Abstract
Background
Patients undergoing cardiac surgeries with cardiopulmonary bypass (on‐pump) have a high risk for acute kidney injury (AKI). We tested ABT‐719, a novel α‐melanocyte‐stimulating hormone analog, for prevention of AKI in postoperative cardiac surgery patients.
Methods and Results
This phase 2b randomized, double‐blind, placebo‐controlled trial included adult patients with stable renal function undergoing high‐risk on‐pump cardiac surgery in the United States and Denmark. Participants received placebo (n=61) or cumulative ABT‐719 doses of 800 (n=59), 1600 (n=61), or 2100 μg/kg (n=59). Primary outcome was development of AKI based on Acute Kidney Injury Network (AKIN) criteria, measured utilizing preoperative creatinine value and maximum value within 48 hours and urine output within the first 42 hours postsurgery. Secondary outcomes included incidence of AKI based on maximal changes from baseline in novel AKI biomarkers over a 72‐hour period after clamp release and length of intensive care unit stays through 90 days postsurgery. A total of 65.5%, 62.7%, and 69.6% of patients in the 800‐, 1600‐, and 2100‐μg/kg groups, respectively, developed AKI (stages 1, 2, and 3 combined) versus 65.5% in the placebo group (for each pair‐wise comparison with placebo, P=0.966, 0.815, and 0.605, respectively). Adverse events occurred at a similar rate in all treatment groups.
Conclusions
ABT‐719 treatment did not lower AKI incidence using AKIN criteria, influence the elevations of novel biomarkers, or change 90‐day outcomes in patients after cardiac surgery.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique Identifier: NCT01777165.
Keywords: acute kidney injury, cardiopulmonary bypass, clinical trial, kidney, renal, renal function, α‐melanocyte‐stimulating hormone
Subject Categories: Cardiovascular Surgery, Revascularization, Biomarkers, Nephrology and Kidney
Introduction
Cardiac‐surgery–associated acute kidney injury (AKI) results in high resource utilization, in‐hospital mortality, permanent loss of renal function, and poor long‐term survival.1, 2, 3, 4 Patients undergoing cardiac surgery procedures performed with cardiopulmonary bypass (CPB; on‐pump) have a high risk of developing AKI.5
In the CPB setting, a potential etiology of cardiac‐surgery–related AKI is ischemia/reperfusion (I/R) injury, which can result in tubular epithelial and vascular endothelial damage.6, 7, 8 α‐Melanocyte‐stimulating hormone (α‐MSH) is an endogenous hormone that inhibits inflammatory, cytotoxic, and apoptotic pathways, thus preventing renal injury caused by I/R‐induced AKI.9, 10, 11 Additionally, α‐MSH has direct protective effects on the kidney, which may result from stimulation of the melanocortin receptors (MCRs) 1 and 3 in the outer renal medulla.12 The apical membrane of collecting ducts, including the principal cells in the cortical collecting duct, also express MCR1 and may play a protective role in I/R injury.11
ABT‐719 (formerly AP214 acetate), a novel synthetic α‐MSH analog with 6 lysine residues at the amino terminus, binds to MCRs 1, 3, 4, and 5 with high specificity.13 Preclinical studies in models of systemic and local inflammation demonstrate that ABT‐719 has anti‐inflammatory and organ‐protective effects.14, 15, 16, 17 In a phase 2 trial (N=77; CS007; NCT01256372), patients receiving ABT‐719 800 μg/kg (n=26) had reduced incidence of AKI defined by the Acute Kidney Injury Network (AKIN)18 and Risk, Injury, Failure, Loss, and End‐Stage Kidney Disease (RIFLE)19 criteria (Table 1), as well as 90‐day composite outcomes after cardiac surgery.20 We sought to substantiate these results by evaluating a higher range of ABT‐719 doses.
Table 1.
Staging Criteria for AKI
| Stage | Serum Creatinine | Urine Output |
|---|---|---|
| AKIN | ||
| 1 | Increase ≥0.3 mg/dL (≥26.5 μmol/L) OR increase to 1.5 to 2.0‐fold from baseline | <0.5 mL/kg per hour for 6 hours |
| 2 | >2.0 to 3.0‐fold from baseline | <0.5 mL/kg per hour for 12 hours |
| 3 | SeruT >3.0‐fold from baseline OR ≥4.0 mg/dL (≥354 μmol/L) with an acute increase ≥0.5 mg/dL (44 μmol/L) OR need for RRT | Anuria for 12 hour OR need for RRT |
| KDIGO | ||
| 1 | 1.5 to 1.9 times baseline OR ≥0.3 mg/dL (≥26.5 μmol/L) increase | <0.5 mL/kg per hour for 6 to 12 hours |
| 2 | 2.0 to 2.9 times baseline | <0.5 mL/kg per hour for ≥12 hours |
| 3 | 3.0 times baseline OR increase in serum creatinine to ≥4.0 mg/dL (≥353.6 μmol/L) OR initiation of renal replacement therapy OR, in patients <18 years, decrease in eGFR to <35 mL/min per 1.73 m2 | |
AKI indicates acute kidney injury; AKIN, Acute Kidney Injury Network; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; OR, odds ratio; RRT, renal replacement therapy.
