Introduction
Chronic kidney disease (CKD) affects 13% of the US population.1 Although a significant proportion of these patients progress to end‐stage renal disease (ESRD) requiring renal replacement therapy (RRT)2 or renal transplantation, cardiovascular disease remains the most common cause of mortality and accounts for 53% of all deaths with a known cause in patients on dialysis.3 Critically, cardiovascular disease also remains the leading cause of death after renal transplantation. Appropriate management of cardiovascular disease in this very high‐risk population is of paramount importance.
Pathobiological processes that underpin the progression and severity of cardiovascular disease in CKD include accelerated atherosclerosis and continuous reduction in left ventricular (LV) function as renal function declines.1 While on hemodialysis, these processes accelerate. Importantly, the risk of developing pulmonary hypertension (PH) also rises proportionately to the duration of hemodialysis.4 In contrast to dialysis, renal transplantation can help prevent the progression of pathological cardiovascular processes. Renal transplantation can potentially reverse myocardial damage that is thought to result from prolonged exposure to uremic toxins and improve LV systolic function.5, 6, 7
In this review, we provide a contemporary overview of the pre‐ and perioperative cardiovascular evaluation of patients with ESRD who are considered suitable candidates for renal transplantation. In addition, we review the evidence‐based guidelines on optimal management of cardiovascular disease in patients with advanced CKD with particular focus on coronary artery disease (CAD), congestive heart failure (CHF), valvular disease, and PH. The overall aim is to identify the subset of patients who may maximally benefit from renal transplantation. Finally, we provide evidence‐based recommendations for diagnosis, management, and application in clinical practice.
CAD in Patients With ESRD
CAD is highly prevalent in patients with ESRD largely because of the presence of comorbidities such as hypertension, diabetes mellitus, dyslipidemia, obesity, and tobacco use.8 The incidence of CAD in patients initiating dialysis is up to 38%, with a relative risk of 5‐ to 20‐fold that of the general population.9 The uremic environment may also contribute to the higher prevalence and accelerated progression of CAD.1, 10 Moreover, atherosclerosis is an inflammatory process.11, 12 Patients with ESRD have high levels of C‐reactive protein and proinflammatory cytokines,1, 10, 13, 14 which predisposes them to plaque formation. Endothelial dysfunction and high oxidative stress further drive atherosclerosis and are exacerbated in the setting of the activated renin–angiotensin–aldosterone system in CKD and ESRD.1, 10, 13, 14 Moreover, therapies for secondary prevention of CAD such as statins and angiotensin‐converting enzyme (ACE) inhibitors may have diminished clinical benefit in ESRD.12, 15
Coronary plaques in patients with ESRD exhibit extensive heterotopic calcification.16 On computed tomography coronary angiography in young patients with ESRD, a disproportionate incidence of high calcium scores is detected with the probability of coronary artery calcification increasing with longer durations of dialysis.16 Calcification occurs in smooth muscle cells in the media or in the neointima of atherosclerotic plaques, contributing to vascular stiffness and death from CAD.17 In addition to increased plaque complexity, the clinical presentation of CAD is also different. Patients with advanced CKD are more likely to present with acute coronary syndrome as the first manifestation of CAD, as opposed to angina in patients without renal disease.18
Noninvasive Imaging to Assess CAD
Many sets of guidelines aim to guide cardiovascular evaluation in renal transplantation candidates, but there is no universal consensus on an optimal approach. The 2014 American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation in the general population undergoing noncardiac surgery do not recommend testing for asymptomatic patients with a functional capacity considered to be moderate (defined as ≥4 metabolic equivalents).19 Testing in patients with poor functional capacity (<4 metabolic equivalents) or unknown functional status is recommended to be based on combined clinical and surgical risk factors, with noninvasive tests performed for patients at elevated risk19. However, it is unclear whether these recommendations should be applied to potential candidates for transplantation.20 A study of 204 candidates for renal transplantation reported that 80% of patients with no active cardiac conditions had a functional status of ≥4 metabolic equivalents,20, 21 which in part reflects the relatively younger age of transplant candidates. Consequently, a functional status of ≥4 metabolic equivalents is not a reliable predictor of CAD in this population.20, 21 Instead, the 2012 AHA/ACC scientific statement regarding cardiac evaluation in renal transplantation candidates recommends that the decision to proceed with noninvasive stress testing in patients with no active cardiac conditions should be based on the presence of multiple risk factors for CAD most relevant to the transplantation population, regardless of functional status. These risk factors include diabetes mellitus, prior cardiovascular disease, >1 year on dialysis, left ventricular hypertrophy (LVH), age >60 years, smoking, hypertension, and dyslipidemia.20, 22 Although the specific number of risk factors to proceed with stress testing remains to be determined, the AHA/ACC guidelines suggest the presence of ≥3 risk factors as a reasonable threshold for noninvasive testing.20 Guidelines from the Kidney Disease Outcomes Quality Initiative (KDOQI) recommend annual evaluation for CAD in diabetic patients on the waiting list for transplantation if the initial evaluation for CAD at the start of dialysis is negative. In high‐risk patients without diabetes mellitus on the transplantation waitlist (≥2 traditional risk factors, known history of CAD, peripheral vascular disease, and LV ejection fraction [LVEF] ≤40%), evaluation for CAD every 24 months is recommended. Patients on hemodialysis with an LVEF ≤40% or those with new symptoms of concern regarding ischemic heart disease are recommended to be evaluated continuously for CAD.23, 24
Noninvasive testing with electrocardiogram (ECG), transthoracic echocardiogram, pharmacological stress echocardiography, and nuclear imaging (with single photon emission computed tomography [SPECT] or cardiac positron emission tomography [PET]) are suggested as the first steps in investigating for presence of CAD. Patients should have baseline ECGs to evaluate for Q waves, ST‐T changes, T wave inversions, and left bundle branch block, which previously have been shown to be predictive of CAD.25 Exercise ECG is not recommended, given abnormal baseline ECGs and overall poor exercise tolerance in this patient population. A baseline transthoracic echocardiogram performed at dry weight is also important because it can help identify impaired LVEF and wall motion abnormalities, which may be signs of prognostically significant CAD.26 A normal cardiac stress test has a high negative predictive value for cardiovascular events27, 28 in the perioperative and follow‐up periods, as shown in a study of renal transplant candidates undergoing preoperative SPECT.29 A hybrid SPECT/computed tomography scan assesses for ischemia and coronary artery calcification, which is highly prevalent in patients with ESRD.30 Coronary artery calcium score, however, does not independently provide significant incremental prognostic value in predicting mortality or nonfatal myocardial infarction in ESRD.30 These findings may be explained by the differences in distribution of calcium within the coronary artery in ESRD, as shown by intravascular imaging.31 Patients with ESRD have a higher prevalence of intimal calcium without greater lipid arc or thin‐cap fibroatheroma, which are markers of vulnerable plaque.31
Presence of inducible ischemia on dobutamine stress echocardiogram (DSE) has been shown to be predictive of future cardiac events and all‐cause mortality.27, 32 Although the accuracy of dobutamine stress echocardiogram and SPECT in detecting obstructive CAD (≥70% stenosis) in renal transplantation candidates was not statistically different in a meta‐analysis,33 the presence of concentric and eccentric LVH, common in ESRD, may affect the accuracy of dobutamine stress echocardiogram.34 PET imaging assesses not only myocardial blood flow but also coronary flow reserve, which can provide additional insights into early stages of atherosclerosis and microvascular dysfunction.35, 36 Recently, coronary flow reserve assessed by cardiac PET has been shown to provide incremental risk stratification for cardiovascular and all‐cause mortality in patients on dialysis, even in the absence of overt cardiovascular disease.36 For the highest risk patients, PET may be advantageous because it has superior sensitivity for detecting CAD.35, 36 In addition, PET exposes patients to far less radiation than SPECT, an important consideration given the potential need for repeated stress testing during the recipient waiting period. Overall, it is important to consider both the local availability of these tests and the expertise in interpreting them when deciding which test is best suited to evaluate for ischemia in this group of patients.
