Abstract
Background
Results from observational and genetic epidemiological studies suggest that lower serum homocysteine levels are associated with lower incidence of cardiovascular disease (CVD). Numerous randomized controlled trials have investigated the efficacy of lowering homocysteine with folic acid supplementation for CVD risk, but conflicting results have been reported.
Methods and Results
Three bibliographic databases (Medline, Embase, and the Cochrane Database of Systematic Reviews) were searched from database inception until December 1, 2015. Of the 1933 references reviewed for eligibility, 30 randomized controlled trials involving 82 334 participants were included in the final analysis. The pooled relative risks of folic acid supplementation compared with controls were 0.90 (95% CI 0.84–0.96; P=0.002) for stroke, 1.04 (95% CI 0.99–1.09; P=0.16) for coronary heart disease, and 0.96 (95% CI 0.92–0.99; P=0.02) for overall CVD. The intervention effects for both stroke and combined CVD were more pronounced among participants with lower plasma folate levels at baseline (both P<0.02 for interaction). In stratified analyses, a greater beneficial effect for overall CVD was seen in trials among participants without preexisting CVD (P=0.006 for interaction) or in trials with larger reduction in homocysteine levels (P=0.009 for interaction).
Conclusions
Our meta‐analysis indicated a 10% lower risk of stroke and a 4% lower risk of overall CVD with folic acid supplementation. A greater benefit for CVD was observed among participants with lower plasma folate levels and without preexisting CVD and in studies with larger decreases in homocysteine levels. Folic acid supplementation had no significant effect on risk of coronary heart disease.
Keywords: cardiovascular disease prevention, folate, stroke prevention
Subject Categories: Cardiovascular Disease, Diet and Nutrition
Introduction
McCully postulated in 1969 that homocysteine affected atherosclerotic processes.1 Since that time, prospective observations and genetic studies have suggested a causal role of blood homocysteine in the development of cardiovascular disease (CVD).2, 3, 4, 5, 6 Observational studies indicated that for each 5‐μmol/L rise in serum homocysteine levels, there was a 32% increased risk of ischemic heart diseases and a 59% increased risk of stroke.4 The potential causal role of homocysteine in CVD was supported by Mendelian randomization studies using the gene encoding methylenetetrahydrofolate reductase (MTHFR), an enzyme involved in homocysteine metabolism, as an instrumental variable. Compared with people who were homozygous for the wild‐type allele (CC) of MTHFR, those who were homozygous for the mutant allele (TT) had 1.93‐μmol/L5 or 25%6 higher homocysteine concentrations, a 26% higher risk of stroke,5 and a 16% higher risk of coronary heart disease (CHD).6
Evidence from these studies provided the rationale for conducting randomized controlled trials (RCTs) of folic acid supplementation and CVD prevention, given that supplementation with folic acid is an inexpensive and effective method of lowering blood homocysteine concentrations.7, 8 The introduction in 1998 of mandatory fortification of enriched cereal grain products with folic acid in North America, with the original impetus to reduce the occurrence of neural tube birth defects,9 was associated with a significant reduction of the mean homocysteine concentration by ≈7% in middle‐aged and older adults.7 RCTs documented that daily dietary supplementation with 0.5 to 5 mg folic acid reduced plasma homocysteine concentrations by ≈25%.8
The inconsistent results of the effects of folic acid supplementation on CVD risk called into question the causal relationship between increased homocysteine and CVD risk.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Several meta‐analyses of RCTs have been conducted to summarize the available evidence, but the pooled results have been inconclusive.20, 21, 22, 23 With the recent publications of several new RCTs,24, 25, 26 we performed a meta‐analysis of RCTs to quantify the relationship between folic acid supplementation and CVD risk.
Methods
Search Strategy
Medline, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Cochrane Database of Systematic Reviews were searched to identify eligible trials published from database inception until December 1, 2015, following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.27, 28 The computer‐based searches combined terms related to the exposure (eg, folic acid, folate) and outcomes (eg, CVD, stroke, CHD), without any language restriction. The definitions of CVD, stroke, and CHD were somewhat heterogeneous among trials, but all trials reported hard clinical end points. We also conducted a manual search for unpublished results of ongoing trials, presentations at significant scientific meetings, and the references listed in the identified publications. Two investigators (Y.L. and T.H.) independently screened abstracts. Discrepancies in eligibility for inclusion were resolved by discussions among investigators.
Selection Criteria
Studies that met the following criteria were included: (1) The study was an RCT; (2) the study reported ≥1 hard disease end point of CVD, CHD, or stroke; (3) the number of events for CVD, CHD, or stroke that occurred during the studies was reported for both intervention and control groups; and (4) the intervention consisted of folic acid supplementation (with or without additional B vitamins).
Patient Involvement
No patients were involved in setting the research question or the outcome measures or in the design and implementation of the study. There are no plans to involve patients in dissemination.
Data Extraction, Synthesis, and Methodological Quality Assessment
According to the standard protocol, all data from eligible trials were independently extracted in duplicate by 2 investigators (Y.L. and T.H.) and reviewed by a third investigator (Y.Z.). A predesigned data extraction form was used to extract relevant information. This included information on study design, baseline characteristics of participants, supplementation strategies, intervention effects on homocysteine and outcomes, mean age of participants at baseline (median if mean value not available), sex proportion, plasma folate and homocysteine levels at baseline, net changes and percentage changes of homocysteine before and after folic acid supplementation, the dosage of folic acid supplementation, intervention with and without other B vitamins, types of controls (placebo, usual care, untreated, or low dose of B vitamins), study location (countries) and mandatory folic acid fortification in the study location, preexisting disease status at baseline, the names of RCTs if available, and any systematically recorded outcomes that occurred during the scheduled treatment period (eg, events in intervention and control groups and reported hazard ratios).
The risk of biases for each RCT (selection, performance, detection, attrition, reporting, and other biases) was assessed using the Cochrane risk‐of‐bias assessment tool29; 7 criteria were evaluated. The risk of bias was rated as low, high, or unclear for each criterion.
For each selected trial, net change in mean homocysteine was calculated as the change in mean homocysteine level from baseline to postintervention in the intervention group minus that change in the control group. Percentage change was defined as the net change divided by the mean baseline homocysteine level averaged across groups. If information on homocysteine was available only for the treatment group,13, 30 net change was calculated as preintervention concentrations minus postintervention concentrations in the treatment group. For RCTs of factorial design,13, 14, 31 participants receiving folic acid supplementation were compared with the placebo group if data on all factors were available in the publications13, 31; otherwise, all trial participants receiving folic acid supplementation were compared with all participants not receiving it regardless of other factorial interventions.14 For trials with multiple publications32, 33 at different follow‐up periods, results for clinical outcomes with the longest follow‐up from the primary publication were extracted.33
Multivariate‐adjusted relative risks (RRs) assessing the effects of folic acid supplementation on the risk of CVD, CHD, and/or stroke were extracted from each RCT if they were reported in the publications; otherwise, we calculated the RRs based on the number of events in each group. CHD events included nonfatal myocardial infarction and fatal coronary disease; stroke events included nonfatal and fatal stroke. The number of CVD events was obtained directly from the trial reports or as the composite of nonfatal myocardial infarction, nonfatal stroke, and vascular death.
Meta‐Analysis
We assessed the overall effect of folic acid supplementation on the risk of CVD, CHD, or stroke. RRs and corresponding standard errors were logarithmically transformed to stabilize variance and normalize the distribution. The inverse variance weighted method was used to combine summary measures using random‐effects models to minimize the effects of between‐study heterogeneity. Fixed‐effect models were used in subsidiary analyses.
We conducted stratified analyses by a number of factors, including duration of folic acid supplementation (<3 or ≥3 years), the magnitude of decrease in homocysteine concentration (<20%, 20–29.9%, or ≥30%), mandatory folic acid grain fortification (yes, partially, or no), folic acid with or without vitamin B6 or B12 (yes or no), control group with or without low‐dose B vitamin (yes or no), preexisting renal disease status (yes or no), and CVD status (yes or no). For the stratified analysis by existing renal disease, we extracted data from the renal HOPE‐2 study,34 which was a post hoc analysis of a subgroup of participants who had renal diseases at baseline of the HOPE‐2 trial.17
The P value of the Cochrane Q statistic and I2 index was used to evaluate between‐study heterogeneity.35 The potential for publication bias was evaluated by using Egger and Begg tests with funnel plots of the natural log of the RR versus its standard error.36 Sensitivity analyses were done to assess the influence of each individual trial by omitting the trial that had the largest effect on the overall result one by one. All analyses were conducted using Stata 10 software (StataCorp LP). A 2‐tailed P value of <0.05 was considered statistically significant.
Results
The initial literature search identified 1933 abstracts (Figure 1). After screening based on titles and abstracts, 74 articles were selected for detailed evaluation of their full texts. Of those, 30 RCTs met our inclusion criteria and were included in our analysis. Thirteen RCTs were conducted in European countries (United Kingdom, Norway, Netherlands, Italy, Germany, Switzerland, and France), 9 were conducted in the Americas (United States, Canada, and Brazil), 4 were conducted in Asia (China, India, and the Philippines), and 1 was conducted in Australia and New Zealand. Another 3 RCTs were conducted in multiple countries across continents, including the Vitamin Intervention for Stroke Prevention (VISP)19 in 3 countries across 2 continents, the 2 Heart Outcomes Prevention Evaluation (HOPE)17 studies in 13 countries across 3 continents, and the VITAmins TO Prevent Stroke trial (VITATOPS)37 in 20 countries across 4 continents (Tables 1 and 2).37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51
Figure 1.
