balance, and patients with diabetic neuropathy are characterized not only by a low 30/15 ratio but also by a lack of or a severely overall blunted rise in HR from the supine to the standing position.10 Thus, the change in HR following getting up from the supine to the upright position (∆HR) might be a simple test to assess the degree of autonomic dysfunction in patients with chronic HF, which may also predict prognosis, but this has not yet been investigated.
Therefore, we have prospectively measured ∆HR in patients with chronic HF included in the Trial of Intensified Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME‐CHF)11, 12 to describe the association of ∆HR with clinical characteristics in an elderly HF cohort and to evaluate its prognostic value.
Methods
Study Population and Protocol
This is a post‐hoc analysis of TIME‐CHF (isrctn.org identifier ISRCTN43596477). Design11 and main results12 of TIME‐CHF have been published previously. In brief, TIME‐CHF was a randomized, controlled multicenter trial comparing an N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP)‐guided vs a symptom‐guided management strategy in patients with chronic HF aged ≥60 years with symptoms corresponding to New York Heart Association (NYHA) ≥II, HF‐related hospitalization within 12 months prior to inclusion, and an age‐adjusted elevated NT‐proBNP plasma concentration (>400 ng/L in those <75 years, >800 ng/L in those ≥75 years). Patients with both reduced (n=499) and preserved (n=123) left ventricular ejection fraction (LVEF) were included between January 2003 and December 2006. The study was approved by the local ethics committees, and all participants provided written informed consent.
For the present analysis, patients in sinus rhythm and without any pacemaker (bradypacing, cardiac resynchronization; patients with defibrillators and pure VVI backup pacing were not excluded) throughout the trial were eligible (n=327). HR was measured in the supine position after at least 1 minute of supine rest. HR in the upright position was measured immediately after getting up by pulse palpation or an automated blood pressure monitor, and ΔHR was calculated as the difference between HR in the upright and supine positions. Thus, a positive value for ΔHR indicates a rise in HR upon standing, whereas a negative value indicates a fall in HR after getting up. This measurement of the HR at rest and after getting up was part of the study protocol, and data were collected in a prospective manner. The present analysis is based on the 321 patients with complete data on HR in the supine and upright positions. Patients were followed in the outpatient clinics after 1, 3, 6, 12, and 18 months. Medical treatment was prescribed in accordance with predefined escalation rules either to reduce symptoms to NYHA ≤II or also to reduce NT‐proBNP below the age‐specific target level (<400 ng/L in patients <75 years, <800 ng/L in those ≥75 years) as described in the design paper of TIME‐CHF.11 The primary endpoint of TIME‐CHF was 18‐month survival free of any hospitalization. Secondary endpoints included survival and survival free of HF‐hospitalization at 18 months. For the present analysis, survival free of HF‐hospitalization was the primary endpoint, and the other two were secondary endpoints.
Statistical Analysis
Descriptive statistics are expressed as mean±standard deviation, or median (interquartile range) for continuous variables and as numbers and percentages for categorical variables. Distribution of continuous data was assessed using Kolmogorov‐Smirnov tests. First, the association between ΔHR and outcomes was examined using univariate Cox regression. Then, best cutoff for ΔHR to predict death or HF hospitalization based on log‐rank testing was performed. Characteristics of patients with ΔHR above and below this cutoff were compared by chi‐squared tests for categorical variables and by t tests or Mann‐Whitney U tests for continuous variables, as appropriate. Kaplan‐Meier curves were constructed for