Abstract
Background
Early age (<45 years) at menopause has been postulated to be associated with increased cardiovascular disease risk; however, evidence of its relation with heart failure (HF) incidence is limited. We examined whether age at menopause is associated inversely with HF incidence in the Atherosclerosis Risk In Communities (ARIC) study and summarized all existing data in a meta‐analysis.
Methods and Results
In ARIC, data were obtained from 5629 postmenopausal women (mean age 56 years, 26% with bilateral oophorectomy) without HF. During a median follow‐up of 21.4 years, 965 incident HF events occurred. In a Cox regression model adjusted for reproductive health and HF risk factors, the hazard ratios for incident HF across categories of age at menopause (<45, 45–49, 50–54, and ≥55 years) were 1.32, 1.17, 1.00 (referent), and 1.12, respectively. Compared with women with later onset of menopause (aged ≥45 years), those with early menopause had elevated HF risk (hazard ratio 1.20, 95% CI 1.01–1.43). For the meta‐analysis, we searched Medline and Embase for articles published through December 2015 that prospectively evaluated age at menopause and HF risk. Summarized estimates from the 3 included studies (3568 events) showed higher HF risk among women with early menopause compared with those with later menopause (hazard ratio 1.33, 95% CI 1.15–1.53).
Conclusions
These results provided evidence that early age at menopause is associated with a modestly greater risk of HF. Identification of women with early menopause offers a window of opportunity to implement interventions that will improve overall cardiovascular health during the postmenopausal years.
Keywords: epidemiology, heart failure, menopause, women's health
Subject Categories: Heart Failure, Epidemiology, Women
Introduction
It is controversial whether an association exists between menopause and incident cardiovascular disease (CVD) beyond age and cardiovascular risk factors, and the underlying mechanisms for such an association are poorly understood. Numerous reports,1, 2, 3 with few exceptions,4 document elevated risk of CVD in women with early natural or surgical menopause (before age 45 years). Some have proposed that the elevated CVD risk among women with early menopause may be explained by estrogen deficiency,1, 2 but emerging evidence shows that women who undergo early menopause tend to have adverse CVD risk factors long before menopause.5
Despite heart failure (HF) accounting for 35% of all CVD mortality among women,6 investigations of the relation between early menopause and incident HF are limited. A few short‐term studies with small sample sizes suggest that early onset of menopause is related to greater likelihood of left ventricular (LV) dysfunction,7, 8, 9 which, in itself, raises the risk of overt HF.10, 11 Recent studies from the Swedish Mammography Cohort12 and the Multi‐Ethnic Study of Atherosclerosis (MESA)13 reported 40% and 66% greater risk of HF, respectively, in women with early versus later menopause. The former study assessed a homogeneous cohort of white, naturally menopausal women, and that limits generalization of the findings to other races. The latter study recorded few events during a short follow‐up and failed to distinguish between types of menopause, and that may have led to misclassification because women with hysterectomy and ovarian conservation were considered to have surgical menopause. Further evidence is needed to clarify the association of early menopause with HF.
The aim of this study was to assess the association of age at menopause with incident HF in a biracial cohort of postmenopausal women enrolled in the Atherosclerosis Risk In Communities (ARIC) study. We also pooled our results with prior prospective studies in a meta‐analysis to put our findings in context and to provide a higher level of evidence.
Methods
Study Population
The ARIC study is a multicenter prospective cohort study that, from 1987 to 1989, enrolled and examined 15 792 participants (8710 women) aged 45 to 64 years from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Only black participants were recruited in Jackson, whereas participants at the other field centers reflected the underlying populations of mostly white residents in the Minneapolis and Washington County sites and white and black residents in Forsyth County. Since baseline, 4 follow‐up examinations have been conducted: 1990 to 1992 (visit 2), 1993 to 1995 (visit 3), 1996 to 1998 (visit 4), and 2011 to 2013 (visit 5). Approximately 85% of surviving cohort members or their proxies continue to respond to the semiannual follow‐up telephone calls. Details of the study design and methods are described elsewhere.14 Local institutional review boards approved the ARIC protocol, and all participants provided written informed consent. The present analysis included all women who reached natural or surgical menopause before ARIC visits 1 through 4, with the visit at which a woman first reported being postmenopausal considered as baseline.
Measures
At each ARIC follow‐up visit, standardized protocols were used to collect information from participants about demographics, anthropometrics, lifestyle and behavioral factors, medical history, cardiovascular risk factors, biomarkers, and medication use. Women were also asked about history of gynecological surgeries and their reproductive milestones: age at menarche, parity, use of oral contraceptives, and postmenopausal hormone therapy.
Consistent with previous ARIC reports, we excluded women who underwent hysterectomy without bilateral oophorectomy before age 55 years that was not preceded by natural menopause, as age at menopause could not be determined precisely for such women.15, 16 At each ARIC visit, the remaining women were considered to be postmenopausal if they reported not having had a menstrual period within the 2 years before the visit. This definition is conservative and ensured that perimenopausal women were not classified as postmenopausal. Among postmenopausal women, those who reported menstrual cessation due to bilateral oophorectomy were classified as having surgical menopause, whereas those who reported cessation of menstrual bleeding that was not preceded by hysterectomy were classified as having natural menopause. Age at menopause was defined as the age at last menstrual period or bilateral oophorectomy. Consistent with previous reports,2, 13 women with onset of menopause before age 45 years were classified as having early menopause.
