Abstract
Background
Even if sodium sensitivity represents a risk factor at any blood pressure (BP) level, limited evidence is available that it may influence cardiovascular control in normotensives, particularly in white individuals. Therefore, the aim of the study was to investigate whether sodium sensitivity alters hemodynamic or autonomic responses to salt in normotensives.
Methods and Results
We evaluated the Sodium‐Sensitivity Index (SS‐Index) in 71 white normotensives after 5 days of high‐ and low‐sodium diets. We measured BP continuously at the end of each period, estimating hemodynamic indices from BP waveform analysis, and autonomic indices from heart rate (HR) and BP variability. According to the SS‐Index distribution, we defined 1 sodium‐sensitive group (SS, with SS‐Index >15 mm Hg/[mmol·day]), 1 sodium‐resistant group, (unresponsive to sodium load with −15≤ SS‐Index ≤+15), and 1 inverse sodium‐sensitive group, responsive to sodium by decreasing BP, with SS‐Index <−15). We compared the effects of the diets among groups, and correlated autonomic/hemodynamic indices with the SS‐Index. After sodium loading, a significant decrease in systemic peripheral resistances, HR, spectral indices of BP modulation, and a significant increase of indices of HR vagal modulation were found in the inverse sodium‐sensitive group but not in SS normotensives. Moreover, the highest SS‐Indices were associated with the lesser vagal HR decelerations.
Conclusions
Our data suggest that salt sensitivity in white normotensive individuals is associated with impaired vasodilation and altered autonomic response to dietary salt. Such dysfunction may critically contribute to induce a BP response to dietary salt.
Keywords: autonomic function, baroreflex, blood pressure spectral analysis, heart rate variability, peripheral resistance, salt intake, salt‐sensitive
Subject Categories: Autonomic Nervous System, Hemodynamics, Diet and Nutrition, Risk Factors
Introduction
Salt‐sensitive hypertensive individuals display a higher rate of cardiovascular events than salt‐resistant hypertensive individuals.1 A similar trend characterizes salt‐sensitive normotensives, who also have a significant increase in mortality rate over time.2 However, few studies investigated the mechanisms relating the increased cardiovascular risk to sodium sensitivity. Sodium loading/depletion maneuvers may unveil alterations in cardiovascular control associated with sodium sensitivity possibly involved in the increased rate of cardiovascular events. In this regard, while it has been shown that salt‐sensitive hypertensive patients respond to sodium loading with either a blunted sympathetic deactivation3 or an impaired cardiac parasympathetic activation,4, 5 limited evidence of an altered autonomic cardiovascular modulation is available in normotensive subjects.
A study in mostly normotensive black Americans, undergoing a few days of dietary salt loading, showed a significant decrease in systemic vascular resistance in sodium‐resistant individuals, while such a response was largely impaired in sodium‐sensitive individuals.6 This finding suggests the hypothesis that an autonomic‐mediated reduction in peripheral vascular resistance, in response to sodium loading, might be weakened or absent in sodium‐sensitive normotensives. However, to the best of our knowledge, no data are available on the dependence of hemodynamic or autonomic responses to salt loading on the degree of sodium sensitivity in white normotensive individuals.
The present study specifically addressed the above open issue. We tested the hypothesis that an altered hemodynamic or autonomic response to dietary salt manipulation is detectable in white normotensive individuals as a function of their sensitivity to sodium. This was done by deriving information on hemodynamic control from pulse waveform analysis and by assessing spontaneous heart rate (HR) and blood pressure (BP) variability during different levels of sodium intake in healthy normotensives with different degrees of sodium sensitivity, as quantified in a continuous fashion by the sodium sensitivity index (SS‐Index).
Methods
Subjects and Data Collection
The experimental protocol conformed to the Declaration of Helsinki and was approved by the ethics committee of Don C. Gnocchi Foundation. Seventy‐one normotensive volunteers (45 females), recruited among medical students and residents attending our University Hospital, were included. Inclusion criteria were a normotensive status, confirmed by clinic BP measurements and 24‐hour ambulatory BP monitoring, and the absence of history and of any physical or laboratory evidence of cardiovascular disease. No endurance or professional athletes were enrolled. Volunteers received a detailed explanation of the study and gave informed consent. All participants followed a low‐sodium (30 mmol NaCl per day) and a high‐sodium (200 mmol NaCl per day) diet, each for 5 days, in random order. They were instructed on how to prepare their meals at home, and received the total amount of sodium to be used in their food during the high‐sodium diet from the hospital pharmacy, in separate 1‐g sachets. During both diets, they were asked to refrain from intense physical exercise. On the last day of each diet, the 24‐hour urinary sodium excretion rate (UNaV) was quantified. In the morning of the same day, body weight was assessed and brachial BP was measured on the right arm with subjects in a sitting position for 2 hours, with readings every 15 minutes with an oscillometric ambulatory BP monitoring device (Spacelabs 90207; Spacelabs Healthcare, Redmond, WA). Mean arterial pressure (MAP) was averaged over the 2‐hour recording period. The difference between average MAP values at the end of the high‐salt and low‐salt diets (ΔMAP) was divided by the difference between the corresponding urinary sodium excretion rates (ΔUNaV) to obtain the SS‐Index,7 defined as ΔMAP/ΔUNaV ratio and expressed in mm Hg/(mol·day).