calculating the time‐dependent occurrence of events in patients with ΔHR above and below this cutoff, and for comparison between these groups the log‐rank test was used. To test the independence of the association between ΔHR (as a continuous and dichotomized variable) and outcomes, multivariate Cox regression was performed after testing the proportional hazard assumption. A stepwise backward model was used. To account for the number of events, the number of covariates was limited to those with the strongest association with the dependent variable based on Wald score in addition to the variable of interest ΔHR. For the model with death or HF hospitalization as the dependent variable, the following covariates were included in the model: age, ischemic HF etiology, NT‐proBNP (log10‐transformed), estimated glomerular filtration rate (eGFR), hemoglobin, peripheral arterial obstructive disease (PAOD), NYHA functional class, presence and extent of rales (4 categories), and β‐blocker use at baseline. For the model with death as the dependent variable, the following covariates were included in the model: ischemic HF etiology, NT‐proBNP (log10‐transformed), eGFR, hemoglobin, NYHA class, and presence and extent of rales. The results of these models are presented in the tables. We also tested whether adjustment of this final model for sex, body mass index, and diabetes changed the findings, and the results of these analyses are mentioned in the text. Interactions between ΔHR and patient characteristics were analyzed using Cox regression with ΔHR and a second covariate and the interaction term forced into a model. In particular, the interactions between ΔHR and age, resting heart rate, NT‐pro‐BNP‐guided therapy (ie, allocation to the NT‐proBNP‐guided arm), LVEF stratum (LVEF <45% vs ≥45%), diabetes, and baseline β‐blocker use were tested 1 at a time. To illustrate the prognostic value of the multivariate model, we constructed a 5‐point score including the following items: ΔHR ≤3 bpm, ischemic HF etiology, eGFR <47 mL/min per 1.73 m2, no β‐blocker use at baseline, and the presence of rales at baseline. One point was allocated for each item. Thus, the maximal score was 5 points, and the minimal score was 0 points. Because there were few patients with 0, 4, and 5 score points, the following 3 risk categories were built for comparison using Kaplan‐Meier curves and log‐rank tests and Cox regression, respectively: 0 or 1 point, 2 points, and 3, 4, or 5 points.
To also assess the prognostic value of changes in ΔHR over time we performed a landmark analysis in which patients with available ΔHR measurements at baseline and 6 months and without event during the first 6 months were included. Within this population, the association between the change in ΔHR from baseline to month 6 and death or HF hospitalization during the following 12 months was assessed. Bootstrapping (1000 bootstrap samples) was used to calculate 95% confidence intervals (95% CI) and P‐values in Cox regression analyses. The level of statistical significance was set at a 2‐tailed probability value ≤0.05. For interactions a P<0.1 was considered relevant. Statistical analysis was performed using the IBM® SPSS® for Windows® software (version 22.0, SPSS® Inc, Chicago, IL).
Results
Patient Characteristics
The study population consisted of 321 patients (57% men) with a mean age of 76±8 years and a mean LVEF of 34±12% (Table 1). The mean HR in the supine position in the entire population was 74±13 bpm. The mean HR after getting up was 78±13 bpm, and the mean ∆HR was 5±6 bpm.
Table 1.
Baseline Characteristics of the Entire Study Population and Patients With ∆HR >3 bpm vs ≤3 bpm
| All (n=321) | ∆HR >3 bpm (n=211) | ∆HR ≤3 bpm (n=110) | P Value | |
|---|---|---|---|---|
| Age, y | 76±8 | 76±8 | 77±7 | 0.17 |
| Male sex | 182 (57%) | 119 (56%) | 63 (57%) | 0.91 |
| Body mass index, kg/m2 | 25±4 | 25±4 | 26±4 | 0.13 |
| Ischemic heart failure etiology | 219 (68%) | 136 (64%) | 83 (75%) | 0.06 |
| LVEF ≥45% | 53 (16%) | 33 (16%) | 20 (18%) | 0.64 |