Coronary Angiography and Revascularization
Although coronary angiography is usually reserved for patients with evidence of ischemia on noninvasive imaging to determine their need for preoperative revascularization, it is also reasonable to consider coronary angiography in renal transplantation candidates at high risk of CAD despite normal stress tests. It is important to identify prognostically important CAD that may require revascularization prior to transplantation.37 Evidence of atherosclerotic vascular disease involving other vascular beds, particularly peripheral arterial disease, may help identify patients with advanced coronary atherosclerosis.38, 39, 40, 41 Since peripheral arterial disease is highly associated with CAD, it may be reasonable to pursue left heart catheterization in patients with peripheral arterial disease despite negative stress tests. Similarly, patients with cardiac autonomic dysfunction, autonomic neuropathy, and retinopathy are at increased risk of CAD.42, 43, 44 The Detection of Ischemia in Asymptomatic Diabetics (DIAD) study found that cardiac autonomic dysfunction was a major predictor of inducible ischemia.42 Furthermore, some diabetic patients with retinopathy have been found to have reduced coronary flow reserve and cardiovascular disease.43, 44 Taken together, renal transplant candidates with diabetes mellitus as the primary etiology for CKD who have normal stress tests may represent a particularly high‐risk group that should be considered for coronary angiography, given their high pretest probability for CAD, as myocardial perfusion imaging has a high false‐negative rate in this population.37
Revascularization With Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention
Observational studies in patients with ESRD who undergo revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) surgery have shown similar long‐term outcomes.45, 46, 47, 48, 49 A recent retrospective analysis of >13 000 patients with CKD treated with CABG or PCI revealed that in the first 3 months after surgery, patients who underwent CABG had a higher risk of progression to ESRD and a higher mortality rate compared with those who underwent PCI.50 This study used more contemporary interventional approaches such as drug‐eluting stents (DESs) as opposed to older generation bare metal stents (BMSs) which helped improve postprocedural cardiovascular outcomes.50 After the first 6 months, however, CABG portended improved survival. An observational study evaluating >21 000 patients with CKD and multivessel CAD undergoing PCI or CABG revealed improved 5‐year survival rates in patients who received CABG51; however, these results do not apply to patients with single‐ or double‐vessel CAD. It is important to note that this study did not take into account LV systolic dysfunction, which places patients at a higher risk of sustaining a cardiac event in the perioperative and postoperative periods. It is possible that patients who underwent PCI as opposed to CABG for multivessel disease had a high operative risk that precluded surgical intervention. The KDOQI guidelines recommend CABG for significant left main or 3‐vessel CAD.24
Although randomized controlled trials to determine the overall mortality benefit of revascularization with PCI in patients with ESRD are lacking, nonrandomized data have suggested that PCI can reduce mortality and lead to greater cardiac event–free survival after transplantation.52 The ongoing ISCHEMIA‐CKD study (ClinicalTrials.gov identifier NCT01985360) will provide critical data for evidence‐based management of CAD in patients with CKD. A retrospective analysis of 1460 renal transplant candidates revealed that patients who underwent preoperative coronary revascularization with PCI had significantly improved 5‐year survival after renal transplantation compared with patients who were medically managed.52 Despite similar indications for revascularization in patients with ESRD, this high‐risk group of patients was significantly less likely to be revascularized compared with patients with normal renal function,20, 53 in particular those patients with CKD not yet requiring RRT, as contrast‐induced nephropathy can precipitate the need for dialysis. To preserve renal function, our group has recently reported the safety and feasibility of cardiac revascularization with PCI guided by intravascular imaging and coronary physiology without utilizing radiocontrast in patients with advanced CKD (stages 4–5).54 This strategy may be adapted in centers with expertise in intravascular imaging and physiology and may lead to increased provision of PCI while protecting against contrast‐induced nephropathy and need for RRT. Unfortunately, even in the setting of acute myocardial infarction, patients with CKD are less likely to be revascularized than patients with normal renal function,55 despite similar health‐related quality of life compared with patients without CKD in the post–myocardial infarction period. In addition, patients with CKD were less likely to be prescribed guideline‐recommended therapy including aspirin, statins, and ACE inhibitors at discharge,55 which may contribute to higher cardiovascular morbidity and mortality. Medical treatment aimed at improving health status after acute myocardial infarction should focus on all patients and be based on current guidelines, regardless of CKD status. The decision to pursue revascularization (CABG or PCI) or to treat medically should be made after a multidisciplinary discussion among interventional cardiologists, nephrologists, and cardiothoracic surgeons and should be individualized to each patient, given the current lack of evidence to guide therapy.56
DES Versus BMS
The choice of the types of stents used for PCI in patients with CKD and ESRD presents a clinical dilemma. Compared with patients with normal renal function, restenosis rates in patients with CKD and ESRD are significantly higher.51, 53, 57, 58, 59 Several studies have shown that the implantation of a DES is associated with lower rates of target vessel revascularization compared with patients who receive a BMS. Nevertheless, the risk of restenosis with a DES in patients with ESRD compared with patients with normal renal function is still higher.51, 53, 57, 58, 59 A randomized multicenter study evaluating the efficacy of everolimus‐eluting stents versus BMSs of identical size and implanted in the same patient showed a reduction in ischemia‐driven target vessel revascularization in patients with CKD who received DESs.60 Nevertheless, it is important to note that a BMS may be preferred in patients in whom renal transplantation is planned within 6 to 12 months, such as those planned to receive living donor transplants, to limit the duration of dual antiplatelet therapy (DAPT). More novel stents that are polymer‐ and carrier‐free but are drug‐coated have been shown to be superior to BMSs with respect to requiring target vessel revascularization on only 1 month of DAPT.61 This type of stent confers the benefit of a DES with an improved safety profile over a BMS, with the additional advantage of a short course of DAPT, which may be ideal for patients with ESRD.61 Aggressive medical therapy is also an option, given the high restenosis rates in patients with CKD and ESRD.62, 63, 64 These risks must be weighed against the observed improved survival rate after PCI versus medical therapy alone in patients with ESRD.65
Duration of DAPT
The 2014 ACC/AHA guidelines recommend that in patients undergoing urgent noncardiac surgery performed <4 to 6 months after BMS or DES implantation, DAPT should be continued unless the relative risk of bleeding outweighs the benefit of prevention of stent thrombosis.19 Moreover, DAPT should be continued for at least 6 months in patients with DESs if the risk of surgical delay is greater than the risk of DES thrombosis. Importantly, the ACC/AHA guidelines also recommend that perioperative management of DAPT should be discussed by a multidisciplinary team including the operating surgeon, cardiologist, anesthesiologist, and patient to weigh the risks of bleeding and stent thrombosis in an individualized fashion. Important factors to consider in perioperative DAPT management are the type, number, and size of stents versus the risk of delaying renal transplantation in favor of prolonging DAPT to prevent stent thrombosis. A newer generation of DESs with enhanced biocompatibility and reduced thrombogenicity may require only 1 to 6 months of DAPT depending on the type of stent, but more evidence is needed.61, 66, 67 These considerations highlight the fact that DAPT should be tailored to the individual patient. For example, patients requiring multivessel PCI with the use of a DES for lesions involving coronary ostia, long lesions, bifurcation lesions, chronic total occlusions, or for cases in which the minimal stent area achieved is small, may benefit from a prolonged course of DAPT. In contrast, patients with high bleeding risk or single‐vessel disease who undergo PCI with a DES for simpler coronary lesions (AHA type A/B1) with a large minimal stent area achieved by imaging guidance may require shorter duration of DAPT.68, 69 Moreover, it is important to note that patients with CKD have less platelet inhibition by clopidogrel.70 Prasugrel and ticagrelor have not been well studied in CKD, and ticagrelor has a relative contraindication in patients with CKD. Data from a few recent clinical series show similar outcomes of renal transplantation performed on DAPT soon after DES implantation. Patients on DAPT or aspirin alone compared with patients not receiving antiplatelet therapy did not have a statistically significant increase in bleeding risk, requirement for blood transfusion, or reoperation.71, 72 These studies should be interpreted with caution because the long‐term effects of increased periprocedural bleeding and the need for transfusion on graft survival are lacking.
Effects of Renal Transplantation on Incidence of Acute Coronary Syndrome
Renal transplantation decreases the incidence of acute coronary syndrome compared with maintenance dialysis.73, 74, 75 Renal transplantation is also independently associated with a lower risk of acute coronary syndrome in patients with ESRD secondary to diabetes mellitus compared with patients maintained on hemodialysis.74 Furthermore, transplantation is associated with a 17% lower adjusted risk of acute coronary syndrome compared with patients remaining on the waiting list for transplantation regardless of the etiology of ESRD.74 In the posttransplantation period, the risk of ischemic heart disease persists, albeit attenuated, compared with maintenance dialysis; acute graft failure, LVH, and traditional cardiovascular risk factors are the major predictors of ischemic events.23 Furthermore, immunosuppressive therapy with calcineurin inhibitors and steroids required in transplanted patients can induce diabetes mellitus and worsen glycemic control, hypertension, and dyslipidemia.1 Consequently, it is important to continue with aggressive risk modification to maintain the cardiovascular benefit of the normalized renal function after renal transplantation.
Recommendations for Management of CAD
The following recommendations take an institutional approach to the management of cardiovascular disease in patients with advanced CKD based on a comprehensive review of the literature and the currently available guidelines.
Given the importance of preexistent CAD for outcomes after renal transplantation, transplantation candidates should have a thorough evaluation for CAD prior to inclusion on the waiting list, as outlined in Figure 1. Careful clinical history and baseline ECG should be performed in all patients. We perform echocardiography to assess ventricular dimensions and function, recognizing that no studies have specifically addressed appropriateness and cost‐effectiveness of this universal approach in transplant candidates. Moreover, given the presence of multiple risk factors for CAD in this patient population, noninvasive testing with dobutamine stress echocardiogram or, preferably, nuclear stress imaging with SPECT or PET are the initial tests that we use to screen for the presence of CAD. Negative results should be interpreted in the context of the pretest probability in individual patients, especially in patients with diabetes mellitus with autonomic dysfunction and microvascular complications.37, 42, 43, 44 Patients with multiple risk factors for CAD (≥3 risk factors: diabetes mellitus, prior cardiovascular disease, >1 year on dialysis, LVH, peripheral arterial disease, age >60 years, smoking, hypertension, dyslipidemia) should be considered for further imaging or cardiac catheterization despite a negative stress test in some instances. In such patients, noninvasive imaging with PET is a prudent second‐line investigation if coronary angiography is to be avoided because of advanced CKD and risk of progression to RRT. A normal PET stress test with abnormal multivessel coronary flow reserve is also a consideration for coronary angiography.36 Repeated evaluation is recommended on an annual basis in patients at high risk, with reevaluation every 3 years for low‐risk patients.
Figure 1.
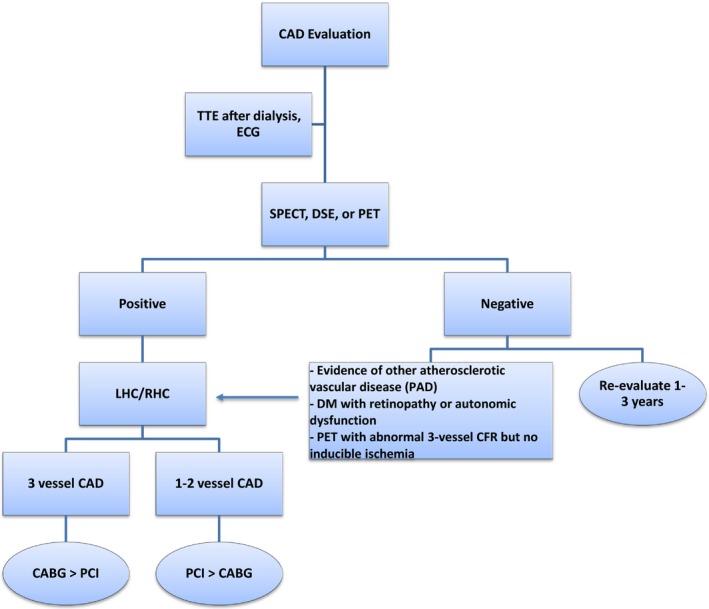
Algorithm for evaluation and treatment of CAD. CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; CFR, coronary flow reserve; DM, diabetes mellitus; DSE, dobutamine stress echocardiography; ECG, electrocardiogram; LHC, left heart catheterization; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PET, positron emission tomography; RHC, right heart catheterization; SPECT, single photon emission computed tomography; TTE, transthoracic echocardiogram.
