Abstract
Background
Depression is a relapsing and remitting disease. Prior studies on the association between depressive symptoms and incident cardiovascular disease (CVD) have been limited by single measurements, and few if any have examined both incident coronary heart disease and stroke in a large biracial national cohort. We aimed to assess whether time‐dependent depressive symptoms conferred increased risk of incident CVD.
Methods and Results
Between 2003 to 2007, 22 666 black and white participants (aged ≥45 years) without baseline CVD in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study were recruited. Cox proportional hazards regression analyses assessed the association between up to 3 measurements of elevated depressive symptoms (4‐item Center for Epidemiologic Studies Depression Scale score ≥4) and incident coronary heart disease, stroke, and CVD death adjusting for age, sex, region, income, health insurance, education, blood pressure, cholesterol, medication, obesity, diabetes mellitus, kidney disease, C‐reactive protein, corrected QT interval, atrial fibrillation, left ventricular hypertrophy, smoking, alcohol, physical inactivity, medication adherence, and antidepressant use. The participants’ average age was 63.4 years, 58.8% were female, and 41.7% black. Time‐varying depressive symptoms were significantly associated with CVD death (adjusted hazard ratio 1.30, 95% CI 1.04–1.63), with a trend toward significance for fatal and nonfatal stroke (adjusted hazard ratio 1.26, 95% CI 0.99–1.60) but not fatal and nonfatal coronary heart disease (adjusted hazard ratio 1.11, 95% CI 0.89–1.38). Race did not moderate the association between depressive symptoms and CVD.
Conclusions
Proximal depressive symptoms were associated with incident fatal and nonfatal stroke and CVD death even after controlling for multiple explanatory factors, further supporting the urgent need for timely management of depressive symptoms.
Keywords: cardiovascular disease, depression, epidemiology
Subject Categories: Quality and Outcomes
Introduction
A growing body of evidence suggests that depressive symptoms may confer an increased risk for incident cardiovascular disease (CVD).1, 2, 3, 4 Nevertheless, few if any previous studies have analyzed both coronary heart disease (CHD) and stroke simultaneously in a large biracial national sample, and almost all studies have been limited to single measurements of depressive symptoms.5 Single measurements do not account for the fact that depression is a relapsing and remitting disease, and cumulative measurements may be limited by selection bias through differential loss to follow‐up.
The mechanisms underlying the association between depressive symptoms and CVD have proven difficult to fully elucidate.4 Although successive meta‐analyses have shown that depressive symptoms increase the risk of incident CHD and CHD death, with pooled hazard ratios (HRs) estimated at 1.6 and 1.8,2, 3, 6 there has been concern about incomplete risk factor adjustment in the majority of studies included in prior meta‐analyses and limited use of time‐dependent variables.2 Similarly, although depressive symptoms have been considered an inconsistent risk factor for incident cerebrovascular disease,7, 8, 9 a recent meta‐analysis found pooled HRs of 1.5 and 1.6 for total and fatal stroke; again, only 6 of 28 studies simultaneously controlled for alcohol, body mass index, and smoking status, and 1 controlled for medication adherence.10 In addition to unmeasured confounders, research also remains inconsistent as to whether race truly moderates the association between depressive symptoms and incident CVD.11, 12, 13, 14 Prior studies suggest that racial and ethnic minorities face unique barriers to depression treatment in the United States,15 and as such, it is unclear how race and time‐dependent depressive symptoms interact to influence CVD events.
Given that time‐varying analyses may better elucidate the timing of the association between depressive symptoms and CVD, the aim of this study was to assess whether time‐varying depressive symptoms confer increased risk of stroke and CHD, adjusting for potential explanatory factors in a large national diverse cohort. We secondarily sought to assess whether depressive symptoms differentially predicted incident stroke and CHD in black and white participants.
Methods
Study Procedures
Details of the REasons for Geographic And Racial Differences in Stroke (REGARDS) study have been described previously.16 In brief, REGARDS is a national cohort study of stroke incidence and cognitive decline in black and white patients aged ≥45 years living in the United States and stratified to reflect specific race, sex, and geographic strata. CHD outcomes were ascertained from a REGARDS ancillary study. Participants were recruited by mail using commercially available lists of US residents, followed by a telephone call and a subsequent home visit at which time patients gave consent and were enrolled. Between January 2003 and October 2007, 30 183 black and white adults were enrolled. Of these, 484 (1.6%) were lost to follow‐up, and 171 (0.6%) were missing baseline depressive symptom measurements (Figure 1). Our current analysis was limited to 22 666 participants without existing CVD at baseline (6862 had stroke, CHD, peripheral vascular disease, or aneurysm). The REGARDS study protocol was approved by the institutional review boards of the participating centers.
Figure 1.