| NT‐proBNP‐guided therapy | 156 (49%) | 101 (48%) | 55 (50%) | 0.73 |
| Medical history | ||||
| Hypertension | 226 (70%) | 150 (71%) | 76 (69%) | 0.70 |
| Diabetes | 119 (37%) | 69 (33%) | 50 (45%) | 0.03 |
| Stroke | 25 (8%) | 13 (6%) | 14 (13%) | 0.05 |
| Chronic obstructive lung disease | 67 (21%) | 42 (20%) | 25 (23%) | 0.85 |
| Peripheral arterial obstructive disease | 63 (20%) | 37 (18%) | 26 (24%) | 0.65 |
| Smoking | 50 (16%) | 33 (16%) | 17 (15%) | 1.0 |
| Clinical characteristics | ||||
| Heart rate, bpm | 74±13 | 73±12 | 75±13 | 0.16 |
| ∆HR, bpm | 5±6 | 8±5 | −1±4 | <0.001 |
| Supine systolic blood pressure, mm Hg | 120 (110‐132) | 120 (109‐130) | 124 (110‐136) | 0.11 |
| Upright systolic blood pressure, mm Hg | 113 (100‐129) | 112 (100‐126) | 115 (100‐133) | 0.13 |
| NYHA (II/III/IV) | 92 (29%)/193 (60%)/36 (11%) | 59 (28%)/129 (61%)/23 (11%) | 33 (30%)/64 (58%)/13 (12%) | 0.88 |
| LVEF (%) | 34±12 | 34±13 | 34±12 | 0.97 |
| Orthopnea (no/<20°/20°‐30°/>30°) | 103 (32%)/118 (37)/76 (24%)/23 (7%) | 69 (33%)/75 (36%)/52 (23%)/14 (7%) | 34 (31%)/43 (39%)/24 (22%)/9 (8%) | 0.85 |
| Edema (no/ankle/<1/2 lower leg/>1/2 lower leg) | 209 (65%)/51 (16%)/31 (10%)/28 (9%) | 149 (71%)/31 (15%)/18 (8%)/12 (6%) | 60 (55%)/20 (18%)/13 (12%)/16 (15%) | 0.01 |
| Rales (no/basal/<1/3 lung/>1/3 lung) | 176 (55%)/98 (31%)/40 (12%)/6 (2%) | 118 (56%)/61 (29%)/26 (12%)/5 (2%) | 88 (53%)/37 (34%)/14 (13%)/1 (1%) | 0.68 |
| Jugular venous pressure (normal/>4 cm H2O/positive hepatojugular reflux/congested) | 112 (36%)/88 (28%)/64 (20%)/49 (15%) | 118 (56%)/61 (29%)/26 (12%)/5 (2%) | 88 (53%)/37 (34%)/14 (13%)/1 (1%) | 0.47 |
| NT‐proBNP, ng/L | 3920 (1773‐7068) | 3645 (1709‐6799) | 4638 (2078‐7538) | 0.07 |
| Potassium, mmol/L | 4.2±0.5 | 4.2±0.5 | 4.2±0.5 | 0.91 |
| Hemoglobin level, g/L | 130±18 | 131±18 | 127±18 | 0.07 |
| Serum creatinine, μmol/L | 104 (85‐136) | 104 (85‐139) | 105 (83‐135) | 0.82 |
| eGFR, mL/min per 1.73 m2 | 55±20 | 55±21 | 54±19 | 0.47 |
| QRS width, ms | 114 (97‐136) | 114 (96‐136) | 114 (99‐136) | 0.82 |
Data are given as numbers and percentages, mean±standard deviation, or median (interquartile range). eGFR indicates estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal‐pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Univariate Association Between ∆HR and Outcomes
There were 100 (31%) patients who experienced the primary endpoint of HF hospitalization or death. There were 61 deaths (19%), and 187 (58%) patients experienced the endpoint of death or any hospitalization. When expressed as a continuous variable, a higher ∆HR was associated with a lower risk of death or HF hospitalization (hazard ratio 0.95 [95% CI 0.92‐0.99] per 1 bpm increase, P=0.01) and death (hazard ratio 0.95 [95% CI 0.90‐1.00] per 1 bpm increase, P=0.05). There was no significant association between ∆HR and all‐cause hospitalization or death (hazard ratio 0.98 [95% CI, 0.96‐1.01] per 1 bpm increase, P=0.25). The optimal threshold for ∆HR to identify subjects experiencing death or HF hospitalization was a ΔHR ≤3 bpm with only marginally less discriminative value for both ≤2 and ≤4 bpm (data not shown). As shown in Figure 1, patients with ∆HR ≤3 bpm had significantly worse HF hospitalization‐free survival, survival, and hospitalization‐free survival compared to those with ∆HR >3 bpm. Thus, in the following, patients with ΔHR ≤3 bpm and those with ΔHR >3 bpm are compared for descriptive purposes.
Figure 1.

Heart failure (HF) hospitalization‐free survival (A), survival (B), and hospitalization‐free survival (C) in patients with baseline ∆HR >3 bpm vs baseline ∆HR ≤3 bpm. CI indicates confidence interval.
Baseline Characteristics of Patients With ΔHR ≤3 bpm Vs ΔHR >3 bpm
As shown in Tables 1 and 2, patients with ∆ HR ≤3 bpm were more likely to have diabetes, more often had peripheral edema, and were less likely to be on digoxin than those with ∆HR >3 bpm. Otherwise, there were no significant differences in baseline characteristics and HF medication.
Table 2.