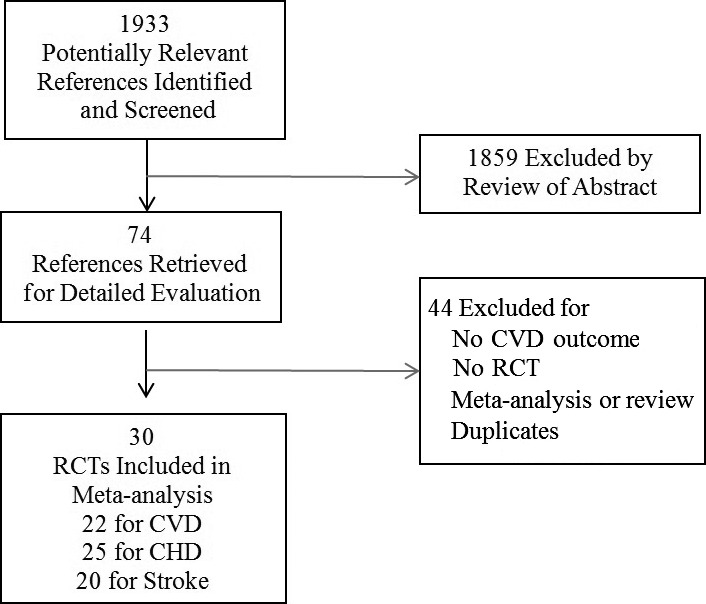
Flow diagram of study selection process. CHD indicates coronary heart disease; CVD, cardiovascular disease; RCT, randomized controlled trial.
Table 1.
Characteristics of Participants in the 30 Randomized Controlled Trials of FA Supplementation
| Sources | N | Treatment, y | Age, y | Male, % | Folate, nmol/L | Homocysteine (μmol/L) | FA Dosage, mg/day | FA Plus Vitamin Bc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Net Changesa | % | ||||||||
| Mark, 199638 | 3318 | 6.0 | 54 | 44 | NR | NR | NR | NR | 0.8 | Yes |
| Baker, 200239 | 1882 | 1.7 | NR | NR | 14.5 | 11.2 | −1.5b | −13.4b | 5 | No |
| Schnyder, 200218 | 553 | 1.0 | 63 | 81 | NR | 11.2 | −2.9 | −25.7 | 1 | Yes |
| Righetti, 200340 | 81 | 1.0 | 64 | 56 | 6.1 | 50.3 | −26.0d | −51.7 | 5/15 | No |
| Lange, 200441 | 636 | 0.7 | 61 | 77 | NR | 12.6 | −3.6 | −28.6 | 1.2 | Yes |
| Liem, 200442 | 283 | 1.0 | 59 | 69 | NR | NR | NR | NR | 5 | No |
| Toole, 200419 | 3680 | 2.0 | 66 | 63 | 26.0d | 13.4 | −2.3 | −17.2 | 2.5 | Yes |
| Wrone, 200443 | 510 | 2.0 | 60 | 50 | 47.4 | 32.9 | −3.6 | −10.9 | 5/15 | Yes |
| Liem, 200532, 33 | 593 | 3.5 | 65 | 78 | 16.0 | 12.1 | −2.6 | −21.5 | 0.5 | No |
| Bonaa, 200631 | 2815e | 3.3 | 63 | 74 | 11.5 | 13.1 | −3.8 | −27.5 | 0.8 | No |
| Lonn, 200617 | 5522 | 5.0 | 69 | 72 | 27.4 | 12.2 | −3.2 | −26.2 | 2.5 | Yes |
| Righetti, 200644 | 88 | 2.4 | 64 | 55 | 15.6 | 34.6 | −15.1 | −43.6 | 2.5/5 | Yes |
| Zoungas, 200645 | 315 | 3.6 | 56 | 68 | NR | 27.0 | −4.7 | −17.4 | 15 | No |
| Cole, 200746 | 1021 | 7.0 | 57 | 64 | 23.8 | 9.8 | NR | NR | 1 | No |
| Jamison, 200716 | 2056 | 3.2 | 66 | 98 | 15.6 | 24.1 | −5.9 | −24.5 | 40 | Yes |
| Vianna, 200730 | 186 | 2.0 | 49 | 59 | 10.0 | 23.5 | −13.0b | −55.3b | 4.29 | No |
| Albert, 200810 | 5442 | 7.3 | 63 | 0 | NR | 12.3f | −2.3 | −18.5 | 2.5 | Yes |
| Ebbing, 200813 | 2324e | 3.2 | 62 | 80 | 10.0d | 10.8 | −3.2b | −29.6b | 0.8 | Yes |
| Potena, 200847 | 51 | 1.0 | 54 | 84 | NR | 17.9 | NR | NR | 15 | No |
| Hodis, 200948 | 506 | 3.1 | 61 | 61 | 21.4 | 9.7 | −2.1 | −21.6 | 5 | Yes |
| Imasa, 200949 | 240 | 0.5 | 59 | 58 | NR | 12.8 | NR | NR | 1 | Yes |
| Armitage, 201011 | 12 064 | 6.7 | 64 | 83 | 16.8 | 13.5 | −3.8 | −28.0 | 2 | Yes |
| Galan, 201014 | 2501 | 4.7 | 61 | 79 | 15.2 | 12.8 | −2.9 | −22.7 | 0.56g | Yes |
| Heinz, 201050 | 650 | 2.1 | 61 | 58 | 14.1 | 29.0 | −8.6 | −30.0 | 2.1 | Yes |
| House, 201051 | 238 | 2.7 | 60 | 75 | 35.1 | 15.5 | −4.8 | −31.0 | 2.5 | Yes |
| VITATOPS, 201037 | 8164 | 3.4 | 63 | 64 | NR | 14.3 | −4.0 | −30.0 | 2.0 | Yes |
| Bostom, 201112 | 4110 | 4.0 | 52 | 63 | NR | 16.4 | −4.4 | −26.8 | 5 | Yes |
| Lamas, 201324 | 1708 | 4.6 | 65 | 82 | NR | NR | NR | NR | 0.8 | Yes |
| Sharma, 201325 | 100 | 0.5 | 49 | 66 | NR | 31.0 | −17.9 | −57.7 | 2.5 | Yes |
| Huo, 201526 | 20 702 | 4.5 | 60 | 41 | 8.1 | 12.5 | NR | NR | 0.8 | No |
FA indicates folic acid; NR, not reported.
Net change indicates change in treatment group (preintervention minus postintervention) minus change in control group (preintervention minus postintervention).
If information was available only for intervention, net change denotes preintervention minus postintervention.
FA plus vitamin B indicates FA supplementation with vitamin B6 or B12.
Value was estimated from a graph.
Combined 2 factors with FA vs placebo control: 1 factor with vitamin B6 only was not included.
Baseline homocysteine was estimated based on net homocysteine changes and percentage of homocysteine changes.
Used 5‐methyltetrahydrofolate.
Table 2.
Characteristics of the 30 Randomized Controlled Trials of FA Supplementation
| Sources | Countries | FA Fortification | Preexisting Disease | Control | Name of RCT |
|---|---|---|---|---|---|
| Mark, 199638 | China | No | Esophageal dysplasia | Placebo | Linxian Trial |
| Baker, 200239 | UK | No | CHD | Placebo | CHAOS |
| Schnyder, 200218 | Switzerland | No | CHD | Placebo | Swiss Heart Study |
| Righetti, 200340 | Italy | No | ESRD | Usual care | — |
| Lange, 200441 | Germany, Netherlands | No | CHD | Placebo | — |
| Liem, 200442 | Netherlands | No | CHD | Usual care | FOLARDA |
| Toole, 200419 | USA, Canada, Scotland | Partial | Stroke | Low‐dose vitamin B | VISP |
| Wrone, 200443 | USA | Yes | ESRD | Low‐dose vitamin B | — |
| Liem, 200532, 33 | Netherlands | No | CHD | Usual care | Goes extension study |
| Bonaa, 200631 | Norway | No | MI | Placebo | NORVIT |
| Lonn, 200617 | 13 countries | Partial | CHDa | Placebo | HOPE‐2 |
| Righetti, 200644 | Italy | No | ESRD | Usual care | — |
| Zoungas, 200645 | Australia, New Zealand | Partial | ESRD | Placebo | ASFAST |
| Cole, 200746 | USA, Canada | Yes | Adenomas | Placebo | Polyp Prevention Study |
| Jamison, 200716 | USA | Yes | ESRD | Placebo | HOST |
| Vianna, 200730 | Brazil | No | ESRD | Placebo | — |
| Albert, 200810 | USA | Yes | CVDb | Placebo | WAFACS |
| Ebbing, 200813 | Norway | No | CVD | Placebo | WENBIT |
| Potena, 200847 | Italy | No | Heart transplant | Placebo | — |
| Hodis, 200948 | USA | Yes | Atherosclerosis | Placebo | BVAIT Research |
| Imasa, 200949 | Philippines | No | CHD | Placebo | — |
| Armitage, 201011 | UK | No | CHD | Placebo | SEARCH |
| Galan, 201014 | France | No | CVD | Placebo | SU.FOL.OM3 |
| Heinz, 201050 | Germany | No | ESRD | Low‐dose vitamin B | — |
| House, 201051 | Canada | Yes | Nephropathy | Placebo | DIVINe |
| VITATOPS, 201037 | 20 countries | Partial | Stroke | Placebo | VITATOPS |
| Bostom, 201112 | USA, Canada, Brazil | Yes | CKD | Low‐dose vitamin B | FAVORIT |
| Lamas, 201324 | USA/Canada | Yes | CHD | Placebo | TACT |
| Sharma, 201325 | India | No | CKD | Placebo | — |
| Huo, 201526 | China | NO | Hypertension | Usual care | CSPPT |
ASFAST indicates Atherosclerosis and Folic Acid Supplementation Trial; BVAIT, B‐Vitamin Atherosclerosis Intervention Trial; CHAOS, Cambridge Heart Antioxidant Study; CHD, coronary heart disease; CKD, chronic kidney disease; CSPPT, China Stroke Primary Prevention Trial; CVD, cardiovascular disease; DIVINe, Diabetic Intervention with Vitamins to Improve Nephropathy; ESRD, end‐stage renal disease; FA, folic acid; FAVORIT, Folic Acid for Vascular Outcome Reduction in Transplantation; FOLARDA, FOLic Acid on Risk Diminishment after Acute myocardial infarction; HOPE‐2, The Heart Outcomes Prevention Evaluation; HOST, Homocysteinemia in Kidney and End Stage Renal Disease; MI, myocardial infarction; NORVIT, Norwegian Vitamin Trial; SEARCH, Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; SU.FOL.OM3, Supplementation en Folates et Omega‐3 trial; TACT, Trial to Assess Chelation Therapy; VISP, Vitamin Intervention for Stroke Prevention; VITATOPS, The VITAmins TO Prevent Stroke trial; WAFACS, Women's Antioxidant and Folic Acid Cardiovascular Study; WENBIT, Western Norway B Vitamin Intervention Trial.