Patients with evidence of ischemia on stress test should be referred for left heart catheterization to identify prognostically significant CAD. Revascularization by PCI or CABG for 3‐vessel disease should be pursued if indicated. The choice to place a BMS or a DES should be individualized to each patient. BMSs or polymer‐ and carrier‐free DESs may be used in patients who require more urgent renal transplantation and a shorter course of DAPT.61 Stent placement with intravascular imaging guidance is recommended to optimize the intervention as imaging guidance has been shown to result in a larger final minimal stent area, minimizing the risk of restenosis and stent thrombosis.69
CHF in Patients With ESRD
It is estimated that up to 36% of all patients with ESRD have CHF at the initiation of dialysis76—12 to 36 times higher than the rate in the general population.9 Another 25% of patients on dialysis develop de novo CHF with an incidence of 7% per year.77 The underlying causes of CHF in patients with ESRD at the initiation of dialysis are similar to those in the general population including advancing age, diabetes mellitus, and ischemic heart disease.9, 77 More specific to CKD, toxins from the uremic milieu may affect myocardial contractility and function,7 and anemia secondary to CKD is associated with a higher incidence of CHF in this population.76 Chronic volume overload and poorly controlled hypertension are also major risk factors for CHF in patients with CKD and ESRD. Therefore, it is important to control hypertension and volume status through diuresis and dialysis to reduce the risk of incident CHF.
Management of CHF in Patients With CKD
Medical treatment of CHF in patients with advanced CKD is similar to patients without renal disease. A meta‐analysis of 8 studies conducted in patients with CKD (stages 3–5) and CHF showed that beta blocker therapy lowered all‐cause and cardiovascular mortality with an increased risk of bradycardia and hypotension.78 Nevertheless, there is a paucity of data regarding beta blocker therapy in patients with ESRD on dialysis. The clinical use of ACE inhibitors in this population is also variable, perhaps due to the potential adverse effects on renal function in patients with advanced CKD who are not yet on RRT. ACE inhibition, however, has been shown to be effective at preventing progression of CKD in patients with an estimated glomerular filtration rate of ≥20 mL/min.79 A drop in estimated glomerular filtration rate of >25% or development of hyperkalemia (>5.5 mmol/L) is an indication for discontinuing therapy.79
LVH is present in 75% of patients with ESRD12 and often is accompanied by cardiac fibrosis, increasing the risk of developing LV dysfunction and ventricular arrhythmias, which are significant causes of morbidity and mortality in this patient population.80, 81 Although many pathological processes drive the development of LVH in patients with ESRD, adequate volume and afterload reduction remain the primary practical targets for preventing and alleviating LVH. Strategies such as salt restriction, ACE inhibition, and use of loop diuretics should be adopted early in the onset of CKD to prevent LVH. Moreover, the usual thrice‐weekly hemo‐ or peritoneal dialysis sessions are inadequate for managing hypervolemia and increased afterload. The Frequent Hemodialysis Network has found that longer, more frequent sessions of RRT are required to decrease LV mass.82 In patients with ESRD, small studies investigating the effect of ACE inhibition on LVH have shown variable results.12, 76 The Fosinopril in Dialysis study (FOSIDIAL), a randomized controlled trial conducted to evaluate the efficacy of fosinopril in helping prevent major adverse cardiac events in patients on dialysis, found no statistically significant difference between the 2 arms in reducing the risk of major adverse cardiac events.83 However, this study was underpowered because of a small sample size, and fewer than expected major adverse cardiac events occurred in the study groups. In the absence of specific data from sufficiently powered randomized controlled trials for treatment of CHF with ACE inhibitors in patients on dialysis, this group of drugs is currently recommended based on the extrapolation of data from patients without ESRD.76 Larger scale studies are needed to investigate the effect of ACE inhibition on LV dimension and function and on clinical outcomes of CHF in patients with ESRD. Renal transplantation has been shown to consistently reduce LVH in dialysis patients after transplant.80, 81, 84, 85 This suggests that the most effective method of treating LVH and the associated impaired LV dysfunction is restoring renal function.80, 81
Device Therapy for Primary Prevention of Sudden Cardiac Death
Severe LV systolic dysfunction (LVEF <35%) in patients with ESRD raises a question about primary prevention of fatal cardiac arrhythmias with device therapy. The utility of implantable cardioverter‐defibrillators (ICDs) has not been well studied in patients with CKD and ESRD, who historically have been excluded from clinical trials investigating ICD use in patients with CHF. Patients who meet the criteria for ICD placement for primary prevention often are not offered these devices because of lower life expectancy, higher rates of device complications, and other comorbidities.86 Patients with advanced CKD and ESRD tend to have higher rates of acute and chronic complications from device placement and higher mortality unrelated to cardiac arrhythmias.86 A study of a cohort of >9500 patients on chronic dialysis implanted with ICDs revealed that 11% of patients died of an infection at 1.4 years of follow‐up, with most infections occurring 1 year after device implantation.87 The incidence of device infection in patients with ESRD is 2 to 5 times greater than in patients without ESRD,88, 89 and this may be the result of frequent bloodstream access for hemodialysis.88, 89 Device extraction is usually recommended as part of the treatment for device infection, but patients with ESRD are often treated medically, perhaps because they are too ill to safely sustain a procedure.88 A decision analysis model analyzing the benefits of ICD therapy found that in patients with mild to moderate CKD (stages 1 and 2), ICD implantation reduces mortality, with patients with more advanced CKD having a higher procedural risk and decreased life expectancy. In contrast, ICD implantation in patients aged <65 years with stage 5 CKD deemed favorable results.86
Subcutaneous ICDs (S‐ICDs) present a novel alternative to ICDs, with potential wide clinical application in patients with ESRD. S‐ICDs are approved by the US Food and Drug Administration, do not require transvenous leads, and were recently studied in patients with ESRD on dialysis for both primary and secondary prevention. A retrospective study found that patients on chronic dialysis who received an S‐ICD had no device‐related infections over a mean follow‐up of 7 months.87 Moreover, a reduced risk of central venous stenosis and hematogenous and endocardial bacterial infections was noted in a recent case series.89 This result was later confirmed in other studies.90 The incidence of appropriate shocks delivered by S‐ICD was significantly higher in the dialysis cohort compared with the nondialysis group, with a low risk of inappropriate shocks.87 Given the high prevalence of CHF in the dialysis population, S‐ICD appears to be an appropriate alternative to transvenous ICD for primary and secondary prevention. Evaluation by an electrophysiologist experienced in S‐ICD implantation should be pursued for renal transplant candidates in whom LVEF remains <35% despite optimization of medical therapy.
Effects of Renal Transplantation on LV Systolic Function
Patients on dialysis with systolic heart failure often are not referred for renal transplantation because of concern about perioperative mortality and the increased risk of cardiovascular events after transplantation. Recent studies, however, have indicated that not only is the risk of perioperative death low, but improvement in LV systolic function is also frequently observed.7, 87, 91 In a study of >100 patients with ESRD and mean LVEF values of 31.6±6.7 undergoing renal transplantation, LV function improved in 86% of the patients, increasing to a mean of 47.2±10.7 at 6 months, with continued improvement to 52.2±12.0 at 12 months after transplantation.7 New York Heart Association (NYHA) functional class also improved. Prior to transplant, 0% of patients reported a functional class of NYHA class 1. This number increased from 0% to 73% in the post–renal transplantation period, with only 24% of patients reporting a functional class of NYHA class 4.7 Time spent on dialysis was the only significant predictor of improvement in LVEF. Patients with longer durations of dialysis therapy were less likely to have normalization of LVEF (defined as ≥40%) after transplantation.
The 3‐year survival rate of patients on dialysis after diagnosis of CHF is reported as only 17%.77, 92 Moreover, the median survival of patients with systolic dysfunction is 38 months compared with 66 months in patients with normal systolic function.92 In a study of nearly 3700 patients with ESRD, LVEF was the best predictor of mortality, with a 2.7% morality increase for each 1% decrease in LVEF for patients awaiting renal transplantation.93 Similarly, a study of >60 000 patients with renal transplantation found that although there was a modest risk of cardiovascular mortality early in the postoperative period, the cardiac death rate dropped significantly 3 months after transplantation compared with patients who remained on the waiting list.73 In patients with diabetes mellitus, LV end‐systolic diameter and indexes of fiber shortening on echocardiography were predictors of survival, with LV end‐systolic diameter >4.0 cm associated with 30% survival at 3 years versus 69% in those with normal LV end‐systolic diameter. Furthermore, no significant impact on survival with renal transplantation was observed in patients with LV end‐systolic diameter ≥6 cm, LV posterior wall thickness ≥1.6 cm, or LVEF ≤43%.94 In a more recent prospective study of >200 renal transplant recipients, age, LV end‐systolic diameter ≥3.5 cm, maximal wall thickness ≥1.4 cm, and mitral annular calcification were shown to be independent predictors of mortality.95 Thus, it is recommended that renal transplantation be considered early for patients with CHF or for those at risk of developing CHF because the beneficial effects of transplantation diminish with a prolonged course of dialysis.7 KDOQI guidelines recommend that patients should be evaluated with an echocardiogram at the initiation of dialysis once dry weight is achieved, ideally 1 to 3 months after the initiation of dialysis, and at 3‐year intervals thereafter24 to assess LVEF, structural abnormalities, and valvular disease.20 Although many studies have demonstrated that patients with low LVEF can safely undergo renal transplantation7, 92, 96—most notably, a report of 11 patients with LVEF ≤20%7—there is currently no consensus regarding the minimum LVEF required to safely undergo renal transplantation.