Blood pressure was measured using a random‐zero sphygmomanometer with participants seated after 5 minutes of rest. The average of the second and third consecutive measurements was used for analysis. Body mass index (BMI) was calculated by dividing weight in kilograms by height in square meters. Waist circumference was measured at the level of the umbilicus. Sports‐related physical activity was assessed by the Baecke questionnaire.17 Fasting plasma total and high‐density lipoprotein cholesterol were determined by enzymatic methods. Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula, which incorporates both cystatin C and creatinine.18 Diabetes mellitus was defined as a fasting blood glucose level ≥7.0 mmol/L, nonfasting blood glucose ≥11.1 mmol/L, a self‐reported physician diagnosis of diabetes mellitus, or current use of antidiabetic medication.
Prevalent HF, for exclusion, was defined by self‐reported use of HF medication or evidence of manifest HF by Gothenburg criteria stage 319 for women who entered the analytic sample at visit 1 or incident HF hospitalization prior to the visit of entry for those who entered the analytic cohort during follow‐up. Prevalent coronary heart disease (CHD) was ascertained from medical history or electrocardiographic evidence of myocardial infarction or coronary revascularization at visit 1 or an incident‐adjudicated CHD event prior to the visit of entry for those who entered the analytic cohort during follow‐up.
Incident HF Ascertainment
Incident HF occurring after visit 1 (1987–1989) up until December 31, 2012, was defined as the first hospitalization that included an International Classification of Diseases, 9th Revision (ICD‐9) discharge code of 428 (428.0–428.9) among the primary or any secondary diagnosis or a death certificate with an ICD‐9 code of 428 or an ICD‐10 code of I50 among any of the listed or underlying causes of death.20
Statistical Analysis
Of the 8710 women enrolled from 1987 to 1989, 7294 women who reported having undergone menstrual cessation due to natural menopause, hysterectomy, or bilateral oophorectomy during ARIC visits 1 to 4 were eligible for this analysis. We excluded women who underwent hysterectomy without bilateral oophorectomy before age 55 years that was not preceded by natural menopause (n=897). In addition, women with missing age at menopause (n=382) and prevalent HF (n=355) and the few who reported races other than black or white (n=31) were excluded, resulting in an analytic sample of 5629 postmenopausal women.
Baseline data (at entry into the analytic cohort) were expressed as means (±SD) for continuous variables and as percentages for categorical variables, stratified by age at menopause (<45, 45–49, 50–54, and ≥55 years). The Kaplan–Meier method was used to estimate cumulative incidence of HF by categories of age at menopause, and the log‐rank test was used to test for differences between survival curves. Cox proportional hazards regression was used to estimate the hazard ratio (HR) of HF in relation to age at menopause modeled in 2 ways: dichotomized as the presence or absence of early menopause and as an ordinal categorical variable, defined above. Because HF rates increase with advancing biological age, attained age in years was used as the time scale for all time‐to‐event analyses. Because of the variable lengths of follow‐up for participants, models that accounted for left truncation of the data as a result of the staggered entry were used. Five models with progressive degrees of adjustment were used. Model 1 adjusted for age, race, and ARIC field center. Model 2 also adjusted for established HF risk factors. Model 3 further adjusted for reproductive health measures. To assess whether the observed association was mediated by CHD, we further adjusted for interim CHD, defined as CHD that occurred before HF, and modeled it as a time‐varying covariate (model 4). Because of the long follow‐up duration, we also modeled longitudinally assessed BMI, systolic blood pressure, and the use of antihypertensive medication and hormone therapy as time‐dependent covariates in model 5. Linear trends in HRs across categories of age at menopause were tested by modeling the median of each category as an ordinal variable in the Cox models. A potential nonlinear relation between age at menopause and incident HF was evaluated by using restricted cubic splines. Wald chi‐square tests were used to formally test for multiplicative interactions of race, smoking status, BMI, hormone therapy use, and type of menopause with age at menopause in relation to incident HF. No statistically significant 2‐way interaction of age at menopause with type of menopause (natural or surgical) was identified (P=0.1819); therefore, we pooled the data. A 2‐tailed P<0.05 was considered to be statistically significant. We tested and confirmed the validity of the proportional hazards assumption using interaction terms between categories of age at menopause and the log of time, which in this case was attained age. All analyses were performed using SAS software version 9.4 (SAS Institute).
Meta‐Analysis
Data Sources and Study Selection
We performed a systematic literature search in Medline and Embase and evaluated all studies that assessed the association of age at menopause with the risk of HF published up to December 31, 2015, using the following keywords: age, early menopause, menopause, postmenopause, bilateral oophorectomy, and heart failure. This yielded 44 and 39 publications from Medline and Embase, respectively. We also reviewed the reference lists of retrieved articles. Studies were included (1) if they had a cohort design, (2) if the main exposure of interest was age at menopause or early menopause, (3) if the end point of interest was incident HF, and (4) if they reported adjusted relative risk or HR and 95% CI for the association of age at menopause with HF incidence or mortality. Studies that did not meet these criteria or that did not provide enough data to allow calculation of the effect estimates were excluded. If multiple reports were obtained from the same study population, the most recent report was chosen. Two studies that met the inclusion criteria and the present study were included in the meta‐analysis (Figure 1).
Figure 1.