Finger BP was recorded beat‐by‐beat for 2 hours from the mid finger of the left hand by the Portapres model‐2 device (Finapres Medical Systems B.V., Amsterdam, the Netherlands) simultaneously with the brachial right‐arm cuff. Finger BP was obtained in 68 of the 71 volunteers; technical problems prevented the recording in 3 cases. Finger BP waveforms were sampled at 100 Hz. Beat‐by‐beat values of systolic BP, diastolic BP, and pulse interval (PI) were derived from the finger BP waveforms. Since we previously observed a significant proportional bias between finger and brachial BP measures associated with the sodium depletion maneuver,8 the brachial measures of systolic and diastolic BP, averaged over the 2‐hour recordings, were used to calibrate the finger BP waveform. After such calibration, the average of systolic and diastolic beat‐by‐beat BP series from Portapres coincided with the corresponding brachial BP measures. The beat‐by‐beat BP series were visually inspected by an expert operator to remove premature beats and artifacts, and the recordings of 1 subject were discarded because a high number of artifacts made them unsuitable for spectral analysis. Thus, data of 67 volunteers (41 females) were available for BP waveform and for HR and BP variability analysis.
Hemodynamic parameters were derived from BP waveform analysis through Model‐Flow analysis (see details of the Model‐Flow method and of validation studies versus “gold standard” methods in Data S1). Hemodynamic parameters were the following: left ventricular ejection time, systemic vascular resistances (SVR), aortic characteristic impedance, arterial compliance, stroke volume, and cardiac output. Autonomic indices were calculated from the analysis of HR and BP variability (Data S1). Frequency‐domain indices of HR variability were the high‐frequency power of PI (PI HF), index of vagal modulations of HR, and the ratio between low‐frequency (LF) and HF powers of PI (PI LF/HF powers ratio), which is an index of the cardiac sympatho/vagal balance.9 Frequency‐domain indices of BP variability were the LF power of diastolic BP, reported to reflect sympathetically mediated vasomotor oscillations generally assumed to be induced by baroreflex resonance10, 11; the very‐low frequency power of diastolic BP that quantifies long‐term fluctuations mainly of vasomotor origin; and the sensitivity of baroreflex control of HR estimated by the transfer function method over the LF (BRS LF) and HF (BRS HF) bands. Additionally, we calculated the number of PI increases per minute larger than 50 ms (NN50+, time‐domain index of cardiac parasympathetic modulation under the hypothesis that bursts of vagal outflow on the sinus node produce PI lengthening of more than 50 ms)12; and the short‐term scale coefficient α1 through detrended fluctuation analysis of PI, which is a complexity‐domain index of cardiac sympatho/vagal balance.13, 14
Statistics
The association between SS‐Index and hemodynamic or autonomic indices was assessed by the nonparametric Kendall's rank correlation coefficient, τ, separately after each diet.
Traditionally, individuals are classified on the basis of their response to sodium tests in those that increase BP, or sodium sensitive (SS), and in those that do not increase BP, even if this latter group has a heterogeneous response, with some individuals presenting even a marked depressor response to salt loading.15 Table S1 (see Data S2) summarizes results following this dichotomous approach. More recently, it has been proposed to further divide individuals who do not increase BP after sodium loading in a genuine sodium‐resistant (SR) group, actually unresponsive to sodium, and in an inverse sodium‐sensitive (ISS) group, that respond to high dietary salt decreasing BP.16 Following this trichotomous approach, we defined SR as those individuals with SS‐Index between −15 and +15 mm Hg/(mol·day), ISS those with SS‐Index lower than −15 mm Hg/(mol·day), and SS those with SS‐Index greater than 15 mm Hg/(mol·day). Figure S1 (see Data S3) illustrates the SS‐Index distribution and the 3 sodium‐sensitivity classes in our normotensive population.
The effects of sodium loading on ISS, SR, and SS groups were assessed by longitudinal analysis of mean response profiles, assuming no specific covariance structure of the repeated measures.17 Analysis factors were the “SS condition” (ie, ISS, SR, and SS) and “diet,” the latter with 2 repeated measures, 1 at the low‐salt diet and 1 at the high‐salt diet. Statistical significance of the difference between diets was evaluated with “a posteriori” contrasts after correction for multiple comparisons with the false discovery rate procedure.18 A proper transformation was applied if the hypothesis of normality was rejected (Shapiro–Wilk test). Frequency domain indices were log‐transformed, reducing their skewness.19 Cardiac output and stroke volume were also log‐transformed. No transformation was required for NN50+ and α1, as previously reported in healthy volunteers.20 Analysis was performed with “R: A Language and Environment for Statistical Computing” software package (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2015), setting the statistical significance threshold at P=0.05.
Results
Table 1 reports the general characteristics of our population. The 3 groups had similar age and 24‐hour ambulatory BP level during habitual diet. Female sex was more common, with their prevalence at 69%, 60%, and 59% in the ISS, SR, and SS groups, respectively, without significant differences among groups. The body mass index was slightly higher in SS than in ISS. The percentage of individuals with positive family history for hypertension was almost double in the SS (41%) than in the ISS (21%) group, although this difference did not reach statistical significance.
Table 1.
