Abstract
Background
Although an association between human herpesvirus (HHV) infection and atherosclerosis has been suggested, the data supporting such an association are controversial and, in most cases, are based on serological evidence or on the presence of cell‐associated HHV DNA, which do not report about actual viral replication. We quantified the DNA of all 8 types of HHVs in plasma, in which their presence is evidence of viral replication.
Methods and Results
Using quantitative real‐time polymerase chain reaction, we evaluated the presence of HHV DNA in blood samples obtained at the time of hospitalization from 71 patients with acute coronary syndrome, 26 patients with stable coronary artery disease, and 53 healthy volunteers and in atherosclerotic plaques of 22 patients with peripheral artery disease who underwent endarterectomy. HHV‐5 (cytomegalovirus [CMV]) was the only HHV with a level that was higher in acute coronary syndrome patients than in the control group and that correlated with the level of high‐sensitivity C‐reactive protein. The numbers of effector memory T cells positively correlated with the numbers of CMV genome copies in carotid arteries plaques, whereas the numbers of central memory T cells negatively correlated with CMV copy numbers.
Conclusions
Of all HHV levels, only CMV was higher in patients with stable coronary artery disease and acute coronary syndrome than in the healthy group, and its load correlated with the level of high‐sensitivity C‐reactive protein. The level of CMV in atherosclerotic plaques correlated with the state of immunoactivation of lymphocytes in plaques, suggesting that the reactivation of CMV may contribute to the immune activation associated with the progression of atherosclerosis.
Keywords: acute coronary syndrome, atherosclerosis, human herpesvirus, immune system, myocardial infarction, polymerase chain reaction, virus
Subject Categories: Acute Coronary Syndromes, Atherosclerosis, Translational Studies
Introduction
Atherosclerosis of coronary arteries, the major cause of acute coronary syndrome (ACS), often may progress for years and even decades and suddenly result in the rupture of an atherosclerotic plaque, leading to myocardial infarction.1, 2, 3, 4 Despite the long history of investigation,5, 6 the triggers and mechanisms of such sudden ruptures remain largely unknown.
Since the 19th century, it has been suspected that the development of atherosclerotic plaques and their rupture are associated with inflammation caused by infection.7 The list of different infectious agents that have been implicated in atherosclerosis development and progression has kept growing and includes bacterial (oral, gut, dental, and bronchoalveolar)8 and different viral infections.9, 10, 11 Based on the slow development of atherosclerosis, a candidate pathogen should be not overly pathogenic and should commonly infect humans, causing slow but persistent inflammation. Among known human viruses, human herpesviruses (HHVs) fit these criteria. Studies in animal models have demonstrated that HHVs are capable of contributing to the development of atherosclerosis‐type pathologies.12 Data on the involvement of HHVs in human atherosclerosis are far less conclusive13 and are predominantly based on epidemiological studies. These data do not allow assessment of actual viral infection in patients because antibodies (and cell‐associated DNA) may provide evidence of past infections rather than infections that are active at the time of analysis.14, 15
In this study, we investigated the relation between HHV infection and cardiovascular disease at two levels. At the whole‐body level, we investigated the association of the amounts of DNA in blood plasma, a marker of productive viral infection,16, 17 and the clinical status of the patients. At the single atherosclerotic plaque level, we investigated the association of the presence of HHVs and T lymphocyte activation. We found that among all HHVs, only HHV‐5 (cytomegalovirus [CMV]) was associated with coronary artery disease (CAD), and the levels of CMV DNA in plasma from patients with different forms of CAD and from healthy volunteers correlated with the levels of high‐sensitivity C‐reactive protein (hs‐CRP). Moreover, in atherosclerotic plaques from the carotid arteries of patients undergoing carotid endarterectomy, the numbers of CMV DNA copies correlated with the immunoactivation of T lymphocytes in the same plaques.
Materials and Methods
Patients With CAD
A total of 53 healthy volunteers (27 men and 26 women, mean age 61.3±12.3 years) and 97 patients with CAD were enrolled consecutively in our study: 71 of the CAD patients were diagnosed with ACS (45 men and 26 women, mean age 64.4±9.7 years), and the other 26 had stable CAD (SCAD; 17 men and 9 women, mean age 66.3±10.6 years), according to the current guidelines.18, 19, 20 Among ACS patients, 47 had myocardial infarction with or without ST‐segment elevation; unstable angina pectoris was diagnosed in 24 patients. Healthy volunteers were recruited as 1 control for 2 cases in our study. Detailed inclusion and exclusion criteria are presented in Table 1.
Table 1.
Inclusion and Exclusion Criteria for Patients With Coronary Artery Disease and Healthy Volunteers
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| ACS patients | STEMI and non–ST‐segment elevation ACS diagnosis at the moment of admission according to the guidelines | Age <40 years, bleeding or necessity of blood/plasma transfusion, acute stroke, pregnancy, acute infections, neoplasms |
| SCAD patients |
Clinical signs of angina of effort or positive treadmill test and/or stenosis >50% on invasive coronary angiography |
|
| Healthy volunteers | No signs of possible atherosclerosis according to treadmill test, echocardiography, and ultrasound of peripheral arteries |
ACS indicates acute coronary syndrome; SCAD, stable coronary artery disease; STEMI, ST‐segment elevation myocardial infarction.
In both patients and healthy volunteers, we analyzed traditional CAD risk factors according to the existing criteria: sex, age, dyslipidemia (fasting low‐density lipoprotein cholesterol level >160 mg/dL or total cholesterol level >200 mg/dL and high‐density lipoprotein cholesterol level <40 mg/dL), obesity (body mass index ≥30), arterial hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg).21
For negative control of HHV infection, we used cord blood samples from 5 parturient women.
Patients With Peripheral Artery Disease Who Underwent Carotid Endarterectomy
We analyzed 18 atherosclerotic plaques from 17 patients (12 men and 5 women, mean age 62.5±13.4 years) with peripheral artery disease who underwent carotid endarterectomy. The degree of carotid artery stenosis varied from 70% to 90% (median 80%, interquartile range [IQR] 75–90%). Four patients (24%) suffered from transient ischemic attack or stroke within 5 years before the surgery, and half of all plaque specimens (50%) were ruptured according to macroscopic evaluation. We divided each sample into 2 parts. One was used for flow cytometry analysis of T cells, and the other was used for real‐time polymerase chain reaction (RT‐PCR) for evaluation of herpesviral DNA.
Local ethics committees approved the study protocol. All participants provided written informed consent.