Recommendations for Management of CHF
All patients under evaluation should have baseline echocardiography at dry weight. For patients with an LVEF <35% not yet on RRT, right and left heart catheterization should be performed to assess for ischemic heart disease and targets for revascularization, with PCI or CABG performed if indicated. In patients with CKD who are not yet on RRT, ultra–low‐contrast angiography followed by staged low‐ or no‐contrast PCI97 should be considered if feasible. Treatments such as beta blockers and ACE inhibitors or angiotensin receptor blockers should be initiated to prevent cardiac remodeling and to improve LVEF (Figure 2). Side effects such as hypotension, electrolyte abnormalities, and bradycardia should be monitored closely once therapy has begun. Importantly, many angiotensin receptor blockers are not dialyzed and are preferred over ACE inhibitors, which are dialyzable. If no improvement in cardiac contractility is achieved and LVEF remains <35% despite optimal medical therapy, the benefits and risks of ICD and S‐ICD for primary prevention should be discussed with the patient. Patients with normal LVEF should be reevaluated by echocardiography within 3 years. Cardiac evaluation should be performed annually for patients with LV systolic dysfunction and more frequently for patients with LVEF <35% for titration of medical therapy based on guidelines for management of patients with severe LV dysfunction.98
Figure 2.
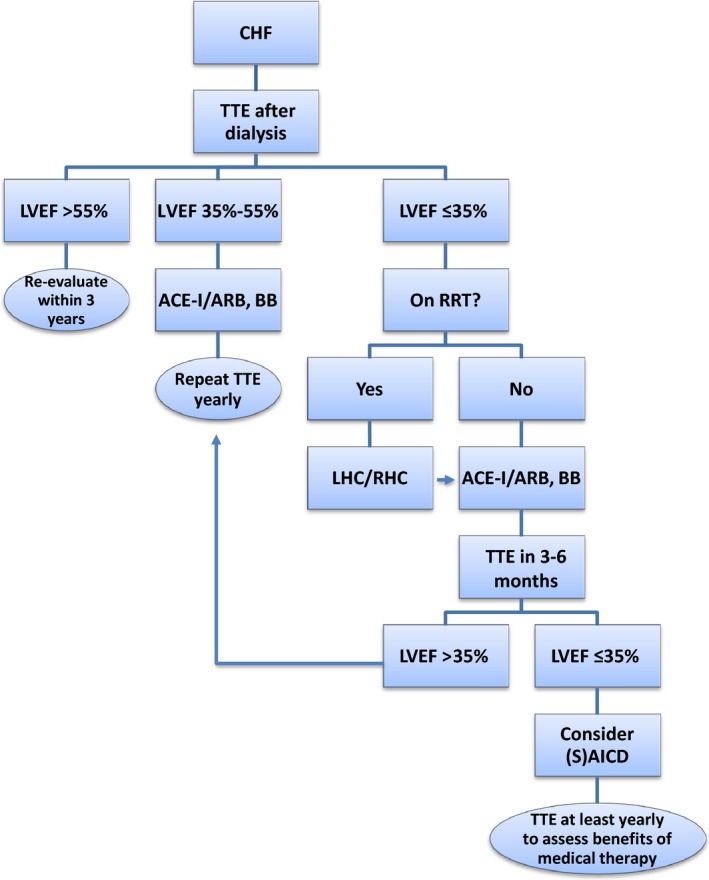
Algorithm for evaluation and treatment of CHF. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; RRT, renal replacement therapy; (S)AICD indicates (subcutaneous) automated implantable cardioverter‐defibrillator; TTE, transthoracic echocardiogram.
Valvular Disease in Patients With ESRD
Valvular abnormalities are very prevalent in patients with ESRD and often pose barriers to renal transplantation. Degenerative valvular calcification is more prevalent and progresses faster in ESRD than in the general population, likely because of abnormal calcium and phosphate metabolism, secondary hyperparathyroidism, and vitamin D and calcium supplementation.99, 100, 101 These metabolic abnormalities lead to increased calcium deposition in the mitral annulus and aortic valve. Consequently, the incidence of aortic valve calcification (AVC) is nearly twice that in the general population and has a direct relationship with time spent on dialysis.99, 100, 101 In patients on dialysis, AVC is often severe and can lead to rapidly progressing aortic stenosis (AS)20. Notably, the rate of AS progression in ESRD is twice the rate in the general population (0.23‐cm2 reduction in valve area per year compared with 0.05–0.1 cm2/year).20 Severe AVC leads to the development of premature AS. One study demonstrated severe AVC at a mean age of 52 years in up to 28% of patients with ESRD on dialysis who had trileaflet aortic valves.20 The premature development of AVC and AS was associated with longer time spent on dialysis, higher serum calcium and phosphate, and high calcium phosphate product.101
Similar to accelerated AVC, 36% of patients had mitral annular calcification that was associated with age, age at initiation of dialysis, calcium phosphate product, and time spent on dialysis.101 Progressive mitral annular calcification can cause functional impairment by encroachment to the mitral leaflets, leading to mitral regurgitation and/or mitral stenosis. To assess mitral valve function, echocardiography should be performed at dry weight because functional mitral regurgitation will improve with improved hemodynamics24 and often resolves after renal transplantation without further intervention.102 It is also important to differentiate primary and secondary mitral regurgitation to determine appropriate treatment strategies. Primary mitral regurgitation may benefit from mitral valve repair or replacement, as per the AHA/ACC 2014 guidelines, whereas secondary mitral regurgitation should be treated by addressing the underlying cause, as identified by transthoracic echocardiogram.103
Transcatheter Versus Surgical Therapy of Valvular Disease
The overall management of valvular disease in candidates for renal transplantation is similar to that for patients without CKD.20 In a retrospective analysis of >35 000 patients with ESRD, patients with valvular heart disease were less likely to undergo renal transplantation104. Patients that received corrective valvular surgery were successfully transplanted at rates similar to patients without valvular disease. Transplantation was associated with the halting of valvular disease progression, particularly AS.104 Although surgical replacement with either a bioprosthetic or mechanical valve has been shown to confer a reduction in mortality in patients who survive the perioperative period, perioperative mortality with cardiac surgery in patients with ESRD remains extremely high and limits the surgical options in this patient population.105, 106, 107, 108 According to the Society of Thoracic Surgeons risk model, aortic valve replacement (AVR) in patients with ESRD is associated with significantly high perioperative mortality, with an odds ratio of 2.8 for operative mortality defined as death within the same hospitalization as surgery and within 30 days after discharge.109 Similarly, mitral valve repair is associated with an odds ratio of 4.59 for operative mortality.109 These high rates of perioperative mortality should be considered against the high mortality from uncorrected valvular disease. Symptomatic AS without definitive treatment is associated with 75% mortality in “all comers” within 3 years, and the risk is likely even higher in patients with ESRD.110
Transcatheter AVR (TAVR) may be an attractive alternative for patients with ESRD and severe AS. In a study of 8 renal transplant recipients who underwent TAVR prior to transplantation, mortality at 12 months was 0%, with 1 reported cardiovascular event (stroke), compared with 30‐day mortality of 11.2% and 1‐year mortality of 16.7% in patients who underwent surgical AVR (SAVR).111 Although patients with ESRD were not included in the initial clinical trials evaluating TAVR, case reports have not found any absolute contraindication to TAVR, especially in those who may otherwise be denied renal transplantation.111, 112 In the new 2014 AHA/ACC guidelines for the management of patients with valvular heart disease, TAVR has a class 1 indication for patients who have prohibitive risk for SAVR and post‐TAVR survival >12 months.103 TAVR is a reasonable alternative to SAVR in renal transplantation candidates.
The management of asymptomatic severe AS remains uncertain and controversial because no randomized control trials have compared AVR, either SAVR or TAVR, with conservative medical therapy. A recent meta‐analysis of 2486 patients with severe asymptomatic AS found a 3.5‐fold higher mortality rate in patients who were treated with a watchful waiting strategy compared with early AVR.113 These results, however, had many potential confounders. Patients with asymptomatic severe AS may be referred for stress testing to determine whether symptoms are unmasked by strenuous exercise.103, 113 Current guidelines recommend AVR in patients with asymptomatic severe AS and an LVEF <50% who have a decreased systolic opening of a calcified aortic valve with an aortic valve velocity ≥4.0 m/s or mean pressure gradient ≥40 mm Hg.103 Nevertheless, patients with advanced CKD and ESRD represent a population that is at high risk for rapid progression of AS.20, 99, 100, 101 LVH, reduced LVEF, and PH are all linked to a higher risk of adverse events in patients with AS and are highly prevalent in patients with advanced CKD and ESRD.113, 114, 115, 116 With these considerations in mind, it may be reasonable to correct severe asymptomatic AS prior to renal transplantation with SAVR or TAVR.
Recommendations for Management of Valvular Disease
Patients with clinically significant valvular abnormalities should be considered for definitive management prior to transplantation. TAVR should be used as an alternative to SAVR in patients at high or intermediate risk for surgery.117, 118 The decision to proceed with TAVR or SAVR should be made in consultation with a cardiac surgeon and an interventional cardiologist (Figure 3). Mitral valve surgery should be performed only after documentation of severe valve dysfunction on echocardiography following right heart catheterization showing normal filling pressures.
Figure 3.