General Characteristics of Normotensive Volunteers by SS‐Index Classes: Mean (SD)
| Classes | ISS (N=29) | SR (N=25) | SS (N=17) | P Value |
|---|---|---|---|---|
| SS‐Index range, mm Hg/(mol·day) | −98.2: −15.3 | −14.5: +14.3 | +15.1: +123.0 | |
| ΔMAP, mm Hga | −6.4 (3.7) | 0.0 (2.0) | 6.5 (3.9) | |
| Sex, F/M | 20/9 | 15/10 | 10/7 | 0.72 |
| Age, y | 27.3 (6.4) | 26.0 (3.8) | 29.6 (6.8) | 0.053 |
| Body mass index, kg/m2 b | 21.4 (2.6)c | 22.1 (2.4) | 23.9 (2.5) | <0.01 |
| ΔWeight, kgd | 1.4 (1.1) | 1.2 (1.6) | 0.9 (1.4) | 0.34 |
| Family history of hypertension | 21% | 36% | 41% | 0.28 |
| 24‐h systolic BP, mm Hge | 115.0 (7.7) | 116.5 (8.6) | 116.2 (11.7) | 0.82 |
| 24‐h diastolic BP, mm Hge | 68.6 (5.6) | 69.2 (6.1) | 69.8 (8.6) | 0.84 |
| 24‐h HR, bpme | 73.5 (9.0) | 70.7 (9.2) | 71.5 (10.5) | 0.53 |
BP indicates blood pressure; HR, heart rate; ISS, inverse sodium‐sensitive; SR, sodium resistant; SS, sodium sensitive.
ΔMAP, difference between high‐salt and low‐salt conditions in mean arterial pressure averaged over the 2‐h recording period at the end of the diet.
Measures at the end of the low‐sodium diet.
Means a significant difference vs SS (Tukey's post‐hoc).
ΔWeight, difference between body weights at the end of the high‐salt and low‐salt diets.
Data from 24‐h ambulatory BP monitoring during habitual diet; P calculated by χ2 test for sex and family history of hypertension, by 1‐way ANOVA for all the other variables.
Figure 1 shows the effect of the sodium loading/depletion maneuver on BP and HR. With respect to the low‐salt diet, the high‐salt diet decreased BP in the ISS group (systolic BP: from 115.3±11.0 to 108.5±11.2; MAP: from 85.1±9.3 to 78.7±9.2; diastolic BP: from 71.3±9.5 to 64.9±8.3 mm Hg, mean±SD), increased BP in the SS group (systolic BP: from 112.8±9.2 to 119.0±9.2; MAP: from 80.7±7.7 to 87.2±8.2; diastolic BP: from 67.0±6.9 to 72.6±7.6), and did not affect the SR group (systolic BP: from 114.7±10.4 to 114.2±11.4; MAP: from 82.3±7.2 to 82.2±7.8; diastolic BP: from 68.9±6.2 to 68.8±6.7). The high‐salt diet also decreased HR significantly in ISS (from 71.9±10.9 to 67.0±8.6 bpm) and SR (from 73.2±10.9 to 69.6±13.4) groups, but not in the SS group (from 69.1±12.1 to 67.9±9.5).
Figure 1.
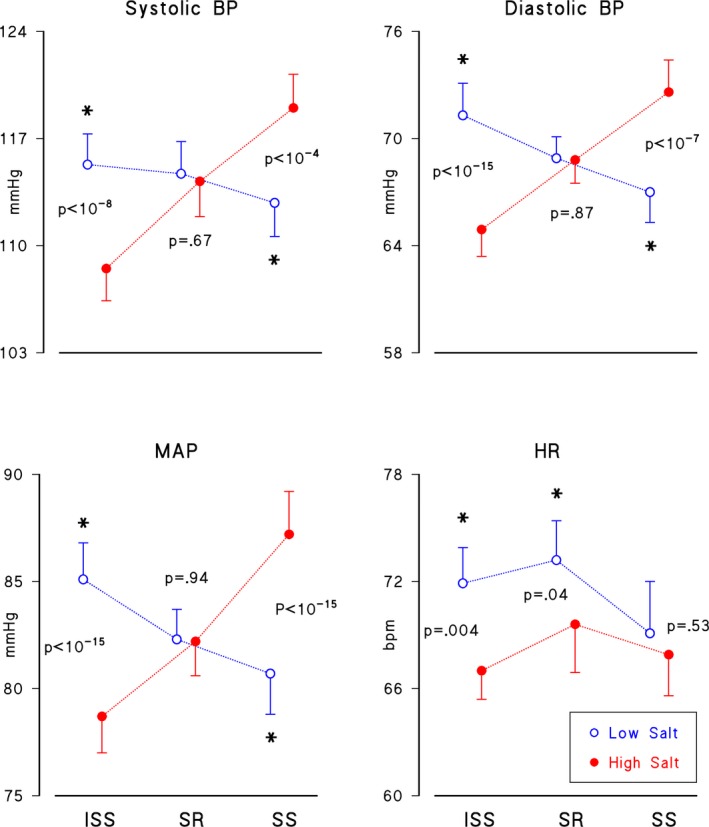
Brachial measures of blood pressure (BP) and heart rate (HR). Mean (SEM) after low‐salt (open circles) and high‐salt (solid circles) diets in 3 groups of normotensive volunteers, defined on the basis of SS‐Index: a sodium‐sensitive group, SS, with SS‐Index >15 mm Hg/(mmol·day), and 2 sodium‐resistant groups: 1 unresponsive to sodium load, SR, with −15≤ SS‐Index ≤15; and 1 responding to sodium load with a BP decrease, ISS, with SS‐index <−15. ISS indicates inverse sodium sensitive; MAP, mean arterial pressure. The “*” marks significant differences between diets.