Flow Cytometry
To isolate T cells from atherosclerotic plaques without stripping off cell surface markers, we digested plaques with an enzymatic cocktail, according to the method we developed previously.22 Briefly, after surgery, atherosclerotic plaques were collected in RPMI 1640 (Gibco, Life Technologies, MA, USA) and kept at room temperature until processing within 2 hours of surgery. Plaques were then washed extensively in RPMI medium to remove the contaminating peripheral blood–derived mononuclear cells. Atherosclerotic plaques were dissected from the healthy tissue, cut into cubes of 2 mm per side, and digested by an enzymatic mixture: RPMI with collagenase IV (Sigma‐Aldrich, MO, USA) at 1 mg/mL in the presence DNase I (Sigma‐Aldrich) at 0.2 mg/mL. Plaque fragments were digested by placing 10 to 15 blocks of tissues in Eppendorf tubes (Eppendorf, Germany) for 1 hour at 37°C in an incubator shaker; they were filtered through a nylon 40‐μm filter, washed twice in 1x PBS (Gibco, Life Technologies), and stained for live/dead discrimination and with mixtures of antibodies. We stained isolated leukocytes with combinations of monoclonal antibodies against markers characterizing naïve T cells (CD45+ CD3+ CD45RA+ CD197+ CD27+ CD28+); central memory T cells (Tcm cells; CD45+CD3+CD45RA−CD197+CD27+CD28+); effector memory T cells (Tem cells; CD45+CD3+CD45RA+/−CD197−), including cells that were differentiated early (CD45+CD3+CD45RA−CD197−CD27+CD28+), intermediate (CD45+CD3+CD45RA−CD197−CD27+/−CD28+), and late (CD45+CD3+CD45RA+/−CD197−CD27−CD28−); and activated T cells (CD25+, CD38+, or HLA‐DR+). Data were acquired with flow cytometer BD FACSCanto II and BD FACSAria II (BD Biosciences, NJ, USA) by means of BD FACSDiva software version 6.1.3 and analyzed using FlowJo software versions 9.3.3 and 9.4 (FlowJo LLC, OR, USA).
DNA Extraction From Plaques
Plaques were washed in RPMI medium and dissected into 2‐mm cubic blocks. We grouped 4 tissue blocks weighing ≈25 mg each and extracted DNA from them using the QIAamp tissue DNA extraction kit (Qiagen, Germany), to which we added proteinase K to obtain higher amounts of extracted DNA and eluted the DNA into 300‐μL aliquots. The DNA concentration and its quality were measured on a NanoDrop spectrophotometer (NanoDrop Products; Thermo Fisher Scientific, MA, USA).
DNA Extraction From Blood
Peripheral blood samples were obtained from patients during the first 24 hours of hospitalization and from healthy controls at the time of examination. Blood was centrifuged at 2500g for 10 minutes; plasma samples were separated, aliquoted, and kept at −80°C. Peripheral blood mononuclear cells were collected according to the previously described method.22 After plasma was decanted, the red blood cells were lysed with a lysis solution (BioLegend, CA, USA), centrifuged at 400g for 5 minutes, and resuspended in PBS. We extracted viral DNA from plasma using the standard spin protocol (QIAamp DNA Blood Mini Kit; Qiagen).
Measurement of hs‐CRP
Plasma hs‐CRP was measured with an automated analyzer (Dimension Xpand Plus; Siemens, Germany) using C‐Reactive Protein Flex Reagent (no. DF37; Siemens, CA, USA).
Quantitative Multiplex RT PCR
Detection of the 8 types of HHV was performed by means of multiplexed quantitative RT‐PCR and expressed as copy number of HHV DNA per microliter of plasma. HHVs that were measured in peripheral blood mononuclear cells and carotid artery plaques were counted as HHV copy numbers per 106 cells. To estimate amounts of genomic DNA, we used primers and probes for endogenous human retrovirus (ERV‐3).23 RT‐PCR was performed on a Bio‐Rad thermal cycler (CFX96 Touch C1000 thermal cycler; Bio‐Rad Laboratories,) using specific primer sets and probes (TaqMan; Thermo Fisher Scientific). We performed PCR using 2 sets of 5 simultaneous reactions with 4 HHV primer probe sets (HHV‐1, HHV‐3, HHV‐5, and HHV‐7) and human genomic DNA sequence ERV‐3 and another set of HHVs (HHV‐2, HHV‐4, HHV‐6, and HHV‐8) with the same human genomic DNA sequence (ERV‐3). Calibrated DNA standards for each HHV were obtained from Advanced Biotechnologies, Inc (MD, USA); human genomic DNA obtained from Novagen (Millipore, MA, USA) was used as the human DNA standard. Amplification of DNA was carried out with equimolar mixtures of primers and probes for each target (4 HHVs and 1 ERV3) using PCR multiplex ToughMix (Quanta Bioscience, MA, USA). The primer and probe sequences are shown in Table 2.
Table 2.
Sequences of HHV Primers and Probes
| Sequence Name | Sequence | 5′ Modificationa | 3′ Modification |
|---|---|---|---|
| ERV‐3 probe | Aacatgggagaccaatggccatggg | Quasar 705 | BHQ‐2 |
| ERV‐3 forward | Gttgcttcatgttatgtctgtgg | ||
| ERV‐3 reverse | Ggcggttagtgtgaaattatcttg | ||
| HHV‐1 probe | Atgaccatgtcggtgaccttggc | Cy5 | BHQ‐2 |
| HHV‐1 forward | Ggaacacaaggccaagaaga | ||
| HHV‐1 reverse | Gttgggaacttgggtgtagtt | ||
| HHV‐2 probe | Tcccttcgaggaggtgatcgacaa | FAM | BHQ‐1 |
| HHV‐2 forward | Caccgctactcccagtttatg | ||
| HHV‐2 reverse | Cggtggtctccatgttgtt | ||
| HHV‐3 probe | Tgttatatgacgacaccgtctcgcg | HEX | BHQ‐1 |
| HHV‐3 forward | Gattatgcccgcaaacttgtag | ||
| HHV‐3 reverse | Atggcccgtctattccattc | ||
| HHV‐4 probe | Tccgtcatctccgtcatcaccctc | Quasar 670 | BHQ‐2 |
| HHV‐4 forward | Tagatttgcctccctggtttc | ||
| HHV‐4 reverse | Catctccatcacctccttcatc | ||
| HHV‐5 probe | Tacctggagtccttctgcgagga | CAL Fluor Red 610 | BHQ‐2 |
| HHV‐5 forward | Aaccaagatgcaggtgatagg | ||
| HHV‐5 reverse | Agcgtgacgtgcataaaga | ||
| HHV‐6 probe | Ttattccttcgggtgtgccgtctg | CAL Fluor Red 610 | BHQ‐2 |
| HHV‐6 forward | Atcgaaacgcctacacagaat | ||
| HHV‐6 reverse | Ccagagcggcatcgatattta | ||
| HHV‐7 probe | Gagaacatcgctctaactggatca | FAM | BHQ‐1 |
| HHV‐7 forward | Agcggtacctgtaaaatcatcca | ||
| HHV‐7 reverse | Aacagaaacgccacctcgat | ||
| HHV‐8 probe | Ttggtggtatatagatcaagttc | HEX | BHQ‐1 |
| HHV‐8 forward | Gtccagacgatatgtgcgc | ||
| HHV‐8 reverse | Actccaaaatatcggccgg |
BHQ‐1 indicates Black Hole Quencher 1; BHQ‐2, Black Hole Quencher 2; HHV, human herpesvirus.
FAM, HEX, Cy5, Quasar 705, Quasar 670, and CAL Fluor Red 610 are fluorescent dyes.
The specificity of the PCR reaction was confirmed with purified HHVs as positive controls and human cord blood DNA as the negative control: All primer/probe sets amplified their cognate DNA, and there was no amplification of cord blood DNA. Examples of standard curves for both combinations of primers and probes are shown in Figures 1 and 2.
Figure 1.

A, Standard's amplification for HHV‐1, HHV‐3, HHV‐5, HHV‐7, and ERV‐3 multiplex combination. B, Standard's curve for the first multiplex combination. FAM, HEX, Cy5, Quasar 705, and Cal Red 610 are fluorescent dyes.