With a history of vascular disease (coronary, cerebrovascular, or peripheral vascular) or diabetes and additional risk factors for atherosclerosis.
With a reported history of CVD or at least 3 cardiac risk factors (hypertension, high cholesterol, diabetes mellitus, parental history of premature MI, obesity, and current cigarette use).
Characteristics of the Selected RCTs
The selected 30 RCTs enrolled 82 334 participants with a mean age of 50 years, and 53% were male. The average folic acid supplementation duration was 3.2 years (<2 years in 7 trials, 2–4 years in 18 trials, and ≥5 years in 5 trials). The dosage of folic acid in the intervention groups ranged from 0.5 to 15 mg/day except in 1 RCT16 among patients with end‐stage renal disease with a dosage of 40 mg/day.
All 30 trials included participants with preexisting conditions (Tables 1 and 2). Sixteen trials enrolled participants with prior CVD, 10 trials included participants with renal diseases (chronic kidney disease, end‐stage renal disease, or diabetes nephropathy), 1 included participants with hypertension,26 1 included participants with atherosclerosis,48 1 included participants with esophageal dysplasia,38 and 1 included participants with history of colorectal adenomas.46 Eighteen trials were conducted in nonfortified regions, 4 were conducted in partly fortified regions, and 8 were conducted in mandatorily fortified populations. Twenty trials applied folic acid supplementation only, and 10 trials applied folic acid supplementation in combination with vitamin B6 and/or B12. The control groups in 4 trials were treated with low‐dose vitamin B, whereas another 26 trials used placebo or usual care for participants in the control group.
In considering the form of supplementation, only the SU.FOL.OM3 trial14 from France used 5‐methyltetrahydrofolate, and all others used folic acid. The change in homocysteine levels from the beginning to the end of the folic acid supplementation was reported in 23 trials (Table 1). All 23 trials showed a reduction in homocysteine levels, with net changes ranging from −1.5 to −26.0 μmol/L (percentage changes from −10.9% to 57.7%) (Table 1).
Effect of Folic Acid Supplementation on Risk of Stroke
In 20 trials, 3164 stroke events were reported among 77 816 participants (Figure 2). Across all 20 trials, the average incident rate for stroke was 3.8% (1509 events in 39 825 participants) in the folic acid supplementation group and 4.4% (1655 events in 37 911 participants) in the control group. Three individual studies observed a significantly reduced risk of stroke with supplementation of folic acid (Figure 2), including the HOPE‐2 trial17 conducted in 13 countries, the SU.FOL.OM3 trial14 conducted in France, and the CSPPT trial26 conducted in China. None of the 20 trials reported significant association between folic acid supplementation and increased risk of stroke (Figure 2).
Figure 2.
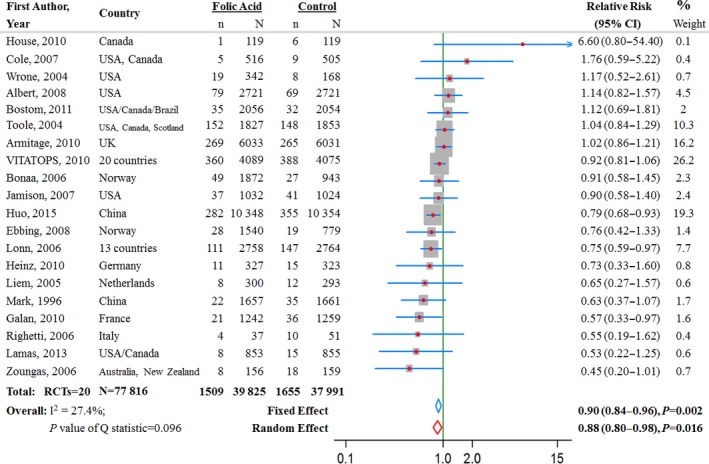
Relative risk estimates for stroke (folic acid supplementation vs control) by individual trials and pooled results. RCT indicates randomized controlled trial.
The pooled RR for the 20 trials on stroke comparing folic acid supplementation with controls was 0.90 (95% CI 0.84–0.96, P=0.002) from the fixed‐effect model and 0.88 (95% CI 0.80–0.98, P=0.02) from the random‐effects model (Figure 2.
Subgroup analyses revealed a more pronounced intervention effect among populations with lower baseline plasma folate levels (baseline folate <16 nmol/L in 7 RCTs: RR 0.79, 95% CI 0.69–0.89, P<0.0001; baseline folate ≥16 nmol/L in 7 RCTs: RR 0.97, 95% CI 0.86–1.08, P=0.57; P=0.02 for interaction) (Table 3). There was no evidence of heterogeneity in terms of significant intervention effects of folic acid supplementation on risk of stroke across other subgroups (all P>0.05 for interaction) (Table 3).
Table 3.
Pooled Relative Risk of Stroke, CHD, and CVD by Subgroups of RCTs Defined by Characteristics of Participants and Study Design
| Subgroups | No. of RCTs | No. of Cases | Relative Risk (95% CI), P Value | I2 | P for Heterogeneity | |
|---|---|---|---|---|---|---|
| Within Subgroup | Between Subgroups | |||||
| Stroke | ||||||
| Continent | ||||||
| North America | 6 | 297 | 1.06 (0.84–1.33), P=0.65 | 28 | 0.23 | 0.07 |
| European | 7 | 774 | 0.92 (0.80–1.06), P=0.25 | 10 | 0.35 | |
| Multicontinent | 4 | 1373 | 0.92 (0.83–1.02), P=0.12 | 33 | 0.21 | |
| Asia | 2 | 694 | 0.78 (0.67–0.90), P=0.001 | 0 | 0.42 | |
| Australia | 1 | 26 | 0.45 (0.20–1.01), P=0.05 | |||
| Grain fortification | ||||||
| No | 9 | 1468 | 0.85 (0.77–0.94), P=0.002 | 19 | 0.27 | 0.15 |
| Partial | 4 | 1332 | 0.90 (0.82–1.00), P=0.05 | 55 | 0.08 | |
| Yes | 7 | 364 | 1.07 (0.87–1.32), P=0.54 | 14 | 0.32 | |
| Preexisting renal disease | ||||||
| No | 12 | 2661 | 0.91 (0.84–0.98), P=0.01 | 34 | 0.12 | 0.32 |
| Yes | 8 | 406 | 1.01 (0.83–1.23), P=0.91 | 34 | 0.16 | |
| Preexisting strokea | ||||||
| No | 10 | 953 | 0.81 (0.72–0.93), P=0.002 | 25 | 0.21 | 0.09 |
| Yes | 10 | 2211 | 0.93 (0.86–1.01), P=0.08 | 28 | 0.19 | |
| Mean age at baseline, y | ||||||
| ≤60 | 7 | 835 | 0.81 (0.71–0.93), P=0.003 | 47 | 0.08 | 0.12 |
| ≥61 | 13 | 2329 | 0.92 (0.85–1.00), P=0.049 | 13 | 0.32 | |
| Intervention regimens | ||||||
| Folic acid only | 5 | 773 | 0.79 (0.69–0.92), P=0.001 | 11 | 0.35 | 0.06 |
| Plus vitamin B6/B12 | 15 | 2391 | 0.93 (0.86–1.00), P=0.06 | 28 | 0.15 | |
| Control regimens | ||||||
| Placebo or usual care | 16 | 2744 | 0.87 (0.81–0.94), P<0.001 | 36 | 0.07 | 0.09 |
| Low‐dose vitamin B | 4 | 420 | 1.04 (0.86–1.25), P=0.70 | 0 | 0.82 | |
| Duration of treatment | ||||||
| <3 years | 5 | 374 | 1.02 (0.84–1.24), P=0.85 | 22 | 0.28 | 0.17 |
| ≥3 years | 15 | 2790 | 0.88 (0.82–0.95), P=0.001 | 31 | 0.12 | |
| Baseline folate level | ||||||
| <16 nmol/L | 7 | 935 | 0.79 (0.69–0.89), P<0.001 | 0 | 0.86 | 0.018 |
| ≥16 nmol/L | 7 | 1160 | 0.97 (0.86–1.08), P=0.57 | 41 | 0.12 | |
| Folic acid dosage | ||||||
| <2.5 mg/day | 11 | 2239 | 0.88 (0.81–0.95), P=0.002 | 23 | 0.23 | 0.36 |
| ≥2.5 mg/day | 9 | 925 | 0.94 (0.83–1.07), P=0.36 | 41 | 0.09 | |
| Baseline homocysteine | ||||||
| <15 μmol/L | 11 | 2839 | 0.90 (0.84–0.97), P=0.006 | 37 | 0.11 | 0.97 |
| ≥15 μmol/L | 7 | 245 | 0.90 (0.70–1.16), P=0.41 | 30 | 0.20 | |
| Homocysteine changes | ||||||
| <4 μmol/L | 9 | 1467 | 0.95 (0.85–1.05), P=0.28 | 28 | 0.20 | 0.73 |
| 4 to <5 μmol/L | 4 | 848 | 0.92 (0.81–1.05), P=0.22 | 57 | 0.07 | |
| ≥5 μmol/L | 3 | 118 | 0.81 (0.57–1.17), P=0.27 | 0 | 0.68 | |