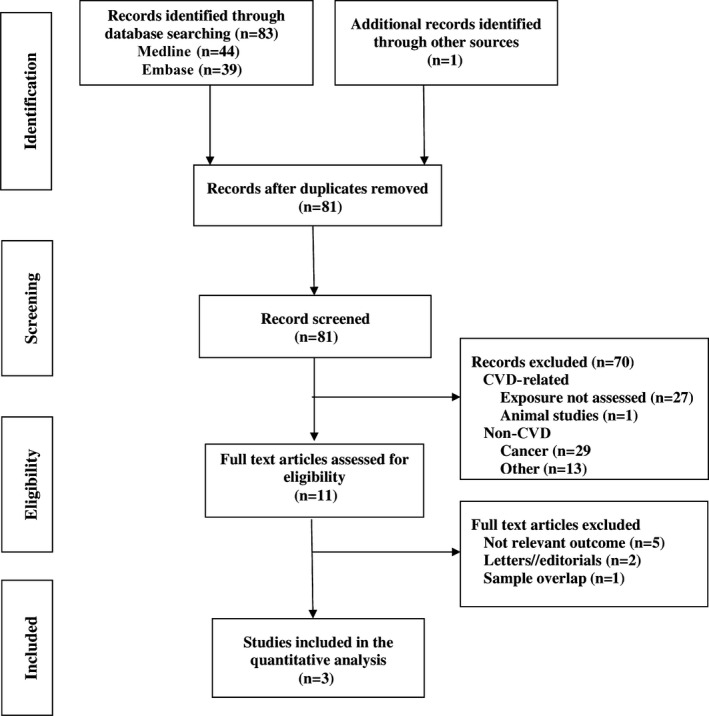
Flowchart of study selection for the meta‐analysis. CVD indicates cardiovascular disease.
Data Extraction and Analysis
The following information was recorded for each study using a standardized form: author, year of publication, location, study design, sample size, study duration, number of events, and number of women with early menopause. Effect estimates from fully adjusted models were extracted. Standard errors were calculated from the CIs provided. Summary estimates across all studies were calculated using DerSimonian and Laird's random‐effects method weighting individual study risk estimates by the inverse of their variance.21 Heterogeneity among studies was investigated using Cochran's Q test and I 2 with a P value of <0.10 to reject the null hypothesis that estimates from the various studies are homogeneous. Publication bias was assessed using funnel plots, Begg's adjusted rank correlation,22 and Egger’ regression asymmetry tests23 at the P<0.10 level of significance. Analyses were conducted using the R software (version 3.2.2; R Foundation for Statistical Computing).24
Results
Of the 5629 postmenopausal women, 1468 (26.1%) reported undergoing surgical menopause. Overall, 1611 women (28.6%) reached menopause before age 45 years, with the median age of menopause recorded as 49 and 44 years for women with natural and surgical menopause, respectively. The distributions of characteristics of participants stratified by categories of age at menopause are presented in Table 1. The average age at menopause was higher for white than black participants and showed positive associations with age, education, systolic blood pressure, waist circumference, age at menarche, and lipid‐lowering medication use and negative associations with parity, hormone therapy use, high‐density lipoprotein cholesterol, and estimated glomerular filtration rate.
Table 1.
Characteristics of Participants According to Age at Menopause in the ARIC Study
| Characteristics | Age at Menopause, y | P trend | |||
|---|---|---|---|---|---|
| <45 | 45–49 | 50–54 | ≥55 | ||
| n | 1611 | 1870 | 1795 | 353 | |
| Age, y | 54.8 (5.6) | 55.4 (4.6) | 57.2 (3.5) | 60.1 (2.7) | 0.001 |
| Race, black, % | 37.5 | 28.1 | 20.5 | 28.0 | 0.001 |
| Education (beyond high school), % | 28.4 | 32.3 | 34.8 | 36.0 | 0.001 |
| Smoking status, % | 0.001 | ||||
| Former | 22.9 | 25.8 | 24.1 | 25.3 | |
| Current | 29.5 | 24.5 | 17.7 | 8.0 | |
| Alcohol intake, g/d | 2.5 (7.4) | 2.9 (6.9) | 3.0 (7.6) | 2.1 (5.3) | 0.406 |
| Heart rate, bpm | 67.2 (10.6) | 67.3 (10.0) | 67.1 (9.7) | 67.0 (9.9) | 0.644 |
| Systolic blood pressure, mm Hg | 121.4 (19.8) | 121.4 (20.1) | 122.2 (19.0) | 126.6 (19.9) | 0.001 |
| Hypertension medication use, % | 33.0 | 31.4 | 29.3 | 36.5 | 0.360 |
| Diabetes mellitus, % | 13.8 | 12.4 | 10.6 | 14.8 | 0.108 |
| Body mass index, kg/m2 | 28.0 (6.1) | 27.9 (6.1) | 28.0 (6.1) | 28.7 (6.0) | 0.064 |
| Waist circumference, cm | 96.2 (15.6) | 96.2 (15.6) | 96.2 (15.8) | 98.8 (15.6) | 0.005 |
| Sports index | 2.3 (0.7) | 2.4 (0.8) | 2.4 (0.8) | 2.4 (0.7) | 0.065 |
| Parity, % | 0.001 | ||||
| 0 | 10.5 | 9.1 | 7.7 | 7.4 | |
| 1 | 14.3 | 11.7 | 10.8 | 9.6 | |
| 2 | 25.5 | 24.9 | 24.8 | 20.4 | |
| ≥3 | 49.7 | 54.3 | 56.6 | 62.6 | |
| Age at menarche, y | 12.8 (1.7) | 12.9 (1.7) | 13.0 (1.7) | 13.1 (3.0) | 0.002 |
| Oral contraceptives (ever used), % | 37.8 | 43.6 | 42.8 | 32.9 | 0.441 |
| Hormone therapy (current use), % | 25.8 | 23.6 | 17.8 | 13.9 | 0.001 |
| Bilateral oophorectomy, % | 48.9 | 22.5 | 11.8 | 13.6 | 0.001 |
| HDL cholesterol, mmol/L | 1.54 (0.5) | 1.52 (0.5) | 1.51 (0.4) | 1.47 (0.4) | 0.004 |
| Total cholesterol, mmol/L | 5.76 (1.1) | 5.74 (1.1) | 5.68 (1.1) | 5.67 (1.1) | 0.091 |
| Lipid‐lowering medication use, % | 3.8 | 3.4 | 4.5 | 5.4 | 0.101 |
| eGFR, mL/min/1.73 m2 | 103.8 (17.3) | 101.9 (15.8) | 98.8 (14.9) | 94.7 (16.0) | 0.001 |
Values are mean (SD) for continuous variables and percentages for categorical variables. ARIC indicates Atherosclerosis Risk In Communities; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein.