Figure 2.

A, Standard's amplification for HHV‐2, HHV‐4, HHV‐6, HHV‐8, and ERV‐3 multiplex combination. B, Standard's curve for the second multiplex combination. FAM, HEX, Cy5, Quasar 705, and Cal Red 610 are fluorescent dyes.
A flowchart of all experiments is provided in Figure 3.
Figure 3.

A flowchart of the experiment for HHV detection in plasma of healthy volunteers, in patients with CAD, and in atherosclerotic plaques from carotid arteries of patients undergoing CEA. Analysis of HHV DNA correlation with T‐cell subsets in atherosclerotic plaques. ACS indicates acute coronary syndrome; CAD, coronary artery disease; CEA, carotid endarterectomy; HHV, human herpesvirus; PCR, polymerase chain reaction; SCAD, stable coronary artery disease.
Statistical Analyses
The data obtained in the present study were not normally distributed, according to the Shapiro‐Wilk test, and are represented as medians and IQRs. Because distributions were not normal, we used the Mann–Whitney rank test for comparisons of 2 groups; for >2 groups, the nonparametric Kruskal–Wallis 1‐way ANOVA was used. For comparison of 2 dependent groups, we used the Wilcoxon matched‐pair test. To assess between‐group effects, we used a multiple‐comparisons rank test and receiver operating characteristic curve analysis. For the analysis of categorical parameters, we used the chi‐square test with 3×2 frequency tables. For the analysis of relations between different HHVs and hs‐CRP, the Spearman correlation coefficient was estimated. For the age distribution, we made the assumption of its normality and analyzed this distribution using the t test for 2 groups and ANOVA for multiple comparison. We also performed binomial multiple logistic regression analysis for the assessment of multiple predictors of CAD development. For this analysis, we used dichotomized age ≥55 years for men and ≥65 years for women (risk factor for CAD24) and hs‐CRP level ≥2 mg/L (marker of active inflammation25, 26) and all other binominal factors as predictors. Statistical analysis was performed using Statistica 10.0 (Statsoft, Dell, OK, USA) and SPSS Statistics 21.0 (IBM Corp, NY, USA). Values of P<0.05 were considered statistically significant.
Results
Analysis of Plasma
Using RT‐PCR, we evaluated DNA of all 8 types of HHV in plasma samples from 71 patients with ACS, 26 patients with SCAD, and 53 healthy volunteers. Statistical analyses showed that the control group was not different from other groups in terms of age and sex characteristics. Patients with CAD had risk factors such as hypertension, obesity, and diabetes mellitus significantly more frequently than healthy controls. Baseline characteristics of all patient groups are presented in Table 3.
Table 3.
Clinical Characteristics of Patients With Coronary Artery Disease and Healthy Volunteers
| ACS Patients | SCAD Patients | Healthy Volunteers | P Value | |
|---|---|---|---|---|
| n | 71 | 26 | 53 | |
| Age, y, mean | 64.4 | 66.3 | 61.3 | 0.116 |
| Age, y, SD | 9.7 | 10.6 | 12.3 | |
| Men, % | 63.4 | 65.4 | 50.9 | 0.298 |
| Smoking, % | 28.2 | 11.5 | 20.8 | 0.205 |
| Dyslipidemia, % | 42.3 | 19.2 | 32.1 | 0.096 |
| Obesity, % | 45.1 | 23.1 | 15.1 | 0.001a |
| Hypertension, % | 90.1 | 92.3 | 47.2 | 0.000a |
| Diabetes mellitus, % | 31.0 | 19.2 | 1.9 | 0.000a |
ACS indicates acute coronary syndrome; SCAD, stable coronary artery disease.
Differences are statistically significant at P<0.05.
We evaluated the frequencies of the presence and the number of copies of HHV DNA in peripheral blood mononuclear cells and found that both frequencies of detection and DNA copy numbers for all 8 types of HHV did not differ significantly among the 3 groups of patients (Table 4).
Table 4.
Frequencies of Detection of HHV DNA in Peripheral Blood Mononuclear Cells in Patients With Coronary Artery Disease and Healthy Volunteers
| ACS Patients | SCAD Patients | Healthy Volunteers | |
|---|---|---|---|
| HHV‐1 | 0 (0/71) | 0 (0/26) | 0 (1/53) |
| HHV‐2 | 4.23 (3/71) | 0 (0/26) | 3.77 (2/53) |
| HHV‐3 | 0 (0/71) | 0 (0/26) | 1.89 (1/53) |
| HHV‐4 | 29.58 (21/71) | 30.77 (8/26) | 39.62 (21/53) |
| HHV‐5 | 0 (0/71) | 3.85 (1/26) | 3.77 (2/53) |
| HHV‐6 | 0 (0/71) | 15.38 (4/26) | 7.55 (4/53) |
| HHV‐7 | 36.62 (26/71) | 42.31 (11/26) | 32.08 (17/53) |
| HHV‐8 | 4.23 (3/71) | 0 (0/26) | 1.89 (1/53) |
Data are presented as percentage (number of positive patients/total number of patients). For all HHVs, the differences between groups were not significant (P>0.05). ACS indicates acute coronary syndrome; HHV, human herpesvirus; SCAD, stable coronary artery disease.
To evaluate productive viral infection, we measured HHV DNA in plasma.16, 17 Although control cord blood samples were negative for the presence of HHVs in our measurements, we set an arbitrary level of 100 copies of viral DNA per microliter of plasma as a threshold for positivity.
The frequencies of the presence of HHV DNA in the plasmatic fractions are presented in Figure 2. The most frequent HHV was CMV, which was found in the plasma of ≈77% of the patients. Others were less frequent, with higher percentages of HHV‐8 and HHV‐7 and the lowest percentage for HHV‐1, which was hardly detected in our samples.
We compared viral frequencies in plasma from participants in different groups and found a significant difference for only CMV regarding viral presence in the ACS, SCAD, and control groups (77.08%, 55.56%, and 46.15%, respectively; P=0.011). This difference was mainly caused by the difference between the ACS group and the control group (P=0.013). For all other HHVs, the differences between different groups were not significant (Figure 4).
Figure 4.

Frequencies (as percentages) of HHVs detected in blood plasma samples in groups of healthy volunteers (green), SCAD patients (blue), and ACS patients (red). Positivity was defined as HHV DNA at amounts >100 copies per microliter of plasma. *Differences are statistically significant at P<0.05. ACS indicates acute coronary syndrome; HHV, human herpesvirus; SCAD, stable coronary artery disease.
We compared HHV loads in samples from the 3 groups (Table 5) and again found significant differences between groups only in the amounts of CMV DNA. According to a multiple‐comparison test, the amount of CMV DNA was significantly higher in ACS patients than in healthy volunteers (median CMV DNA copy number 213.15 [IQR 101.21–436.67] versus 82.10 [IQR 18.58–188.67]; P=0.012); however, there was no statistical difference in the amounts of CMV DNA between samples from SCAD patients and healthy volunteers (median CMV DNA copy number 109.32 [IQR 39.05–355.23] versus 82.10 [IQR 18.58–188.67]; P>0.05). Consequently, using multiple‐comparison rank methods, we found a statistically significant difference in CMV DNA copy numbers between healthy volunteers and patients with ACS but not with SCAD.
Table 5.