Figure 2 shows left ventricular ejection time and other hemodynamic parameters from model‐flow analysis. The high‐salt diet increased left ventricle ejection time in all groups (+10.8, +17.8, and +13.8 ms in ISS, SR, and SS, respectively). However, it induced opposite changes of SVR and arterial compliance in ISS and SS, without affecting SR (P<0.001 for factors interaction). In fact, after the high‐salt diet, SVR decreased in ISS (from 1.22±0.34 to 1.07±0.20 mm Hg×s/mL), tended to increase in SS (from 1.00±0.27 to 1.12±0.31) without substantial changes in SR (from 1.03±0.19 to 1.07±0.26), while arterial compliance increased in ISS (from 2.16±0.43 to 2.33±0.45 mL/mm Hg) and decreased in SS (from 2.45±0.56 to 2.31±0.58), also in this case without substantial changes in SR (from 2.35±0.38 to 2.38±0.41). Aortic characteristic impedance decreased in ISS only.
Figure 2.

Hemodynamic parameters. Mean (SEM) after low‐salt and high‐salt diets in inverse sodium‐sensitive (ISS), sodium‐resistant (SR), and sodium‐sensitive (SS) individuals; SVR indicates systemic vascular resistances. The “*” marks significant differences between diets.
Considering HR variability (Figure 3), the high‐salt diet decreased spectral and complexity‐based indices of cardiac sympatho/vagal balance (PI LF/HF decreased by −21%, −20%, and −16% and α1 decreased by −6%, −4%, and −7% in ISS, SR, and SS groups, respectively). The high‐salt diet also increased time‐domain and spectral indices of vagal HR modulation (+32%, +36%, and +24% for NN50+, +51%, +54%, and +38% for PI HF, in ISS, SR, and SS groups, respectively). The vagal HR modulation tended to decrease with the severity of sodium sensitivity and the effect of sodium loading appeared less marked in the SS group.
Figure 3.

Autonomic indices of HR variability. Left: frequency‐ and complexity‐domain indices of cardiac sympatho/vagal balance (PI LF/HF and α1); right: frequency‐ and time‐domain indices of vagal modulations of HR (PI HF and NN50+), in inverse sodium‐sensitive (ISS), sodium‐resistant (SR), and sodium‐sensitive (SS) individuals. α1 indicates short‐term scale coefficient of PI; HF indicates high frequency; HR, heart rate; LF, low frequency; NN50+, number of PI increases per minute larger than 50 ms; PI, pulse interval. Spectral indices are represented in logarithmic scale. The “*” marks significant differences between diets.
Figure 4 shows that the high‐salt diet increased the baroreflex sensitivity in all groups (BRS LF: +22%, +38%, and +23%; BRS HF: +29%, +48%, and +28% in ISS, SR, and SS groups). The high‐salt diet also decreased the LF power of diastolic BP in ISS (−24%), in SR (−52%), and in SS (−24%) groups, with the change reaching the significance threshold in ISS and SR groups only. Sodium loading also decreased the diastolic BP fluctuations of longer term (very‐low frequency power), significantly in the ISS group (−32%), and close to the significance level in the SR group (−26%). By contrast, very‐low frequency power of diastolic BP was only marginally influenced by the sodium diets in the SS group (−6%).
Figure 4.
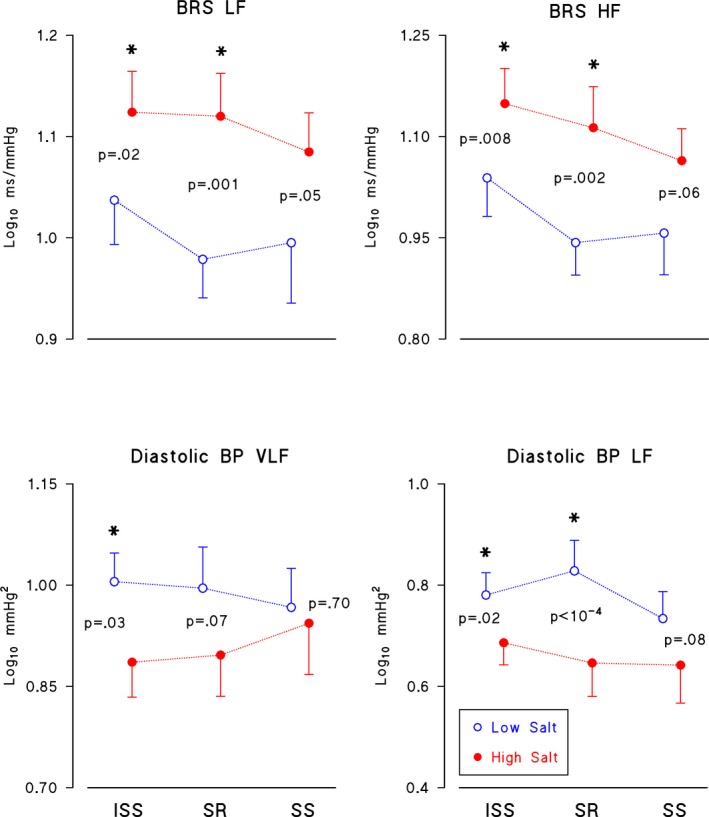
Autonomic indices of BP variability. Indices of baroreflex sensitivity (BRS LF and BRS HF) and of sympathetic modulations of vascular tone (diastolic BP VLF and LF) represented in logarithmic scale, in inverse sodium sensitive (ISS), sodium resistant (SR), and sodium sensitive (SS) individuals. BP indicates blood pressure; HF, high frequency; LF, low frequency; VLF, very‐low frequency.