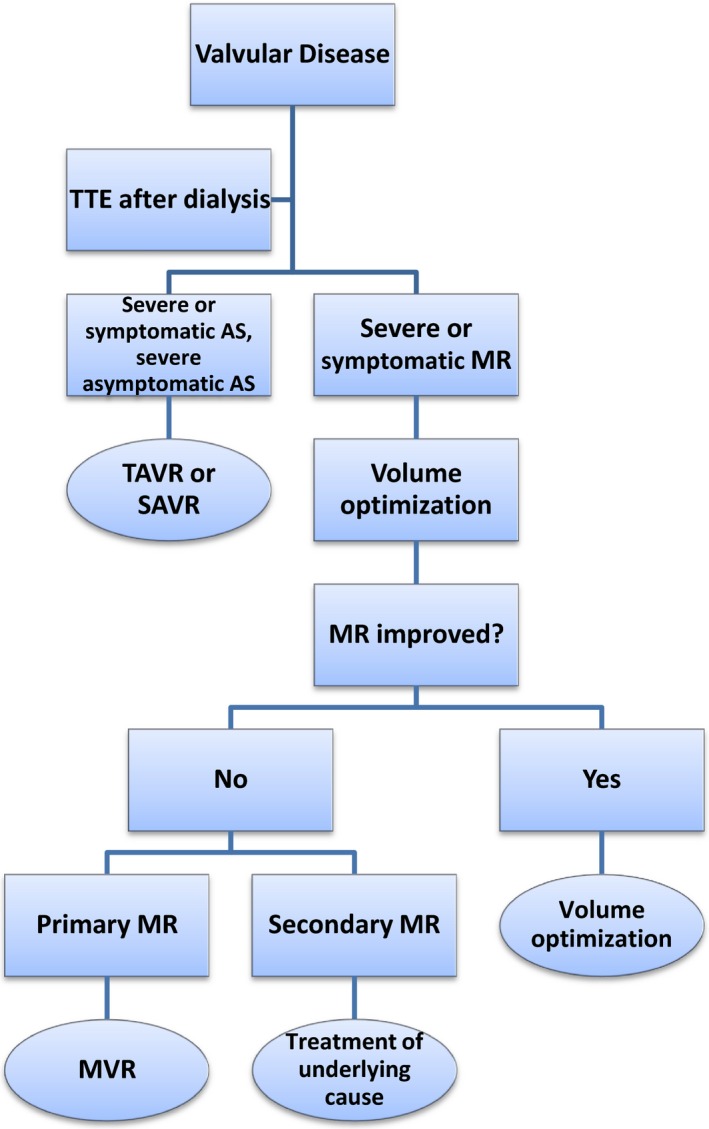
Algorithm for evaluation and treatment of valvular disease. AS indicates aortic stenosis; MR, mitral regurgitation; MVR, mitral valve repair/replacement; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; TTE, transthoracic echocardiogram.
PH in Patients With ESRD
PH is common in patients with ESRD, and multiple studies have estimated the prevalence to be 26% to 48% depending on the mean age of the population studied and the time spent on dialysis.4, 119 The majority of PH observed in patients receiving RRT occurs in patients with arteriovenous fistulae (AVF) for hemodialysis. Patients on peritoneal dialysis also have a higher incidence of PH compared with the general population.4, 119 Several factors place patients with ESRD at risk for the development of PH: placement of AVF, chronic hypervolemia, and anemia. These risk factors can lead to a state of high cardiac output, which can further contribute to the development of PH. It is essential to dialyze patients to their dry weights to prevent chronic volume overload and reduce the risk of development of PH, which is frequently observed in this patient population.120, 121 Compression of AVF for 1 minute has been shown to decrease cardiac output and pulmonary arterial pressure and may be a useful diagnostic maneuver to determine the reversibility of PH.122 Given the massive capacitance of the pulmonary vasculature, increased cardiac output alone might not be the only driving force for the development of PH in patients on dialysis.120 Endothelial dysfunction caused by decreased nitric oxide production may also play a role122. It has been shown that patients with PH on dialysis have reduced serum levels of nitric oxide both before and after hemodialysis compared with patients on dialysis without PH.122 This suggests that the uremic environment may reduce the capacitance of the pulmonary vasculature, predisposing patients on dialysis with high cardiac outputs to the development of PH.119, 122
Treatment of PH
Development of PH is associated with significant morbidity and mortality.121, 122, 123, 124, 125 Patients on dialysis with PH have significantly lower survival rates than their counterparts without PH, with respective survival rates of 78.6% versus 96.5% at 1 year, 42.9% versus 78.8% at 3 years, and 25.5% versus 66.4% at 5 years.126 Thus, patients with ESRD and severe PH should be referred to a PH specialist for PH‐specific therapies. Unfortunately, therapeutic options for patients with ESRD and PH are limited. Treatments such as phosphodiesterase type 5 inhibitors or endothelin receptor antagonists have not been studied specifically in patients with ESRD and PH. Surgical reduction of AVF should be considered in patients with very high cardiac output in whom improvements in cardiac output and PH by temporary AVF closure has been shown.121, 122, 126, 127 An AVF flow rate ≥2 L/min and cardiac output of ≥8 L/min place patients at high risk of high‐output cardiac failure.128, 129 The definitive treatment for PH in this population is renal transplantation if the etiology is secondary to high cardiac output from AVF. These patients should be considered for renal transplantation as soon as possible.123, 124, 126
Evidence‐based guidelines for the perioperative management of patients with PH are lacking because the AHA/ACC practice guidelines for noncardiac surgery do not list PH as an independent risk factor for postoperative complications.125 Several small studies, however, have suggested that PH is a risk factor for increased peri‐ and postoperative morbidity and mortality. According to the AHA/ACC recommendations specifically addressing cardiac disease evaluation among kidney transplantation candidates, right heart catheterization is reasonable to pursue to confirm echocardiographic evidence of elevated pulmonary arterial pressures.20 Right heart catheterization is also warranted to assess the severity of PH before transplantation to determine whether there is an association with a state of high cardiac output.20, 125 Consultation with a PH specialist should also be considered early because therapy with phosphodiesterase type 5 inhibitors or endothelin receptor antagonists may be needed to facilitate renal transplantation in patients with refractory PH not secondary to AVF‐dependent high cardiac output.121 During surgery, systemic hypotension or abrupt increases in pulmonary artery pressures can cause right ventricular overload and lead to right ventricular systolic dysfunction and decreased cardiac output. Therefore, intraoperative invasive hemodynamic monitoring of pulmonary circulation should be considered.125 Nevertheless, renal transplantation has been shown to be curative for PH under certain circumstances. If the pulmonary pressures do not preclude a surgical procedure, renal transplantation should be pursued aggressively to improve morbidity and mortality in this group of patients.
Recommendations for Management of PH
Evidence of PH on echocardiogram (≥40 mm Hg) should be confirmed with repeat echocardiography following hemodialysis to ensure that PH is not simply caused by volume overload (Figure 4). If pulmonary artery pressures remain elevated despite optimization of volume status by dialysis, right heart catheterization to assess severity and potential etiology of PH should be performed. Severe PH (mean pulmonary artery pressure ≥40 mm Hg) in the setting of elevated pulmonary capillary wedge pressure (≥18 mm Hg) should be treated with more aggressive diuresis to optimize volume status, at times requiring inpatient admission to perform daily dialysis. When PH is present in the absence of elevated pulmonary capillary wedge pressure but with high cardiac output (>8 L/min), attention should be paid to the AVF. Evidence of decreased cardiac output and improved pulmonary pressures acutely during AVF occlusion in the catheterization laboratory are suggestive of AVF as the etiology of PH, and surgical revision should be considered. Patients with PH with normal left atrial pressures and normal cardiac output should undergo reversibility testing with intravenous and with or without inhaled vasodilators to determine the potential response to medical therapy. Patients with severe PH should be referred to a PH specialist for help with perioperative management. A multidisciplinary approach for perioperative management should be considered, including consultation with anesthesiology to help determine the optimal intraoperative plan of care.125
Figure 4.
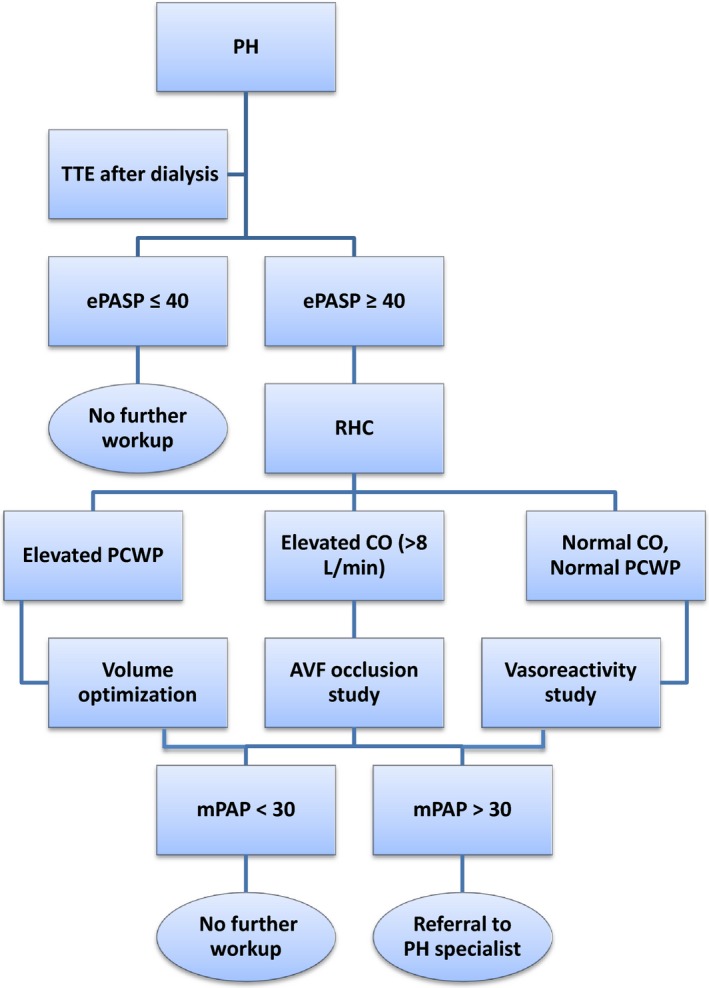
Algorithm for evaluation and treatment of PH. AVF indicates arteriovenous fistula; CO, cardiac output; ePASP, estimated pulmonary artery systolic pressure (echocardiography); mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; RHC, right heart catheterization; TTE, transthoracic echocardiogram.
Conclusions
Cardiovascular disease processes are highly prevalent and have major negative impacts on clinical outcomes in patients with advanced CKD. Nevertheless, optimal cardiovascular management in this population remains challenging due to the absence of data from randomized clinical trials, from which this high‐risk group continues to be excluded. Encouraging data on improvement of cardiovascular outcomes after successful renal transplantation with appropriate cardiovascular workup and management highlights the urgent need for clinical trials to investigate a wide array of unresolved clinical issues related to cardiovascular pathologies in advanced CKD.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003648 doi: 10.1161/JAHA.116.003648)
References
- 1. Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;53:2129–2140. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention, Division of Diabetes Translation . National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States. 2014. Available at: https://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf. Accessed June 24, 2016.