HHV DNA Copy Numbers in Plasma of Patients of Different Groups
| Type of HHV | ACS Patients | SCAD Patients | Healthy Volunteers | P Value |
|---|---|---|---|---|
| HHV‐1a | ||||
| HHV‐2 | 29.39 (6.19–160.48) | 24.85 (6.17–153.45) | 50.37 (0.74–203.31) | 0.857 |
| HHV‐3 | 2.01 (0.94–4.48) | 1.22 (0.68–3.46) | 1.36 (0.51–2.53) | 0.075 |
| HHV‐4 | 0.125 (0.03–1.57) | 0.25 (0.03–0.82) | 0.07 (0.02–0.70) | 0.621 |
| HHV‐5 | 213.15 (101.21–436.67) | 109.32 (39.05–355.23) | 82.10 (18.58–188.67) | 0.014b |
| HHV‐6 | 2.50 (1.24–9.56) | 2.17 (0.88–8.45) | 6.93 (2.59–22.51) | 0.053 |
| HHV‐7 | 71.30 (33.79–256.36) | 75.93 (18.62–544.87) | 29.29 (6.00–122.05) | 0.123 |
| HHV‐8 | 95.12 (32.64–374.75) | 116.73 (2.21–289.24) | 74.91 (0.00–344.06) | 0.407 |
HHV DNA copy numbers are presented as median (interquartile range) in microliters of plasma. ACS indicates acute coronary syndrome; HHV, human herpesvirus; SCAD, stable coronary artery disease.
Not enough data for statistical comparison.
Differences are statistically significant at P<0.05.
To determine the threshold level of viral load in plasma for the patients with acute coronary events and with all CAD types, we performed a receiver operating characteristic analysis. The receiver operating characteristic curves were statistically significant in both cases (Figures 5 and 6). We defined a threshold level giving equal numbers of false‐negative and false‐positive results: 142 CMV DNA copies for ACS patients and 114 CMV DNA copies for ACS and SCAD patients combined. The area under the curve, however, was larger in the first case than in the second, and the sum of false‐negative and false‐positive results was lower for the threshold of 142. Based on this analysis, we determined that patients with ACS with high probability will have a CMV load of >142 DNA copies per microliter of plasma.
Figure 5.
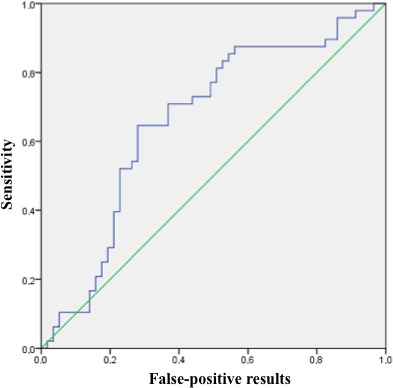
Receiver operating characteristic (ROC) curve for the cytomegalovirus (CMV) DNA load in patients with acute coronary syndrome compared with patients with stable coronary artery disease and healthy controls. The area under the ROC curve was 0.662 (95% CI 0.56–0.77, P=0.004). With the threshold of 142 copies of CMV DNA per microliter of plasma, specificity was 64.9% and sensitivity was 64.6%.
Figure 6.
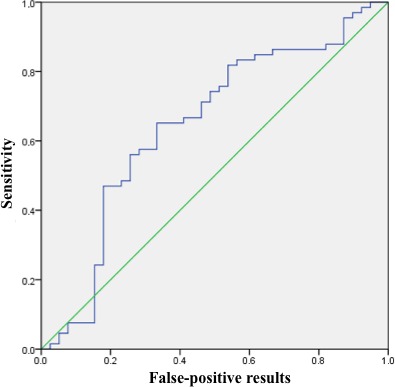
Receiver operating characteristic (ROC) curve for the cytomegalovirus (CMV) DNA load in all patients with coronary artery disease compared with healthy controls. The area under the ROC curve was 0.646 (95% CI 0.53–0.76, P=0.013). With the threshold of 114 copies of CMV DNA per microliter of plasma, specificity was 64.1% and sensitivity was 65.2%.
Except for CMV, the viral loads of all other HHVs did not differ significantly in plasma of participants from different groups (Table 5).
Because our patient groups differed regarding several risk factors, we analyzed whether CMV load correlated with them and found that CMV plasma loads were higher in hypertensive participants (median CMV DNA copy number 168.09 [IQR 54.10–419.18] versus 100.34 [IQR 15.57–172.76]; P=0.016). Although CMV‐productive infection did not correlate with other risk factors, it correlated with the hs‐CRP level, which, as expected, was significantly higher in patients with ACS than in controls (median 4.79 mg/L [IQR 1.71–14.7 mg/L] versus 1.10 mg/L [IQR 0.80–3.87 mg/L]; P=0.00002). CMV load correlated with hs‐CRP levels in both patient and control groups (Table 6).
Table 6.
Spearman Rank Order Correlation Between hs‐CRP Level and CMV in Patients and Controls
| Variables | Patients, n | Spearman Rank, R | t (N−2)a | P Value |
|---|---|---|---|---|
| hs‐CRP (mg/L) and CMV DNA copy numbers | 103 | 0.249 | 2.586 | 0.011b |
CMV indicates cytomegalovirus; hs‐CRP, high‐sensitivity C‐reactive protein.
Denotes correlation level.
Correlations are statistically significant at P<0.05.
Due to the correlations of CMV load with risk factors found in our study, it was crucial to establish whether CMV‐productive infection is an independent risk factor for ACS development or it depends on other risk factors, such as obesity, hypertension, and diabetes mellitus. Toward this goal, we investigated whether HHV frequencies and loads correlated with the CAD risk factors. Statistical analysis showed no such correlation. Moreover, we performed a multiple binomial logistic regression analysis of CAD development with such predictors as CAD risk factors, hs‐CRP level, and CMV presence in plasma. We found that age, arterial hypertension, diabetes mellitus, and CMV independently predicted the development of ACS in the multiple regression model (Figure 7). As for SCAD, the only risks factors persisting in the multiple regression model were arterial hypertension and hs‐CRP level (Table 7).
Figure 7.
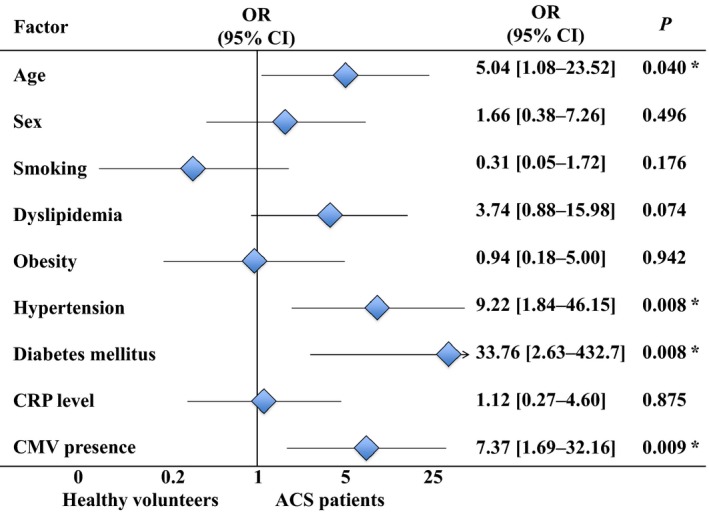
Odds ratios for binomial coronary artery disease risk factors (with age ≥55 years for men and ≥65 years for women), high‐sensitivity CRP level (≥2 mg/L), and CMV presence in the multiple logistic regression model for ACS patients and healthy volunteers. *Differences are statistically significant at P<0.05. ACS indicates acute coronary syndrome; CMV, cytomegalovirus; CRP, C‐reactive protein; OR, odds ratio.