The SS‐Index also allows describing the severity of sodium sensitivity in a continuous fashion, without introducing predefined classes of salt sensitivity. In this regard, Figure 5 shows mean values of SVR, cardiac output, and MAP by quintiles of SS‐Index, separately after the 2 diets. Interestingly, SVR progressively decreased and cardiac output progressively increased from the lower to the upper quintile after the low‐salt diet while, after the high‐salt diet, SVR appeared uniformly distributed and the increase of cardiac output with SS‐Index was much less pronounced. MAP was substantially higher in the 2 upper quintiles after the high‐salt diet while, after the low‐salt diet, the highest value was associated with the lower quintile. Table 2 reports the Kendall's associations between SS‐Index and all the other variables of the study by diets. As expected, BP increased with SS‐Index after the high‐salt diet. Indices of cardiovascular hemodynamics were also associated with SS‐Index but only after the low‐salt diet: stroke volume, cardiac output, and arterial compliance increased while SVR and aortic characteristic impedance decreased with SS‐Index. As regards HR and BP variability, SS‐Index was negatively associated with vagal HR modulation after the high‐salt diet: NN50+ decreased significantly with SS‐Index, and the same trend, close to the significance threshold (P=0.07), characterized the frequency‐domain index of vagal HR modulation, PI HF.
Figure 5.

Dependence of SVR, cardiac output, and MAP on SS‐Index. Mean and SEM by quintiles of the SS‐Index distribution, plotted separately after the low‐salt diet (upper panels) and the high‐salt diet (lower panels). SS‐Index range for each quintile is reported on the lower right panel. MAP indicates mean arterial pressure; SVR, systemic vascular resistances.
Table 2.
Correlation Between SS‐Index and Hemodynamic or Autonomic Variables (Kendall's τ and Significance P) at the End of Each Diet
| Low‐Salt Diet | High‐Salt Diet | |||
|---|---|---|---|---|
| τ | P Value | τ | P Value | |
| Systolic BP | −0.09 | 0.29 | 0.24a | 0.003 |
| Diastolic BP | −0.14 | 0.08 | 0.31a | <0.001 |
| MAP | −0.18 | 0.02a | 0.29a | <0.001 |
| Pulse pressure | −0.08 | 0.29 | 0.08 | 0.30 |
| HR | −0.09 | 0.27 | 0.03 | 0.68 |
| Model flow analysis | ||||
| Left ventricular ejection time | −0.02 | 0.77 | 0.03 | 0.76 |
| Stroke volume | 0.19a | 0.02 | 0.05 | 0.51 |
| Cardiac output | 0.17a | 0.04 | 0.10 | 0.23 |
| SVR | −0.21a | 0.01 | 0.03 | 0.68 |
| Arterial compliance | 0.18a | 0.03 | 0.02 | 0.82 |
| Aortic characteristic impedance | −0.19a | 0.02 | −0.14 | 0.10 |
| HR and BP variability analysis | ||||
| PI HF | −0.09 | 0.26 | −0.15 | 0.07 |
| PI LF/HF | 0.08 | 0.33 | 0.13 | 0.12 |
| Diastolic BP LF | −0.05 | 0.53 | 0.03 | 0.70 |
| Diastolic BP VLF | −0.06 | 0.45 | −0.05 | 0.53 |
| BRS HF | −0.025 | 0.77 | −0.04 | 0.65 |
| BRS LF | −0.07 | 0.38 | −0.12 | 0.16 |
| NN50+ | −0.12 | 0.15 | −0.21a | 0.013 |
| α1 | 0.10 | 0.22 | 0.10 | 0.24 |
α1 indicates short‐term scale coefficient of PI; BP, blood pressure; BRS, baroreflex sensitivity; HF, high frequency; HR, heart rate; LF, low frequency; MAP, mean arterial pressure; NN50+, number of PI increases per minute larger than 50 ms; PI, pulse interval; SVR, systemic vascular resistances; VLF, very‐low frequency.
Correlations significant at P<0.05.
Discussion
This study investigated whether hemodynamic and autonomic responses to salt intake depend on the degree of salt sensitivity, for the first time in a population of white, young, normotensive volunteers and by adopting a classification in 3 rather than in 2 classes: ISS, SR, and SS. In fact, the SS‐Index distribution emphasized the oversimplification of the traditional dichotomous approach in sensitive/resistant individuals to sodium loading, because normotensive subjects traditionally classified as sodium resistant do not all respond to sodium loading in the same way.
The BP decrease induced by sodium loading in the ISS group appeared to be a consequence of a dramatic decrease of vascular resistances. By contrast, vascular resistances did not decrease in SR and SS groups. In this regard, SR normotensives seemed more similar to SS than to ISS individuals, and after the low‐salt diet peripheral resistances in both SR and SS groups were about 20% lower than in the ISS group.