- 3. United States Renal Data System . 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 4. Bozbas SS, Akcay S, Altin C, Bozbas H, Karacaglar E, Kanyilmaz S, Sayin B, Muderrisoglu H, Haberal M. Pulmonary hypertension in patients with end‐stage renal disease undergoing renal transplantation. Transplant Proc. 2009;41:2753–2756. [DOI] [PubMed] [Google Scholar]
- 5. Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9:1018–1022. [DOI] [PubMed] [Google Scholar]
- 6. Mall G, Huther W, Schneider J, Lundin P, Ritz E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant. 1990;5:39–44. [DOI] [PubMed] [Google Scholar]
- 7. Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S, Fink JC, Fisher ML, Bartlett ST, Weir MR. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end‐stage renal disease. J Am Coll Cardiol. 2005;45:1051–1060. [DOI] [PubMed] [Google Scholar]
- 8. Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end‐stage renal disease therapy. Kidney Int. 1995;47:186–192. [DOI] [PubMed] [Google Scholar]
- 9. Stack AG, Bloembergen WE. A cross‐sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis. 2001;38:992–1000. [DOI] [PubMed] [Google Scholar]
- 10. Stenvinkel P, Alvestrand A. Inflammation in end‐stage renal disease: sources, consequences, and therapy. Semin Dial. 2002;15:329–337. [DOI] [PubMed] [Google Scholar]
- 11. Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. [DOI] [PubMed] [Google Scholar]
- 12. Tai DJ, Lim TW, James MT, Manns BJ, Tonelli M, Hemmelgarn BR. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta‐analysis. Clin J Am Soc Nephrol. 2010;5:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wanner C, Metzger T. C‐reactive protein a marker for all‐cause and cardiovascular mortality in haemodialysis patients. Nephrol Dial Transplant. 2002;17(suppl 8):29–32; discussion 39‐40. [DOI] [PubMed] [Google Scholar]
- 14. Xing D, Hage FG, Chen YF, McCrory MA, Feng W, Skibinski GA, Majid‐Hassan E, Oparil S, Szalai AJ. Exaggerated neointima formation in human C‐reactive protein transgenic mice is IgG Fc receptor type I (Fc gamma RI)‐dependent. Am J Pathol. 2008;172:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen‐Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 16. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary‐artery calcification in young adults with end‐stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. [DOI] [PubMed] [Google Scholar]
- 17. Hruska K, Mathew S, Lund R, Fang Y, Sugatani T. Cardiovascular risk factors in chronic kidney disease: does phosphate qualify? Kidney Int Suppl. 2011;121:S9–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Go AS, Bansal N, Chandra M, Lathon PV, Fortmann SP, Iribarren C, Hsu CY, Hlatky MA. Chronic kidney disease and risk for presenting with acute myocardial infarction versus stable exertional angina in adults with coronary heart disease. J Am Coll Cardiol. 2011;58:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila‐Roman VG, Gerhard‐Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77–e137. [DOI] [PubMed] [Google Scholar]
- 20. Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, Kasiske BL, Carithers RL, Ragosta M, Bolton K, Auerbach AD, Eagle KA. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60:434–480. [DOI] [PubMed] [Google Scholar]
- 21. Friedman SE, Palac RT, Zlotnick DM, Chobanian MC, Costa SP. A call to action: variability in guidelines for cardiac evaluation before renal transplantation. Clin J Am Soc Nephrol. 2011;6:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abbud‐Filho M, Adams PL, Alberu J, Cardella C, Chapman J, Cochat P, Cosio F, Danovitch G, Davis C, Gaston RS, Humar A, Hunsicker LG, Josephson MA, Kasiske B, Kirste G, Leichtman A, Munn S, Obrador GT, Tibell A, Wadstrom J, Zeier M, Delmonico FL. A report of the Lisbon Conference on the care of the kidney transplant recipient. Transplantation. 2007;83:S1–S22. [DOI] [PubMed] [Google Scholar]
- 23. Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16:496–506. [DOI] [PubMed] [Google Scholar]
- 24. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–S153. [PubMed] [Google Scholar]
- 25. Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ. Identifying high‐risk asymptomatic diabetic patients who are candidates for screening stress single‐photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45:43–49. [DOI] [PubMed] [Google Scholar]
- 26. Chang SM, Hakeem A, Nagueh SF. Predicting clinically unrecognized coronary artery disease: use of two‐ dimensional echocardiography. Cardiovasc Ultrasound. 2009;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bergeron S, Hillis GS, Haugen EN, Oh JK, Bailey KR, Pellikka PA. Prognostic value of dobutamine stress echocardiography in patients with chronic kidney disease. Am Heart J. 2007;153:385–391. [DOI] [PubMed] [Google Scholar]
- 28. Patel RK, Mark PB, Johnston N, McGeoch R, Lindsay M, Kingsmore DB, Dargie HJ, Jardine AG. Prognostic value of cardiovascular screening in potential renal transplant recipients: a single‐center prospective observational study. Am J Transplant. 2008;8:1673–1683. [DOI] [PubMed] [Google Scholar]
- 29. Patel AD, Abo‐Auda WS, Davis JM, Zoghbi GJ, Deierhoi MH, Heo J, Iskandrian AE. Prognostic value of myocardial perfusion imaging in predicting outcome after renal transplantation. Am J Cardiol. 2003;92:146–151. [DOI] [PubMed] [Google Scholar]
- 30. Moody WE, Lin EL, Stoodley M, McNulty D, Thomson LE, Berman DS, Edwards NC, Holloway B, Ferro CJ, Townend JN, Steeds RP. Prognostic utility of calcium scoring as an adjunct to stress myocardial perfusion scintigraphy in end‐stage renal disease. Am J Cardiol. 2016;117:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chin C, Matsumura M, Yamamoto M, Song L, Galougahi K, Bhatti N, Brinkman M, Mintz G, Ali Z. Book of abstracts 2016: unique coronary lesion characteristics in haemodialysis patients as assessed by OCT. EuroPCR. 2016.
- 32. Wong CF, Little MA, Vinjamuri S, Hammad A, Harper JM. Technetium myocardial perfusion scanning in prerenal transplant evaluation in the United Kingdom. Transplant Proc. 2008;40:1324–1328. [DOI] [PubMed] [Google Scholar]
- 33. Wang LW, Fahim MA, Hayen A, Mitchell RL, Lord SW, Baines LA, Craig JC, Webster AC. Cardiac testing for coronary artery disease in potential kidney transplant recipients: a systematic review of test accuracy studies. Am J Kidney Dis. 2011;57:476–487. [DOI] [PubMed] [Google Scholar]
- 34. Yuda S, Khoury V, Marwick TH. Influence of wall stress and left ventricular geometry on the accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 2002;40:1311–1319. [DOI] [PubMed] [Google Scholar]
- 35. Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–640. [DOI] [PubMed] [Google Scholar]
- 36. Shah NR, Charytan DM, Murthy VL, Skali Lami H, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Foster CR, Hainer J, Gaber M, Klein J, Dorbala S, Blankstein R, Di Carli MF. Prognostic value of coronary flow reserve in patients with dialysis‐dependent ESRD. J Am Soc Nephrol. 2016;6:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Welsh RC, Cockfield SM, Campbell P, Hervas‐Malo M, Gyenes G, Dzavik V. Cardiovascular assessment of diabetic end‐stage renal disease patients before renal transplantation. Transplantation. 2011;91:213–218. [DOI] [PubMed] [Google Scholar]
- 38. Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. [DOI] [PubMed] [Google Scholar]
- 39. Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–1181. [DOI] [PubMed] [Google Scholar]
- 40. Aronow WS, Ahn C. Prevalence of coexistence of coronary artery disease, peripheral arterial disease, and atherothrombotic brain infarction in men and women > or = 62 years of age. Am J Cardiol. 1994;74:64–65. [DOI] [PubMed] [Google Scholar]
- 41. Duran NE, Duran I, Gurel E, Gunduz S, Gol G, Biteker M, Ozkan M. Coronary artery disease in patients with peripheral artery disease. Heart Lung. 2010;39:116–120. [DOI] [PubMed] [Google Scholar]
- 42. Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N, Engel S, Ratner RE, Iskandrian AE. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. [DOI] [PubMed] [Google Scholar]
- 43. Akasaka T, Yoshida K, Hozumi T, Takagi T, Kaji S, Kawamoto T, Morioka S, Yoshikawa J. Retinopathy identifies marked restriction of coronary flow reserve in patients with diabetes mellitus. J Am Coll Cardiol. 1997;30:935–941. [DOI] [PubMed] [Google Scholar]
- 44. Hiller R, Sperduto RD, Podgor MJ, Ferris FL III, Wilson PW. Diabetic retinopathy and cardiovascular disease in type II diabetics. The Framingham Heart Study and the Framingham Eye Study. Am J Epidemiol. 1988;128:402–409. [DOI] [PubMed] [Google Scholar]
- 45. Herzog CA, Ma JZ, Collins AJ. Long‐term outcome of renal transplant recipients in the United States after coronary revascularization procedures. Circulation. 2004;109:2866–2871. [DOI] [PubMed] [Google Scholar]
- 46. Rinehart AL, Herzog CA, Collins AJ, Flack JM, Ma JZ, Opsahl JA. A comparison of coronary angioplasty and coronary artery bypass grafting outcomes in chronic dialysis patients. Am J Kidney Dis. 1995;25:281–290. [DOI] [PubMed] [Google Scholar]
- 47. Ivens K, Gradaus F, Heering P, Schoebel FC, Klein M, Schulte HD, Strauer BE, Grabensee B. Myocardial revascularization in patients with end‐stage renal disease: comparison of percutaneous transluminal coronary angioplasty and coronary artery bypass grafting. Int Urol Nephrol. 2001;32:717–723. [DOI] [PubMed] [Google Scholar]