Table 7.
Multiple Logistic Regression Analysis of CAD Risk Factors, hs‐CRP Level, and Impact of CMV Frequency Comparing SCAD Patients and Healthy Volunteers
| Factor | Age | Sex | Smoking | Dyslipidemia | Obesity | Hypertension | DM | hs‐CRP | CMV |
|---|---|---|---|---|---|---|---|---|---|
| OR | 0.85 | 3.78 | 0.09 | 0.56 | 1.12 | 43.70 | 51.37 | 24.97 | 0.43 |
| Lowest 95% CL | 0.12 | 0.56 | 0.01 | 0.06 | 0.08 | 1.73 | 0.98 | 1.69 | 0.05 |
| Highest 95% CL | 5.75 | 25.35 | 1.09 | 5.19 | 15.78 | 1101.83 | 2697.63 | 368.74 | 3.45 |
| P value | 0.861 | 0.167 | 0.058 | 0.599 | 0.930 | 0.023a | 0.051 | 0.020a | 0.421 |
ORs are given for binomial CAD risk factors (with age ≥55 years for men and ≥65 years for women), hs‐CRP level (≥2 mg/L), and CMV presence in the multiple regression model comparing stable CAD patients and healthy volunteers. CAD indicates coronary artery disease; CL, confidence level; CMV, cytomegalovirus; DM, diabetes mellitus; hs‐CRP, high‐sensitivity C‐reactive protein; OR, odds ratio.
Differences are statistically significant at P<0.05.
Analysis of Plaques
Using an original protocol for isolation of lymphocytes from atherosclerotic plaques, we previously found that plaques are enriched with activated CD4 and especially with CD8 T lymphocytes.22 In the current study, we investigated whether T lymphocyte activation and differentiation correlated with the presence of HHVs in individual atherosclerotic plaques from 22 patients who underwent endarterectomy.
As reported earlier22 and confirmed in this study, both CD4+ and CD8+ T lymphocytes in plaques were highly activated compared with these cells in blood, as shown by the expression of the CD25, CD38, and HLA‐DR activation markers. Furthermore, there were significantly more Tem cells and fewer naïve T cells and Tcm cells in plaques than in blood from the same patients (Table 8).
Table 8.
T‐Cell Activation and Differentiation in Atherosclerotic Plaques and in Blood
| Type of T Cells | Plaques | Blood | P Value |
|---|---|---|---|
| HLA‐DR+ % CD4+ | 37.00 (23.70–46.10) | 9.13 (5.55–16.60) | 0.006a |
| CD38+ % CD4+ | 24.40 (19.00–45.80) | 39.40 (30.35–58.00) | 0.004a |
| CD25+ % CD8+ | 9.23 (3.02–37.40) | 1.75 (1.27–7.71) | 0.001a |
| HLA‐DR+ % CD8+ | 48.90 (32.10–54.35) | 22.90 (13.05–33.55) | 0.008a |
| Naïve % CD4+ (CD45+CD3+CD4+CD45RA+CD197+CD27+CD28+) | 2.09 (1.26–3.66) | 18.85 (5.75–25.50) | 0.003a |
| Tcm % CD4+ (CD45+CD3+CD4+CD45RA−CD197+CD27+CD28+) | 24.60 (19.35–33.70) | 50.55 (38.65–62.40) | 0.001a |
| Tem % CD4+ (CD45+CD3+CD4+CD45RA+/−CD197−) | 41.30 (31.50–50.50) | 21.20 (17.60–30.35) | 0.001a |
| Tem ID % CD4+ (CD45+CD3+CD4+CD45RA−CD197−CD27+/−CD28+) | 62.04 (49.01–66.11) | 38.69 (23.20–44.00) | 0.002a |
| Tem LD % CD4+ (CD45+CD3+CD4+CD45RA+/−CD197−CD27−CD28−) | 5.11 (1.99–8.98) | 29.30 (11.25–45.65) | 0.013a |
| Naïve % CD8+ (CD45+CD3+CD4−CD45RA+CD197+CD27+CD28+) | 1.70 (0.93–3.35) | 4.05 (2.24–8.36) | 0.030a |
| Tem % CD8+ (CD45+CD3+CD4−CD45RA+/−CD197−) | 69.10 (56.70–77.40) | 80.20 (71.00–87.20) | 0.015a |
| Tem ID % CD8+ (CD45+CD3+CD4−CD45RA−CD197−CD27+/−CD28+) | 39.61 (28.00–45.20) | 19.76 (14.00–32.15) | 0.023a |
| Tem LD % CD8+ (CD45+CD3+CD4−CD45RA+/−CD197−CD27−CD28−) | 23.30 (11.40–37.70) | 61.45 (27.15–69.10) | 0.006a |
Presented are percentages (%) of CD4 and CD8 T cells expressing particular markers. Median (interquartile range) is shown for fractions of different subtypes of T lymphocytes in blood and carotid atherosclerotic plaques from the same patients. ID indicates intermediately differentiated; LD, late‐differentiated; naïve indicates naïve T cells; Tcm, central memory T cells; Tem, effector memory T cells.
Differences are statistically significant at P<0.05.
We evaluated HHV DNA in the same plaques in which we measured T lymphocyte status and found that the most frequent HHVs were HHV‐3 and CMV (at least 100 copies of HHV DNA were found in 88% and 82% of plaques, respectively). Other HHVs were detected less often (Figure 8). For CMV, the viral load varied within an IQR of 446.92 to 18 508.48, with a median of 4973.53 DNA copies per 106 cells.
Figure 8.

Frequencies (as percentages) of HHVs detected in carotid artery plaque samples. Positivity was defined as HHV DNA at amounts >100 copies per 106 cells. HHV indicates human herpesvirus.
Comparison of HHV load and T lymphocyte status revealed a strong positive correlation between the level of CMV DNA in carotid artery plaques and the fraction of intermediately differentiated CD4+ and CD8+ Tem cells (Spearman R=0.642, P=0.004, and R=0.591, P=0.010, respectively) (Figures 9 and 10) as well as the fraction of all CD4+ Tem lymphocytes (Spearman R=0.516, P=0.028). In addition, there was a negative correlation between CMV load and the fraction of CD4+ and CD8+ Tcm lymphocytes (Spearman R=−0.673, P=0.002, and R=0.520, P=0.027, respectively) (Figures 11 and 12).
Figure 9.

Correlation coefficient with 95% CI according to Spearman rank test between CMV DNA loads and fractions of intermediately differentiated CD4+ Tem cells in atherosclerotic plaques. Correlation is statistically significant at P<0.05. CMV indicates cytomegalovirus; ID, intermediately differentiated; Tem, effector memory T cells.
Figure 10.