Medication at Baseline in the Entire Study Population and Patients With ∆HR >3 bpm vs ≤3 bpm
| All (n=321) | ∆HR >3 bpm (n=211) | ∆HR ≤3 bpm (n=110) | P Value | |
|---|---|---|---|---|
| β‐Blocker | 261 (81%) | 171 (81%) | 90 (82%) | 0.87 |
| Target dose, median (IQR)a | 25 (6.25‐37.5) | 25 (6.25‐50) | 12.5 (2.3‐37.5) | 0.26 |
| ACE inhibitor or ARB | 303 (94%) | 199 (94%) | 104 (95%) | 0.93 |
| Target dose, median (IQR)a | 50 (25‐67) | 50 (25‐67) | 50 (25‐67) | 1.00 |
| Mineralocortcoid receptor blocker | 135 (42%) | 92 (44%) | 43 (39%) | 0.44 |
| Dose | 0 (0‐25) | 0 (0‐25) | 0 (0‐25) | 0.12 |
| Loop diuretic | 298 (93%) | 197 (93%) | 101 (92%) | 0.61 |
| dose, median (IQR)b | 40 (20‐80) | 40 (20‐80) | 40 (20‐80) | 0.73 |
| Digoxin | 23 (7%) | 20 (10%) | 3 (3%) | 0.03 |
ACE inhibitor or ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker.
Indicates percentage of target dose patients were receiving.
A dose of 10 mg of torasemide is equivalent to 40 mg of furosemide.
Multivariate Association Between ∆HR and Outcomes
In Tables 3 and 4, univariate and multivariate predictors of HF hospitalization‐free survival and survival are shown with models with ΔHR as a dichotomized variable (ΔHR ≤3 bpm vs >3 bpm). As shown in Table 3, ∆HR ≤3 bpm was an independent predictor of HF hospitalization or death along with ischemic HF etiology, lower eGFR, presence and extent of rales at baseline, and no β‐blocker use at baseline. When used as a continuous variable, ΔHR (HR 0.95 [95% CI, 0.91‐0.99] per bpm increase; P=0.02) was also an independent predictor of HF hospitalization or death along with the same covariates as in the model with ΔHR as a dichotomized variable (data not shown). Adjustment of the multivariate models for sex, body mass index, and diabetes did not significantly change the results (data not shown).
Table 3.
Multivariate Predictors of HF Hospitalization or Death in a Model With ΔHR as a Dichotomized Variable
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| ΔHR ≤3 bpm | 1.79 (1.19‐2.75) | 0.004 | 1.75 (1.18‐2.61) | 0.004 |
| Age, per year | 1.06 (1.03‐1.08) | 0.002 | ||
| Ischemic HF etiology | 3.28 (2.00‐6.62) | 0.001 | 2.75 (1.69‐5.77) | 0.001 |
| Log10 NT‐proBNP | 2.94 (1.81‐4.78) | 0.001 | ||
| Heart rate, per bpm | 1.02 (0.999‐1.03) | 0.06 | ||
| eGFR, per mL/(min·1.73 m2) | 0.98 (0.96‐0.99) | 0.001 | 0.98 (0.96‐0.99) | 0.001 |
| Hemoglobin, per g/dL | 0.98 (0.97‐0.99) | 0.001 | ||
| Hypertension | 1.56 (1.03‐2.68) | 0.05 | ||
| COPD | 1.62 (1.01‐2.42) | 0.03 | ||
| PAOD | 1.90 (1.19‐2.92) | 0.002 | ||
| NYHA class | 1.61 (1.17‐2.24) | 0.004 | ||
| Edemaa | 1.33 (1.12‐1.56) | 0.002 | ||
| Orthopneaa | 1.49 (1.17‐2.00) | 0.001 | ||
| Ralesa | 0.60 (0.40‐0.93) | 0.01 | 1.36 (1.06‐1.75) | 0.01 |
| β‐Blocker use at baseline | 1.45 (0.99‐2.21) | 0.06 | 0.63 (0.40‐1.00) | 0.04 |
| Spironolactone use at baseline | 0.41 (0.05‐1.04) | 0.09 | ||
| Digoxin use at baseline | 1.33 (1.12‐1.56) | 0.002 | ||
95% CI indicates 95% confidence interval; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal‐pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PAOD, peripheral arterial obstructive disease.