| Homocysteine changes | ||||||
| <20% | 4 | 501 | 1.03 (0.87–1.23), P=0.71 | 33 | 0.21 | 0.39 |
| 20.0–29.9% | 8 | 1137 | 0.90 (0.80–1.01), P=0.07 | 18 | 0.29 | |
| ≥30% | 4 | 795 | 0.91 (0.80–1.04), P=0.18 | 34 | 0.21 | |
| Risk of bias29 | ||||||
| Low | 9 | 2552 | 0.90 (0.83–0.97), P=0.005 | 238 | 0.12 | 0.95 |
| Medium | 8 | 564 | 0.89 (0.76–1.05), P=0.17 | 40 | 0.11 | |
| High | 3 | 48 | 0.82 (0.46–1.46), P=0.50 | 26 | 0.26 | |
| CHD | ||||||
| Continent | ||||||
| North America | 6 | 1008 | 0.98 (0.87–1.11), P=0.76 | 3 | 0.40 | 0.50 |
| European | 11 | 1335 | 1.07 (0.999–1.14), P=0.05 | 8 | 0.37 | |
| Multicontinent | 4 | 3424 | 0.99 (0.89–1.10), P=0.46 | 0 | 0.90 | |
| Asia | 3 | 90 | 1.08 (0.73–1.61), P=0.70 | 0 | 0.80 | |
| Australia | 1 | 42 | 1.23 (0.70–2.17), P=0.47 | |||
| Grain fortification | ||||||
| No | 14 | 3514 | 1.07 (1.00–1.14), P=0.05 | 0 | 0.58 | 0.39 |
| Partial | 4 | 1201 | 0.99 (0.89–1.11), P=0.86 | 0 | 0.84 | |
| Yes | 7 | 1184 | 1.00 (0.89–1.12), P=0.93 | 0 | 0.48 | |
| Preexisting renal diseases | ||||||
| No | 16 | 4600 | 1.05 (1.00–1.11), P=0.07 | 0 | 0.59 | 0.47 |
| Yes | 9 | 716 | 0.99 (0.86–1.15), P=0.95 | 0 | 0.72 | |
| Preexisting CHDa | ||||||
| No | 10 | 680 | 1.00 (0.86–1.16), P=0.98 | 0 | 0.68 | 0.62 |
| Yes | 15 | 5219 | 1.04 (0.99–1.10), P=0.13 | 0 | 0.55 | |
| Mean age at baseline, y | ||||||
| ≤60 | 9 | 373 | 1.15 (0.94–1.40), P=0.17 | 0 | 0.91 | 0.28 |
| ≥61 | 15 | 5491 | 1.03 (0.94–1.40), P=0.33 | 0 | 0.60 | |
| Intervention regimens | ||||||
| Folic acid only | 7 | 689 | 1.13 (0.98–1.31), P=0.10 | 0 | 0.66 | 0.21 |
| Plus vitamin B6/B12 | 18 | 5210 | 1.02 (0.97–1.08), P=0.36 | 0 | 0.67 | |
| Control regimens | ||||||
| Placebo or usual care | 21 | 5434 | 1.04 (0.99–1.09), P=0.15 | 0 | 0.57 | 0.80 |
| Low‐dose vitamin B | 4 | 465 | 1.01 (0.85–1.21), P=0.88 | 0 | 0.75 | |
| Duration of treatment | ||||||
| <3 years | 11 | 537 | 1.07 (0.91–1.26), P=0.39 | 20 | 0.25 | 0.66 |
| ≥3 years | 14 | 5362 | 1.03 (0.98–1.09), P=0.23 | 0 | 0.91 | |
| Baseline folate level | ||||||
| <16 nmol/L | 8 | 1172 | 1.03 (0.92–1.15), P=0.64 | 7 | 0.38 | 0.89 |
| ≥16 nmol/L | 7 | 3429 | 1.04 (0.97–1.11), P=0.26 | 0 | 0.57 | |
| Folic acid dosage | ||||||
| <2.5 mg/day | 13 | 3786 | 1.06 (1.00–1.13), P=0.05 | 0 | 0.72 | 0.16 |
| ≥2.5 mg/day | 12 | 2113 | 0.99 (0.91–1.07), P=0.77 | 0 | 0.63 | |
| Baseline homocysteine | ||||||
| <15 μmol/L | 15 | 5153 | 1.05 (0.99–1.10), P=0.09 | 0 | 0.49 | 0.42 |
| ≥15 μmol/L | 8 | 609 | 0.98 (0.84–1.14), P=0.78 | 0 | 0.65 | |
| Homocysteine changes | ||||||
| <4 μmol/L | 12 | 4835 | 1.05 (0.99–1.10), P=0.11 | 11 | 0.33 | 0.19 |
| 4 to <5 μmol/L | 4 | 462 | 1.08 (0.90–1.30), P=0.38 | 0 | 0.69 | |
| ≥5 μmol/L | 4 | 366 | 0.87 (0.71–1.06), P=0.17 | 0 | 0.97 | |
| Homocysteine changes | ||||||
| <20% | 5 | 890 | 1.03 (0.90–1.17), P=0.69 | 15 | 0.32 | 0.94 |
| 20.0–29.9% | 10 | 4442 | 1.04 (0.98–1.10), P=0.17 | 14 | 0.31 | |
| ≥30% | 5 | 331 | 1.00 (0.82–1.24), P=0.97 | 0 | 0.71 | |
| Risk of bias29 | ||||||
| Low | 9 | 4679 | 1.04 (0.98–1.10), P=0.21 | 0 | 0.72 | 0.998 |
| Medium | 12 | 1104 | 1.04 (0.92–1.17), P=0.54 | 25 | 0.19 | |
| High | 4 | 116 | 1.05 (0.75–1.47), P=0.79 | 0 | 0.44 | |
| CVD | ||||||
| Continent | ||||||
| North America | 5 | 1080 | 0.91 (0.81–1.02), P=0.09 | 0 | 0.58 | 0.01 |
| European | 10 | 4420 | 1.01 (0.95–1.07), P=0.71 | 19 | 0.24 | |
| Multicontinent | 1 | 3413 | 0.94 (0.89–1.01), P=0.08 | 0 | 0.79 | |
| Asia | 1 | 729 | 0.80 (0.69–0.92), P=0.002 | |||
| Australia | 1 | 97 | 0.87 (0.58–1.31), P=0.51 | |||
| South America | 1 | 38 | 0.81 (0.46–1.43), P=0.47 | |||
| Grain fortification | ||||||
| No | 12 | 5187 | 0.92 (0.84–1.02), P=0.39 | 46 | 0.04 | 0.51 |
| Partial | 4 | 2963 | 0.93 (0.87–1.00), P=0.05 | 0 | 0.85 | |
| Yes | 6 | 1589 | 0.93 (0.85–1.03), P=0.15 | 0 | 0.61 | |
| Preexisting renal diseases | ||||||
| No | 13 | 7340 | 0.97 (0.93–1.01), P=0.15 | 35 | 0.10 | 0.38 |
| Yes | 9 | 1503 | 0.92 (0.84–1.02), P=0.104 | 8 | 0.37 | |
| Preexisting CVDa | ||||||
| No | 10 | 2082 | 0.86 (0.79–0.94), P<0.001 | 0 | 0.61 | 0.006 |
| Yes | 12 | 7657 | 0.98 (0.94–1.03), P=0.44 | 2 | 0.43 | |
| Mean age at baseline, y | ||||||
| ≤60 | 7 | 1544 | 0.89 (0.81–0.98), P=0.02 | 19 | 0.30 | 0.12 |
| ≥61 | 15 | 8195 | 0.97 (0.93–1.01), P=0.13 | 14 | 0.29 | |
| Intervention regimens | ||||||
| Folic acid only | 8 | 1627 | 0.90 (0.82–0.99), P=0.03 | 30 | 0.19 | 0.18 |
| Plus vitamin B6/B12 | 14 | 8112 | 0.97 (0.93–1.01), P=0.12 | 8 | 0.37 | |
| Control regimens | ||||||
| Placebo or usual care | 18 | 8465 | 0.95 (0.91–0.99), P=0.03 | 26 | 0.15 | 0.80 |
| Low‐dose vitamin B | 4 | 1274 | 0.97 (0.87–1.08), P=0.54 | 0 | 0.40 | |
| Duration of treatment | ||||||
| <3 years | 8 | 8808 | 0.92 (0.81–1.03), P=0.14 | 0 | 0.44 | 0.45 |
| ≥3 years | 14 | 931 | 0.96 (0.92–1.00), P=0.05 | 30 | 0.14 | |
| Baseline folate level | ||||||
| <16 nmol/L | 9 | 2316 | 0.90 (0.83–0.97), P=0.006 | 33 | 0.16 | 0.016 |
| ≥16 nmol/L | 6 | 4767 | 1.01 (0.95–1.07), P=0.78 | 0 | 0.59 | |
| Folic acid dosage | ||||||
| <2.5 mg/day | 9 | 6486 | 0.96 (0.92–1.01), P=0.12 | 56 | 0.02 | 0.57 |
| ≥2.5 mg/day | 13 | 3253 | 0.94 (0.88–1.00), P=0.07 | 0 | 0.84 | |
| Baseline homocysteine | ||||||
| <15 μmol/L | 11 | 8104 | 0.97 (0.93–1.01), P=0.16 | 35 | 0.12 | 0.15 |
| ≥15 μmol/L | 9 | 1338 | 0.89 (0.81–0.99), P=0.03 | 0 | 0.52 | |
| Homocysteine changes | ||||||
| <4 μmol/L | 10 | 6121 | 1.01 (0.96–1.06), P=0.67 | 0 | 0.80 | 0.009 |
| 4 to <5 μmol/L | 3 | 1938 | 0.93 (0.85–1.01), P=0.08 | 0 | 0.66 | |
| ≥5 μmol/L | 5 | 649 | 0.82 (0.71–0.94), P=0.005 | 0 | 0.93 | |
| Homocysteine changes | ||||||
| <20% | 4 | 1059 | 0.98 (0.87–1.10), P=0.67 | 0 | 0.72 | 0.05 |
| 20.0–29.9% | 9 | 6063 | 1.00 (0.95–1.05), P=0.93 | 0 | 0.55 | |
| ≥30% | 5 | 1059 | 0.88 (0.81–0.96), P=0.006 | 0 | 0.68 | |
| Risk of bias29 | ||||||
| Low | 9 | 7820 | 0.97 (0.93–1.02), P=0.20 | 48 | 0.05 | 0.18 |
| Medium | 8 | 1648 | 0.91 (0.93–1.02), P=0.05 | 0 | 0.73 | |
| High | 5 | 271 | 0.83 (0.68–1.02), P=0.08 | 0 | 0.58 | |
CHD indicates coronary heart disease; CVD, cardiovascular disease; RCT, randomized controlled trial.
RCTs that reported stroke, CHD, or CVD at baseline among some or all participants were classified as having preexisting stroke, CHD, or CVD, respectively. The RCT47 conducted among participants with heart transplant was classified as those with preexisting CHD/CVD, whereas the RCT48 among participants with subclinical atherosclerosis was classified as those without preexisting CVD.