Methods
Study Design
This was a phase 2b randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study designed to evaluate the safety and efficacy of ABT‐719 in preventing AKI associated with high‐risk, predefined on‐pump cardiac surgeries (ClinicalTrials.gov identifier: NCT01777165). Patients were stratified by screening estimated glomerular filtration rate (eGFR; 16–45 mL/min per 1.73 m2 and >45 mL/min per 1.73 m2), and 3 categories for type of surgery and were randomized into 4 treatment groups: ABT‐719 800, 1600, and 2100 μg/kg and placebo. The study was designed to enroll up to 240 patients in the United States and Denmark. ABT‐719 treatment in the phase 2 CS007 study resulted in a 46% relative risk reduction (RRR) of developing a positive AKIN score. Expecting sparse data and using a Fisher's exact test with a 0.050 1‐sided significance level, a sample size of 60 patients per treatment group was determined to have 94% power to detect an RRR of 46% in developing AKI, assuming a 65% rate of the primary endpoint in the placebo group without adjustment for multiple comparisons. A baseline visit occurred within 24 hours of surgery, with daily follow‐up visits up to day 7 (depending on the length of hospitalization); 3 additional follow‐up visits occurred approximately 30, 60, and 90 days postsurgery.
As shown in Table 2, treatments were administered as 6 intravenous infusions: (1) before skin incision (within 10 minutes); (2) before aortic cross‐clamp release, but ≥1 hour after the first dose; (3) 6 hours (±30 minutes) after clamp release; (4) 12 hours (±30 minutes) after clamp release; (5) 24 hours (±60 minutes) after clamp release; and (6) 48 hours (±60 minutes) after clamp release. Infusions were given over a 10‐minute period.
Table 2.
Dosing Schedule for Randomized Groups
| ABT‐719 Dose (μg/kg) | Before Skin Incision | At Clamp Release | After Clamp Release | |||
|---|---|---|---|---|---|---|
| 6 hours | 12 hours | 24 hours | 48 hours | |||
| 800 | 200 | 400 | 200 | 0 | 0 | 0 |
| 1600 | 300 | 600 | 300 | 200 | 200 | 0 |
| 2100 | 300 | 600 | 300 | 300 | 300 | 300 |
Skin incision refers to the cardiac surgery.
An independent data monitoring committee monitored the safety and tolerability of treatment with ABT‐719. The study protocol and informed consent were reviewed and approved by the institutional review board or independent ethics committee at each study site, and the study was compliant with the Declaration of Helsinki. The US Food and Drug Administration was consulted and provided feedback on the study protocol. All subjects gave informed consent.
Eligibility Criteria and Recruitment
Enrolled patients were ≥18 years old and provided written informed consent before any study‐specific procedures were performed (Figure 1). Eligible patients were required to have stable renal function and must have been undergoing a predefined on‐pump cardiac surgery with a high‐risk of developing AKI in 1 of 3 categories: (1) coronary artery bypass grafting (CABG) and surgery of ≥1 cardiac valves (valve[s] surgery) or surgery of the aortic root or ascending part of the aorta combined with CABG and/or valve(s) surgery; (2) surgery of >1 cardiac valve(s) or surgery of the aortic root or ascending part of the aorta; or (3) patients undergoing either CABG or single‐valve surgery were required to have chronic kidney disease (CKD; eGFR 16–59 mL/min per 1.73 m2). Patients with an eGFR ≤15 mL/min per 1.73 m2, requiring reoperation of ≥1 valves within 3 months of the original surgery, with a diagnosis of endocarditis, or with a known AKI during the 4 weeks before the study were excluded.
Figure 1.
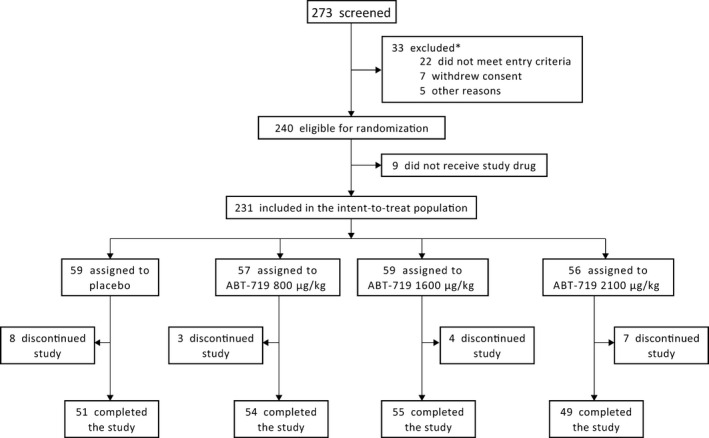
Disposition of patients in CONSORT flow diagram. *Reasons for exclusion were not mutually exclusive.
Efficacy and Safety Analyses
Primary and secondary outcomes
The primary efficacy endpoint was evaluated from the modified intent‐to‐treat analysis set (ie, all randomized patients who received ≥1 infusion of study drug). The primary endpoint was development of AKI defined by the AKIN criteria, utilizing the preoperative creatinine value, and the maximum value within 48 hours of surgery and the urine output within the first 42 hours postsurgery. The AKIN criteria were the most contemporary criteria available at the time of the initial study design; the Kidney Disease: Improving Global Outcomes (KDIGO) criteria (Table 1) were published during the course of protocol development. Major secondary efficacy endpoints included the percentage of patients who developed ≥1 of the following composite endpoints: death, need for any renal replacement therapy (RRT) during the 90‐day postoperative period, or ≥25% reduction in eGFR from baseline to 90 days postsurgery (major adverse kidney events [MAKE])21; percentage of patients who developed AKI as defined by the RIFLE19 or KDIGO22 criteria; maximal changes from baseline in novel AKI biomarkers over a 72‐hour period after clamp release; and length of intensive care unit (ICU) and hospital stays. Per the study protocol, the aforementioned endpoints were only followed out to 90 days postsurgery. Information regarding perfusion methods was not collected as part of this study.