During a median follow‐up of 21.4 years, 965 incident HF events and 755 CHD events occurred. The incidence of HF according to age at menopause of <45, 45 to 49, 50 to 54, and ≥55 years was 10.7, 8.5, 7.3, and 10.7 events per 1000 person‐years, respectively (Table 2); corresponding estimates for CHD were 15.5, 13.8, 11.1, and 13.3 events per 1000 person‐years, respectively. In Kaplan–Meier cumulative incidence analysis, a younger age at menopause was significantly associated with higher incidence of HF (Figure 2). Compared with women who reached menopause at age 50 to 54 years, the HRs for incident HF were 1.38, 1.16, and 1.08 for women who reached menopause at age <45, 45 to 49, and ≥55 years, respectively (Table 2). Additional adjustment for reproductive health factors and interim CHD did not significantly alter the observed associations with the HRs for incident HF, observed to be 1.32, 1.17, and 1.12 for women who reached menopause at age <45, 45 to 49, and ≥55 years, respectively, compared with women who reached menopause at age 50 to 54 years. A 1‐year increment in age at menopause was not significantly inversely associated with the incidence of HF (HR 0.99, 95% CI 0.98–1.00, P=0.16). No statistically significant (P>0.05) 2‐way interactions of age at menopause with race, smoking status, BMI, waist circumference, systolic blood pressure, diabetes mellitus, hormone therapy use, or type of menopause were identified.
Table 2.
Hazard Ratios and 95% CIs for the Association of Age at Menopause With Incident HF in the ARIC Study, 1987 to 2012
| Events | n | Incidence Ratea | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|---|---|---|
| Age at menopause, y | ||||||||
| <45 | 336 | 1611 | 10.7 (9.6–11.9) | 1.47 (1.25–1.74) | 1.38 (1.16–1.63) | 1.35 (1.11–1.63) | 1.32 (1.09–1.60) | 1.39 (1.12–1.74) |
| 45–49 | 313 | 1870 | 8.5 (7.6–9.5) | 1.22 (1.03–1.44) | 1.16 (0.97–1.37) | 1.15 (0.96–1.38) | 1.17 (0.98–1.40) | 1.26 (1.02–1.56) |
| 50–54 | 250 | 1795 | 7.3 (6.5–8.3) | 1 (Referent) | 1 (Referent) | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| ≥55 | 66 | 353 | 10.7 (8.4–13.6) | 1.16 (0.88–1.52) | 1.08 (0.82–1.43 | 1.14 (0.86–1.51) | 1.12 (0.85–1.49) | 1.26 (0.88–1.81) |
| P trend | 0.001 | 0.001 | 0.007 | 0.012 | 0.019 | |||
| Early menopause | ||||||||
| Age ≥45 y | 629 | 4018 | 8.2 (7.6–8.9) | 1 (Referent) | 1 (Referent) | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| Age <45 y | 336 | 1611 | 10.7 (9.6–11.9) | 1.32 (1.15–1.51) | 1.27 (1.11–1.46) | 1.23 (1.06–1.44) | 1.21 (1.03–1.41) | 1.20 (1.01–1.43) |
ARIC indicates Atherosclerosis Risk In Communities; HF indicates heart failure.
Model 1: Hazard ratio with 95% CI from Cox proportional hazards model adjusted for age and race by field center. Model 2: Model 1 was additionally adjusted for education level (less than high school, high school, beyond high school), smoking status (never, former, current), alcohol intake (continuous), body mass index (continuous), waist circumference (continuous), systolic blood pressure (continuous), antihypertensive medication use (yes or no), heart rate (continuous), diabetes mellitus status (yes or no), estimated glomerular filtration rate (continuous), total and high‐density lipoprotein cholesterol (continuous). Model 3: Model 2 was additionally adjusted for age at menarche (continuous), oral contraceptive use (ever, never), parity, hormone therapy use (never, former, current), and type of menopause (natural, surgical). Model 4: Model 3 was additionally adjusted for coronary heart disease occurring before heart failure incidence as a time‐varying covariate. Model 5: Model 4 was additionally adjusted for time‐varying covariates of systolic blood pressure, body mass index, and use of antihypertensive medication and hormone therapy during the 25 years of follow‐up.
Unadjusted incidence rate per 1000 person‐years with 95% CI.
Figure 2.