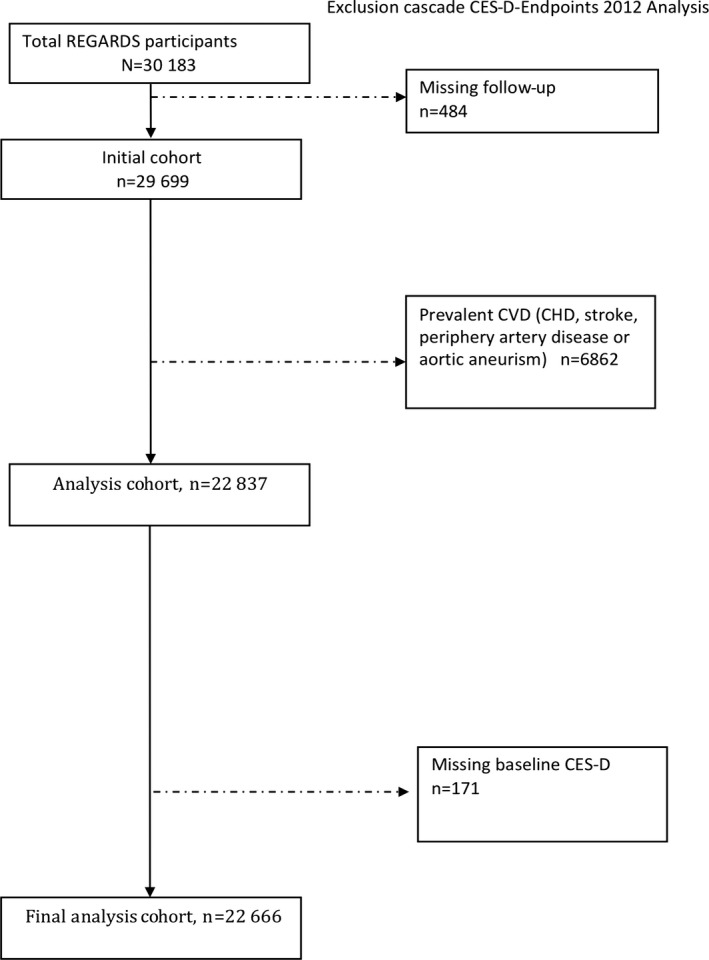
Cohort flow diagram. Numbers of participants originally recruited for the REGARDS study, lost to follow‐up, missing baseline depressive symptoms, excluded from the analysis due to existing baseline CVD, and in the final analysis. CES‐D indicates Center for Epidemiologic Studies Depression Scale; CHD, coronary heart disease; CVD, cardiovascular disease; REGARDS, REasons for Geographic And Racial Differences in Stroke.
Baseline data were collected through computer‐assisted telephone interviews, an in‐home examination, and self‐administered questionnaires. Trained research staff conducted telephone interviews to collect demographic data and education. Following telephone interview, patients had an in‐home visit during which physical measurements, a resting electrocardiogram, medication inventory, phlebotomy, and urine were collected. Medication use was measured by self‐report or pill bottle review during the in‐home visit.
Primary Outcomes
The primary outcomes for these analyses were (1) incident CHD events, defined as definite or probable nonfatal or fatal myocardial infarction or acute CHD death events; (2) incident stroke events, defined as probable nonfatal or fatal stroke; (3) incident CVD death, defined as death from CHD, stroke, heart failure, sudden cardiac death, vascular pathology, and other CVD causes. Living participants or their proxies were followed up every 6 months by telephone, with retrieval of medical records for reported hospitalizations or physician visits. Data were collected on suspected stroke events that required hospitalization and on physician evaluations for stroke‐like symptoms detected using the Questionnaire for Verifying Stroke‐Free Status.17 The adjudication process validated stroke occurrence and classified events by stroke “subtype” and severity using the National Institutes of Health Stroke Scale. Stroke was defined as rapid onset of a persistent neurological deficit attributable to an obstruction or rupture of the arterial system (including stroke occurring during a procedure such as angiograph or surgery) that causes a deficit not known to be secondary to brain trauma; that lasts >24 hours, unless death supervenes; and that has a demonstrable lesion compatible with acute stroke on computed tomography or magnetic resonance imaging.16, 18 For CHD events, medical records were examined for the presence of signs and symptoms of ischemia, cardiac enzymes, electrocardiogram changes consistent with ischemia, or myocardial infarction based on the Minnesota code; myocardial infarctions were adjudicated as being definite or probable, based on published guidelines.19, 20 Deaths were detected by report of next of kin or through online services (eg, Social Security Death Index) or the National Death Index.16 Death certificates, medical records, and autopsy reports were obtained to adjudicate cause of death and cardiovascular outcomes.
Depressive Symptoms
The primary predictor was time‐varying depressive symptoms. The 4‐item Center for Epidemiologic Studies Depression Scale (CES‐D) was used to assess the presence of depressive symptoms. This scale asks participants to rate the number of days over the past week in which they (1) felt depressed, (2) felt lonely, (3) had crying spells, and (4) felt sad. Response options included <1 day (no points), 1 to 2 days (1 point), 3 to 4 days (2 points), and 5 to 7 days (3 points). Cronbach's α for the CES‐D in the total sample was 0.80 and among black and white participants was 0.81 and 0.79, respectively. Elevated depressive symptoms were defined as a summed score of ≥4.21 The reliability and validity of the 4‐item CES‐D is similar to the original 20‐item instrument.22 The CES‐D was administered 3 times: (1) during the initial telephone interview, (2) at 5 years after baseline measurement, and (3) at 2 years after the second measurement. CES‐D scores measured after an end point or after the end of follow‐up were not eligible to be included in the time‐varying analysis.