Effect of Folic Acid Supplementation on Risk of CHD
In 25 trials, 5899 CHD events were reported among 78 192 participants (Figure 3). Across all 25 trials, the average incidence rate for CHD was 7.7% (3091 events in 40 004 participants) in the folic acid supplementation group and 7.4% (2808 events in 38 188 participants) in the control group. Only 1 individual study (Lange et al41) observed a significantly increased risk of CHD with supplementation of folic acid (Figure 3). The pooled RR for CHD comparing folic acid supplementation with the control group was 1.04 (95% CI 0.99–1.09, P=0.16) in both the fixed‐ and random‐effects models (Figure 3). The result did not differ significantly across subgroups (all P>0.15 for interaction) (Table 3).
Figure 3.
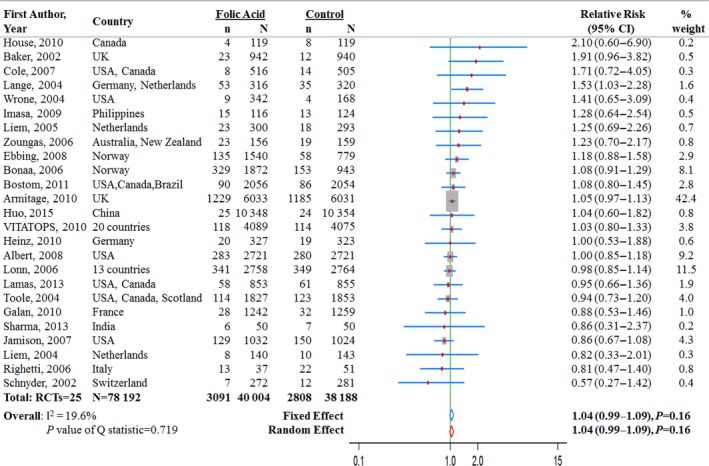
Relative risk estimates for coronary heart disease (folic acid supplementation vs control) by individual trials and pooled results. RCT indicates randomized controlled trial.
Effect of Folic Acid Supplementation on Risk of CVD
In 22 trials, 8739 CVD events were reported among 74 346 participants (Figure 4). Across all 22 trials, the average incident rate for CVD was 12.8% (4890 events in 38 097 participants) in the folic acid supplementation group and 13.4% (4849 events in 36 249 participants) in the control group. Two studies26, 37 observed a significantly reduced risk of CVD with supplementation of folic acid (Figure 4). One was the VITATOPS study,37 which was conducted in 20 countries, and the other was the CSPPT trial,26 which was conducted among 20 702 participants. The pooled RR for CVD comparing folic acid supplementation with control was 0.96 (95% CI 0.92–0.99, P=0.02) from the fixed‐effects model and 0.94 (95% CI 0.90–0.99, P=0.02) from the random‐effects model (Figure 4.
Figure 4.
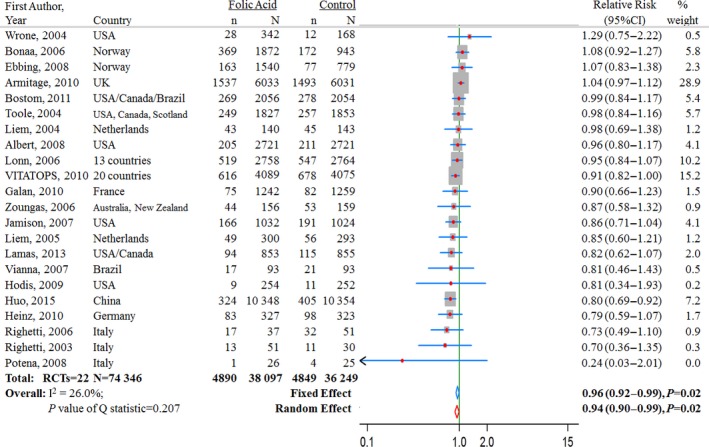
Relative risk estimates for cardiovascular diseases (folic acid supplementation vs control) by individual trials and pooled results. RCT indicates randomized controlled trial.
Among the 22 RCTs for CVD, 10 RCTs were conducted among patients without preexisting CVD, and 12 RCTs were conducted among patients with preexisting CVD. Subgroup analyses revealed a significant difference in the intervention effect between trials with and without preexisting CVD (without preexisting CVD in 10 RCTs: RR 0.86, 95% CI 0.79–0.94, P<0.0001; with preexisting CVD in 12 RCTs: RR 0.98, 95% CI 0.94–1.03, P=0.44; P=0.006 for interaction). In further stratification analysis of the 10 RCTs without preexisting CVD, folic acid supplementation significantly reduced the risk of CVD by 10% (8 RCTs: RR 0.90, 95% CI 0.81–0.99; P=0.035) among patients with renal diseases and by 20% (2 RCTs: RR 0.80, 95% CI 0.69–0.92; P=0.002) among participants with neither CVD nor renal disease. In our stratified analysis by preexisting renal disease shown in Table 3, we also included the result of the renal HOPE‐2 trial and the post hoc analysis of the HOPE‐2 trial among participants with preexisting renal disease (most also had preexisting CHD34); the pooled RR by further including this RCT was 0.99 (9 RCTs: RR 0.99, 95% CI 0.86–1.15, P=0.95).
Subgroup analyses revealed more pronounced intervention effects among populations with lower baseline folate levels (baseline folate <16 nmol/L in 9 RCTs: RR 0.90, 95% CI 0.83–0.97, P<0.0001; baseline folate ≥16 nmol/L in 6 RCTs: RR 1.01, 95% CI 0.95–1.07, P=0.78; P=0.016 for interaction) (Table 3). A larger magnitude of homocysteine reduction was associated with a greater reduction in the risk of CVD (change in homocysteine by 4 μmol/L in 10 RCTs: RR 1.01, 95% CI 0.96–1.06; P=0.67; by 4 to <5 μmol/L in 3 RCTs: RR 0.93, 95% CI 0.85–1.01; P=0.08; by ≥5 μmol/L in 5 RCTs: RR 0.82, 95% CI 0.71–0.94; P=0.005; P=0.009 for interaction). There was no significant difference in the intervention effect for other subgroups (Table 3); however, the benefits of folic acid supplementation for stroke and CVD appear to be stronger in Asian populations than in European or North American populations. We did not observe a significant difference of intervention effect according to the dosage of folic acid supplementation, as shown in Table 3. We also examined the intervention effect stratified by average baseline age of participants and duration of follow‐up but did not observe a clear pattern of intervention effect on CVD in the joint categories of these 2 variables (P=0.22 for interaction).
Assessment of Heterogeneity, Publication Bias, and Sensitivity Analysis
There was no evidence of between‐study heterogeneity in any of the analyses (Figures 2, 3, 4). Visual examination of Begg funnel plots for all analyses were moderately symmetrical, providing little evidence of publication bias. This was further supported by the results of the Egger test, which were nonsignificant in all analyses (all P>0.05) (Figure 5). We did not observe any difference of intervention effects between trials with different risks of bias (Tables 3 and 4).
Figure 5.
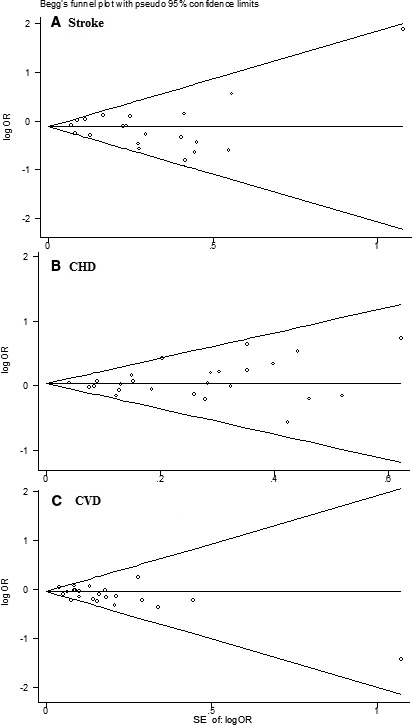
Funnel plot of data from the meta‐analysis of the effects of folic acid supplementation for preventing stroke (A), CHD (B) and CVD. CHD indicates coronary heart disease; CVD, cardiovascular disease; OR, odds ratio.
Table 4.
Methodological Quality Summary of Authors' Judgments About Each Methodological Item
| Sources | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Researchers | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias | Overall Risk of Biasa |
|---|---|---|---|---|---|---|---|---|
| Mark, 199638 | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | M |
| Baker, 200239 | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Unclear | M |
| Schnyder, 200218 | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | M |
| Righetti, 200340 | Yes | Unclear | No | No | Yes | Yes | Yes | H |
| Lange, 200441 | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | M |
| Liem, 200442 | Unclear | Unclear | No | Yes | Yes | Yes | Unclear | H |
| Toole, 200419 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | M |
| Wrone, 200443 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Liem, 200532, 33 | Yes | Unclear | No | Yes | Yes | Yes | Unclear | H |
| Bonaa, 200631 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Lonn, 200617 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Righetti, 200644 | Yes | Yes | No | Unclear | Yes | Yes | Yes | H |
| Zoungas, 200645 | Unclear | Unclear | Yes | Yes | Yes | Yes | Unclear | M |
| Cole, 200746 | Yes | Yes | Yes | Yes | No | No | Yes | H |
| Jamison, 200716 | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | M |
| Vianna, 200730 | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | M |
| Albert, 200810 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Ebbing, 200813 | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | M |
| Potena, 200847 | Yes | Unclear | No | Unclear | Yes | Yes | Unclear | H |
| Hodis, 200948 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | M |
| Imasa, 200949 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | M |
| Armitage, 201011 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Galan, 201014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Heinz, 201050 | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | M |
| House, 201051 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | M |
| VITATOPS, 201037 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Bostom, 201112 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| Lamas, 201324 | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | M |
| Sharma, 201325 | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Unclear | M |
| Huo, 201526 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
H indicates high; L, low; M, medium.
Please refer to the Cochrane risk‐of‐bias tool for definitions and judgments for each item. The response to each item is “yes” (low risk of bias), “no” (high risk of bias), or “unclear” (insufficient information).