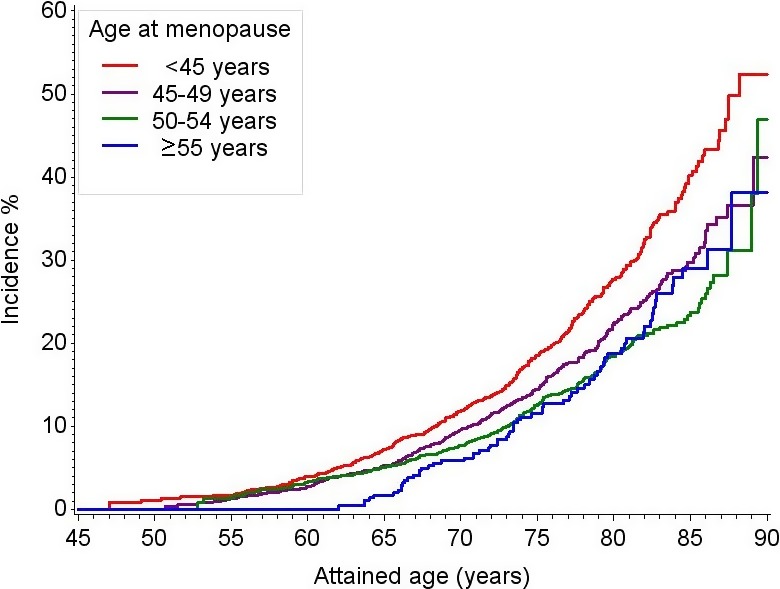
Kaplan–Meier cumulative incidence estimates of HF according to age at menopause, ARIC, 1987 to 2012. Log‐rank test P=0.001. ARIC indicates Atherosclerosis Risk In Communities; HF, heart failure.
We also performed analyses based on the presence or absence of early menopause (Table 2). Compared with women with later onset of menopause (at age ≥45 years), women who experienced early menopause (at age <45 years) had a greater risk of HF after adjusting for education level, smoking status, alcohol intake, BMI, waist circumference, systolic blood pressure, antihypertensive medication use, heart rate, diabetes status, estimated glomerular filtration rate, and total and high‐density lipoprotein cholesterol (HR 1.27, 95% CI 1.11–1.46; model 2). In the most fully adjusted model (model 5), compared with women with later onset of menopause, women with early menopause had a 20% elevated risk of HF (HR 1.20, 95% CI 1.01–1.43). Further adjustments for a history of cancer diagnosis did not significantly influence the observed positive association between early menopause and incident HF (data not shown). The relation of age at menopause with incident HF was reasonably linear (P=0.026), with results from restricted cubic spline Cox regression analysis confirming this observation (Figure 3).
Figure 3.
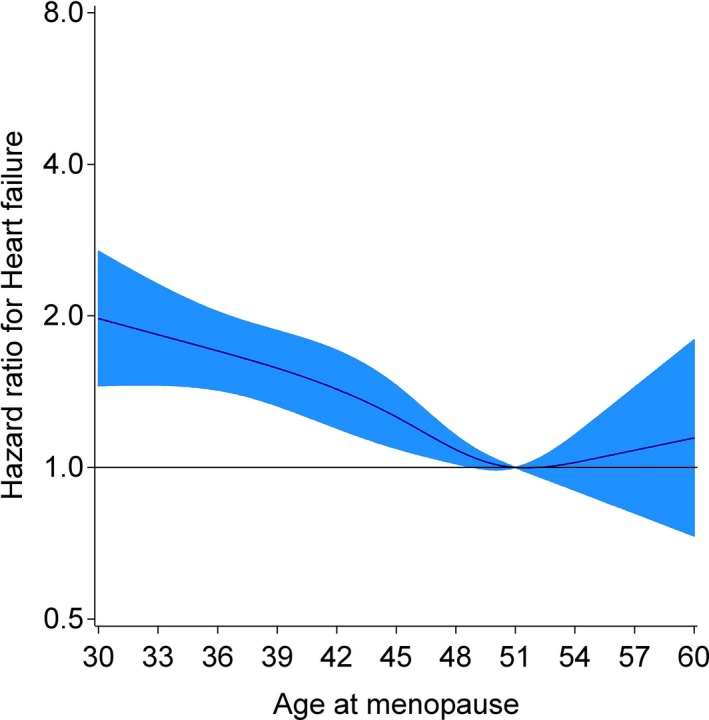
Restricted cubic spline depicting relationship between age at menopause and incident HF adjusted for age, race, and center. Reference value was 51 years, the average for age at natural menopause in developed countries. The 95% CIs are gray. Knots set at fifth, 35th, 65th, and 95th percentiles of age at menopause. HF indicates heart failure.
Meta‐Analysis
Table 3 summarizes the characteristics of the 3 prospective studies included in the meta‐analysis. In all 3 studies, age at menopause was self‐reported, whereas ICD code or clinical adjudication (based on patient charts) was used for diagnosing HF incidence. The Swedish Mammography Cohort12 and the MESA13 were the 2 other prospective studies identified that evaluated the association of age at menopause with the incidence of HF, and those studies respectively reported 40% and 66% greater risk of HF in women with early versus later onset of menopause. In total, including the present study, 3568 HF events were included in the meta‐analysis. Overall, a 1‐year increment in age at menopause was minimally inversely associated with the risk of HF (HR 0.98, 95% CI 0.97–0.995, P=0.009). Compared with women with later onset of menopause, women who experienced early menopause (at age <45 years) had a significantly greater risk of HF (HR 1.33, 95% CI 1.15–1.53) (Figure 4). We did not observe evidence of heterogeneity of the results (Q=2.60, I 2=22.9%, P=0.27). Finally, we were unable to detect the presence of publication bias in our analyses (Begg test P=0.60, Egger's bias=1.23, P=0.67), possibly because of the few number of studies included in the quantitative review.25
Table 3.
Characteristics of Studies That Assessed the Relation of Age at Menopause With Incident HF
| Author | Study | Location | Design | Sample | Events | Early Menopause, n | Follow‐up, y |
|---|---|---|---|---|---|---|---|
| Ebong et al (2014)13 | Multi‐Ethnic Study of Atherosclerosis | United States | Prospective cohort | 2947 | 71 | 730 | 8.5 (median) |
| Rahman et al (2015)12 | Swedish Mammography Cohort | Sweden | Prospective cohort | 22 256 | 2532 | 1571 | 13 (mean) |
| Appiah et al (current study) | Atherosclerosis Risk In Communities | United States | Prospective cohort | 5629 | 965 | 1611 | 21.4 (median) |
HF indicates heart failure.