- 48. Chertow GM, Normand SL, Silva LR, McNeil BJ. Survival after acute myocardial infarction in patients with end‐stage renal disease: results from the cooperative cardiovascular project. Am J Kidney Dis. 2000;35:1044–1051. [DOI] [PubMed] [Google Scholar]
- 49. Hemmelgarn BR, Southern D, Culleton BF, Mitchell LB, Knudtson ML, Ghali WA. Survival after coronary revascularization among patients with kidney disease. Circulation. 2004;110:1890–1895. [DOI] [PubMed] [Google Scholar]
- 50. Charytan DM, Li S, Liu J, Herzog CA. Risks of death and end‐stage renal disease after surgical compared with percutaneous coronary revascularization in elderly patients with chronic kidney disease. Circulation. 2012;126:S164–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chang TI, Shilane D, Kazi DS, Montez‐Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kahn MR, Fallahi A, Kim MC, Esquitin R, Robbins MJ. Coronary artery disease in a large renal transplant population: implications for management. Am J Transplant. 2011;11:2665–2674. [DOI] [PubMed] [Google Scholar]
- 53. El‐Menyar A, Zubaid M, Sulaiman K, Singh R, Al Thani H, Akbar M, Bulbanat B, Al‐Hamdan R, Almahmmed W, Al Suwaidi J. In‐hospital major clinical outcomes in patients with chronic renal insufficiency presenting with acute coronary syndrome: data from a registry of 8176 patients. Mayo Clin Proc. 2010;85:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karimi Galougahi K, Zalewski A, Leon MB, Karmpaliotis D, Ali ZA. Optical coherence tomography‐guided percutaneous coronary intervention in pre‐terminal chronic kidney disease with no radio‐contrast administration. Eur Heart J. 2016;37:1059. [DOI] [PubMed] [Google Scholar]
- 55. Navarro MA, Gosch KL, Spertus JA, Rumsfeld JS, Ho PM. Chronic kidney disease and health status outcomes following acute myocardial infarction. J Am Heart Assoc. 2016;5:e002772 doi: 10.1161/JAHA.115.002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choi HY, Park HC, Ha SK. How do we manage coronary artery disease in patients with CKD and ESRD? Electrolyte Blood Press. 2014;12:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saltzman AJ, Stone GW, Claessen BE, Narula A, Leon‐Reyes S, Weisz G, Brodie B, Witzenbichler B, Guagliumi G, Kornowski R, Dudek D, Metzger DC, Lansky AJ, Nikolsky E, Dangas GD, Mehran R. Long‐term impact of chronic kidney disease in patients with ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS‐AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4:1011–1019. [DOI] [PubMed] [Google Scholar]
- 58. Tsai TT, Messenger JC, Brennan JM, Patel UD, Dai D, Piana RN, Anstrom KJ, Eisenstein EL, Dokholyan RS, Peterson ED, Douglas PS. Safety and efficacy of drug‐eluting stents in older patients with chronic kidney disease: a report from the linked CathPCI Registry‐CMS claims database. J Am Coll Cardiol. 2011;58:1859–1869. [DOI] [PubMed] [Google Scholar]
- 59. Green SM, Selzer F, Mulukutla SR, Tadajweski EJ, Green JA, Wilensky RL, Laskey WK, Cohen HA, Rao SV, Weisbord SD, Lee JS, Reis SE, Kip KE, Kelsey SF, Williams DO, Marroquin OC. Comparison of bare‐metal and drug‐eluting stents in patients with chronic kidney disease (from the NHLBI Dynamic Registry). Am J Cardiol. 2011;108:1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tomai F, Ribichini F, De Luca L, Petrolini A, Ghini AS, Weltert L, Spaccarotella C, Proietti I, Trani C, Nudi F, Pighi M, Vassanelli C. Randomized comparison of Xience V and multi‐link vision coronary stents in the same multivessel patient with chronic kidney disease (RENAL‐DES) study. Circulation. 2014;129:1104–1112. [DOI] [PubMed] [Google Scholar]
- 61. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrie D, Naber C, Lipiecki J, Richardt G, Iniguez A, Brunel P, Valdes‐Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC. Polymer‐free drug‐coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–2047. [DOI] [PubMed] [Google Scholar]
- 62. Szczech LA, Reddan DN, Owen WF, Califf R, Racz M, Jones RH, Hannan EL. Differential survival after coronary revascularization procedures among patients with renal insufficiency. Kidney Int. 2001;60:292–299. [DOI] [PubMed] [Google Scholar]
- 63. Azar RR, Prpic R, Ho KK, Kiernan FJ, Shubrooks SJ Jr, Baim DS, Popma JJ, Kuntz RE, Cohen DJ. Impact of end‐stage renal disease on clinical and angiographic outcomes after coronary stenting. Am J Cardiol. 2000;86:485–489. [DOI] [PubMed] [Google Scholar]
- 64. Williams ME. Coronary revascularization in diabetic chronic kidney disease/end‐stage renal disease: a nephrologist's perspective. Clin J Am Soc Nephrol. 2006;1:209–220. [DOI] [PubMed] [Google Scholar]
- 65. Yasuda K, Kasuga H, Aoyama T, Takahashi H, Toriyama T, Kawade Y, Iwashima S, Yamada S, Kawahara H, Maruyama S, Yuzawa Y, Ishii H, Murohara T, Matsuo S. Comparison of percutaneous coronary intervention with medication in the treatment of coronary artery disease in hemodialysis patients. J Am Soc Nephrol. 2006;17:2322–2332. [DOI] [PubMed] [Google Scholar]
- 66. Urban P, Abizaid A, Chevalier B, Greene S, Meredith I, Morice MC, Pocock S. Rationale and design of the LEADERS FREE trial: a randomized double‐blind comparison of the BioFreedom drug‐coated stent vs the Gazelle bare metal stent in patients at high bleeding risk using a short (1 month) course of dual antiplatelet therapy. Am Heart J. 2013;165:704–709. [DOI] [PubMed] [Google Scholar]
- 67. Kereiakes DJ, Meredith IT, Windecker S, Lee Jobe R, Mehta SR, Sarembock IJ, Feldman RL, Stein B, Dubois C, Grady T, Saito S, Kimura T, Christen T, Allocco DJ, Dawkins KD. Efficacy and safety of a novel bioabsorbable polymer‐coated, everolimus‐eluting coronary stent: the EVOLVE II Randomized Trial. Circ Cardiovasc Interv. 2015;8:e002372. [DOI] [PubMed] [Google Scholar]
- 68. Binder RK, Luscher TF. Duration of dual antiplatelet therapy after coronary artery stenting: where is the sweet spot between ischaemia and bleeding? Eur Heart J. 2015;36:1207–1211. [DOI] [PubMed] [Google Scholar]
- 69. Zhang Y, Farooq V, Garcia‐Garcia HM, Bourantas CV, Tian N, Dong S, Li M, Yang S, Serruys PW, Chen SL. Comparison of intravascular ultrasound versus angiography‐guided drug‐eluting stent implantation: a meta‐analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855–865. [DOI] [PubMed] [Google Scholar]
- 70. Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, Caillard S, Campia U, Moulin B, Gachet C, Ohlmann P. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011;57:399–408. [DOI] [PubMed] [Google Scholar]
- 71. Malone J, Singh G, Armstrong E, DeMattos A, Laird J, Low R, Nishimura M, Rogers J, Southard J, Wong G. Abstract 17044: percutaneous coronary intervention and dual antiplatelet therapy are safe prior to kidney transplantation. Circulation. 2014;130:A17044. [Google Scholar]
- 72. Bailey PDAH, Lubetzky M, Kayler LK. Outcomes of kidney transplant recipients on dual antiplatelet therapy. Austin J Nephrol Hypertens. 2015;2:1040. [Google Scholar]
- 73. Meier‐Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end‐stage renal disease. Am J Transplant. 2004;4:1662–1668. [DOI] [PubMed] [Google Scholar]
- 74. Hypolite IO, Bucci J, Hshieh P, Cruess D, Agodoa LY, Yuan CM, Taylor AJ, Abbott KC. Acute coronary syndromes after renal transplantation in patients with end‐stage renal disease resulting from diabetes. Am J Transplant. 2002;2:274–281. [DOI] [PubMed] [Google Scholar]
- 75. Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol. 2006;17:900–907. [DOI] [PubMed] [Google Scholar]
- 76. Schreiber BD. Congestive heart failure in patients with chronic kidney disease and on dialysis. Am J Med Sci. 2003;325:179–193. [DOI] [PubMed] [Google Scholar]
- 77. Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–890. [DOI] [PubMed] [Google Scholar]
- 78. Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V. Effects of beta‐adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:1152–1161. [DOI] [PubMed] [Google Scholar]
- 79. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. [DOI] [PubMed] [Google Scholar]
- 80. Glassock RJ, Pecoits‐Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4(suppl 1):S79–S91. [DOI] [PubMed] [Google Scholar]
- 81. Glassock RJ, Pecoits‐Filho R, Barbareto S. Increased left ventricular mass in chronic kidney disease and end‐stage renal disease: what are the implications? Dial Transplant. 2010;39:16–19. [Google Scholar]
- 82. Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In‐center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zannad F, Kessler M, Lehert P, Grunfeld JP, Thuilliez C, Leizorovicz A, Lechat P. Prevention of cardiovascular events in end‐stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–1324. [DOI] [PubMed] [Google Scholar]
- 84. Bignelli AT, Barberato SH, Aveles P, Abensur H, Pecoits‐Filho R. The impact of living donor kidney transplantation on markers of cardiovascular risk in chronic kidney disease patients. Blood Purif. 2007;25:233–241. [DOI] [PubMed] [Google Scholar]
- 85. Ferreira SR, Moises VA, Tavares A, Pacheco‐Silva A. Cardiovascular effects of successful renal transplantation: a 1‐year sequential study of left ventricular morphology and function, and 24‐hour blood pressure profile. Transplantation. 2002;74:1580–1587. [DOI] [PubMed] [Google Scholar]
- 86. Amin MS, Fox AD, Kalahasty G, Shepard RK, Wood MA, Ellenbogen KA. Benefit of primary prevention implantable cardioverter‐defibrillators in the setting of chronic kidney disease: a decision model analysis. J Cardiovasc Electrophysiol. 2008;19:1275–1280. [DOI] [PubMed] [Google Scholar]