Safety analysis
Patients were asked to report all adverse events (AEs) at the 30‐day follow‐up visit; AEs that were spontaneously reported by the patient at the day 60 and day 90 follow‐up visits were recorded. AEs and serious AEs were categorized by Medical Dictionary for Regulatory Activities (MedDRA) system organ class (SOC) and preferred term. The composite endpoint of major adverse cardiac events (MACE), which included patients with cardiac disorders, irregular heart rates, and nervous system disorders based on the MedDRA SOC and preferred term classification, was assessed for up to 120 days postsurgery.
Statistical Analysis
Efficacy analyses were conducted on the modified intent‐to‐treat analysis set (i.e., all randomized patients who received ≥1 infusion of study drug). Safety analyses were conducted on the safety analysis set, which also includes all randomized patients who received ≥1 infusion of study drug. The primary efficacy analysis was pair‐wise comparisons between each ABT‐719 dose group (shown in Table 2) versus placebo in the percentage of patients who developed AKI as defined by the AKIN scoring criteria, using the Cochran–Mantel–Haenszel test to control for stratification factors (serum creatinine [SCr]‐based eGFR and 3 categories for type of surgery) through 48 hours postsurgery (48 hours for SCr and 42 hours for urine output). Dose responses among the ABT‐719 dose groups in the primary efficacy endpoint were evaluated using a Cochran–Armitage test for trend. The Cochran–Mantel–Haenszel test was also used to analyze AKI incidence as defined by RIFLE and KDIGO, 90‐day composite/MAKE, and AKI biomarker elevations. Treatment effects were further evaluated using ANCOVA models, with treatment group as a factor and baseline as a covariate for AKI biomarkers. Per protocol, ANOVA was used to analyze ICU stay and days spent in the hospital. A post‐hoc analysis of ICU stay and days spent in the hospital was also run using the Kruskal–Wallis test. AKI biomarker elevations were evaluated according to 1‐fold (1×) or 2‐fold (2×) elevations from baseline to the peak values measured within 72 hours, given the wide range of baseline values expected with these markers. P values are reported without adjustment for multiplicity
Results
Patients
Disposition
Patient disposition is shown in Figure 1. A total of 240 patients were randomized and 9 dropped out before receiving study drug. All 231 patients included for analysis received at least 1 dose of study drug. Twenty‐two patients, all of whom received 1 or more doses of study drug, discontinued the study; the most common reason for discontinuation was AEs (n=11; 4.8%). A total of 209 patients completed the study receiving all dose administrations of study drug, including 51, 54, 55, and 49 patients from the placebo, 800‐, 1600‐, and 2100‐μg/kg ABT‐719 groups, respectively (Figure 1).
Demographics and baseline characteristics
Baseline characteristics, demographics, and comorbidities were similar among patients in the 4 treatment groups (Table 3). Mean age was 69.1 years, 71.4% were men, the mean eGFR calculated from the preoperative creatinine value was 70.6 mL/min per 1.73 m2, 29.0% had a history of diabetes mellitus, 27.3% had a history of heart failure, and the mean time on CPB was 130.9 minutes.
Table 3.
Demographics and Baseline Characteristics
| Characteristic | Placebo (n=59) | ABT‐719 | ||
|---|---|---|---|---|
| 800 μg/kg (n=57) | 1600 μg/kg (n=59) | 2100 μg/kg (n=56) | ||
| Age, y | 71.1 (9.4) | 68.9 (10.2) | 67.7 (12.5) | 68.6 (10.6) |
| Male, n (%) | 46 (78.0) | 40 (70.2) | 43 (72.9) | 36 (64.3) |
| Weight, kg | 86.4 (15.1) | 84.1 (16.8) | 84.1 (17.1) | 90.9 (19.6) |
| Race, n (%) | ||||
| White | 56 (94.9) | 53 (93.0) | 56 (94.9) | 50 (89.3) |
| Black | 3 (5.1) | 4 (7.0) | 1 (1.7) | 5 (8.9) |
| Asian | 0 | 0 | 1 (1.7) | 0 |
| Other | 0 | 0 | 1 (1.7) | 1 (1.8) |
| Systolic blood pressure, mm Hg | 117.6 (28.4) | 115.0 (26.8) | 109.8 (24.6) | 114.8 (23.2) |
| Diastolic blood pressure, mm Hg | 57.9 (14.3) | 58.2 (13.0) | 57.0 (10.7) | 57.3 (11.9) |
| eGFR, mL/min per 1.73 m2 | 69.5 (21.6)a | 72.2 (23.8)a | 70.1 (20.8)a | 70.6 (20.7) |
| Serum creatinine, mg/dL | 1.1 (0.39) | 1.1 (0.41) | 1.1 (0.34) | 1.1 (0.42) |
| Hemoglobin, g/dL | 13.0 (1.8)a | 12.6 (1.9) | 12.6 (2.0) | 12.7 (2.0) |
| Albumin, g/L | 3.7 (0.4) | 4.4 (5.0) | 3.8 (0.5) | 4.5 (6.1) |
| Comorbidities at baseline, n (%) | ||||
| Diabetes mellitus | 16 (27.1) | 16 (28.1) | 18 (30.5) | 17 (30.4) |
| CKD | 23 (39.0) | 22 (38.6) | 23 (39.0) | 19 (33.9) |
| Hypertension | 47 (79.7) | 46 (80.7) | 44 (74.6) | 43 (76.8) |
| Heart failure | 15 (25.4) | 20 (35.1) | 15 (25.4) | 13 (23.2) |
| Duration of CPB, minutes | 134.9 (80.6)a | 130.1 (54.1)a | 128.8 (51.7)a | 129.8 (53.8)a |
| Blood lossb, mL | ||||
| Mean (SD) | 589.7 (1037.9) | 463.5 (485.7) | 680.1 (1039.6) | 534.5 (643.7) |
| Median | 250.0 | 300.0 | 450.0 | 300.0 |
CKD indicates chronic kidney disease; CPB, cardiopulmonary bypass; eGFR, estimated glomerular filtration rate; SD, standard deviation. Data are presented as mean (SD) unless otherwise noted.