Figure 4.
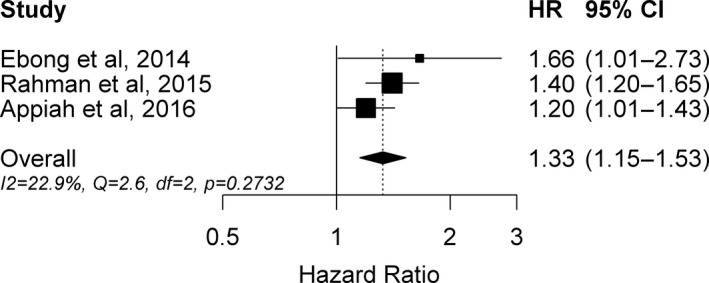
Forest plot of hazard ratios and 95% CIs for the association of early menopause (at age <45 years) versus later menopause (at age ≥45 years) with incident HF. Appiah et al is the current study. HF indicates heart failure.
Discussion
Results from this large prospective observational cohort of white and black women enrolled in the ARIC study with a median of 21 years of follow‐up showed a generally inverse association between age at menopause and incident HF; however, the modestly elevated risk of HF was mainly limited to women who reached menopause before age 45 years (HR 1.20). This observation was confirmed by a meta‐analysis pooling our data with 2 other prospective studies12, 13 that found women with early menopause to have elevated HF risk (HR 1.33). It is worth noting that, similar to other investigations,12 we observed a higher incidence of HF among women who reached menopause after age 55 years compared with women who reached menopause at 50 to 54 years, but this result did not attain statistical significance in either crude or multivariable adjusted models. HF affects about 2.4 million American women,6 and the prevalence has been projected to increase by 46% by the end of 2030.26 With ≈10% all women experiencing natural menopause before age 45 years27 and two‐thirds of all surgical menopause occurring before age 50 years,28 identification of women with early menopause as a population at high risk of HF provides a window of opportunity for intervention to prevent the onset of HF.
Menopause is characterized by physiological changes that influence several organs and systems, with the cardiovascular system among the most predominantly affected.8 Although early age at menopause is widely believed to be associated with greater risk for CVD events, its relation with incident HF has not been investigated in detail. Previous small studies reported that, compared with premenopausal women, postmenopausal women had more LV systolic and diastolic dysfunction,7, 8, 9, 29 an important predictor of overt HF.10, 11 A study among 72 postmenopausal women and 71 age‐matched premenopausal women observed that menopause negatively affected myocardial velocities and myocardial performance.8 Similarly, other case–control studies reported that, compared with premenopausal women, postmenopausal women had higher relative wall thickness and concentric LV geometric remodeling independent of high blood pressure30 and lower peak early diastolic velocity E wave and mitral E/A ratio, indicative of impaired LV diastolic filling.31 In a study of 50 postmenopausal women who reported ages at menopause ranging from 29 to 55 years, Kaur et al9 observed significant impairment in LV systolic function among women who reached menopause before age 40 years compared with those with onset of menopause at age ≥50 years.
Emerging epidemiological evidence suggests that early menopause is positively associated with incident HF. After following 22 256 postmenopausal women enrolled in the Swedish Mammography Cohort for an average of 13 years, Rahman et al12 observed a 40% elevated risk of HF among women who experienced early natural menopause (at age 40–45 years) compared with those who reached menopause at age 50 to 54 years. Smoking was found to modify the association by augmenting HF risk beyond expectations among women with onset of menopause between ages 46 and 49 years. Likewise, results from 2947 postmenopausal women enrolled in MESA13 with a median follow‐up of 8.5 years showed a 66% greater risk of HF in women with early menopause (at age <45 years) compared with women with onset of menopause at age ≥45 years. Consistent with these results, we also observed a 30% greater risk of HF among women with onset of menopause before age 45 years compared with those who reached menopause at age 50 to 54 years. This elevated HF risk among women with early menopause was reduced to 19% when we compared them with all women with onset of menopause at age ≥45 years and adjusted for other HF risk factors. It is well known that smoking decreases the age of natural menopause by ≈1 to 2 years32 and by as much as 9 years in women with certain genetic variants.33 In contrast to the Swedish Mammography Cohort, the present study and MESA did not identify any interaction of early menopause with smoking status, and we did not observe race, hormone therapy, general adiposity, or type of menopause modifying the association of early menopause with incident HF.
The underlying mechanisms by which early menopause may influence HF risk remain elusive. Because early menopause leads to reduced exposure to endogenous estrogens, it has been postulated that such women experience less lifetime cardioprotective effect of ovarian estrogen. This argument was first observed among premenopausal women with bilateral oophorectomy, among which those who used exogenous hormones had lower incidence of CVD events compared with those without hormone therapy use.34 Estrogen deficiency after menopause predisposes women to adverse levels of risk factors for HF,35 and evidence from animal models suggests that reduced estrogen levels may directly or indirectly contribute to diastolic dysfunction and hypertensive heart diseases in postmenopausal women. Specifically, the loss of ovarian estrogens leads to chronic activation of the renin–angiotensin–aldosterone system and the nitric oxide synthase system as well as other related intracellular signaling pathways, in part via the membrane G protein–coupled receptor 30.35, 36 This can result in endothelial dysfunction, inflammation, and immune dysfunction, which may lead to collagen synthesis, LV remodeling, and diastolic dysfunction,35, 36, 37 the latter being a major cause of HF among women.38 Despite these reported cardioprotective roles of hormone therapy in experimental studies, there are contrasting reports about the influence of hormone therapy for the prevention of CVD end points among postmenopausal women. On the one hand, some controlled primary prevention trials show slower progression of subclinical CVD39, 40 and lower risk of CVD mortality41 among postmenopausal women on hormone therapy compared with those on placebo. On the other hand, other randomized trials have observed no benefits of hormone therapy in the primary42, 43 or secondary44, 45, 46 prevention of CVD end points in postmenopausal women. Although a few trials among recently menopausal women show slower progressions of carotid artery intima–media thickness in women taking estradiol,47 others show no effect of estradiol or other hormone therapies on vascular endothelial function,48 carotid artery intima–media thickness,49 or coronary artery calcium and total stenosis.47 These equivocal results cast doubt about a cardioprotective role for estrogen in CVD development among postmenopausal women.