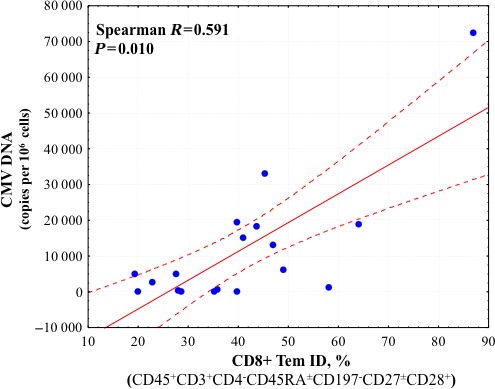
Correlation coefficient with 95% CI according to Spearman rank test between CMV DNA loads and fractions of intermediately differentiated CD8+ Tem cells in atherosclerotic plaques. Correlation is statistically significant at P<0.05. CMV indicates cytomegalovirus; ID, intermediately differentiated; Tem, effector memory T cells.
Figure 11.
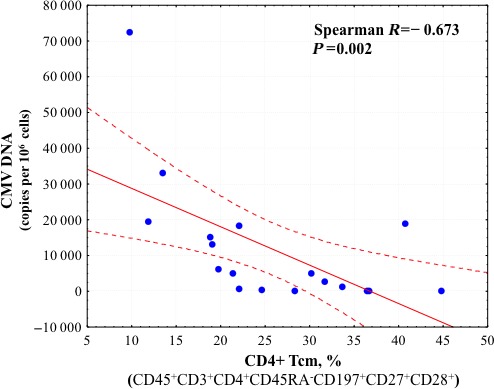
Correlation coefficient with 95% CI according to Spearman rank test between CMV DNA load and fraction of CD4+ Tcm cells in atherosclerotic plaques. Correlation is statistically significant at P<0.05. CMV indicates cytomegalovirus; Tcm, central memory T cells.
Figure 12.
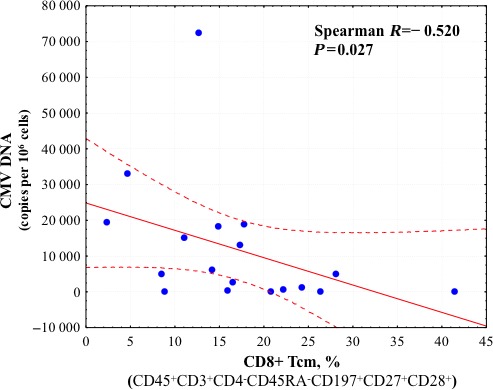
Correlation coefficient with 95% CI according to Spearman rank test between CMV DNA load and fraction of CD8+ Tcm cells in atherosclerotic plaques. Correlation is statistically significant at P<0.05. CMV indicates cytomegalovirus; Tcm, central memory T cells.
In summary, we found that (1) HHV DNA was present in plasma of both ACS and SCAD patients and in that of healthy controls; (2) HHV DNA copy numbers of all HHVs did not differ significantly between the SCAD and control groups; (3) the presence and load of HHV‐5 (CMV), but not other HHVs, were higher in plasma of patients with ACS than in healthy volunteers; (4) CMV load in patients and controls correlated with the levels of hs‐CRP in these participants; and (5) the copy numbers of CMV DNA in carotid artery plaques correlated with the lymphocyte differentiation levels in the same plaques.
Discussion
It is well established that immunoactivation is associated with the destabilization of atherosclerotic plaques.4 We previously found that both the spectrum of lymphocytes and their activation status in plaques are different from those in blood.22 In particular, plaques are enriched in CD8 and CD4 T lymphocytes, which express activation markers CD25 and HLA‐DR and are effector memory rather than naïve. These lymphocyte phenotypes may indicate the presence of a specific antigen toward which these lymphocytes are reactive.
Herpesviruses, which are among the most ancient and abundant viruses in humans, have been considered to be associated with atherosclerosis. Although some continue to be highly pathogenic,27 others are moderately pathogenic, and some (HHV‐6, HHV‐7) have generally lost their pathogenicity for immunocompetent persons. Although it was initially thought that HHVs could go into latency, it was found later that some are frequently reactivated and resuppressed by the immune system.28 The ubiquity and the ability to cycle between dormancy and replication make HHVs candidates for the role of constant triggers of immunoactivation that is closely related to the development of atherosclerosis. Early in 1980s, it was shown in chickens infected with Marek disease herpesvirus12 that diet‐induced experimental atherosclerosis developed at a higher rate than in control animals. Evidence of the involvement of HHVs in the development of atherosclerosis and restenosis after coronary angioplasty and in the acceleration of atherosclerosis development in heart transplant recipients has also been reported.29 Furthermore, CMV antibody levels have been linked to CAD‐related mortality in the general population.14
Data on the involvement of HHVs in general and of CMV in particular in human atherosclerosis are based predominantly on serological evidence. Seropositivity for CMV (or the level of CMV immunoglobulin G antibodies) has been reported to be associated with cardiovascular disease.30, 31 Furthermore, positive serology for HHV‐1 has been found to be associated with a higher risk of myocardial infarction or cardiovascular death.32, 33 In autopsies of aortic tissues that were identified histologically as atherosclerotic, DNA of HHV‐1, HHV‐4, and CMV were present more frequently than in nonatherosclerotic tissues.34, 35 In other studies, however, no differences were found between healthy persons and patients with early signs of atherosclerosis regarding the presence of anti–HHV‐1, anti–HHV‐2, and anti‐CMV antibodies36 and HHV‐1, HHV‐4, and HHV‐5 DNA in blood monocytes of patients with CAD.13 These contradictions may stem from the fact that neither serology nor DNA in cells give information about whether HHVs continue to replicate at the time of analysis. To study productive CMV infection, Gredmark et al studied HHV‐5 RNA expression in monocytes isolated from peripheral blood and found that productive CMV infection occurs in 15% of ACS patients and 10% of SCAD patients but in only 2% of healthy persons.37
In this study, we undertook another approach to evaluate productive HHV infection. Rather than focusing on HHV replication in particular cells, we evaluated DNA of all HHV types in plasma, into which the productively infected cells may release viruses.17 This analysis revealed all HHVs in plasma of healthy controls and patients with SCAD and ACS. Unexpectedly, this included HHV‐8, which was found at a higher frequency than what is usually reported for white populations (12.9%) but was well within the reported frequency in elderly populations in endemic regions (46.4%) and in Russian populations affected by several conditions.38 We next investigated the frequencies of the HHV genomes for different groups: except for CMV, we did not find differences among the 3 groups regarding the frequency of HHV presence.
We analyzed CMV in plasma not only qualitatively (present or not present) but also quantitatively by measuring HHV load with RT‐PCR for HHV DNA. We found that for CMV, the DNA copy numbers in plasma positively correlated with ACS. There were no such correlations for other HHVs. Moreover, there was no difference in viral load, including that for CMV, between SCAD patients and healthy volunteers. Consequently, the increased viral load for CMV was restricted to the acute stage of the disease.