- 87. El‐Chami MF, Levy M, Kelli HM, Casey M, Hoskins MH, Goyal A, Langberg JJ, Patel A, Delurgio D, Lloyd MS, Leon AR, Merchant FM. Outcome of subcutaneous implantable cardioverter defibrillator implantation in patients with end‐stage renal disease on dialysis. J Cardiovasc Electrophysiol. 2015;26:900–904. [DOI] [PubMed] [Google Scholar]
- 88. Guha A, Maddox WR, Colombo R, Nahman NS Jr, Kintziger KW, Waller JL, Diamond M, Murphy M, Kheda M, Litwin SE, Sorrentino RA. Cardiac implantable electronic device infection in patients with end‐stage renal disease. Heart Rhythm. 2015;12:2395–2401. [DOI] [PubMed] [Google Scholar]
- 89. Saad TF, Hentschel DM, Koplan B, Wasse H, Asif A, Patel DV, Salman L, Carrillo R, Hoggard J. Cardiovascular implantable electronic device leads in CKD and ESRD patients: review and recommendations for practice. Semin Dial. 2013;26:114–123. [DOI] [PubMed] [Google Scholar]
- 90. Dhamija RK, Tan H, Philbin E, Mathew RO, Sidhu MS, Wang J, Saour B, Haqqie SS, Beathard G, Yevzlin AS, Salman L, Boden WE, Siskin G, Asif A. Subcutaneous implantable cardioverter defibrillator for dialysis patients: a strategy to reduce central vein stenoses and infections. Am J Kidney Dis. 2015;66:154–158. [DOI] [PubMed] [Google Scholar]
- 91. Siedlecki A, Foushee M, Curtis JJ, Gaston RS, Perry G, Iskandrian AE, de Mattos AM. The impact of left ventricular systolic dysfunction on survival after renal transplantation. Transplantation. 2007;84:1610–1617. [DOI] [PubMed] [Google Scholar]
- 92. Karthikeyan V, Chattahi J, Kanneh H, Koneru J, Hayek S, Patel A, Goggins M, Ananthasubramaniam K. Impact of pre‐existing left ventricular dysfunction on kidney transplantation outcomes: implications for patient selection. Transplant Proc. 2011;43:3652–3656. [DOI] [PubMed] [Google Scholar]
- 93. Hage FG, Smalheiser S, Zoghbi GJ, Perry GJ, Deierhoi M, Warnock D, Iskandrian AE, de Mattos AM, Aqel RA. Predictors of survival in patients with end‐stage renal disease evaluated for kidney transplantation. Am J Cardiol. 2007;100:1020–1025. [DOI] [PubMed] [Google Scholar]
- 94. Weinrauch LA, D'Elia JA, Monaco AP, Gleason RE, Welty F, Nishan PC, Nesto RW. Preoperative evaluation for diabetic renal transplantation: impact of clinical, laboratory, and echocardiographic parameters on patient and allograft survival. Am J Med. 1992;93:19–28. [DOI] [PubMed] [Google Scholar]
- 95. Sharma R, Chemla E, Tome M, Mehta RL, Gregson H, Brecker SJ, Chang R, Pellerin D. Echocardiography‐based score to predict outcome after renal transplantation. Heart. 2007;93:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Melchor JL, Espinoza R, Gracida C. Kidney transplantation in patients with ventricular ejection fraction less than 50 percent: features and posttransplant outcome. Transplant Proc. 2002;34:2539–2540. [DOI] [PubMed] [Google Scholar]
- 97. Ali ZA, Karimi Galougahi K, Nazif T, Maehara A, Hardy MA, Cohen DJ, Ratner LE, Collins MB, Moses JW, Kirtane AJ, Stone GW, Karmpaliotis D, Leon MB. Imaging‐ and physiology‐guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J. 2016. doi: 10.1093/eurheartj/ehw078. Available at: Available at: http://eurheartj.oxfordjournals.org/content/early/2016/03/07/eurheartj.ehw078. Accessed July 27, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 99. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic‐valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. [DOI] [PubMed] [Google Scholar]
- 100. Urena P, Malergue MC, Goldfarb B, Prieur P, Guedon‐Rapoud C, Petrover M. Evolutive aortic stenosis in hemodialysis patients: analysis of risk factors. Nephrologie. 1999;20:217–225. [PubMed] [Google Scholar]
- 101. Maher ER, Pazianas M, Curtis JR. Calcific aortic stenosis: a complication of chronic uraemia. Nephron. 1987;47:119–122. [DOI] [PubMed] [Google Scholar]
- 102. Melchor JL, Gracida C, Orihuela O. Improvement of mitral dysfunction after kidney transplantation. Transplant Proc. 2002;34:396–397. [DOI] [PubMed] [Google Scholar]
- 103. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 104. Abbott KC, Hshieh P, Cruess D, Agodoa LY, Welch PG, Taylor AJ, Yuan CM. Hospitalized valvular heart disease in patients on renal transplant waiting list: incidence, clinical correlates and outcomes. Clin Nephrol. 2003;59:79–87. [DOI] [PubMed] [Google Scholar]
- 105. Flameng WJ, Herijgers P, Szecsi J, Sergeant PT, Daenen WJ, Scheys I. Determinants of early and late results of combined valve operations and coronary artery bypass grafting. Ann Thorac Surg. 1996;61:621–628. [DOI] [PubMed] [Google Scholar]
- 106. Suehiro S, Shibata T, Sasaki Y, Murakami T, Hosono M, Fujii H, Kinoshita H. Cardiac surgery in patients with dialysis‐dependent renal disease. Ann Thorac Cardiovasc Surg. 1999;5:376–381. [PubMed] [Google Scholar]
- 107. Thourani VH, Sarin EL, Kilgo PD, Lattouf OM, Puskas JD, Chen EP, Guyton RA. Short‐ and long‐term outcomes in patients undergoing valve surgery with end‐stage renal failure receiving chronic hemodialysis. J Thorac Cardiovasc Surg. 2012;144:117–123. [DOI] [PubMed] [Google Scholar]
- 108. Kogan A, Medalion B, Kornowski R, Raanani E, Sharoni E, Stamler A, Sahar G, Snir E, Porat E. Cardiac surgery in patients on chronic hemodialysis: short and long‐term survival. Thorac Cardiovasc Surg. 2008;56:123–127. [DOI] [PubMed] [Google Scholar]
- 109. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. [DOI] [PubMed] [Google Scholar]
- 110. Carabello BA. Evaluation and management of patients with aortic stenosis. Circulation. 2002;105:1746–1750. [DOI] [PubMed] [Google Scholar]
- 111. Fox H, Buttner S, Hemmann K, Asbe‐Vollkopf A, Doss M, Beiras‐Fernandez A, Moritz A, Zeiher AM, Scheuermann E, Geiger H, Fichtlscherer S, Hauser IA, Lehmann R. Transcatheter aortic valve implantation improves outcome compared to open‐heart surgery in kidney transplant recipients requiring aortic valve replacement. J Cardiol. 2013;61:423–427. [DOI] [PubMed] [Google Scholar]
- 112. Rau S, Wessely M, Lange P, Kupatt C, Steinbeck G, Fischereder M, Schonermarck U. Transcatheter aortic valve implantation in dialysis patients. Nephron Clin Pract. 2012;120:c86–c90. [DOI] [PubMed] [Google Scholar]
- 113. Généreux P, Stone GW, O'Gara PT, Marquis‐Gravel G, Redfors B, Giustino G, Pibarot P, Bax JJ, Bonow RO, Leon MB. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2016;67:2263–2288. [DOI] [PubMed] [Google Scholar]
- 114. Marechaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV, Pibarot P. Usefulness of exercise‐stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 116. Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone G. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–307. [DOI] [PubMed] [Google Scholar]
- 117. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 118. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 119. Casas‐Aparicio G, Castillo‐Martinez L, Orea‐Tejeda A, Abasta‐Jimenez M, Keirns‐Davies C, Rebollar‐Gonzalez V. The effect of successful kidney transplantation on ventricular dysfunction and pulmonary hypertension. Transplant Proc. 2010;42:3524–3528. [DOI] [PubMed] [Google Scholar]
- 120. Di Lullo L, Floccari F, Rivera R, Barbera V, Granata A, Otranto G, Mudoni A, Malaguti M, Santoboni A, Ronco C. Pulmonary hypertension and right heart failure in chronic kidney disease: new challenge for 21st‐century cardionephrologists. Cardiorenal Med. 2013;3:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lentine KL, Villines TC, Axelrod D, Kaviratne S, Weir MR, Costa SP. Evaluation and management of pulmonary hypertension in kidney transplant candidates and recipients: concepts and controversies. Transplantation. doi: 10.1097/TP.0000000000001043. Available at: http://journals.lww.com/transplantjournal/pages/articleviewer.aspx?year=9000&issue=00000&article=97476&type=abstract. Accessed July 27, 2016. [DOI] [PubMed] [Google Scholar]
- 122. Yigla M, Abassi Z, Reisner SA, Nakhoul F. Pulmonary hypertension in hemodialysis patients: an unrecognized threat. Semin Dial. 2006;19:353–357. [DOI] [PubMed] [Google Scholar]
- 123. Abedini M, Sadeghi M, Naini AE, Atapour A, Golshahi J. Pulmonary hypertension among patients on dialysis and kidney transplant recipients. Ren Fail. 2013;35:560–565. [DOI] [PubMed] [Google Scholar]
- 124. Ramasubbu K, Deswal A, Herdejurgen C, Aguilar D, Frost AE. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: prevalence and clinical significance. Int J Gen Med. 2010;3:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Minai OA, Yared JP, Kaw R, Subramaniam K, Hill NS. Perioperative risk and management in patients with pulmonary hypertension. Chest. 2013;144:329–340. [DOI] [PubMed] [Google Scholar]
- 126. Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, Nakhoul F. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–975. [DOI] [PubMed] [Google Scholar]
- 127. Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, Reisner SA. Pulmonary hypertension in patients with end‐stage renal disease. Chest. 2003;123:1577–1582. [DOI] [PubMed] [Google Scholar]
- 128. Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23:282–287. [DOI] [PubMed] [Google Scholar]
- 129. Ye WL, Fang LG, Ma J, Li XM. Long‐term effects of arteriovenous fistula on cardiac structure and function in non‐diabetic hemodialysis patients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013;35:95–101. [DOI] [PubMed] [Google Scholar]