In the sensitivity analysis, we did a metaregression analysis of the continuous variables that might potentially affect the treatment effects. We found no statistically significant dose‐response relationships between CVD outcomes and dosage of folic acid, baseline homocysteine levels, baseline mean age, and percentage of men in the trial, average follow‐up time, and number of events in the study, suggesting that none of these factors had a significant impact on the overall meta‐analysis (data not shown). We observed an inverse relation between degree of homocysteine reduction and intervention effect on CVD (Figure 6) with a significant dose‐response association (P=0.037 for metaregression after adjustment of baseline mean age and percentage of men in the trial). We did not, however, observe significant dose‐response associations between degree of homocysteine reduction and risk of stroke or CHD. The exclusion of any single study from the analyses did not appreciably change the summary RRs and between‐study heterogeneity (Table 5).
Figure 6.
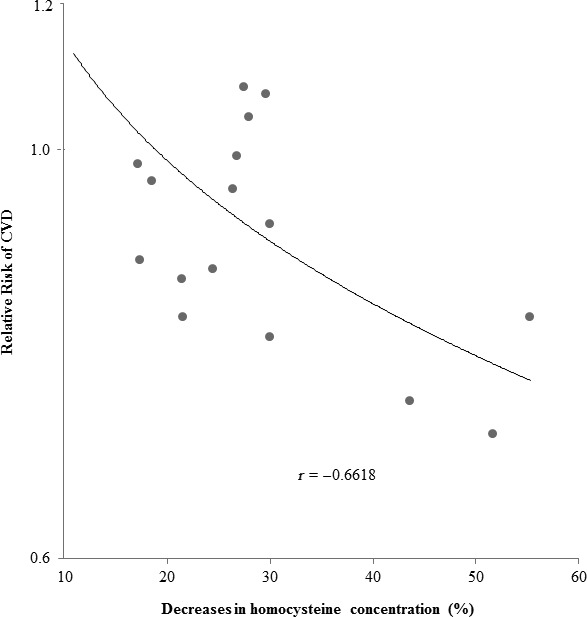
Relative risk of CVD in relation to percentage decreases in homocysteine concentration based on 16 trials with full records of homocysteine changes after the intervention. CVD indicates cardiovascular disease.
Table 5.
Sensitivity Analyses by Omitting 1 RCT Each Time
| The Omitted RCT | RR (95% CI), P S, I2, P H | ||
|---|---|---|---|
| Stroke | CHD | CVD | |
| Albert, 200810 | 0.89 (0.82–0.95), P S=0.001, I2=29, P H=0.12 | 1.04 (0.99–1.10), P S=0.14, I2=0, P H=0.68 | 0.96 (0.92–0.99), P S=0.02, I2=23, P H=0.17 |
| Armitage, 201011 | 0.87 (0.81–0.94), P S<0.001, I2=27, P H=0.13 | 1.03 (0.96–1.10), P S=0.43, I2=0, P H=0.68 | 0.92 (0.88–0.99), P S=0.001, I2=0, P H=0.56 |
| Baker, 200239 | — | 1.03 (0.98–1.09), P S=0.20, I2=0, P H=0.83 | — |
| Bonaa, 200631 | 0.89 (0.83–0.96), P S=0.002, I2=34, P H=0.07 | 1.03 (0.98–1.09), P S=0.22, I2=0, P H=0.68 | 0.95 (0.91–0.99), P S=0.009, I2=15, P H=0.26 |
| Bostom, 201112 | 0.89 (0.83–0.96), P S=0.001, I2=32, P H=0.09 | 1.04 (0.98–1.09), P S=0.18, I2=0, P H=0.67 | 0.95 (0.92–0.99), P S=0.02, I2=22, P H=0.17 |
| Cole, 200746 | 0.89 (0.83–0.96), P S=0.001, I2=31, P H=0.10 | 1.04 (0.99–1.09), P S=0.18, I2=0, P H=0.68 | — |
| Ebbing, 200813 | 0.90 (0.84–0.96), P S=0.002, I2=34, P H=0.08 | 1.03 (0.98–1.09), P S=0.21, I2=0, P H=0.72 | 0.95 (0.92–0.99), P S=0.02, I2=21, P H=0.19 |
| Galan, 201014 | 0.90 (0.84–0.97), P S=0.003, I2=27, P H=0.13 | 1.04 (0.99–1.09), P S=0.14, I2=0, P H=0.69 | 0.96 (0.92–0.99), P S=0.03, I2=23, P H=0.17 |
| Heinz, 201050 | 0.90 (0.84–0.96), P S=0.002, I2=34, P H=0.08 | 1.04 (0.99–1.09), P S=0.16, I2=0, P H=0.67 | 0.96 (0.92–1.00), P S=0.03, I2=18, P H=0.23 |
| Hodis, 200948 | — | — | 0.96 (0.92–0.99), P S=0.02, I2=23, P H=0.17 |
| House, 201051 | 0.89 (0.83–0.96), P S=0.001, I2=25, P H=0.16 | 1.04 (0.99–1.09), P S=0.17, I2=0, P H=0.74 | — |
| Huo, 201526 | 0.92 (0.85–0.995), P S=0.04, I2=26, P H=0.14 | 1.04 (0.99–1.09), P S=0.16, I2=0, P H=0.67 | 0.97 (0.93–1.01), P S=0.12, I2=0, P H=0.48 |
| Imasa, 200949 | — | 1.04 (0.99–1.09), P S=0.17, I2=0, P H=0.69 | — |
| Jamison, 200716 | 0.90 (0.83–0.96), P S=0.002, I2=34, P H=0.07 | 1.05 (0.99–1.10), P S=0.09, I2=0, P H=0.80 | 0.96 (0.92–1.00), P S=0.04, I2=19, P H=0.21 |
| Lamas, 201324 | 0.90 (0.84–0.96), P S=0.002, I2=31, P H=0.10 | 1.04 (0.99–1.09), P S=0.14, I2=0, P H=0.68 | 0.96 (0.92–1.00), P S=0.03, I2=19, P H=0.21 |
| Lange, 200441 | — | 1.03 (0.98–1.08), P S=0.25, I2=0, P H=0.86 | — |
| Liem, 200442 | — | 1.04 (0.99–1.09), P S=0.15, I2=0, P H=0.68 | 0.96 (0.92–0.99), P S=0.02, I2=23, P H=0.17 |
| Liem, 200532, 33 | 0.90 (0.84–0.96), P S=0.002, I2=33, P H=0.08 | 1.04 (0.99–1.09), P S=0.17, I2=0, P H=0.69 | 0.96 (0.92–1.00), P S=0.03, I2=22, P H=0.18 |
| Lonn, 200617 | 0.91 (0.85–0.98), P S=0.008, I2=29, P H=0.12 | 1.05 (0.99–1.10), P S=0.11, I2=0, P H=0.70 | 0.96 (0.92–1.00), P S=0.03, I2=23, P H=0.17 |
| Mark, 199638 | 0.90 (0.84–0.97), P S=0.003, I2=30, P H=0.11 | — | — |
| Potena, 200847 | — | — | 0.96 (0.92–0.99), P S=0.02, I2=18, P H=0.23 |
| Righetti, 200340 | — | — | 0.96 (0.92–0.99), P S=0.02, I2=20, P H=0.20 |
| Righetti, 200644 | 0.90 (0.84–0.96), P S=0.002, I2=32, P H=0.09 | 1.04 (0.99–1.09), P S=0.14, I2=0, P H=0.71 | 0.96 (0.92–1.00), P S=0.03, I2=18, P H=0.23 |
| Schnyder, 200218 | — | 1.04 (0.99–1.09), P S=0.13, I2=0, P H=0.78 | — |
| Sharma, 201325 | — | 1.04 (0.99–1.09), P S=0.15, I2=0, P H=0.67 | — |
| Toole, 200419 | 0.88 (0.82–0.95), P S=0.001, I2=29, P H=0.12 | 1.04 (0.99–1.10), P S=0.12, I2=0, P H=0.70 | 0.95 (0.92–0.99), P S=0.02, I2=23, P H=0.17 |
| Vianna, 200730 | — | — | 0.96 (0.92–0.99), P S=0.02, I2=22, P H=0.18 |
| VITATOPS, 201037 | 0.89 (0.82–0.96), P S=0.003, I2=34, P H=0.08 | 1.04 (0.99–1.09), P S=0.16, I2=0, P H=0.67 | 0.96 (0.92–1.01), P S=0.09, I2=20, P H=0.21 |
| Wrone, 200443 | 0.89 (0.83–0.96), P S=0.001, I2=33, P H=0.08 | 1.04 (0.99–1.09), P S=0.17, I2=0, P H=0.70 | 0.95 (0.92–0.99), P S=0.02, I2=19, P H=0.21 |
| Zoungas, 200645 | 0.90 (0.84–0.96), P S=0.003, I2=27, P H=0.14 | 1.04 (0.99–1.09), P S=0.17, I2=0, P H=0.69 | 0.96 (0.92–0.99), P S=0.02, I2=22, P H=0.17 |
CHD indicates coronary heart disease; CVD, cardiovascular disease; P H, P value for heterogeneity; P S, P value for significance; RCT, randomized controlled trial; RR, relative risk.
Discussion
Summary of Main Results
In this meta‐analysis we found a modest benefit of 10% reduced risk of stroke with folic acid supplementation compared with the control group. Folic acid supplementation also showed a small but significant benefit or 4% less risk of overall CVD events, with significant heterogeneity according to baseline folate levels, preexisting diseases, or the extent of homocysteine reduction. No significant benefit or harm of folic acid supplementation on the risk of incident CHD was found.
Comparison With Other Meta‐Analyses or Reviews
Compared with the most recent systematic reviews of folic acid supplementation and CVD risk,20, 23, 52 we included 3 new trials24, 25, 26 published during the 2013–2015 period and conducted in 4 countries with 22 510 more participants. Consequently, our comprehensive meta‐analysis summarized the most up‐to‐date evidence. In addition, different from previous meta‐analyses, we included all RCTs of folic acid supplementation and CVD without restrictions on the study sample size, treatment period, or preexisting disease status; however, we conducted stratified analyses to fully consider the impact of these factors on the intervention effect.