Important marker of inflammation during CAD progression is CRP, which is an established risk factor of CAD.39, 40 In this study, we found a positive correlation between CMV and hs‐CRP across all groups, including SCAD patients and healthy controls. This indirect but strong evidence showed that CMV infection is an important cause of the inflammatory process. Whatever the actual trigger of the inflammatory process in atherosclerosis, it is associated with perturbation of the T cell repertoire in blood and in plaques.41 In particular, the expansion of unusual T lymphocytes in peripheral blood (CD4+CD28− T cells) is strongly associated with the recurrence of acute coronary events42 and appears in other diseases,43 such as acute ischemic stroke.44 In contrast, accumulation of intermediately and late‐differentiated CD8 Tem cells in blood is associated with CMV infection.45, 46, 47
With the development of the protocol of flow analysis of individual T cells in plaques, we can now link the status of these T cells to CMV load. We confirmed our earlier report that the fractions of activated T cells in carotid artery plaques of patients undergoing carotid endarterectomy were higher than in blood and demonstrated the enrichment of intermediately differentiated (CD27+/−CD28+) but not late‐differentiated (CD27−CD28−) Tem cells. Furthermore, there were fewer naïve CD4 and CD8 T cells and fewer CD4 Tcm cells in plaques than in blood. The increase of the fraction of Tem cells and the decrease of the fraction of Tcm and naïve cells suggest a more activated T cell phenotype, probably due to an antigen‐driven process48; therefore, plaques seem to represent the sites of a particular high immune activation. Remarkably, the fraction of intermediately differentiated Tem cells positively correlated and the fraction of Tcm cells negatively correlated with the presence of CMV DNA in the same plaque. We also found some other HHVs in plaques; however, their DNA levels did not correlate with T‐cell activation and differentiation profiles. Consequently, our results indicated that plaques with high amounts of CMV (which we found to be associated with ACS) are characterized by high levels of T‐cell activation. Because these plaques were obtained from the carotid arteries, their structure is slightly different from that of the coronary arteries. Although there are apparent limitations in combining plaques at remote sites and differing arterial beds into a single pool, we checked that half of plaques were macroscopically complicated, although all of these patients were without signs of an acute event in the studied vascular area. It is conceivable that CMV contributes to T‐cell activation and may trigger the rupture of these plaques. Although we previously found and confirmed in this study that activated T cells accumulate in plaques, which are infected with CMV, these cells may not be the only ones that play a role in HHV infection. Macrophages and endothelial cells may serve as targets and/or transporters of various HHVs and also may contribute to local and systemic immunoactivation. Further studies are needed to establish the role of these and other cells.
In summary, we found that CMV is strongly associated with the atherosclerotic process and, in particular, with ACS.
Our work has significant limitations. Although the correlations with CMV load seem to be proven, the size of the cohort may have contributed to the fact that we did not find any correlations with HHV‐7 and HHV‐8, which are present at lower frequency in this population. Furthermore, the dispersion in several risk factor frequencies, such as hypertension and diabetes mellitus, between groups was so wide that perhaps there were too few events in some groups to support these variables in the multiple regression model. Although the release of CMV into the blood is accepted as evidence of productive infection, we do not know which specific cells produce CMV and whether these cells are localized in plaques or at other sites.
Although we found an association between CMV in plasma and ACS, it is possible that other pathogens not studied in the present work also may be associated with this syndrome.49 In general, our results support a hypothesis that acute coronary events are synchronized with acute viral replication, possibly because of CMV reactivation, and this reactivation, which may occur both at the systemic level and in plaques, may contribute to plaque destabilization. It is conceivable that preventing the reactivation of CMV may affect the outcome of CAD.
Future studies should shed more light on the relation of ACS in particular and cardiovascular diseases in general to CMV and possibly to other HHVs,50 which may lead to new treatment strategies.
Sources of Funding
This work of Nikitskaya, Lebedeva, Ivanova, Maryukhnich, Shpektor, and Vasilieva was supported by Russian Federation Government grant #14.B25.31.0016. The work of Margolis and Grivel was supported by the Intramural Program of National Institute of Child Health and Human Development.
Disclosures
None.
Acknowledgments
We thank Dr Elena Yarovaya for assistance in statistical analysis.
(J Am Heart Assoc. 2016;5:e003759 doi: 10.1161/JAHA.116.003759)
References
- 1. Ross R, Harker L. Platelets, endothelium, and smooth muscle cells in atherosclerosis. Adv Exp Med Biol. 1978;102:135–141. [DOI] [PubMed] [Google Scholar]
- 2. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Apple FS, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Kristin Newby L, Galvani M, Hamm CW, Uretsky BF, Gabriel Steg P, Wijns W, Bassand JP, Menasché P, Ravkilde J, Magnus Ohman E, Antman EM, Wallentin LC, Armstrong PW, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, De Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 3. Libby P, Bornfeldt KE, Tall AR. Atherosclerosis. Circ Res. 2016;118:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crea F, Liuzzo G. Pathogenesis of acute coronary syndromes. J Am Coll Cardiol. 2013;61:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. [DOI] [PubMed] [Google Scholar]
- 6. Abbas A, Aukrust P, Russell D, Krohg‐Sørensen K, Almås T, Bundgaard D, Bjerkeli V, Sagen EL, Michelsen AE, Dahl TB, Holm S, Ueland T, Skjelland M, Halvorsen B. Matrix metalloproteinase 7 is associated with symptomatic lesions and adverse events in patients with carotid atherosclerosis. PLoS One. 2014;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbert A, Lion G. Arterites infectieuses experimentales. Comptes Rendus Hebdomadaires des Seances et Memoires de la Societe de Biologie 1889;41:583–584. [Google Scholar]
- 8. Buhlin K, Hultin M, Norderyd O, Persson L, Pockley AG, Pussinen PJ, Rabe P, Klinge B, Gustafsson A. Periodontal treatment influences risk markers for atherosclerosis in patients with severe periodontitis. Atherosclerosis. 2009;206:518–522. [DOI] [PubMed] [Google Scholar]
- 9. Lemström KB, Aho PT, Bruggeman CA, Häyry PJ. Cytomegalovirus infection enhances mRNA expression of platelet‐derived growth factor‐BB and transforming growth factor‐beta 1 in rat aortic allografts. Possible mechanism for cytomegalovirus‐enhanced graft arteriosclerosis. Arterioscler Thromb. 1994;14:2043–2052. [DOI] [PubMed] [Google Scholar]
- 10. Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. [DOI] [PubMed] [Google Scholar]
- 11. Mitra S, Drautz‐Moses DI, Alhede M, Maw MT, Liu Y, Purbojati RW, Yap ZH, Kushwaha KK, Gheorghe AG, Bjarnsholt T, Hansen GM, Sillesen HH, Hougen HP, Hansen PR, Yang L, Tolker‐Nielsen T, Schuster SC, Givskov M. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome. 2015;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fabricant CG, Fabricant J, Minick CR, Litrenta MM. Virus‐induced atherosclerosis. J Exp Med. 1978;148:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schlitt A, Blankenberg S, Weise K, Gärtner BC, Mehrer T, Peetz D, Meyer J, Darius H, Rupprecht HJ. Herpesvirus DNA (Epstein‐Barr virus, herpes simplex virus, cytomegalovirus) in circulating monocytes of patients with coronary artery disease. Acta Cardiol. 2005;60:605–610. [DOI] [PubMed] [Google Scholar]