Semiquantitative 4‐point scale (hazard ratio for increase by 1 class).
Table 4.
Multivariate Predictors of Death in a Model With ΔHR as a Dichotomized Variable
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| ΔHR ≤3 bpm | 2.09 (1.23‐3.46) | 0.002 | 2.01 (1.16‐3.47) | 0.003 |
| Age, per year | 1.04 (1.01‐1.08) | 0.02 | ||
| Ischemic HF etiology | 4.16 (1.89‐9.15) | <0.001 | 3.78 (1.84‐11.75) | 0.001 |
| Log10 NT‐proBNP | 3.33 (1.82‐6.08) | <0.001 | ||
| eGFR, per mL/(min·1.73 m2) | 0.98 (0.97‐0.99) | 0.004 | ||
| Hemoglobin, per g/dL | 0.98 (0.97‐0.99) | 0.002 | ||
| NYHA class | 1.74 (1.16‐2.62) | 0.008 | ||
| Edemaa | 1.33 (1.06‐1.67) | 0.01 | ||
| Ralesa | 1.93 (1.47‐2.52) | <0.001 | 2.05 (1.50‐2.75) | 0.003 |
| PAOD | 2.19 (1.29‐3.73) | 0.004 | ||
95% CI indicates 95% confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal‐pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PAOD, peripheral arterial obstructive disease.
Semiquantitative 4‐point scale (hazard ratio for increase by 1 class).
As shown in Table 4, ∆HR ≤3 bpm was an independent predictor of death along with ischemic HF etiology and presence and extent of rales. Patients with ΔHR ≤3 bpm had a more than doubled risk of death compared to those with ΔHR >3 bpm (HR 2.01 [95% CI, 1.16‐3.47] P=0.003). Adjustment of the multivariate models for sex, body mass index, and diabetes did not significantly change the results (data not shown). When expressed as a continuous variable, ∆HR failed to remain in the model as an independent predictor of death (HR 0.95 [95% CI, 0.90‐1.00] per 1 bpm increase; P=0.05). In this model, ischemic HF etiology, lower eGFR, and the presence of rales were independent predictors of death (data not shown).
In Figure 2, the prognostic value of the 5‐point score built from the multivariate model including ΔHR is shown. Patients with a score of 3 points or more had a more than 8‐fold risk of HF hospitalization or death and death compared to those with 0 or 1 point.
Figure 2.
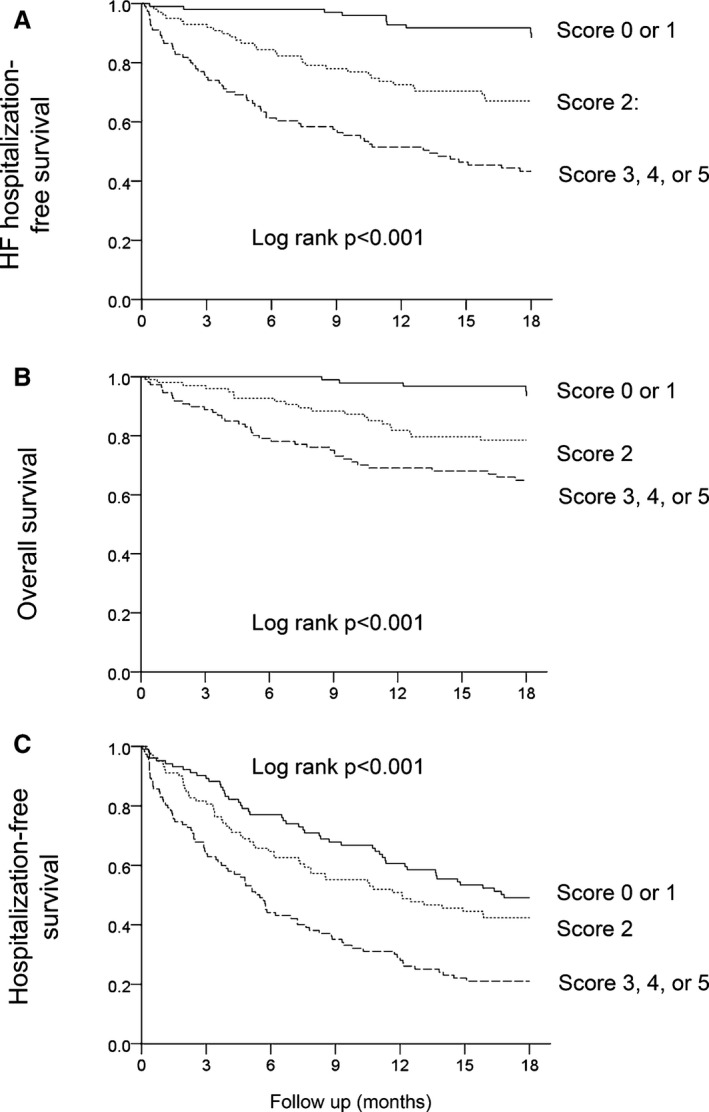
Heart failure (HF) hospitalization‐free survival (A), survival (B), and hospitalization‐free survival (C) in patients with a 5‐point score (ΔHR ≤3 bpm, ischemic HF etiology, eGFR <47 mL/min per 1.73 m2, no β‐blocker use at baseline, and the presence of rales at baseline) of 0 or 1 point (score 0 or 1), a score of 2 points (score 2), and a score of 3, 4, or 5 points (score 3, 4, or 5). Hazard ratios (95% confidence intervals) for a score of 3, 4, or 5 points and a score of 2 points, respectively, with a score a 0 or 1 point as referent are as follows: HF hospitalization‐free survival (A) 8.21 (4.20‐16.08) and 3.72 (1.82‐7.58), survival (B) 8.61 (3.38‐21.96) and 4.60 (1.73‐12.25), and hospitalization‐free survival (C) 2.41 (1.69‐3.44) and 1.29 (0.88‐1.89).