Folic acid supplementation significantly reduced the risk of stroke by 18% in a meta‐analysis in 2007 based on 8 RCTs with at least 10 stroke cases in each group and at least 6 months of follow‐up.22 After that, with more RCTs published, 10 more meta‐analyses of folic acid supplementation and risk of stroke were published with different inclusion criteria.20, 21, 23, 53, 54, 55, 56, 57, 58, 59 Although all of these meta‐analyses suggested a potential beneficial effect of folic acid supplementation on stroke (RRs ranged from 0.82 to 0.96),20, 21, 23, 53, 54, 55, 56, 57, 58, 59 none reached statistical significance, making the findings less conclusive. The results of our meta‐analysis based on 20 RCTs provided similar results and extended the findings of previous meta‐analyses, indicating a 10% significantly lower risk of stroke with folic acid supplementation.
Regarding the intervention effect of folic acid supplementation on risk of CHD, all meta‐analyses,23, 53, 56, 57, 59, 60, 61 including ours, reported a consistent nonsignificant intervention effect. The mechanism for the discrepancy in the folic acid intervention effect on stroke and CHD is unclear. Compared with CHD, which involves mainly large vessels, the underlying pathophysiological mechanisms for stroke are more heterogeneous and involve both cerebral large vessels and small vessels. As reported previously, elevated homocysteine was more likely a risk factor for small vessel diseases62; therefore, the modest beneficial effect of homocysteine reduction by folic acid supplementation on stroke but not on CHD may be driven by its impact on reducing the atherosclerotic processes of small vessels. This hypothesis was indirectly supported by the stronger associations with stroke than CHD, with higher homocysteine levels observed in both prospective cohort and genetic studies.2, 3, 4, 5, 6
Three previous meta‐analyses50, 63, 64 of RCTs indicated a significant intervention effect among patients with renal diseases. One of the meta‐analyses observed an intervention effect of 27% (95% CI 0.56–0.94, P=0.02)50 based on pooled results from 3 RCTs30, 40, 44 among patients on dialysis; another meta‐analysis of 7 RCTs observed an intervention effect of 15% (95% CI 0.76–0.96, P=0.009) among patients with end‐stage renal disease or advanced chronic kidney disease.63 The third meta‐analysis64 based on 10 RCTs among patients with chronic kidney diseases did not observe significant overall intervention effects but found significant intervention effects among subgroups (with preexisting end‐stage renal disease: RR 0.82, 95% CI 0.68–0.99; without folic acid fortification: RR 0.74, 95% CI 0.56–0.96). Our subgroup analysis reported a nonsignificant effect on CVD among patients with renal disease, mainly driven by 1 RCT.34
Comparison of RCTs With Observational and Genetic Studies
Compared with the results from prospective observations,2, 3, 65 the magnitude of the intervention effect on stroke was much lower (only around half), and we did not find any significant effect on CHD. Many factors may contribute to this discrepancy in the results of observational studies and RCTs of folic acid supplementation.
All participants in the RCTs had some preexisting diseases, including CVD, renal disease, atherosclerosis, or hypertension, and they were usually older, whereas participants in epidemiological studies were typically younger and healthier. In our subgroup analysis, the intervention effect of folic acid supplementation on both stroke and CVD was stronger among participants with neither CVD nor renal diseases; the magnitude of 20% reduced risk among this relatively healthy population was comparable to the findings from observational studies. Another explanation was that homocysteine reduction may be beneficial only at early stages of vascular disease manifestation and less effective in established CVD. Hodis and colleagues48 observed that B vitamin supplementation significantly reduced the progression of early‐stage subclinical atherosclerosis and carotid artery intima–media thickness but had no effect on the progression of markers of late‐stage atherosclerosis. Patients with CVD tend to have more serious complications, and thus lifestyle modifications or medications, which may have stronger effects on CVD than homocysteine reduction, might have masked the effect of homocysteine reduction on risk of CVD. The HOPE2 trial observed that participants who did not receive lipid‐lowering drugs had a larger treatment benefit,66 whereas the VITATOPS trial found beneficial effects of B vitamins among patients without antiplatelet treatment.15
It was also possible that the intervention effects of folic acid supplementation are modified by the specific genetic backgrounds of the populations. Previous genetic studies indicated that the MTHFR genotype was associated with a significant difference in homocysteine reduction following the same folic acid supplementation.5, 6 Participants with different MTHFR genotypes also showed significantly different CVD risk5, 6; however, only 3 RCTs measured the MTHFR genotypes of participants.26, 67 The intervention effects of folic acid supplementation were not significant on stroke, CHD, or CVD in both the Norwegian Vitamin Trial (NORVIT) and the Western Norway B Vitamin Intervention Trial (WENBIT). In a post hoc analysis67 combining 2 RCTs, Ebbing and colleagues measured the MTHFR genotype of 976 participants but did not find any significant interaction between the MTHFR genotype and the intervention. The China Stroke Primary Prevention Trial (CSPPT) also did not find a significant interaction between the MTHFR genotype and the intervention on stroke.26 Nevertheless, after further stratifying the intervention effects according to the joint classification of baseline folate level and MTHFR genotype, folic acid intervention significantly reduced stroke risk in those with low folate levels and the CC or CT genotype and in those with high baseline folate together with the TT genotype but not among those with low folate with the TT genotype.26
Strengths and Limitations
Because we included only RCTs in our meta‐analysis, our findings were unlikely to be affected by confounding factors. Other strengths included no evidence of heterogeneity and no detected publication bias. Our sensitivity analysis also showed minimal influence of any individual RCT on the combined results.
Several limitations warrant consideration. First, like any meta‐analysis, our findings may be constrained by the methodological rigor of the included studies. Although we assessed publication bias of the overall analysis with a funnel plot test, we could not completely exclude publication bias. Second, the included trials varied with respect to the characteristics of participants, the duration and intensity of treatment, and other design features; however, our stratified analyses and meta‐regression analyses did not identify any factors that would have influenced the summary estimates of the meta‐analysis. Third, the definitions of the CVD outcomes were somewhat heterogeneous in the selected trials, and that may influence the interpretation of the results; however, we examined the effect of folic acid supplementation on stroke and CHD separately, although there was no sufficient power to examine subtypes of stroke or CHD.
Compliance with treatment could be an important determinant of the outcome of RCTs. Although extent of homocysteine reduction could serve as a surrogate biomarker for the compliance of folic acid supplementation, not all RCTs provided data on homocysteine changes. Among a subgroup of RCTs with data of homocysteine changes, however, we observed a stronger intervention effect on CVD when the magnitude of homocysteine reduction was greater. Because the meta‐analysis was based on the result at the study level instead of individual data, we could not evaluate the intervention effects according to individual homocysteine change or treatment compliance. We found a dose‐response relationship between homocysteine reduction by folic acid supplementation and a reduction in risk of CVD but not of stroke. The lack of a dose‐response relationship between degree of homocysteine reduction and risk of stroke may not support a causal link between folic acid supplementation and stroke. A potential reason for the nonlinear relationship between homocysteine reduction and reduction in stroke risk could be related to threshold effects of high homocysteine levels on risk of stroke; a previous observational study observed significantly increased risk of stroke only in the group with serum homocysteine >18.6 mmol/L (upper quintile).68
Conclusion
Our findings, based on the most comprehensive and up‐to‐date evidence, provide support for a modest benefit of folic acid supplementation for the prevention of stroke. There was a 10% reduced risk of stroke and a 4% reduced risk of overall CVD with folic acid supplementation. A greater benefit for CVD was observed among participants without preexisting CVD or with lower plasma folate levels at baseline and in studies with a larger decrease in homocysteine levels. We did not observe any significant benefit or harm with folic acid supplementation for the risk of CHD.
Sources of Funding
This research is partly supported by Metagenics. The funder had no role in study.
Disclosures
Dr Hu has received research support from Metagenics and served as a consultant for Metagenics. Other authors have indicated no financial conflicts of interest.