It is possible that other processes besides ovarian estrogen deficiency contribute to both early menopause and HF or CVD incidence, and thus the observed association may not be causal. Accumulating evidence suggests that CVD risk factors (and latent CVD) may influence the timing of menopause. Results from the Framingham Heart Study cohort showed that greater levels of cholesterol, relative weight, and blood pressure were each associated with early onset of natural menopause, independent of smoking.5 In contrast, the Coronary Artery Risk Development in Young Adults study, which followed 1045 premenopausal women with an average age of 26 years at baseline for 25 years, observed that trajectories of total cholesterol, triglycerides, BMI, and systolic blood pressure were elevated in women who had earlier onset of both natural and surgical menopause.50 Other studies have also identified genetic predictors of age at menopause33 and early menopause.51 In a follow‐up of the National Health and National Examination Survey, the elevated risk of CVD mortality in women with surgical menopause before age 45 years was limited to those who reported a family history of CHD, suggesting that the relation of surgical menopause and CVD may be explained by susceptibility genes that increase the risk of both outcomes.52 Analogously, the elevated incidence of HF among women with early menopause observed in this and other studies may reflect residual confounding of unmeasured variables.
The strengths of this study include the use of a large population‐based biracial sample, extensive assessment of HF risk factors, conservative definition of postmenopausal status, and 25 years of follow‐up. Potential limitations of this study warrant consideration. First, age at menopause was self‐reported and may be subject to recall error. The precision of recollecting age at menopause appears to decrease as time since menopause increases.53 This may contribute to dilution of the relative hazard estimates, as women who had early menopause may subsequently report an older age at menopause. A woman's accuracy in recalling age at bilateral oophorectomy seems to surpass recall of age at natural menopause.54 Second, HF events were ascertained using ICD codes from hospital discharge and death certificates, which may not capture events occurring in outpatients, although most outpatient HF patients are eventually hospitalized.55 In ARIC, the validity of ICD codes for identifying HF events is high.56 Finally, information on subtypes of HF at diagnosis was not available.
In conclusion, our findings demonstrate a modest association between early age at natural or surgical menopause and greater incidence of HF, which is consistent with previous reports. Owing to the burden of HF in women and the projected increase in HF prevalence, research to establish causality and to understand the mechanisms underlying early onset of menopause and how it may contribute to HF are needed. Such information might contribute to improving the long‐term cardiovascular health of women who experience early menopause.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr. Appiah was supported by National Heart, Lung, and Blood Institute training grant T32HL007779.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions.
(J Am Heart Assoc. 2016;5:e003769 doi: 10.1161/JAHA.116.003769)
References
- 1. Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta‐analysis. Menopause. 2006;13:265–279. [DOI] [PubMed] [Google Scholar]
- 2. Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the multi‐ethnic study of atherosclerosis. Menopause. 2012;19:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr, Roger VL, Melton LJ III, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gong D, Sun J, Zhou Y, Zou C, Fan Y. Early age at natural menopause and risk of cardiovascular and all‐cause mortality: a meta‐analysis of prospective observational studies. Int J Cardiol. 2016;203:115–119. [DOI] [PubMed] [Google Scholar]
- 5. Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PH, Wilson PW, Pearson PL, Grobbee DE. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1983. [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics C ; Stroke Statistics S . Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duzenli MA, Ozdemir K, Sokmen A, Gezginc K, Soylu A, Celik C, Altunkeser BB, Tokac M. The effects of hormone replacement therapy on myocardial performance in early postmenopausal women. Climacteric. 2010;13:157–170. [DOI] [PubMed] [Google Scholar]
- 8. Duzenli MA, Ozdemir K, Sokmen A, Soylu A, Aygul N, Gezginc K, Tokac M. Effects of menopause on the myocardial velocities and myocardial performance index. Circ J. 2007;71:1728–1733. [DOI] [PubMed] [Google Scholar]
- 9. Kaur M, Singh H, Ahuja GK. Cardiac performance in relation to age of onset of menopause. J Indian Med Assoc. 2011;109:234–237. [PubMed] [Google Scholar]
- 10. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuznetsova T, Herbots L, Jin Y, Stolarz‐Skrzypek K, Staessen JA. Systolic and diastolic left ventricular dysfunction: from risk factors to overt heart failure. Expert Rev Cardiovasc Ther. 2010;8:251–258. [DOI] [PubMed] [Google Scholar]
- 12. Rahman I, Akesson A, Wolk A. Relationship between age at natural menopause and risk of heart failure. Menopause. 2015;22:12–16. [DOI] [PubMed] [Google Scholar]
- 13. Ebong IA, Watson KE, Goff DC Jr, Bluemke DA, Srikanthan P, Horwich T, Bertoni AG. Age at menopause and incident heart failure: the multi‐ethnic study of atherosclerosis. Menopause. 2014;21:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15. Nabulsi AA, Folsom AR, Szklo M, White A, Higgins M, Heiss G. No association of menopause and hormone replacement therapy with carotid artery intima‐media thickness. Atherosclerosis risk in communities (ARIC) study investigators. Circulation. 1996;94:1857–1863. [DOI] [PubMed] [Google Scholar]