There were no significant interactions between allocation to treatment strategy (NT‐proBNP–guided vs symptom‐guided), resting heart rate, baseline β‐blocker use, or diabetes with the prognostic impact of ∆HR on outcomes. There was an interaction between age if expressed as a dichotomized variable (<75 vs ≥75 years) and the prognostic impact of ∆HR on HF hospitalization‐free survival (P=0.07 for ∆HR as a continuous variable and P=0.07 for ∆HR as a dichotomized variable) and survival (P=0.04 for ∆HR as a continuous variable and P=0.09 for ∆HR as a dichotomized variable) in that low ∆HR predicted poor outcome in younger but not in older patients. There was, however, no interaction between age expressed as a continuous variable and the prognostic impact of ∆HR. There was also an interaction between the LVEF stratum (LVEF <45% vs ≥45%) and the association of ΔHR (both as continuous or categorical variables) with the endpoint of death or HF hospitalization (P=0.09 and 0.07, respectively) in that the association between lower ΔHR and worse outcomes (death or HF hospitalization) was somewhat stronger for patients with preserved LVEF.
Changes in HR Over Time and Impact on HF‐Hospitalization‐Free Survival
There were 229 patients alive at 6 months and without HF hospitalization during the first 6 months who had available data on ∆HR at baseline and month 6. The mean change in ∆HR from baseline at month 6 was 0±9 bpm (P=0.57 for overall comparison baseline vs month 6). There were 107 patients with a decrease in ∆HR, 27 patients with unchanged ∆HR, and 95 patients with an increase in ∆HR. A more positive change in ∆HR from baseline to month 6 was not significantly associated with a lower risk of HF hospitalization or death (hazard ratio 0.96 [95% CI, 0.92‐1.002] per 1 bpm increase; P=0.07), but patients with an increase in ∆HR from baseline to month 6 by <2 bpm (optimal cutoff; n=138) had a significantly higher risk of HF hospitalization or death than those with an increase in ∆HR from baseline to month 6 by ≥2 bpm (n=91; hazard ratio 2.13 [95% CI 1.12‐5.00]; P=0.027; Figure 3).
Figure 3.

HF hospitalization‐free survival according to the change in ΔHR from baseline to month 6 (Change0→6∆HR; <2 bpm vs ≥2 bpm) after month 6.