(J Am Heart Assoc. 2016;5:e003768 doi: 10.1161/JAHA.116.003768)
References
- 1. McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 2. Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. [DOI] [PubMed] [Google Scholar]
- 3. Homocysteine and risk of ischemic heart disease and stroke: a meta‐analysis. JAMA. 2002;288:2015–2022. [DOI] [PubMed] [Google Scholar]
- 4. Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta‐analysis. BMJ. 2002;325:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD. Homocysteine and stroke: evidence on a causal link from Mendelian randomisation. Lancet. 2005;365:224–232. [DOI] [PubMed] [Google Scholar]
- 6. Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C–>T polymorphism and risk of coronary heart disease: a meta‐analysis. JAMA. 2002;288:2023–2031. [DOI] [PubMed] [Google Scholar]
- 7. Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. [DOI] [PubMed] [Google Scholar]
- 8. Lowering blood homocysteine with folic acid based supplements: meta‐analysis of randomised trials. Homocysteine Lowering Trialists' Collaboration. BMJ. 1998;316:894–898. [PMC free article] [PubMed] [Google Scholar]
- 9. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification‐its history, effect, concerns, and future directions. Nutrients. 2011;3:370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albert CM, Cook NR, Manson JE. Effect of folic acid and B vitamins on cardiovascular disease in women—reply. JAMA. 2008;300:1410. [DOI] [PubMed] [Google Scholar]
- 11. Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R; Study of the Effectiveness of Additional Reductions in C, Homocysteine Collaborative G . Effects of homocysteine‐lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–2494. [DOI] [PubMed] [Google Scholar]
- 12. Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, Selhub J, Jacques PF, Cole E, Gravens‐Mueller L, House AA, Kew C, McKenney JL, Pacheco‐Silva A, Pesavento T, Pirsch J, Smith S, Solomon S, Weir M. Homocysteine‐lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011;123:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygård O. Mortality and cardiovascular events in patients treated with homocysteine‐lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. [DOI] [PubMed] [Google Scholar]
- 14. Galan P, Kesse‐Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hankey GJ, Eikelboom JW, Yi Q, Lees KR, Chen C, Xavier D, Navarro JC, Ranawaka UK, Uddin W, Ricci S, Gommans J, Schmidt R; Group Vts . Antiplatelet therapy and the effects of B vitamins in patients with previous stroke or transient ischaemic attack: a post‐hoc subanalysis of VITATOPS, a randomised, placebo‐controlled trial. Lancet Neurol. 2012;11:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM; Veterans Affairs Site I . Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end‐stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–1170. [DOI] [PubMed] [Google Scholar]
- 17. Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J Jr. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. [DOI] [PubMed] [Google Scholar]
- 18. Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine‐lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the SWISS Heart Study: a randomized controlled trial. JAMA. 2002;288:973–979. [DOI] [PubMed] [Google Scholar]
- 19. Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the vitamin intervention for stroke prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. [DOI] [PubMed] [Google Scholar]
- 20. Zeng R, Xu CH, Xu YN, Wang YL, Wang M. The effect of folate fortification on folic acid‐based homocysteine‐lowering intervention and stroke risk: a meta‐analysis. Public Health Nutr. 2015;18:1514–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, Cooper J, Breteler MM, Bautista LE, Sharma P, Whittaker JC, Smeeth L, Fowkes FG, Algra A, Shmeleva V, Szolnoki Z, Roest M, Linnebank M, Zacho J, Nalls MA, Singleton AB, Ferrucci L, Hardy J, Worrall BB, Rich SS, Matarin M, Norman PE, Flicker L, Almeida OP, van Bockxmeer FM, Shimokata H, Khaw KT, Wareham NJ, Bobak M, Sterne JA, Smith GD, Talmud PJ, van Duijn C, Humphries SE, Price JF, Ebrahim S, Lawlor DA, Hankey GJ, Meschia JF, Sandhu MS, Hingorani AD, Casas JP. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta‐analysis of genetic studies and randomised trials. Lancet. 2011;378:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta‐analysis. Lancet. 2007;369:1876–1882. [DOI] [PubMed] [Google Scholar]
- 23. Yang HT, Lee M, Hong KS, Ovbiagele B, Saver JL. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta‐analysis of randomized controlled trials. Eur J Intern Med. 2012;23:745–754. [DOI] [PubMed] [Google Scholar]
- 24. Lamas GA, Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL. Oral high‐dose multivitamins and minerals after myocardial infarction: a randomized trial. Ann Intern Med. 2013;159:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma M, Mittal N. Prevalence of hyperhomocysteinaemia in chronic kidney disease and effect of supplementation of folic acid and vitamin B12 on cardiovascular mortality. J Indian Acad Clin Med. 2013;14:33–36. [Google Scholar]
- 26. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, Yang T, Xiao C, Zhao G, Dong Q, Zhu D, Ge J, Zhao L, Hu D, Liu L, Hou FF. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 27. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala‐Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vianna ACA, Mocelin AJ, Matsuo T, Morais‐Filho D, Largura A, Delfino VA, Soares AE, Matni AM. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodial Int. 2007;11:210–216. [DOI] [PubMed] [Google Scholar]
- 31. Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. [DOI] [PubMed] [Google Scholar]
- 32. Liem A, Reynierse‐Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol. 2003;41:2105–2113. [DOI] [PubMed] [Google Scholar]
- 33. Liem A, Reynierse‐Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart. 2005;91:1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, Yusuf S, Lonn EM. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease—results of the renal HOPE‐2 study. Nephrol Dial Transplant. 2008;23:645–653. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org. Accessed August 10, 2016. [Google Scholar]
- 36. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. B vitamins in patients with recent transient ischaemic attack or stroke in the vitamins to prevent stroke (VITATOPS) trial: a randomised, double‐blind, parallel, placebo‐controlled trial. Lancet Neurol. 2010;9:855–865. [DOI] [PubMed] [Google Scholar]
- 38. Mark SD, Wang W, Fraumeni JF Jr, Li JY, Taylor PR, Wang GQ, Guo W, Dawsey SM, Li B, Blot WJ. Lowered risks of hypertension and cerebrovascular disease after vitamin/mineral supplementation: the Linxian Nutrition Intervention Trial. Am J Epidemiol. 1996;143:658–664. [DOI] [PubMed] [Google Scholar]
- 39. Baker F, Picton D, Blackwood S, Hunt J, Erskine M, Dyas M, Ashby M, Siva A, Brown MJ. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial. Circulation. 2002;106(suppl II):741. [Google Scholar]
- 40. Righetti M, Ferrario GM, Milani S, Serbelloni P, La Rosa L, Uccellini M, Sessa A. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003;9:PI19–PI24. [PubMed] [Google Scholar]
- 41. Lange H, Suryapranata H, De Luca G, Borner C, Dille J, Kallmayer K, Pasalary MN, Scherer E, Dambrink JH. Folate therapy and in‐stent restenosis after coronary stenting. N Engl J Med. 2004;350:2673–2681. [DOI] [PubMed] [Google Scholar]
- 42. Liem AH, van Boven AJ, Veeger NJ, Withagen AJ, Robles de Medina RM, Tijssen JG, van Veldhuisen DJ. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. Int J Cardiol. 2004;93:175–179. [DOI] [PubMed] [Google Scholar]
- 43. Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end‐stage renal disease. J Am Soc Nephrol. 2004;15:420–426. [DOI] [PubMed] [Google Scholar]
- 44. Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine‐lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24:379–386. [DOI] [PubMed] [Google Scholar]
- 45. Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ. Cardiovascular morbidity and mortality in the atherosclerosis and folic acid supplementation trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–1116. [DOI] [PubMed] [Google Scholar]
- 46. Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown‐Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. [DOI] [PubMed] [Google Scholar]
- 47. Potena L, Grigioni F, Masetti M, Magnani G, Coccolo F, Fallani F, Russo A, Pizzuti M, Scalone A, Bianchi IG, Branzi A. Long‐term effect of folic acid therapy in heart transplant recipients: follow‐up analysis of a randomized study. Transplantation. 2008;85:1146–1150. [DOI] [PubMed] [Google Scholar]
- 48. Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, Selhub J, Alaupovic P, Liu CR, Liu CH, Hwang J, Wilcox AG, Selzer RH. High‐dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomized controlled trial. Stroke. 2009;40:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Imasa MS, Gomez NT, Nevado JB Jr. Folic acid‐based intervention in non‐ST elevation acute coronary syndromes. Asian Cardiovasc Thorac Ann. 2009;17:13–21. [DOI] [PubMed] [Google Scholar]
- 50. Heinz J, Kropf S, Domrose U, Westphal S, Borucki K, Luley C, Neumann KH, Dierkes J. B vitamins and the risk of total mortality and cardiovascular disease in end‐stage renal disease: results of a randomized controlled trial. Circulation. 2010;121:1432–1438. [DOI] [PubMed] [Google Scholar]
- 51. House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, Dresser GK, Spence JD. Effect of B‐vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010;303:1603–1609. [DOI] [PubMed] [Google Scholar]
- 52. Dong H, Pi F, Ding Z, Chen W, Pang S, Dong W, Zhang Q. Efficacy of supplementation with B vitamins for stroke prevention: a network meta‐analysis of randomized controlled trials. PLoS One. 2015;10:e0137533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bønaa KH, Spence JD, Nygård O, Jamison R, Gaziano JM, Guarino P, Bennett D, Mir F, Peto R, Collins R. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause‐specific mortality: meta‐analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170:1622–1631. [DOI] [PubMed] [Google Scholar]
- 54. Huo Y, Qin X, Wang J, Sun N, Zeng Q, Xu X, Liu L, Wang X. Efficacy of folic acid supplementation in stroke prevention: new insight from a meta‐analysis. Int J Clin Pract. 2012;66:544–551. [DOI] [PubMed] [Google Scholar]
- 55. Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine‐lowering therapy with folic acid in stroke prevention: a meta‐analysis. Stroke. 2010;41:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martí‐Carvajal AJ, Solà I, Lathyris D, Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;4:CD006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mei W, Rong Y, Jinming L, Yongjun L, Hui Z. Effect of homocysteine interventions on the risk of cardiocerebrovascular events: a meta‐analysis of randomised controlled trials. Int J Clin Pract. 2010;64:208–215. [DOI] [PubMed] [Google Scholar]
- 58. Miller IER, Juraschek S, Pastor‐Barriuso R, Bazzano LA, Appel LJ, Guallar E. Meta‐analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517–527. [DOI] [PubMed] [Google Scholar]
- 59. Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, Wang C, Xu JF, He J. Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta‐analysis. PLoS One. 2011;6:e25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta‐analysis of randomized controlled trials. JAMA. 2006;296:2720–2726. [DOI] [PubMed] [Google Scholar]
- 61. Zhang C, Wang ZY, Qin YY, Yu FF, Zhou YH. Association between B vitamins supplementation and risk of cardiovascular outcomes: a cumulative meta‐analysis of randomized controlled trials. PLoS One. 2014;9:e107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Undas A, Stepien E, Plicner D, Zielinski L, Tracz W. Elevated total homocysteine is associated with increased platelet activation at the site of microvascular injury: effects of folic acid administration. J Thromb Haemost. 2007;5:1070–1072. [DOI] [PubMed] [Google Scholar]
- 63. Qin X, Huo Y, Langman CB, Hou F, Chen Y, Matossian D, Xu X, Wang X. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: a meta‐analysis. Clin J Am Soc Nephrol. 2011;6:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ji Y, Tan S, Xu Y, Chandra A, Shi C, Song B, Qin J, Gao Y. Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: a meta‐analysis. Neurology. 2013;81:1298–1307. [DOI] [PubMed] [Google Scholar]
- 65. Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta‐analysis. Mayo Clin Proc. 2008;83:1203–1212. [DOI] [PubMed] [Google Scholar]
- 66. Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Homocysteine‐lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365–1372. [DOI] [PubMed] [Google Scholar]
- 67. Ebbing M, Bonaa KH, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njolstad I, Nilsen DW, Refsum H, Tverdal A, Vollset SE, Schirmer H, Bleie O, Steigen T, Midttun O, Fredriksen A, Pedersen ER, Nygard O. Combined analyses and extended follow‐up of two randomized controlled homocysteine‐lowering B‐vitamin trials. J Intern Med. 2010;268:367–382. [DOI] [PubMed] [Google Scholar]
- 68. Bots ML, Launer LJ, Lindemans J, Hoes AW, Hofman A, Witteman JC, Koudstaal PJ, Grobbee DE. Homocysteine and short‐term risk of myocardial infarction and stroke in the elderly: the Rotterdam Study. Arch Intern Med. 1999;159:38–44. [DOI] [PubMed] [Google Scholar]