Our findings support the notion that the disproportion between cardiac output and vascular resistances in SS subjects is determined by their failure to adequately lower vascular resistances during sodium repletion,21 and confirm data collected in a mostly normotensive population of black individuals dichotomously classified in salt‐sensitive and salt‐resistant groups,6 in whom vascular resistances increased in salt‐sensitive and decreased in salt‐resistant individuals after 5 days of high‐salt diet without significant changes in cardiac output. However, by splitting salt‐resistant individuals into the ISS and SR subgroups, we provide evidence that only in ISS normotensives was the unchanged cardiac output after the high‐salt diet the result of a significantly lower HR level, which acted as a compensatory factor against a substantially higher stroke volume.
The role of peripheral resistances in mediating salt‐induced increases of blood pressure is still a matter of lively debate.22, 23 The Guyton's theory concluded that salt loading induces hypertension causing transient large increases in cardiac output, whereas systemic vascular resistance initially remains normal or unchanged.24, 25 A recent review challenged this view, proposing that direct response to sodium load in SS individuals is attributable to an initial inhibitory effect on arteriolar vasodilation that is able to prevent vascular resistance decrease.26 Our observation that the initial hemodynamic pattern after sodium loading is a lack of vasodilation in SS individuals seems to confirm this vasodysfunction theory. However, we also showed that during low‐salt diet the SS‐Index is positively associated with cardiac output and arterial compliance and negatively associated with SVR and characteristic impedance, suggesting a residual positive salt balance in SS subjects during salt depletion and giving support to Guyton's physiology. We may also hypothesize a role played by altered modulations of the renin–angiotensin–aldosterone system or of the sympathetic nervous system, reported to occur in hypertensive SS individuals.3, 27 However, studies in normotensive humans revealed that an increased dietary salt intake (5–7 days of high‐salt regimen) induces a profound reduction in vascular nitric oxide bioavailability, which limits endothelium‐dependent vasodilation.28, 29 Thus, a reduced bioavailability of nitric oxide after 5 days of high‐salt diet might determine the altered response of vascular resistances in SS and SR normotensive subjects. Future studies could validate this speculation by measuring plasma levels of nitric oxide and of interacting cytokines, such as transforming growth factor β1,28 in ISS, SR, and SS groups undergoing a high‐salt diet.
Our data also indicate effects of the sodium loading/depletion maneuver on the autonomic cardiovascular modulation. First, sodium loading increased vagal modulation of HR (PI HF and NN50+) and the HR BRS (Figure 4), with these changes appearing less pronounced in SS individuals, and decreased HR significantly, but not in SS normotensives (Figure 1). These findings suggest an increased vagal modulation of HR after a high‐salt diet, partially blunted in the SS group.
Second, sodium loading decreased indices reflecting cardiac sympatho/vagal balance (PI LF/HF and α1) and vasomotor oscillations considered to reflect sympathetic vascular modulation associated with a baroreflex resonance (diastolic BP LF in Figure 4). In line with these findings, it was observed that a low‐salt diet increases the muscle sympathetic nerve traffic in hypertensive individuals.30
Third, sodium loading significantly decreased slow vasomotor components of diastolic BP variability (Figure 4), likely generated by sympathetic modulation of SVR, only in the ISS group, in which the high‐salt diet also dramatically decreased SVR. This suggests that the preserved adaptation of vascular resistances to dietary sodium intake in ISS individuals may reflect the preserved ability of the autonomic nervous system to modulate SVR.
The effects of sodium diets we reported in normotensive individuals are similar to changes described in hypertensive patients.4, 5 Increased BRS, increased cardiac vagal index, and decreased HR and cardiac sympatho/vagal balance have been reported in hypertensive patients after a high‐salt diet, with these changes being blunted in those hypertensive patients with the more severe degree of sodium sensitivity. Therefore, our results indicate that the same type of cardiac autonomic alterations characterizing sodium sensitivity in hypertensive patients are already detectable in a population of young, healthy, white normotensive individuals, with possible clinical implications for prevention of the adverse cardiovascular effects of sodium loading as well as of an increased sodium sensitivity.
We also identified associations between SS‐Index and several hemodynamic variables, but only after the low‐salt diet (Table 2). This was not reported by Schmidlin et al, probably because of the dichotomous classification of sodium sensitivity, or of the lower sample size.6 We found that after 5 days of a low‐salt diet, individuals with the lowest degree of sodium sensitivity had the highest SVR, the highest aortic impedance, and the lowest arterial compliance. These trends were responsible for a reduction in cardiac afterload with increased sodium sensitivity, and for an increase in the Windkessel effect with increased sodium sensitivity under reduced sodium loading. In this condition of sodium depletion, BP levels did not depend on the SS‐Index significantly, as for systolic and diastolic BP, or even decreased with the severity of sodium sensitivity, as for MAP. By contrast, the high‐salt diet uncoupled SS‐Index from cardiac afterload and Windkessel effect, and concomitantly BP increased significantly with SS‐Index.