Patients with an increase in ∆HR from baseline to month 6 by <2 bpm were characterized by a higher ∆HR (7±6 vs 2±5 bpm; P<0.001) and a lower eGFR (55±21 vs 60±18 mL/min per 1.73 m2; P=0.03) at baseline compared to those with an increase in ∆HR from baseline to month 6 by ≥2 bpm. Otherwise, there were no significant differences in baseline characteristics between groups including medication. Patients with an increase in ∆HR from baseline to month 6 by <2 bpm were less likely to be prescribed a higher dose of spironolactone or to be started on spironolactone at baseline as part of the study protocol (12/138 vs 17/91; P=0.03), and the same tended to be the case at the month 1 visit (9/138 vs 13/91; P=0.05). Otherwise, there were no significant changes in the titration of HF medication from baseline to month 3 (data not shown). Given the possible impact of congestion on ΔHR, we looked at the association between changes in edema and changes in ΔHR. However, the proportion of patients with a reduction in edema severity did not differ between patients with a change in ∆HR from baseline to month 6 by <2 bpm vs ≥2 bpm (reduction in edema 31 vs 23, unchanged edema 98 vs 57, worsening of edema: 9 vs 9; P=0.47).
Discussion
We showed that ΔHR provided prognostic information in patients with chronic HF, particularly in younger patients. The association between ∆HR and a HF hospitalization‐free survival remained statistically significant in the multivariable analysis. We also showed that ΔHR is modifiable and that changes in ΔHR over time also predicted outcomes. These findings may imply that this easy‐to‐perform and easy‐to‐repeat test may be a novel prognostic marker in patients with chronic HF with potential clinical applicability.
∆HR is a simple test that can be performed at the doctor's office within a short time, and its concept as a tool to assess the autonomic response to a physiological stimulus is plausible and biologically intuitive. We must acknowledge that the exact pathophysiological correlate of ∆HR is unknown, and given the methods used in our study, it is likely that we assessed neither the maximum baroreflex‐mediated rise in HR nor the nadir of the relative bradycardia thereafter. Rather, our measurement likely reflects an average HR throughout this period of HR dynamics after getting up or even the more or less stable HR that is established after ~2 minutes at least in healthy subjects.10 Still, we assume that ΔHR as assessed in the present study reflects the integrity or defect, respectively, of the mechanisms responsible for an increased sympathetic outflow to the heart after a person gets up.
We have not demonstrated how well ΔHR reflects established measures of autonomic tone in HF, including HR variability,4 HR recovery,3 norepinephrine spillover,2 baroreflex sensitivity, or muscle sympathetic nerve activity.8 Further studies will be required to better define the pathophysiological correlates of ΔHR. It might, for example, be considered that ΔHR depends on the presence and extent of congestion because congestion could limit the venous capacitance on standing and thereby attenuate the stimulus for the rise in HR with standing. Indeed, patients with lower ΔHR had more severe edema at baseline. However, we were unable to demonstrate an association between changes in edema severity and changes in ΔHR over time. This may, however, be due to the fact that changes in ΔHR were assessed in only a subgroup of patients surviving until month 6 without event, and thus, the sickest patients were excluded from this analysis. There was an association between changes in ΔHR over time and treatment with spironolactone, which may be regarded as indicative that neurohumoral antagonism and/or reduction of congestion had a favorable impact on ΔHR.
We have shown that the prognostic value of ΔHR is independent of established markers of HF severity, in particular eGFR.13 Furthermore, the subgroup analysis suggested that ΔHR is modifiable and that its changes over time are also associated with prognosis. Thus, these observations raise the possibility that ΔHR could be used as a biomarker that is measured serially to assess the effect of treatment. This seems to be particularly promising in patients younger than 75 years. Still, the hypothesis‐generating nature of our study needs to be emphasized, and the findings need confirmation in other cohorts. There was also an interaction between the LVEF stratum and the ability of ΔHR to predict outcomes in that the association between ΔHR and death or HF hospitalization was somewhat stronger in patients with preserved LVEF. Cautious interpretation of this finding is required, however. The group of patients with preserved LVEF in TIME‐CHF was small, and this was particularly true for this post‐hoc analysis for which patients with atrial fibrillation were not eligible.
The spectrum of ΔHR values was relatively narrow, and even in patients with good prognosis, the rise in HR on standing was small. This is not a surprising finding in this population of elderly patients with advanced HF, however. The original study by Ewing et al10 had shown that the magnitude of HR response to standing depends on age and disease status (diabetes, neuropathy). One might argue that this relatively narrow range of ΔHR in HF may limit the applicability of the parameter in practice. Thus, the clinically most meaningful ΔHR cutoff will have to be defined. On the other hand, we were able to show that not only absolute ΔHR cutoff but also individual changes in ΔHR are important.