Covariates
Demographic data included self‐reported age, sex, race (black or white), education (less than high school, high school graduate, some college, and college graduate and higher), annual income (less than $20 000, $20 000–$34 999, $35 000–$74 999, $75 000 and above), insurance status (yes or no), and stroke region (“stroke belt,” defined as the states of Alabama, Arkansas, Louisiana, Mississippi, Tennessee, and the noncoastal regions within the states of North Carolina, South Carolina, and Georgia; “stroke buckle,” defined as coastal regions within the states of North Carolina, South Carolina, and Georgia). Clinical CHD risk factors included diabetes mellitus, defined as fasting blood glucose ≥126 mL/dL, random glucose >200 mL/dL, or oral hypoglycemic or insulin use; systolic and diastolic blood pressures based on the average of 2 standardized blood pressure measurements (continuous variables in mm Hg); body mass index based on measured height and weight; albumin:creatinine ratio; and high‐density lipoprotein and total cholesterol. We also assessed use of aspirin (yes or no), statins (yes or no), and antihypertensive medications (yes or no). Behavioral risk factors included (1) smoking status, based on self‐reported pack‐years of cigarette smoking; (2) physical inactivity, ascertained by asking, “How many times per week do you engage in intense physical activity, enough to work up a sweat?” with response options of “none,” “1 to 3 times per week,” or “4 or more times per week”; (3) alcohol use, ascertained by asking, “How many alcoholic beverages do you drink?” and use based on National Institute on Alcohol Abuse and Alcoholism classifications (none, moderate [1 drink per day for women or 2 drinks per day for men], and heavy [>1 drink per day for women and >2 drinks per day for men])16; and (4) medication nonadherence, assessed with the 4‐item Morisky Medication Adherence Scale (ranging from 0–4, with a score ≥1 indicating medication nonadherence).23 Potential mediators included high‐sensitivity C‐reactive protein, QT intervals corrected for heart rate using the formula QT+(154×[1−(60/heart rate)]), and antidepressant use (serotonin and norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, tricyclic antidepressants; yes or no).
Statistical Analyses
Baseline characteristics of participants with and without elevated depressive symptoms at baseline were compared using chi‐square tests, Student t tests, and Kruskal–Wallis tests, as appropriate.
Cox proportional hazards regression models were constructed to separately analyze the association between depressive symptoms (CES‐D score ≥4) and incident CHD, incident stroke, and CVD death. The end date of follow‐up for this analysis was December 31, 2012. Follow‐up time for each participant was calculated from the date of the in‐home visit to the date of the earliest of the first CHD or stroke event, death, the last telephone follow‐up, or the end of follow‐up. Depressive symptoms were measured with the CES‐D at baseline and again at 5‐ and 7‐year follow‐up. In the analyses, we considered depressive symptoms as a time‐varying exposure, with updates of exposure at 5‐ and 7‐year follow‐up. Consequently, each participant contributed up to 3 measures of CES‐D over follow‐up. Adjusted modeling proceeded in stages, with each model including additional covariates. Model 1 adjusted for demographic factors, including age, sex, region, income, health insurance, education, and traditional CVD risk factors (systolic blood pressure; total cholesterol; high‐density lipoprotein‐cholesterol; use of aspirin, statins, or antihypertensives; body mass index; logarithmically transformed albumin:creatinine ratio; and diabetes status). Model 2 additionally adjusted for behavioral CVD risk factors (pack‐years of cigarette smoking, self‐reported alcohol use, physical inactivity, medication nonadherence). Model 3 adjusted for other physiological explanatory factors including logarithmically transformed high‐sensitivity C‐reactive protein, antidepressant use, and QT interval corrected for heart rate. Model 3 for stroke was additionally adjusted for atrial fibrillation and left ventricular hypertrophy. The analyses were conducted overall and were stratified by race. We also conducted a formal test for interaction with depressive symptoms by race in the fully adjusted models. The proportionality assumption was tested by assessing depressive symptoms by log of follow‐up time interactions and was satisfied for all end points.
Missing data in covariates were imputed using chained equations, and final estimates were derived by bootstrapping across the 5 imputed data sets. Among the 22 666 participants, the following data were missing: 2768 (12%), income; 9 (0.03%), education; 22 (0.09%), health insurance; 831 (4%), diabetes mellitus; 13 (0.06%), aspirin use; 61 (0.3%), statin use; 61 (0.3%), antidepressant use; 242 (1%), antihypertension medication use; 326 (1%), physical activity; 2415 (11%), medication adherence; 146 (0.6%), body mass index; 908 (4%), cholesterol; 1027 (5%), high‐density lipoprotein; 659 (3%), pack‐years smoked; 60 (0.3%), systolic blood pressure; 1000 (4%), albumin:creatinine ratio; 296 (1%), corrected QT interval; 1379 (6%), C‐reactive protein. Analyses were conducted using SAS software version 9.4 (SAS Institute) and Stata version 12 (StataCorp).
Results
Participant Characteristics
Of the 22 666 eligible participants, 2267 (10.0%) had elevated depressive symptoms at baseline (CES‐D score ≥4). Overall, 73.7% of participants completed a second measurement, and 59.9% completed a third measurement. Of patients with elevated depressive symptoms at baseline, 39.2% and 37.5% had elevated depressive symptoms at the second and third measurements, respectively. The participants’ average age was 63.4 years, 58.8% were female, 41.7% were black, 18.6% had diabetes, 32.7% were physically inactive, and 29.2% were nonadherent to their medication regimens. Of the participants who completed the initial baseline scale, patients with elevated depressive symptoms were more likely to be younger, female, and black; have less than a high school education; have an income below $20 000 (39.9% versus 14.4%); be uninsured; have higher systolic blood pressure and total cholesterol; have high‐density lipoprotein; be obese; have diabetes mellitus; have higher self‐reported pack‐years; be physically inactive; report medication nonadherence; have higher C‐reactive protein levels; use antihypertensive medications; and, finally, have a prolonged corrected QT interval at baseline (Table 1).
Table 1.