- 14. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow‐up. Am J Epidemiol. 2010;172:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all‐cause and cardiovascular disease‐related mortality in the United States. PLoS One. 2011;6:e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ross SA, Novak Z, Pati S, Boppana SB. Overview of the diagnosis of cytomegalovirus infection. Infect Disord Drug Targets. 2011;11:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gimeno C, Solano C, Latorre JC, Hernández‐Boluda JC, Clari MA, Remigia MJ, Furió S, Calabuig M, Tormo N, Navarro D. Quantification of DNA in plasma by an automated real‐time PCR assay (cytomegalovirus PCR kit) for surveillance of active cytomegalovirus infection and guidance of preemptive therapy for allogeneic hematopoietic stem cell transplant recipients. J Clin Microbiol. 2008;46:3311–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamm CW, Bassand J‐P, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevatio. Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 19. Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Astin F, Åström‐Olsson K, Budaj A, Clemmensen P, Collet JP, Fox KA, Fuat A, Gustiene O, Hamm CW, Kala P, Lancellotti P, Maggioni AP, Merkely B, Neumann FJ, Piepoli MF, Van De Werf F, Verheugt F, Wallentin L. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 20. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot J‐S, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJM, Zamorano JL, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Valgimigli M, Claeys MJ, Donner‐Banzhoff N, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Husted S, James SK, Kervinen K, Kristensen SD, Maggioni AP, Pries AR, Romeo F, Rydén L, Simoons ML, Steg PG, Timmis A, Yildirir A. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 21. Agostino RBD, Vasan RS, Pencina MJ, Philip A, Cobain M, Massaro JM, Kannel WB, D'Agostino RB, Wolf PA. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 22. Grivel J‐C, Ivanova O, Pinegina N, Blank PS, Shpektor A, Margolis LB, Vasilieva E. Activation of T lymphocytes in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2011;31:2929–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV‐3 real time PCR assay. J Virol Methods. 2001;91:109–117. [DOI] [PubMed] [Google Scholar]
- 24. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck‐Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Rydén L, Sirenko Y, Stanton A, Struijker‐Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 26. Koenig W, Sund M, Fröhlich M, Fischer HG, Löwel H, Döring A, Hutchinson WL, Pepys MB. C‐reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle‐aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984. Circulation. 1999;99:237–242. [DOI] [PubMed] [Google Scholar]
- 27. Oh J‐K, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. 2014;80:384–392. [DOI] [PubMed] [Google Scholar]
- 28. Schädlich HJ, Nekic M, Jeske J, Karbe H. Intrathecal humoral immune reaction in zoster infections. J Neurol Sci. 1991;103:101–104. [DOI] [PubMed] [Google Scholar]
- 29. Nicholson AC, Hajjar DP. Herpesviruses in atherosclerosis and thrombosis: etiologic agents or ubiquitous bystanders? Arterioscler Thromb Vasc Biol. 1998;18:339–348. [DOI] [PubMed] [Google Scholar]
- 30. Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2000;160:2027–2032. [DOI] [PubMed] [Google Scholar]
- 31. Adam E, Melnick JL, Probtsfield JL, Petrie BL, Burek J, Bailey KR, McCollum CH, DeBakey ME. High levels of cytomegalovirus antibody in patients requiring vascular surgery for atherosclerosis. Lancet. 1987;2:291–293. [DOI] [PubMed] [Google Scholar]
- 32. Siscovick DS, Schwartz SM, Corey L, Grayston JT, Ashley R, Wang SP, Psaty BM, Tracy RP, Kuller LH, Kronmal RA. Chlamydia pneumoniae, herpes simplex virus type 1, and cytomegalovirus and incident myocardial infarction and coronary heart disease death in older adults: the Cardiovascular Health Study. Circulation. 2000;102:2335–2340. [DOI] [PubMed] [Google Scholar]
- 33. Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. [DOI] [PubMed] [Google Scholar]
- 34. Horváth R, Cerný J, Benedík J, Hökl J, Jelínková I. The possible role of human cytomegalovirus (HCMV) in the origin of atherosclerosis. J Clin Virol. 2000;16:17–24. [DOI] [PubMed] [Google Scholar]
- 35. Shi Y, Tokunaga O. Herpesvirus (HSV‐1, EBV and CMV) infections in atherosclerotic compared with non‐atherosclerotic aortic tissue. Pathol Int. 2002;52:31–39. [DOI] [PubMed] [Google Scholar]
- 36. Nieto FJ, Adam E, Sorlie P, Farzadegan H, Melnick JL, Comstock GW, Szklo M. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal‐medial thickening, a measure of subclinical atherosclerosis. Circulation. 1996;94:922–927. [DOI] [PubMed] [Google Scholar]
- 37. Gredmark S, Jonasson L, Van Gosliga D, Ernerudh J, Söderberg‐Nauclér C. Active cytomegalovirus replication in patients with coronary disease. Scand Cardiovasc J. 2007;41:230–234. [DOI] [PubMed] [Google Scholar]
- 38. Lacoste V, Kadyrova E, Chistiakova I, Gurtsevitch V, Judde JG, Gessain A. Molecular characterization of Kaposi's sarcoma‐associated herpesvirus/human herpesvirus‐8 strains from Russia. J Gen Virol. 2000;81:1217–1222. [DOI] [PubMed] [Google Scholar]
- 39. Lagrand WK, Visser CA, Hermens WT, Niessen HWM, Verheugt FWA, Wolbink G‐J, Hack CE. C‐reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:96–102. [DOI] [PubMed] [Google Scholar]
- 40. Whelton SP, Roy P, Astor BC, Zhang L, Hoogeveen RC, Ballantyne CM, Coresh J. Elevated high‐sensitivity C‐reactive protein as a risk marker of the attenuated relationship between serum cholesterol and cardiovascular events at older age: the ARIC Study. Am J Epidemiol. 2013;178:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M, Grigore L, Pellegatta F, Pirillo A, Garlaschelli K, Manfredi AA, Catapano AL, Maseri A, Palini AG, Norata GD. Effector memory T cells are associated with atherosclerosis in humans and animal models. J Am Heart Assoc. 2012;1:27–41. doi: 10.1161/JAHA.111.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, Rizzello V, Rebuzzi AG, Rumi C, Maseri A, Crea F. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol. 2007;50:1450–1458. [DOI] [PubMed] [Google Scholar]
- 43. Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28‐ and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nadareishvili ZG, Li H, Wright V, Maric D, Warach S, Hallenbeck JM, Dambrosia J, Barker JL, Baird AE. Elevated pro‐inflammatory CD4+CD28‐ lymphocytes and stroke recurrence and death. Neurology. 2004;63:1446–1451. [DOI] [PubMed] [Google Scholar]
- 45. Sacre K, Carcelain G, Cassoux N, Fillet A‐M, Costagliola D, Vittecoq D, Salmon D, Amoura Z, Katlama C, Autran B. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201:1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van de Berg PJEJ, Yong S‐L, Remmerswaal EBM, van Lier RAW, ten Berge IJM. Cytomegalovirus‐induced effector T cells cause endothelial cell damage. Clin Vaccine Immunol. 2012;19:772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GMA, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland‐Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. [DOI] [PubMed] [Google Scholar]
- 48. Bateman EAL, Ayers L, Sadler R, Lucas M, Roberts C, Woods A, Packwood K, Burden J, Harrison D, Kaenzig N, Lee M, Chapel HM, Ferry BL. T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol. 2012;170:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pesonen E, El‐Segaier M, Persson K, Puolakkainen M, Sarna S, Ohlin H, Pussinen PJ. Infections as a stimulus for coronary occlusion, obstruction, or acute coronary syndromes. Ther Adv Cardiovasc Dis. 2009;3:447–454. [DOI] [PubMed] [Google Scholar]
- 50. Elkind MSV, Hills NK, Glaser CA, Lo WD, Amlie‐Lefond C, Dlamini N, Kneen R, Hod EA, Wintermark M, deVeber GA, Fullerton HJ. Herpesvirus infections and childhood arterial ischemic stroke: results of the VIPS Study. Circulation. 2016;133:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]