At least 1 missing assessment.
Placebo, n=30; ABT‐719 800 μg/kg, n=33; ABT‐719 1600 μg/kg, n=39; ABT‐719 2100 μg/kg, n=33.
Primary Outcome
The percentage of patients who developed AKI according to the AKIN (Figure 2) criteria was similar among treatment groups (Figure 2A). A total of 65.5% (95% CI, 51.4–77.8), 62.7% (95% CI, 49.2–75.0), and 69.6% (95% CI, 55.9–81.2) of patients in the 800‐, 1600‐, and 2100‐μg/kg groups, respectively, developed AKI (stages 1, 2, and 3 combined) compared with 65.5% (95% CI, 51.9–77.5) in the placebo group (pair‐wise comparison with placebo, P=0.966, P=0.815, and P=0.605, respectively). The percentage of patients who developed stage 3 AKI was numerically lower in all ABT‐719 groups (5.5% for 800, 5.1% for 1600, and 5.4% for 2100 μg/kg) compared to the placebo arm (12.1%; Figure 2A); however, none were statistically different from placebo.
Figure 2.
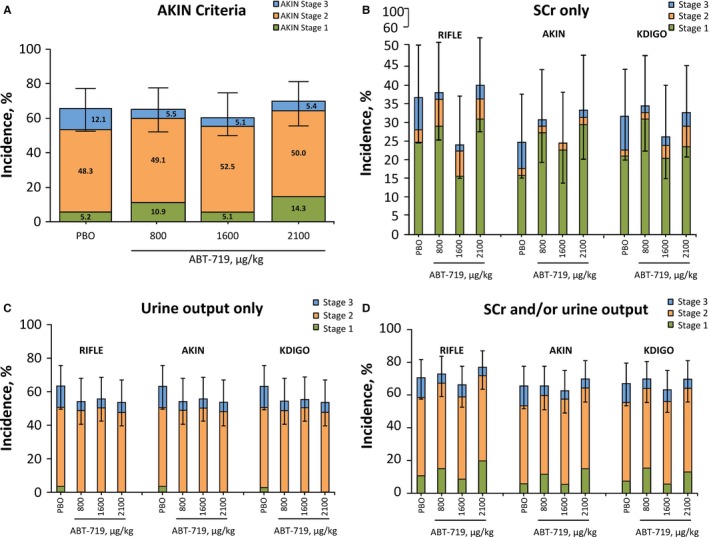
Effect of ABT‐719 treatment on AKI incidence. *AKI incidence was determined by (A) the AKIN criteria, (B) RIFLE, AKIN, and KDIGO criteria using SCr only, (C) RIFLE, AKIN, and KDIGO criteria using urine output only, and (D) RIFLE, AKIN, and KDIGO criteria using both SCr and/or urine output. Any patient receiving RRT was classified as stage 3 AKI according to the AKIN and KDIGO criteria. AKI indicates acute kidney injury; AKIN, Acute Kidney Injury Network; KDIGO, Kidney Disease: Improving Global Outcomes; PBO, placebo; RIFLE, Risk, Injury, Failure, Loss, and End‐Stage Kidney Disease; RRT, renal replacement therapy; SCr, serum creatinine. *95% CI for any stage AKI incidence.
Secondary Outcomes
AKI Incidence by RIFLE and KDIGO criteria
There were no significant differences in the percentage of patients who developed AKI among the treatment groups based on the RIFLE and KDIGO AKI scoring criteria (data not shown). Comparing the incidence of AKI using RIFLE and KDIGO criteria based on SCr only, urine output only, or both SCr and/or urine output did not alter the conclusion (Figure 2B through 2D). However, AKI incidence was relatively higher using the RIFLE criteria compared with KDIGO criteria because the RIFLE criteria include reduction in eGFR (Figure 2B and 2D). The efficacy of treatment with ABT‐719 was further assessed by comparing the percentage of patients who developed ≥1 of the 90‐day composite outcomes (MAKE). Overall, there was no significant difference between patients receiving placebo and ABT‐719 (P=0.250). Compared to the placebo group (n=12; 20.3%; 95% CI, 11.0–32.8), similar percentages of patients in the 800‐μg/kg group (n=7; 12.3%; 95% CI, 5.1–23.7), 1600‐μg/kg group (n=6; 10.2%; 95% CI, 3.8–20.8), and 2100‐μg/kg group (n=11; 19.6%; 95% CI, 10.2–32.4) developed a MAKE event (Figure 3). When events were examined individually, participants needed RRT across all ABT‐719 treatment groups (4 of 172; 2.3%) at a numerically lower, albeit not statistically significant, rate compared to patients in the placebo group (5 of 59; 8.5%; P=0.055; Figure 3).