We also detected a negative association between SS‐Index and NN50+, but only after sodium loading (Table 2), indicating a partially blunted vagal control of HR in individuals with the higher degree of sodium sensitivity, when facing a high‐salt diet. Interestingly, this hypothesis is supported by previous evidence of a blunted vagal modulation of HR in SS normotensive individuals following their habitual diet.31
Our results might also help to clarify recent controversial findings that indicate a U‐shaped association between sodium intake and health outcomes.32 In fact, our findings seem to suggest that an ideal healthy sodium diet, valid for all normotensive individuals, might not exist. In a fraction of our volunteers, the diet with a very low sodium content induced hemodynamic adaptations that are generally associated with increased risk of cardiovascular events. Volunteers with SS‐Index lower than −30 mm Hg/(mol·day) even had higher MAP values after the low‐salt diet, as a consequence of a remarkable increase of BP induced by salt restriction (Figure 5). This would imply that a very low‐sodium diet, likely beneficial or at least not harmful for most normotensive individuals, should be recommended only if inverse sensitivity to sodium can be excluded. Future epidemiological and prospective studies might clarify whether the ISS individuals described in our study significantly contribute to the observed greater incidence of cardiovascular events in populations exposed to extremely low dietary sodium intakes.
Two study limitations should be considered. First, participants in our study, enrolled among students and young medical doctors of our University Hospital on a voluntary basis, might not be fully representative of a white normotensive population, due to their educational level, lifestyle habits, and sex distribution. In case the BP response to sodium intake might depend on sex, the higher prevalence of females among our participants might have biased our results. Therefore, to evaluate whether the imbalance between males and females might have influenced our results, we have also quantified the SVR response to dietary salt by sex in Table 3. The table suggests that differences between sexes may affect the absolute values of SVR (higher in females than in males), but are unlikely to affect the response to dietary salt, because in both sexes SVR decreased in the ISS group and increased in the SS group.
Table 3.
SVR (mm Hg×s/mL) at the End of Each Diet in the 3 Sodium Sensitivity Classes (ISS, SR, and SS) by Sex: Mean (SEM)
| ISS | SR | SS | |
|---|---|---|---|
| Females (N=41) | |||
| Low‐salt | 1.28 (0.07) | 1.09 (0.04) | 1.16 (0.08) |
| High‐salt | 1.12 (0.04) | 1.16 (0.06) | 1.31 (0.08) |
| Males (N=26) | |||
| Low‐salt | 1.10 (0.13) | 0.95 (0.07) | 0.79 (0.06) |
| High‐salt | 0.95 (0.06) | 0.95 (0.09) | 0.87 (0.06) |
ISS indicates inverse sodium‐sensitive; SR, sodium resistant; SS, sodium sensitive; SVR, systemic vascular resistances.
Second, the salt‐sensitivity test, based on high‐ and low‐salt diets, lasted several days. Therefore, we cannot extend our results to individuals classified as ISS, SR, or SS by the sodium loading/depletion maneuver based on saline infusion and diuretics (the so‐called “Indiana protocol”)15 because this maneuver lasts only a few hours and might not elicit the same autonomic and hemodynamic adaptations observed in our study.
In conclusion, our study provides evidence that in SS normotensive subjects, an impaired vasodilatory response to dietary salt loading can be a major pathogenetic factor in determining the pressor effect of dietary salt intake. This alteration seems to be associated with a dysfunction in autonomic cardiovascular regulation, as suggested by a blunted vagal cardiac modulation and a reduced vascular sympathetic inhibition in SS normotensives during high salt intake.
Sources of Funding
This study funded by the Italian Ministry of Health.
Disclosures
None.
Supporting information
Data S1. Hemodynamic parameters from BP waveform analysis and autonomic indices from HRV and BP variability analysis.
Data S2. Hemodynamic and autonomic indices in salt‐resistant and salt‐sensitive groups as classified by traditional criteria.
Data S3. SS‐Index distribution in normotensives and definition of sodium‐sensitivity groups.
Table S1. Hemodynamic and Autonomic Indices in Traditionally Defined Salt‐Resistant and Salt‐Sensitive Groups: Mean (SEM)
Figure S1. SS‐Index distribution over 71 normotensive volunteers. The distribution was decomposed into 3 groups representing inverse sodium sensitive (ISS), sodium‐sensitive (SS), and sodium‐resistant (SR) individuals by setting the thresholds for identifying SR individuals at −15 and +15 mm Hg/(mol·day). This choice, although arbitrary, defines an SR distribution symmetrically centered on 0, and associates the distribution peak occurring at a negative SS‐Index value with the ISS group.