Baseline Characteristics of REGARDS Cohort Members Who Were Free of CVD,a by Baseline Depressive Symptoms (CES‐D)
| Characteristics | Overall (n=22 666) | CES‐D Score ≥4 (n=2267) | CES‐D Score <4 (n=20 399) | P Valueb |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age, y, mean±SD | 63.9±9.3 | 62.2±9.7 | 64.1±9.2 | <0.001 |
| Sex, n (%) | <0.001 | |||
| Female | 13 321 (58.8) | 1675 (73.9) | 11 646 (57.1) | |
| Race, n (%) | <0.001 | |||
| Black | 9444 (41.7) | 1183 (52.2) | 8261 (40.5) | |
| Education, n (%) | <0.001 | |||
| Less than high school | 2466 (10.9) | 484 (21.4) | 1982 (9.7) | |
| High school graduate | 5718 (25.2) | 692 (30.5) | 5026 (24.7) | |
| Some college | 6127 (27.0) | 592 (26.1) | 5535 (27.1) | |
| College graduate and higher | 8343 (36.8) | 498 (22.0) | 7845 (38.5) | |
| Annual household income, n (%) | <0.001 | |||
| Less than $20 000 | 3705 (16.3) | 768 (33.9) | 2937 (14.4) | |
| $20 000 to $34 999 | 5314 (23.4) | 573 (25.3) | 4741 (23.2) | |
| $35 000 to $74 999 | 6965 (30.7) | 443 (19.5) | 6522 (32.0) | |
| $75 000 and above | 3908 (17.2) | 168 (7.4) | 3740 (18.3) | |
| Declined to report | 2771 (12.2) | 315 (13.9) | 2456 (12.0) | |
| No health insurance, n (%) | 1642 (7.3) | 307 (13.5) | 1335 (6.6) | <0.001 |
| Region,c n (%) | <0.001 | |||
| Stroke belt | 7832 (34.6) | 850 (37.5) | 6982 (34.2) | |
| Stroke buckle | 4776 (21.1) | 533 (23.5) | 4243 (20.8) | |
| Not stroke belt or buckle | 10 055 (44.4) | 884 (39.0) | 9171 (45.0) | |
| Physiological risk factors | ||||
| Body mass index, kg/m2, mean±SD | 29.3±6.2 | 30.5±7.0 | 29.2±6.1 | <0.001 |
| Diabetes mellitus,d n (%) | 4051 (18.6) | 560 (25.6) | 3491 (17.8) | <0.001 |
| Systolic blood pressure, mm Hg, mean±SD | 126.8±16.3 | 127.5±17.4 | 126.7±16.2 | 0.0370 |
| Total cholesterol, mg/dL, mean±SD | 195.7±39.0 | 198.2±41.0 | 195.4±38.8 | 0.0014 |
| High‐density lipoprotein, mg/dL, mean±SD | 53.0±16.3 | 54.0±16.3 | 52.9±16.3 | 0.0027 |
| QT interval, corrected for heart rate, ms, mean±SD | 406.0±22.2 | 408.0±21.8 | 405.8±22.2 | <0.001 |
| High‐sensitivity C‐reactive protein, mg/L, median (IQR) | 2.2 [0.9–4.9] | 2.9 [1.2–6.6] | 2.1 [0.9–4.7] | <0.001 |
| Albumin to creatinine ratio, mg/g, median (IQR) | 7.0 [4.5–13.9] | 7.4 [4.8–15.4] | 7.0 [4.5–13.8] | <0.001 |
| Medications | ||||
| Antihypertensive medication use, n (%) | 10 589 (47.2) | 1184 (52.9) | 9405 (46.6) | <0.001 |
| Statin use, n (%) | 5626 (24.9) | 571 (25.3) | 5055 (24.8) | 0.6555 |
| Aspirin use, n (%) | 8282 (36.6) | 800 (35.3) | 7482 (36.7) | 0.1947 |
| Antidepressant use, n (%) | 2913 (12.9) | 600 (26.6) | 2313 (11.4) | <0.001 |
| Behavioral risk factors | ||||
| Self‐reported smoking, pack years, mean±SD | 11.3±20.3 | 12.5±22.0 | 11.2±20.1 | 0.0031 |
| Alcohol use, n (%) | <0.001 | |||
| Heavy | 964 (4.3) | 97 (4.4) | 867 (4.3) | |
| Moderate | 7595 (34.2) | 617 (28.0) | 6978 (34.8) | |
| None | 13 676 (61.5) | 1490 (67.6) | 12 186 (60.8) | |
| Physical inactivity, n (%) | 7303 (32.7) | 997 (44.5) | 6306 (31.4) | <0.001 |
| Medication nonadherence, n (%) | 5916 (29.2) | 773 (37.5) | 5143 (28.3) | <0.001 |
CES‐D indicates Center for Epidemiologic Studies Depression Scale; CVD, cardiovascular disease; IQR, interquartile range; REGARDS, REasons for Geographic And Racial Differences in Stroke.
CVD defined as baseline coronary heart disease, stroke, peripheral artery disease, or aortic aneurysm.
P values from chi‐square or student t tests.
“Stroke belt” is defined as the states of Alabama, Arkansas, Louisiana, Mississippi, Tennessee, and the noncoastal regions within the states of North Carolina, South Carolina, and Georgia. “Stroke buckle” is defined as coastal regions within the states of North Carolina, South Carolina, and Georgia.
Diabetes is defined as fasting blood glucose ≥126 mL/dL, random glucose >200 mL/dL, or oral hypoglycemic or insulin use.