Figure 3.
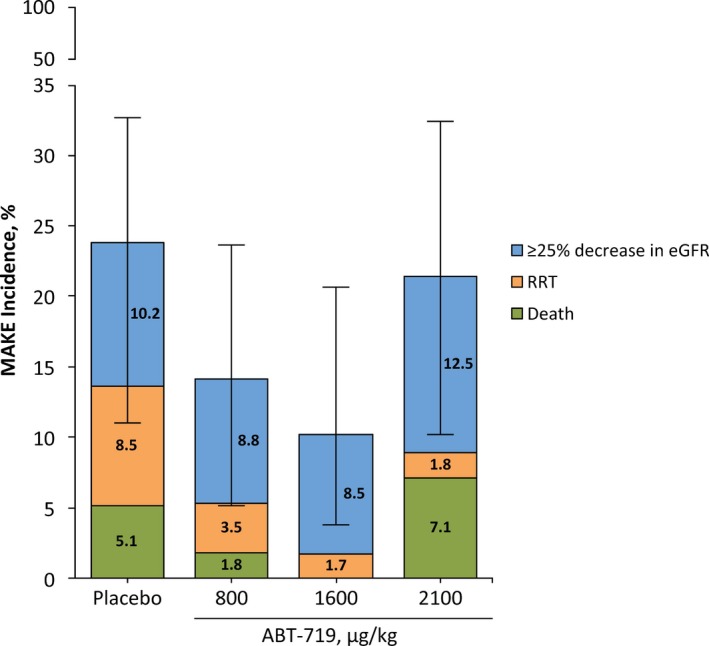
Effect of ABT‐719 treatment on incidence of composite outcomes (MAKE). *Percentage of patients developing at least 1 of the composite events of death, needing RRT during the 90‐day postoperative period or ≥25% reduction in eGFR at day 90. eGFR indicates estimated glomerular filtration rate; MAKE, major adverse kidney events; RRT, renal replacement therapy. *95% CI for any composite event.
Length of hospitalization and ICU stays
The respective median (range) lengths of hospital stay within 90 days after surgery for the ABT‐719 800‐, 1600‐, and 2100‐μg/kg and placebo groups were 8.0 (5.0–90.0), 8.0 (5.0–90.0), 10.0 (5.0–90.0), and 9.0 (5.0–90.0) days. Using ANOVA, P values were P=0.124, P=0.025, and P=0.773 versus placebo, respectively; P values were ≥0.06 using the Kruskal–Wallis test (Figure 4). The respective median (range) lengths of ICU stays for the ABT‐719 800‐, 1600‐, and 2100‐μg/kg and placebo groups were 3.0 (1.0–32.0), 2.0 (2.0–17.0), 4.0 (1.0–39.0), and 3.0 (2.0–47.0) days. Using ANOVA, P values were P=0.258, P=0.073, and P=0.906 versus placebo, respectively; P values were ≥0.09 using the Kruskal–Wallis test (Figure 4).
Figure 4.
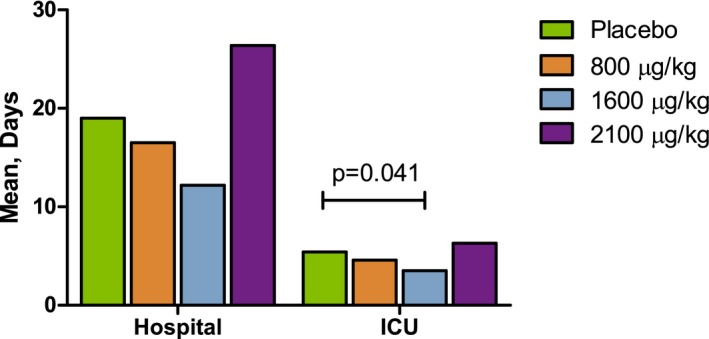
Effect of ABT‐719 treatment on the length of hospitalization. Length of stay in the hospital and intensive care unit (ICU) by 90 days postsurgery. Using the Kruskal–Wallis test for the comparisons between placebo and ABT‐719, P values were ≥0.06 for hospitalization and ≥0.09 for ICU stay.
Changes in biomarkers of tubular injury
Serum and urine samples were collected from study participants over a 72‐hour period after clamp release to assess the expression of biomarkers that are predictive of early‐stage AKI. There were no significant differences in the percentage of patients with 1× and 2× increases from baseline in expression of serum and urine neutrophil gelatinase‐associated lipocalin (NGAL), urine interleukin‐18 (IL‐18), and urine kidney injury molecule‐1 (KIM‐1) among the 4 treatment groups (Figure 5).
Figure 5.
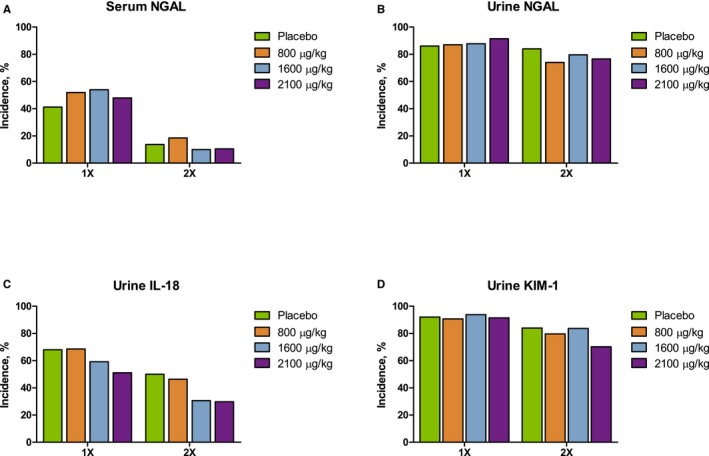
Increase in AKI biomarkers over a 72‐hour period after clamp release. (A) Serum NGAL, (B) urine NGAL, (C) urine IL‐18, and (D) urine KIM‐1. AKI indicates acute kidney injury; IL‐18, interleukin‐18; KIM‐1, kidney injury molecule 1; NGAL, neutrophil gelatinase‐associated lipocalin.