Our study has some limitations in addition to those already discussed. First, the number of patients in this post‐hoc analysis was limited. Thus, our results will need confirmation in a larger population of HF patients, and it will be important to investigate whether treatment responses depend on ΔHR and how patients with low ΔHR must be treated to improve their outcomes. Second, we studied a population with a high proportion of patients on β‐blocker therapy, and the effect of β‐blocker therapy on ΔHR is unknown. An early pathophysiological study has revealed that the above‐described HR response to standing is under vagal control.10 However, adjustment for baseline β‐blocker dosage did not alter the results (data not shown), and there was no interaction between baseline β‐blocker use and the association between ΔHR and outcomes. Importantly, we were able to demonstrate that ΔHR is a prognostic predictor in a population treated with β‐blockers in a high proportion of patients, which represents the situation in real life. Third, ΔHR assessment by manual pulse palpation or an automated blood pressure monitor may be less accurate than continuous ECG monitoring. On the other hand, we showed that ΔHR as a true bedside test has the potential to predict outcomes. Still, the optimal method of ΔHR assessment will have to be defined. Finally, we have not performed a formal assessment of the reproducibility of ΔHR, which will be required before the parameter can be used in clinical practice.
Conclusions
ΔHR as a simple clinical parameter and presumed marker of autonomic function provides independent prognostic information in patients with chronic HF. ΔHR is modifiable, and changes in ΔHR over time are also predictive of outcomes. These findings imply that this easy‐to‐perform and easy‐to‐repeat test may be a novel prognostic marker in patients with chronic HF with potential clinical applicability.
Sources of Funding
This work was supported by the Horten Research Foundation (Lugano, Switzerland; 55% of the study's budget), as well as by smaller unrestricted grants from AstraZeneca Pharma, Novartis Pharma, Menarini Pharma, Pfizer Pharma, Servier, Roche Diagnostics, Roche Pharma, and Merck Pharma.
Disclosures
None.
Supporting information
Appendix S1. TIME‐CHF Investigators.
(J Am Heart Assoc. 2016;5:e003524 doi: 10.1161/JAHA.116.003524)
References
- 1. Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J. 2015;36:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. [DOI] [PubMed] [Google Scholar]
- 3. Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J. 2006;151:851.e7 851.e13 [DOI] [PubMed] [Google Scholar]
- 4. Mortara A, La Rovere MT, Signorini MG, Pantaleo P, Pinna G, Martinelli L, Ceconi C, Cerutti S, Tavazzi L. Can power spectral analysis of heart rate variability identify a high risk subgroup of congestive heart failure patients with excessive sympathetic activation? A pilot study before and after heart transplantation. Br Heart J. 1994;71:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. [DOI] [PubMed] [Google Scholar]
- 6. Brunner‐La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J. 2001;22:1136–1143. [DOI] [PubMed] [Google Scholar]
- 7. Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. [DOI] [PubMed] [Google Scholar]
- 8. Ferguson DW, Berg WJ, Sanders JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol. 1990;16:1125–1134. [DOI] [PubMed] [Google Scholar]
- 9. Ewing DJ, Campbell IW, Clarke BF. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med. 1980;92:308–311. [DOI] [PubMed] [Google Scholar]
- 10. Ewing DJ, Campbell IW, Murray A, Neilson JM, Clarke BF. Immediate heart‐rate response to standing: simple test for autonomic neuropathy in diabetes. BMJ. 1978;1:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunner‐La Rocca HP, Buser PT, Schindler R, Bernheim A, Rickenbacher P, Pfisterer M. Management of elderly patients with congestive heart failure—design of the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME‐CHF). Am Heart J. 2006;151:949–955. [DOI] [PubMed] [Google Scholar]
- 12. Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, Vuillomenet A, Jeker U, Dubach P, Beer H, Yoon SI, Suter T, Osterhues HH, Schieber MM, Hilti P, Schindler R, Brunner‐La Rocca HP. BNP‐guided vs symptom‐guided heart failure therapy: the trial of intensified vs standard medical therapy in elderly patients with congestive heart failure (TIME‐CHF) randomized trial. JAMA. 2009;301:383–392. [DOI] [PubMed] [Google Scholar]
- 13. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. TIME‐CHF Investigators.