Incident Cardiovascular Events
The median follow‐up time was 6.9 years. Among participants with elevated depressive symptoms at any of the 3 assessments, there were 96 (10.7%) CHD events, 81 (12.2%) strokes, and 94 (13.4%) CVD deaths. In the unadjusted analyses, the HR for CHD associated with time‐varying depressive symptoms was 1.15 (95% CI 0.93–1.42), which remained similar after adjusting for demographics, traditional CVD, and other explanatory behavioral and physiological factors (Table 2). The unadjusted HR for fatal and nonfatal stroke with depressive symptoms was 1.35 (95% CI 1.07–1.70). The adjusted HR (aHR) for fatal and nonfatal stroke was 1.31 (95% CI 1.04–1.67) after controlling for demographic and traditional CVD risk factors, 1.30 (1.02–1.65) after adjusting for behavioral factors, and 1.26 (0.99–1.60) after additionally adjusting for other physiological factors. Time‐varying depressive symptoms were significantly associated with CVD death in the unadjusted analysis (HR 1.49, 95% CI 1.20–1.85) and remained so after adjusting for demographics and traditional CVD risk factors (aHR 1.36, 95% CI 1.09–1.70), behavioral risk factors (aHR 1.35, 95% CI 1.08–1.68), and other explanatory physiological factors (aHR 1.30, 95% CI 1.04–1.63) (Figure 2).
Table 2.
Incident Cardiovascular Events and Death Associated With Time‐Varying CES‐D Scoresa
| Time‐Varying Categorical CES‐D (Score ≥4 vs <4), HR (95% CI) | |||
|---|---|---|---|
| Overall | Black | White | |
| CHD | n=895 | n=377 | n=518 |
| Unadjusted | 1.15 (0.93–1.42) | 1.32 (0.99–1.75) | 0.97 (0.70–1.34) |
| Model 1b | 1.16 (0.93–1.43) | 1.24 (0.93–1.66) | 1.02 (0.74–1.42) |
| Model 2c | 1.14 (0.92–1.42) | 1.22 (0.91–1.64) | 1.00 (0.72–1.39) |
| Model 3d | 1.11 (0.89–1.38) | 1.17 (0.87–1.57) | 0.98 (0.70–1.36) |
| Strokee | n=663 | n=299 | n=364 |
| Unadjusted | 1.35 (1.07–1.70)f | 1.34 (0.97–1.84) | 1.29 (0.91–1.82) |
| Model 1b | 1.31 (1.04–1.67)f | 1.28 (0.93–1.77) | 1.30 (0.91–1.85) |
| Model 2c | 1.30 (1.02–1.65)f | 1.28 (0.95–1.77) | 1.29 (0.91–1.84) |
| Model 3d | 1.26 (0.99–1.60)f | 1.28 (0.92–1.78) | 1.23 (0.86–1.76) |
| CVD death | n=702 | n=376 | n=326 |
| Unadjusted | 1.49 (1.20–1.85)f | 1.41 (1.08–1.86)f | 1.40 (0.98–1.98) |
| Model 1b | 1.36 (1.09–1.70)f | 1.35 (1.02–1.80)f | 1.29 (0.90–1.86) |
| Model 2c | 1.35 (1.08–1.68)f | 1.35 (1.01–1.79)f | 1.29 (0.90–1.85) |
| Model 3d | 1.30 (1.04–1.63)f | 1.28 (0.96–1.71) | 1.25 (0.83–1.80) |
HR and 95% CI were estimated by Cox proportional hazards regression models. CES‐D indicates Center for Epidemiologic Studies Depression Scale; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio.
Excludes participants with baseline CVD (history of CHD, stroke, peripheral artery disease, aortic aneurism). All participants’ follow‐up time ended at the time of the end point, death, or last follow‐up. End of follow‐up was December 31, 2012. CES‐D measurements taken after an end point or end of follow‐up were not considered. Missing data in covariates were imputed.
Adjusted for demographics: age, sex, region, income, health insurance, education, and traditional CHD risk factors (systolic blood pressure, total cholesterol, high‐density lipoprotein cholesterol, and medication use [aspirin, statins, any antihypertensive medications], body mass index, log of albumin:creatinine ratio, diabetes mellitus).
Adjusted for model 1 covariates and behavioral risk factors: pack‐years of cigarette smoking, self‐reported alcohol use, physical inactivity, medication adherence. All‐cause mortality models also adjusted for self‐reported physical health (Short Form 12, Physical Component Summary).
Adjusted for model 2 covariates and other physiological factors: log of high‐sensitivity C‐reactive protein, antidepressant use, QT interval corrected for heart rate.
Models of stroke also adjusted for atrial fibrillation and left ventricular hypertrophy.
P<0.05.
Figure 2.
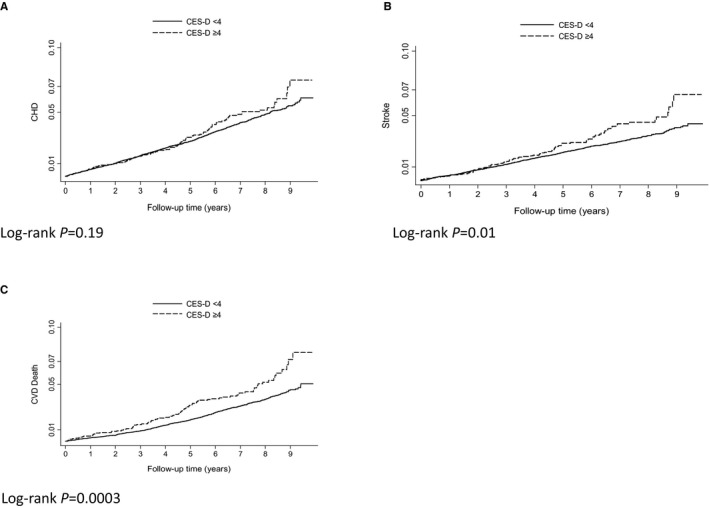
Kaplan–Meier graphs of depression and CVD events and deaths on or before December 31, 2012. Kaplan–Meier curves and log‐rank P values for the relationship between depressive symptoms and fatal and nonfatal CHD (A), fatal and nonfatal stroke (B), and CVD death (C). CES‐D indicates Center for Epidemiologic Studies Depression Scale; CHD, coronary heart disease; CVD, cardiovascular disease.