Safety
Fewer patients in the 800‐ and 1600‐μg/kg groups, but more patients in the 2100‐μg/kg group, developed serious AEs (21.1%, 22.0%, and 48.2%, respectively, vs 44.1% for placebo) and severe AEs (death, life‐threatening, required or prolonged hospitalization, or caused permanent disability; 17.5%, 20.3%, and 42.9%, respectively, vs 30.5% for placebo; Table 4). In addition, numerically, more patients discontinued from the study because of AEs in the placebo (6.8%) and 2100‐μg/kg groups (7.1%) compared to the 800‐ (3.5%) and 1600‐μg/kg groups (1.7%). Hypersensitivity, atrial fibrillation, nausea, and surgery‐related pain were the most common AEs. There were no clinical differences in MACE events among treatment groups through 120 days postsurgery (placebo, 5.1%; ABT‐719 800 μg/kg, 5.3%; ABT‐719 1600 μg/kg, 6.8%; ABT‐719 2100 μg/kg, 12.5%; Table 4).
Table 4.
Summary of Incidence of AEs
| Type of Event, n (%) | Placebo (n=59) | ABT‐719 | ||
|---|---|---|---|---|
| 800 μg/kg (n=57) | 1600 μg/kg (n=59) | 2100 μg/kg (n=56) | ||
| Any AE | 56 (94.9) | 48 (84.2) | 56 (94.9) | 54 (96.4) |
| Any serious AE | 26 (44.1) | 12 (21.1) | 13 (22.0) | 27 (48.2) |
| Any severe AE | 18 (30.5) | 10 (17.5) | 12 (20.3) | 24 (42.9) |
| Any AE leading to discontinuation of study drug | 4 (6.8) | 2 (3.5) | 1 (1.7) | 4 (7.1) |
| MACE 120 | 3 (5.1) | 3 (5.3) | 4 (6.8) | 7 (12.5) |
AE indicates adverse event; MACE 120, major adverse cardiac events up to 120 days after surgery.
Discussion
Treatment with ABT‐719 did not reduce the overall incidence of AKI as assessed using the AKIN, RIFLE, and KDIGO classifications, nor did it influence elevations of novel biomarkers or clinical outcomes through 90 days. NGAL, KIM‐1, and IL‐18 levels were elevated at baseline and further increased in the majority of patients; these elevations did not differ significantly among the groups. The safety profile of ABT‐719 was relatively similar across all treatment groups with numerically higher numbers of adverse events in the placebo and 2100‐μg/kg groups.
Effective prophylactic treatments for AKI are needed. Approximately 5% to 30% of patients develop AKI after cardiac surgery, with 1% to 5% of patients requiring RRT.2, 23, 24, 25 Although the risk of mortality after major cardiac surgery is 2% to 8%, the overall mortality among patients who develop postoperative AKI can exceed 50%, which suggests that AKI may contribute in the causal pathway of postoperative death.26, 27 In the present phase 2b study (M13‐796), the efficacy and safety of ABT‐719 for the prevention and treatment of AKI were analyzed. This was perhaps the fastest enrolling trial for the prevention of AKI after cardiac surgery and was the first to prospectively use both SCr and urine output data (standardized definitions of AKI) in the primary endpoint. However, we failed to confirm the previous phase 2 trial results with this agent and our study serves as a cautious reminder of how early phase 2 trials can yield results that fail to be confirmed in larger, more tightly controlled studies involving a larger number of enrolling sites. Furthermore, our trial illustrates the difficulties in using a primary endpoint that relies on changes in SCr and urine output, given that these measures can be manipulated by use of intravenous fluid and diuretics and do not represent an outcome that is measurable by clinical symptoms or imaging or physiological studies.
Cardiac‐surgery–associated AKI remains a clinical challenge. Risk factors for postoperative AKI include the type of procedure, prolonged bypass time, CKD, preexisting congestive heart failure, diabetes mellitus, female sex, and advanced age.2, 23, 28, 29 I/R injury associated with CPB may trigger strong systemic inflammatory response syndrome,6, 7, 30 resulting in oxidative stress, apoptotic cell death, and/or acute tubular necrosis.31, 32, 33 Because AKI occurs at similar rates in on‐pump and off‐pump cardiac surgery, neurohormonal activation, cholesterol microembolization, and hemodynamic instability are all considered potential contributors to cardiac‐surgery–associated AKI.34 The relative contributions of atheroembolism, I/R, inflammation, and other potential pathogenic mechanisms, including neurohormonal activation and injurious cell signaling, remain poorly understood. We demonstrated that the use of AKIN, RIFLE, and KDIGO criteria demonstrates largely concordant results in this, the first clinical trial in which they have been specified as outcomes.