(J Am Heart Assoc. 2016;5:e003736 doi: 10.1161/JAHA.116.003736)
References
- 1. Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. [DOI] [PubMed] [Google Scholar]
- 2. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. [DOI] [PubMed] [Google Scholar]
- 3. Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt‐sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. [DOI] [PubMed] [Google Scholar]
- 4. Coruzzi P, Parati G, Brambilla L, Brambilla V, Gualerzi M, Novarini A, Castiglioni P, Di Rienzo M. Effects of salt sensitivity on neural cardiovascular regulation in essential hypertension. Hypertension. 2005;46:1321–1326. [DOI] [PubMed] [Google Scholar]
- 5. Minami J, Kawano Y, Ishimitsu T, Takishita S. Blunted parasympathetic modulation in salt‐sensitive patients with essential hypertension: evaluation by power‐spectral analysis of heart‐rate variability. J Hypertens. 1997;15:727–735. [DOI] [PubMed] [Google Scholar]
- 6. Schmidlin O, Sebastian AF, Morris RC Jr. What initiates the pressor effect of salt in salt‐sensitive humans? Observations in normotensive blacks Hypertension. 2007;49:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimura G, Brenner BM. A method for distinguishing salt‐sensitive from non‐salt‐sensitive forms of human and experimental hypertension. Curr Opin Nephrol Hypertens. 1993;2:341–349. [PubMed] [Google Scholar]
- 8. Castiglioni P, Parati G, Di Rienzo M, Brambilla V, Brambilla L, Gualerzi M, Lazzeroni D, Coruzzi P. Blood pressure changes after high‐ and low‐salt diets: are intermittent arm measures and beat‐by‐beat finger measures equivalent? J Hum Hypertens. 2015;27:430–435. [DOI] [PubMed] [Google Scholar]
- 9. Task Force of the European Society of Cardiology, the North American Society of Pacing and Electrophysiology . Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 10. Castiglioni P, Di Rienzo M, Veicsteinas A, Parati G, Merati G. Mechanisms of blood pressure and heart rate variability: an insight from low‐level paraplegia. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1502–R1509. [DOI] [PubMed] [Google Scholar]
- 11. Julien C. The enigma of Mayer waves: facts and models. Cardiovasc Res. 2006;70:12–21. [DOI] [PubMed] [Google Scholar]
- 12. Merati G, Maggioni MA, Invernizzi PL, Ciapparelli C, Agnello L, Veicsteinas A, Castiglioni P. Autonomic modulations of heart rate variability and performances in short‐distance elite swimmers. Eur J Appl Physiol. 2015;115:825–835. [DOI] [PubMed] [Google Scholar]
- 13. Castiglioni P, Parati G, Di Rienzo M, Carabalona R, Cividjian A, Quintin L. Scale exponents of blood pressure and heart rate during autonomic blockade as assessed by detrended fluctuation analysis. J Physiol. 2011;589:355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tulppo MP, Makikallio TH, Seppanen T, Shoemaker K, Tutungi E, Hughson RL, Huikuri HV. Effects of pharmacological adrenergic and vagal modulation on fractal heart rate dynamics. Clin Physiol. 2001;21:515–523. [DOI] [PubMed] [Google Scholar]
- 15. Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–II134. [DOI] [PubMed] [Google Scholar]
- 16. Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens. 2013;22:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 18. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 19. Castiglioni P, Parati G, Omboni S, Mancia G, Imholz BP, Wesseling KH, Di Rienzo M. Broad‐band spectral analysis of 24 h continuous finger blood pressure: comparison with intra‐arterial recordings. Clin Sci (Lond). 1999;97:129–139. [PubMed] [Google Scholar]
- 20. Castiglioni P, Parati G, Lombardi C, Quintin L, Di Rienzo M. Assessing the fractal structure of heart rate by the temporal spectrum of scale exponents: a new approach for detrended fluctuation analysis of heart rate variability. Biomed Tech (Berl). 2011;56:175–183. [DOI] [PubMed] [Google Scholar]
- 21. Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, Connor MJ. Hemodynamic characteristics of sodium‐sensitive human subjects. Hypertension. 1987;9:398–406. [DOI] [PubMed] [Google Scholar]
- 22. Morris RC Jr, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt‐induced hypertension. Circulation. 2016;133:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall JE. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt‐induced hypertension. Circulation. 2016;133:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. [DOI] [PubMed] [Google Scholar]
- 25. Guyton AC. The surprising kidney‐fluid mechanism for pressure control—its infinite gain! Hypertension. 1990;16:725–730. [DOI] [PubMed] [Google Scholar]
- 26. Kurtz TW, Dominiczak AF, DiCarlo SE, Pravenec M, Morris RC Jr. Molecular‐based mechanisms of Mendelian forms of salt‐dependent hypertension: questioning the prevailing theory. Hypertension. 2015;65:932–941. [DOI] [PubMed] [Google Scholar]
- 27. Sharma AM, Schattenfroh S, Thiede HM, Oelkers W, Distler A. Effects of sodium salts on pressor reactivity in salt‐sensitive men. Hypertension. 1992;19:541–548. [DOI] [PubMed] [Google Scholar]
- 28. Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297:F237–F243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens. 2015;24:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grassi G, Dell'Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G. Short‐ and long‐term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation. 2002;106:1957–1961. [DOI] [PubMed] [Google Scholar]
- 31. Buchholz K, Schachinger H, Wagner M, Sharma AM, Deter HC. Reduced vagal activity in salt‐sensitive subjects during mental challenge. Am J Hypertens. 2003;16:531–536. [DOI] [PubMed] [Google Scholar]
- 32. Graudal N, Jurgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low‐ and excessive‐sodium diets are associated with increased mortality: a meta‐analysis. Am J Hypertens. 2014;27:1129–1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Hemodynamic parameters from BP waveform analysis and autonomic indices from HRV and BP variability analysis.
Data S2. Hemodynamic and autonomic indices in salt‐resistant and salt‐sensitive groups as classified by traditional criteria.
Data S3. SS‐Index distribution in normotensives and definition of sodium‐sensitivity groups.
Table S1. Hemodynamic and Autonomic Indices in Traditionally Defined Salt‐Resistant and Salt‐Sensitive Groups: Mean (SEM)
Figure S1. SS‐Index distribution over 71 normotensive volunteers. The distribution was decomposed into 3 groups representing inverse sodium sensitive (ISS), sodium‐sensitive (SS), and sodium‐resistant (SR) individuals by setting the thresholds for identifying SR individuals at −15 and +15 mm Hg/(mol·day). This choice, although arbitrary, defines an SR distribution symmetrically centered on 0, and associates the distribution peak occurring at a negative SS‐Index value with the ISS group.