Assessment of the Potential for Effect Modification by Race
In additional analyses, we further assessed whether race moderated the association between depressive symptoms and CVD. We found that there was no significant interaction between depressive symptoms and race for any of the aforementioned outcomes (Table 2). In Model 1, the P value for the depressive symptoms by race interaction term was 0.21 for CHD, 0.89 for stroke, and 0.77 for CVD death in the overall models adjusted for all covariates. In the race‐stratified analyses, the HRs for depressive symptoms and CHD in black and white participants were 1.32 (95% CI 0.99–1.75; aHR 1.17, 95% CI 0.87–1.57) and 0.97 (95% CI 0.70–1.34; aHR 0.98, 95% CI 0.70–1.36), respectively.
Discussion
In this large biracial cohort of participants free of CVD and enrolled in the REGARDS study, we found that proximate depressive symptoms were associated with a 26% increased risk of incident stroke and a 30% increased risk of incident CVD death. Interestingly, depressive symptoms did not confer increased risk for the outcome of incident CHD. This is one of few large cohort studies to use multiple measurements of depressive symptoms and to more definitively clarify that race does not appear to moderate the relationship between depressive symptoms and CVD, which was shown inconsistently in the previous literature.
Our study is consistent with prior studies demonstrating that depressive symptoms confer an increased risk of stroke,2, 10, 24 including prior meta‐analyses (aHR 1.34, 95% CI 1.17–1.54)10, 25 and a prospective study of stroke risk in middle‐aged women with time‐varying covariates (adjusted odds ratio 1.94, 95% CI 1.37–2.74).26 We expanded on prior literature by accounting for factors such as atrial fibrillation and left ventricular hypertrophy as well as behavioral factors such as medication adherence and physical activity, which were rarely included in prior studies.2, 10 In addition, few studies simultaneously examined the association between depressive symptoms and both stroke and CHD among participants free from underlying CVD. A prior European study of predominantly white men did not adjust for behavioral risk factors or explore physiological factors and found that baseline depressive symptoms increased the risk of stroke over 0 to 5 years only (aHR 1.60, 95% CI 1.1–2.3), whereas only cumulative depressive symptoms led to CHD incidence (1–2 times per case: aHR 1.12, 95% CI 0.7–1.7; 3–4 times: aHR 2.06, 95% CI 1.2–3.7).27 Another study, also conducted in Europe, found that baseline depressive symptoms conferred an increased risk of CHD (aHR 1.47, 95% CI 1.08–1.99) but not stroke (aHR 0.87, 95% CI 0.57–1.32) in a predominantly young (aged 20–55 years) nondiverse group of women.7 Our study of diverse middle‐aged to older participants living in the continental United States adds to the literature by suggesting that elevated depressive symptoms may be an independent proximal risk factor for stroke and CVD death but not for CHD, even after controlling for traditional, behavioral, and other physiological explanatory factors.
Depressive symptoms have been shown to be associated with smoking,28 medication noncompliance,29 and physical inactivity30 as well as diabetes,31 obesity,32 hypertension,33 and inflammation.34 We added to the literature by demonstrating that these factors and others such as antidepressant use35 and corrected QT interval36 do not appear to fully explain the association between depressive symptoms and incident CVD death and stroke in otherwise healthy persons. Other unmeasured mediating factors such as heart rate variability, platelet activation, neurohormonal activation, and endothelial interaction may play a greater role and explain the proximate effects on CVD death.37
Our study supports prior literature showing a proximal association between depressive symptoms and stroke, which has not been shown to be associated with lifetime or prior history of depressive symptoms,9 and highlights the urgent need for early intervention in patients with depressive symptoms. A recent study showed that collaborative depression care in the primary care setting might lead to decreased risk of incident CVD events in older patients38; however, depressive symptoms continue to be suboptimally recognized, diagnosed, and treated in primary care.39, 40 Further research is needed to examine the moderating effect of depression treatment on incident CVD and to improve treatment rates in primary care settings.
We did not find an association between depressive symptoms and our composite outcome of probable myocardial infarction or fatal CHD, although the directionality is concordant with prior literature.2, 3, 6, 41 Nonetheless, time‐dependent analyses may afford more robust findings (aHR 1.15) compared with the previously published null relationship between baseline depressive symptoms and incident CHD in the REGARDS cohort (aHR 0.99, 95% CI 0.66–1.48).42 Prior research has suggested that cumulative depressive symptoms over ≥2 occasions, although subject to selection bias, are better associated with increased risk of CHD as a dose‐response effect than baseline or proximate symptoms.27 Nonetheless, depressive symptoms may be a better marker of CHD prognosis in patients with existing heart disease than of CHD incidence.43
Racial disparities exist for depressive symptom severity, recognition, and treatment15, 44, 45 and CVD outcomes.46 Nevertheless, we found that race did not appear to moderate the association between depressive symptoms and CVD. REGARDS is a large biracial prospective cohort with expert adjudication of outcomes, and that may explain why we were able to better elucidate the potential moderating effect of race shown in prior studies.11, 12, 13, 14 In addition, prior research has suggested that income may mediate the relationship between race and CVD outcomes47 and that stress confers increased CVD events among low‐income persons.48 In our analyses, income did not appear to fully explain the relationship between depressive symptoms and CVD for either black or white participants. Further research is needed to better define the relationship among race, income, depression, and CVD. Nonetheless, we demonstrated that early depressive symptom recognition and treatment should be emphasized, regardless of race.