Changes in SCr levels may take time to evolve and do not become detectable until approximately 50% of kidney function is lost in patients with previously normal GFR. Biomarkers of AKI, such as NGAL, KIM‐1, and IL‐18, may be potentially beneficial for early detection, diagnosis, and prognosis of AKI after cardiac surgery.35, 36 However, in our study and others, these markers are commonly elevated at baseline, and thus a relatively large change from baseline (doubling or tripling) would be needed to detect AKI in this setting.37, 38, 39 In our study, the results in functional and novel injury markers were concordant in demonstrating no benefit of ABT‐719 treatment.
Several studies have shown that postoperative AKI is associated with increased resource utilization, including prolonged hospitalization, longer ICU stays, higher hospital costs, and increased mortality risk.1, 2 We observed that patients in our trial experienced a range of hospital stays, and there was no clear signal that consistently suggested that ABT‐719 was influencing clinical factors (eg, AKI, pain, functional status, and wound healing) related to resource utilization.
Our study has several strengths. First, it was a randomized, multidose, placebo‐controlled study in a relevant patient population at high risk for AKI. Second, the study had appropriate follow‐up and assessed guideline‐recommended definitions of AKI, novel biomarkers, and short‐ and medium‐term clinical outcomes. This trial showed progress in trial design of pharmacological agents for the treatment of cardiac‐surgery–related AKI, and information gathered from this clinical trial will help inform future AKI studies.
Our study has all the limitations inherent in small, randomized trials seeking to find differences in measured outcomes post‐treatment with a novel agent. It is possible that the therapy simply was not biologically active in this application, was given in doses that were too small, or was not given for a sufficient length of time. We attempted to manage limitations by varying both the dose and duration of treatment; however, we could not identify a beneficial signal on the primary endpoint. Additionally, it is possible that we simply did not have a sensitive outcome of AKI and cannot measure renal injury to a sufficiently precise degree to detect benefit or harm of ABT‐719.
Measurement of GFR, using the inulin or isotype method, may have provided a more‐sensitive assessment of renal injury in this cohort. The patient population enrolled in this study was predominantly white and male, which limits the generalizability of the results. We did not collect detailed information regarding the hemoperfusion methods used in each case, and this may be a source of unmeasured confounding. It is possible that the trial was simply too small and that a much larger trial would have delivered a more‐secure result with respect to efficacy of ABT‐719. The relatively large reduction of AKI incidence with 800‐μg/kg ABT‐719 treatment observed in the previous phase 2 trial (NCT01256372) may have been attributed to alpha error attributed to the small study size (n=26 in the 800‐μg/kg dose group). Finally, it may be possible that the demonstrated preclinical efficacy of ABT‐719 in preventing AKI may not translate to the clinical setting because of the differences in the pathophysiology of AKI in humans and animal models.40 Patterns of damage observed in human AKI are not necessarily consistent with those observed in animal models. Future studies of ABT‐719 may require a change from the perioperative dosing schedule utilized in the present study. Preoperative dosing to ensure adequate bioavailability of ABT‐719 may increase the likelihood of reduced AKI incidence.
In summary, treatment with ABT‐719 did not lower AKI incidence using the AKIN criteria, nor did it influence the elevation of novel markers of renal tubular injury or clinical outcomes through 90 days.
Author Contributions
All authors contributed equally to this manuscript to the study design, data collection, analysis, and interpretation, and writing and critical review of the manuscript.
Sources of Funding
AbbVie provided funding for study M13‐796 and was involved in the study design, study execution, collection, analysis, and interpretation of data, and in writing, reviewing, and approval of the report and decision to submit the manuscript for publication. All authors had access to study results, and the lead author vouches for the accuracy and completeness of the data reported. All authors had the final decision to submit the publication. The study was overseen by a data review committee. Dr Peter McCullough had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
McCullough has been a consultant to AbbVie. Bennett‐Guerrero has the following to disclose: consultant/advisory board: AbbVie (<$5000), Quark Pharmaceuticals (<$5000); and research support: AbbVie (paid to employer). Chawla has been a consultant to AbbVie. Beaver has been a consultant to AbbVie. Mehta has the following disclosures: institution received funding from AbbVie for the conduct of the study, research support, and for activities outside of the submitted work. He has received grants from the International Safety Adverse Events Consortium and Thrasos, AM Pharma, Eli Lilly, Ardea, Astute Inc, CSL Behring, GSK, Baxter, Sova, Astellas, Sanofi‐Aventis, Ferring Research, and Isis Pharmaceuticals. Molitoris has been a consultant to AbbVie. Eldred is an employee of AbbVie and owns AbbVie stock. Ball has been an employee of AbbVie and may own AbbVie stock. Lee has been an employee of AbbVie and may own AbbVie stock. Houser has been an employee of AbbVie and may own AbbVie stock. Khan has been an employee of AbbVie and may own AbbVie stock.
Acknowledgments
Assistance with manuscript and figure files was provided by Adebusola Ajibade, PhD, of AbbVie. Formatting assistance was provided by Lisa Havran, Katherine Groschwitz, Todd Waldron, and John E. Fincke of Complete Publication Solutions, LLC (North Wales, PA); this formatting assistance was supported by AbbVie.