Our study had several limitations. We were unable to assess other time‐varying covariates, limiting conclusions regarding mediating effects of behavioral and physiological conditions. In addition, the use of self‐reported covariates may have led to misclassifcation and reporting biases, which may have overestimated our results. In addition, our findings for this biracial US cohort may not be generalizable to other races and nationalities. We were unable to confirm whether depressive symptoms were simply a marker of preclinical CVD, which in turn contributed to CVD death and stroke.27 Finally, we used the short CES‐D measurement for depressive symptoms, but it does not fully measure the breadth of cognitive and somatic symptoms of depression. Research has shown that cognitive symptoms are better recognized by providers but are less likely to predict mortality than somatic symptoms.49 This may have contributed to the nonsignificant association between depressive symptoms and CHD, although the 4‐item CES‐D has been shown to have adequate sensitivity and specificity compared with depressive symptoms on the 20‐item CES‐D and has been used to assess depressive symptoms in prior CVD studies.
In conclusion, time‐varying depressive symptoms were independently associated with incident stroke and CVD death but not with incident CHD in a large cohort of black and white adults living in the continental United States, with no indication that associations were moderated by race. These findings lend support to the belief that depressive symptomatology is an early modifiable risk factor for CVD. Improving depressive symptom screening and treatment has the potential to reduce the burden of incident CVD in the United States.
Author Contributions
Drs Khodneva and Richman had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Moise, Khodneva, Safford; Acquisition of data: Khodneva, Safford; Analysis and interpretation of data: Khodneva, Moise, Richman, Safford; Drafting of the manuscript: Moise, Khodneva Critical revision of manuscript for important intellectual content: Moise, Khodneva, Richman, Shimbo, Kronish, Safford; Statistical analysis: Khodneva; Obtained funding: Safford; Study supervision: Safford.
Sources of Funding
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The REGARDS study was supported by NIH grant 2U01NS041588; REGARDS‐MI study was supported by NIH grants R01 HL080477 and K24 HL111154. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. This work was also supported by funds from National Heart Lung and Blood Institute (3 R01 HL114924‐03S1; HL080477; and K24 HL111154).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003767 doi: 10.1161/JAHA.116.003767)
References
- 1. Glymour MM, Maselko J, Gilman SE, Patton KK, Avendano M. Depressive symptoms predict incident stroke independently of memory impairments. Neurology. 2010;75:2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. [DOI] [PubMed] [Google Scholar]
- 3. Rugulies R. Depression as a predictor for coronary heart disease: a review and meta‐analysis. Am J Prev Med. 2002;23:51–61. [DOI] [PubMed] [Google Scholar]
- 4. Glassman AH, Maj M, Sartorius N. Depression and Heart Disease. Chichester, West Sussex: John Wiley & Sons; 2011. [Google Scholar]
- 5. Ariyo AA, Haan M, Tangen CM, Rutledge JC, Cushman M, Dobs A, Furberg CD. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–1779. [DOI] [PubMed] [Google Scholar]
- 6. Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. [DOI] [PubMed] [Google Scholar]
- 7. Nabi H, Kivimaki M, Suominen S, Koskenvuo M, Singh‐Manoux A, Vahtera J. Does depression predict coronary heart disease and cerebrovascular disease equally well? The Health and Social Support Prospective Cohort Study. Int J Epidemiol. 2010;39:1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salaycik KJ, Kelly‐Hayes M, Beiser A, Nguyen A‐H, Brady SM, Kase CS, Wolf PA. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38:16–21. [DOI] [PubMed] [Google Scholar]
- 9. Surtees PG, Wainwright NWJ, Luben RN, Wareham NJ, Bingham SA, Khaw K‐T. Psychological distress, major depressive disorder, and risk of stroke. Neurology. 2008;70:788–794. [DOI] [PubMed] [Google Scholar]
- 10. Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta‐analysis and systematic review. JAMA. 2011;306:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capistrant BD, Gilsanz P, Moon JR, Kosheleva A, Patton KK, Glymour MM. Does the association between depressive symptoms and cardiovascular mortality risk vary by race? Evidence from the Health and Retirement Study. Ethn Dis. 2013;23:155–160. [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis TT, Guo H, Lunos S, Mendes de Leon CF, Skarupski KA, Evans DA, Everson‐Rose SA. Depressive symptoms and cardiovascular mortality in older black and white adults: evidence for a differential association by race. Circ Cardiovasc Qual Outcomes. 2011;4:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463–471. [DOI] [PubMed] [Google Scholar]
- 14. Glymour MM, Yen JJ, Kosheleva A, Moon JR, Capistrant BD, Patton KK. Elevated depressive symptoms and incident stroke in Hispanic, African‐American, and White older Americans. J Behav Med. 2012;35:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alegria M, Chatterji P, Wells K, Cao Z, Chen CN, Takeuchi D, Jackson J, Meng XL. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Serv. 2008;59:1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 17. Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke‐Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. [DOI] [PubMed] [Google Scholar]
- 18. Kasner SE, Cucchiara BL, McGarvey ML, Luciano JM, Liebeskind DS, Chalela JA. Modified National Institutes of Health Stroke Scale can be estimated from medical records. Stroke. 2003;34:568–570. [DOI] [PubMed] [Google Scholar]