(J Am Heart Assoc. 2016;5:e003549 doi: 10.1161/JAHA.116.003549)
References
- 1. Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long‐term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta‐analysis. Am J Kidney Dis. 2009;53:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dasta JF, Kane‐Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. [DOI] [PubMed] [Google Scholar]
- 3. Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. [DOI] [PubMed] [Google Scholar]
- 4. Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37:2552–2558. [DOI] [PubMed] [Google Scholar]
- 5. Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92:1539–1547. [DOI] [PubMed] [Google Scholar]
- 6. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. [DOI] [PubMed] [Google Scholar]
- 7. Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. [DOI] [PubMed] [Google Scholar]
- 8. Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. [DOI] [PubMed] [Google Scholar]
- 9. Chiao H, Kohda Y, McLeroy P, Craig L, Linas S, Star RA. Alpha‐melanocyte‐stimulating hormone inhibits renal injury in the absence of neutrophils. Kidney Int. 1998;54:765–774. [DOI] [PubMed] [Google Scholar]
- 10. Jo SK, Yun SY, Chang KH, Cha DR, Cho WY, Kim HK, Won NH. alpha‐MSH decreases apoptosis in ischaemic acute renal failure in rats: possible mechanism of this beneficial effect. Nephrol Dial Transplant. 2001;16:1583–1591. [DOI] [PubMed] [Google Scholar]
- 11. Kohda Y, Chiao H, Star RA. alpha‐Melanocyte‐stimulating hormone and acute renal failure. Curr Opin Nephrol Hypertens. 1998;7:413–417. [DOI] [PubMed] [Google Scholar]
- 12. Lee YS, Park JJ, Chung KY. Change of melanocortin receptor expression in rat kidney ischemia‐reperfusion injury. Transplant Proc. 2008;40:2142–2144. [DOI] [PubMed] [Google Scholar]
- 13. Jonassen TEN, Frokiaer J, Nielsen S. Organ protective effect of a novel alphaMSH analogue AP214 in ischemia induced acute renal failure in rats. J Am Soc Nephrol (Abstract). 2006;S17:326A. [Google Scholar]
- 14. Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frokiaer J, Nielsen S, Star RA. AP214, an analogue of alpha‐melanocyte‐stimulating hormone, ameliorates sepsis‐induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kristensen J, Jonassen TE, Rehling M, Tonnesen E, Sloth E, Nielsen S, Frokiaer J. The alpha‐MSH analogue AP214 attenuates rise in pulmonary pressure and fall in ejection fraction in lipopolysaccharide‐induced systemic inflammatory response syndrome in pigs. Clin Physiol Funct Imaging. 2011;31:54–60. [DOI] [PubMed] [Google Scholar]
- 16. Montero‐Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M. The melanocortin agonist AP214 exerts anti‐inflammatory and proresolving properties. Am J Pathol. 2011;179:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simmons MN, Subramanian V, Crouzet S, Haber GP, Colombo JR Jr, Ukimura O, Nielsen S, Gill IS. Alpha‐melanocyte stimulating hormone analogue AP214 protects against ischemia induced acute kidney injury in a porcine surgical model. J Urol. 2010;183:1625–1629. [DOI] [PubMed] [Google Scholar]
- 18. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure ‐ definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan S, Nielsen S, Beckert M, Houser M, Wang D. Effects of ABT‐719 on incidence of acute kidney injury during cardiac surgery procedures. Presented at: American Society of Nephrology Kidney Week 2013 (Abstract), November 2013; Atlanta, GA.
- 21. Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long‐term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 23. Kellerman PS. Perioperative care of the renal patient. Arch Intern Med. 1994;154:1674–1688. [PubMed] [Google Scholar]
- 24. Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in‐hospital mortality and long‐term survival. J Am Soc Nephrol. 2005;16:195–200. [DOI] [PubMed] [Google Scholar]
- 25. Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216; quiz 2208. [DOI] [PubMed] [Google Scholar]
- 26. Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. [DOI] [PubMed] [Google Scholar]
- 27. Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, Paganini EP. ARF after open‐heart surgery: influence of gender and race. Am J Kidney Dis. 2003;41:742–751. [DOI] [PubMed] [Google Scholar]
- 28. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. [DOI] [PubMed] [Google Scholar]
- 29. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. [DOI] [PubMed] [Google Scholar]
- 30. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. [DOI] [PubMed] [Google Scholar]
- 31. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. [DOI] [PubMed] [Google Scholar]
- 32. Parida S, Badhe AS. Cardiac surgery‐associated acute kidney injury. J Anesth. 2013;27:433–446. [DOI] [PubMed] [Google Scholar]
- 33. Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens. 2000;9:427–434. [DOI] [PubMed] [Google Scholar]
- 34. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Vaijyanath P, Reddy S, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Ng J, Chrolavicius S, Yusuf S. Coronary Investigators. Off‐pump or on‐pump coronary‐artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. [DOI] [PubMed] [Google Scholar]
- 35. Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. [DOI] [PubMed] [Google Scholar]
- 36. Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Gorlich D, Kellum JA, Zarbock A. Urinary TIMP‐2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akrawinthawong K, Ricci J, Cannon L, Dixon S, Kupfer K, Stivers D, Alexander P, David S, McCullough PA. Subclinical and clinical contrast‐induced acute kidney injury: data from a novel blood marker for determining the risk of developing contrast‐induced nephropathy (ENCINO), a prospective study. Ren Fail. 2015;37:187–191. [DOI] [PubMed] [Google Scholar]
- 38. Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase‐associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res. 2009;32:91–98. [DOI] [PubMed] [Google Scholar]
- 39. Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV. Blood kidney injury molecule‐1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heyman SN, Rosenberger C, Rosen S. Experimental ischemia‐reperfusion: biases and myths‐the proximal versus distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. [DOI] [PubMed] [Google Scholar]