- 19. Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy S, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall‐Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. [DOI] [PubMed] [Google Scholar]
- 20. Zhang ZM, Prineas RJ, Eaton CB. Evaluation and comparison of the Minnesota Code and Novacode for electrocardiographic Q‐ST wave abnormalities for the independent prediction of incident coronary heart disease and total mortality (from the Women's Health Initiative). Am J Cardiol. 2010;106:18–25.e12. [DOI] [PubMed] [Google Scholar]
- 21. Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53:1117–1125. [Google Scholar]
- 22. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 24. Penninx BH, Beekman AF, Honig A, Deeg DJ, Schoenvers RA, van Eijk JT, Van Tilburg W. Depression and cardiac mortality: results from a community‐based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. [DOI] [PubMed] [Google Scholar]
- 25. Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta‐analysis of prospective studies. Stroke. 2012;43:32–37. [DOI] [PubMed] [Google Scholar]
- 26. Jackson CA, Mishra GD. Depression and risk of stroke in midaged women: a prospective longitudinal study. Stroke. 2013;44:1555–1560. [DOI] [PubMed] [Google Scholar]
- 27. Brunner EJ, Shipley MJ, Britton AR, Stansfeld SA, Heuschmann PU, Rudd AG, Wolfe CD, Singh‐Manoux A, Kivimaki M. Depressive disorder, coronary heart disease, and stroke: dose‐response and reverse causation effects in the Whitehall II cohort study. Eur J Prev Cardiol. 2014;21:340–346. [DOI] [PubMed] [Google Scholar]
- 28. Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- 29. Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA. Depression and diabetes treatment nonadherence: a meta‐analysis. Diabetes Care. 2008;31:2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134:220–231. [DOI] [PubMed] [Google Scholar]
- 31. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta‐analytic review of the literature. Diabetes Care. 2000;23:934–942. [DOI] [PubMed] [Google Scholar]
- 32. Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta‐analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. [DOI] [PubMed] [Google Scholar]
- 33. Nabi H, Chastang JF, Lefevre T, Dugravot A, Melchior M, Marmot MG, Shipley MJ, Kivimaki M, Singh‐Manoux A. Trajectories of depressive episodes and hypertension over 24 years: the Whitehall II prospective cohort study. Hypertension. 2011;57:710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Empana JP, Sykes DH, Luc G, Juhan‐Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation. 2005;111:2299–2305. [DOI] [PubMed] [Google Scholar]
- 35. Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, Robinson JG, Rosal MC, Wenger NK, Wassertheil‐Smoller S. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women's Health Initiative Study. Arch Intern Med. 2009;169:2128–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dekker JM, Crow RS, Hannan PJ, Schouten EG, Folsom AR. Heart rate‐corrected QT interval prolongation predicts risk of coronary heart disease in black and white middle‐aged men and women: the ARIC study. J Am Coll Cardiol. 2004;43:565–571. [DOI] [PubMed] [Google Scholar]
- 37. Burg MM, Edmondson D, Shimbo D, Shaffer J, Kronish IM, Whang W, Alcantara C, Schwartz JE, Muntner P, Davidson KW. The ‘perfect storm’ and acute coronary syndrome onset: do psychosocial factors play a role? Prog Cardiovasc Dis. 2013;55:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stewart JC, Perkins AJ, Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med. 2014;76:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simon GE, VonKorff M. Recognition, management, and outcomes of depression in primary care. Arch Fam Med. 1995;4:99–105. [DOI] [PubMed] [Google Scholar]
- 40. Katon W, von Korff M, Lin E, Bush T, Ormel J. Adequacy and duration of antidepressant treatment in primary care. Med Care. 1992;30:67–76. [DOI] [PubMed] [Google Scholar]
- 41. Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, Wang Y, Xu X, Yin X, Deng J, Li L, Cao S, Lu Z. Depression and the risk of coronary heart disease: a meta‐analysis of prospective cohort studies. BMC Psychiatry. 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sims M, Redmond N, Khodneva Y, Durant RW, Halanych J, Safford MM. Depressive symptoms are associated with incident coronary heart disease or revascularization among blacks but not among whites in the Reasons for Geographical and Racial Differences in Stroke study. Ann Epidemiol. 2015;25:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lichtman JH, Bigger JT Jr, Blumenthal JA, Frasure‐Smith N, Kaufmann PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. [DOI] [PubMed] [Google Scholar]
- 44. Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, Jackson JS. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non‐Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–315. [DOI] [PubMed] [Google Scholar]
- 45. Simpson SM, Krishnan LL, Kunik ME, Ruiz P. Racial disparities in diagnosis and treatment of depression: a literature review. Psychiatr Q. 2007;78:3–14. [DOI] [PubMed] [Google Scholar]
- 46. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Judd SE, Kissela BM, Kittner SF, Lackland DT, Lichtman JH, Lisabeth LD, Machkey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health. 2005;95:1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Redmond N, Richman J, Gamboa CM, Albert MA, Sims M, Durant RW, Glasser SP, Safford MM. Perceived stress is associated with incident coronary heart disease and all‐cause mortality in low but not high‐income participants in the Reasons for Geographic and Racial Differences in Stroke study. J Am Heart Assoc. 2013;2:e000447 doi: 10.1161/JAHA.113.000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, Vaccarino V, Chan PS. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]