- 16. Luoto R, Sharrett AR, Schreiner P, Sorlie PD, Arnett D, Ephross S. Blood pressure and menopausal transition: the atherosclerosis risk in communities study (1987–95). J Hypertens. 2000;18:27–33. [DOI] [PubMed] [Google Scholar]
- 17. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 18. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; Investigators C‐E . Estimating glomerular filtration rate from serum creatinine and cystatin c. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea–validation of a scoring test for clinical‐epidemiological use: the study of men born in 1913. Eur Heart J. 1987;8:1007–1014. [DOI] [PubMed] [Google Scholar]
- 20. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 22. Begg CB, Berlin JA. Publication bias ‐ a problem in interpreting medical data. J Roy Stat Soc A Stat. 1988;151:419–463. [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 25. Palma S, Delgado‐Rodriguez M. Assessment of publication bias in meta‐analyses of cardiovascular diseases. J Epidemiol Community Health. 2005;59:864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG; American Heart Association Advocacy Coordinating C ; Council on Arteriosclerosis T ; Vascular B ; Council on Cardiovascular R ; Intervention ; Council on Clinical C ; Council on E ; Prevention ; Stroke C . Forecasting the impact of heart failure in the united states: a policy statement from the american heart association. Circulation. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subrat P, Santa SA, Vandana J. The concepts and consequences of early ovarian ageing: a caveat to women's health. J Reprod Infertil. 2013;14:3–7. [PMC free article] [PubMed] [Google Scholar]
- 28. Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol. 2011;118:1280–1286. [DOI] [PubMed] [Google Scholar]
- 29. Kaur M, Ahuja GK, Singh H, Walia L, Avasthi KK. Evaluation of left ventricular performance in menopausal women. Indian J Physiol Pharmacol. 2010;54:80–84. [PubMed] [Google Scholar]
- 30. Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Porcellati C. Early cardiac changes after menopause. Hypertension. 1998;32:764–769. [DOI] [PubMed] [Google Scholar]
- 31. Kangro T, Henriksen E, Jonason T, Leppert J, Nilsson H, Sorensen S, Ringqvist I. Effect of menopause on left ventricular filling in 50‐year‐old women. Am J Cardiol. 1995;76:1093–1096. [DOI] [PubMed] [Google Scholar]
- 32. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butts SF, Freeman EW, Sammel MD, Queen K, Lin H, Rebbeck TR. Joint effects of smoking and gene variants involved in sex steroid metabolism on hot flashes in late reproductive‐age women. J Clin Endocrinol Metabol. 2012;97:E1032–E1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. [DOI] [PubMed] [Google Scholar]
- 35. Gorodeski EZ. Is early menopause a risk factor for heart failure? Menopause. 2014;21:558–560. [DOI] [PubMed] [Google Scholar]
- 36. Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol. 2014;306:H628–H640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gardner JD, Brower GL, Voloshenyuk TG, Janicki JS. Cardioprotection in female rats subjected to chronic volume overload: synergistic interaction of estrogen and phytoestrogens. Am J Physiol. 2008;294:H198–H204. [DOI] [PubMed] [Google Scholar]
- 38. Hsich EM, Pina IL. Heart failure in women: a need for prospective data. J Am Coll Cardiol. 2009;54:491–498. [DOI] [PubMed] [Google Scholar]
- 39. Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu Cr CR, Liu Ch CH, Azen SP; Estrogen in the Prevention of Atherosclerosis Trial Research G . Estrogen in the prevention of atherosclerosis. A randomized, double‐blind, placebo‐controlled trial. Ann Intern Med. 2001;135:939–953. [DOI] [PubMed] [Google Scholar]
- 40. de Kleijn MJ, Wilmink HW, Bots ML, Bak AA, van der Schouw YT, Planellas J, Engelen S, Banga JD, Grobbee DE. Hormone replacement therapy and endothelial function. Results of a randomized controlled trial in healthy postmenopausal women. Atherosclerosis. 2001;159:357–365. [DOI] [PubMed] [Google Scholar]
- 41. Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta‐analysis. J Gen Intern Med. 2004;19:791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski‐Wende J, Wallace R, Wassertheil‐Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women's health initiative randomized controlled trial. JAMA. 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 43. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 44. Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, Furberg CD, Kowalchuk GJ, Stuckey TD, Rogers WJ, Givens DH, Waters D. Effects of estrogen replacement on the progression of coronary‐artery atherosclerosis. N Engl J Med. 2000;343:522–529. [DOI] [PubMed] [Google Scholar]
- 45. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (HERS) research group. JAMA. 1998;280:605–613. [DOI] [PubMed] [Google Scholar]
- 46. Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, Faxon DP, Cashin‐Hemphill L, Sanmarco ME, French WJ, Shook TL, Gaarder TD, Mehra AO, Rabbani R, Sevanian A, Shil AB, Torres M, Vogelbach KH, Selzer RH; Women's Estrogen‐Progestin Lipid‐Lowering Hormone Atherosclerosis Regression Trial Research G . Hormone therapy and the progression of coronary‐artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–545. [DOI] [PubMed] [Google Scholar]
- 47. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang‐Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP, Group ER. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kling JM, Lahr BA, Bailey KR, Harman SM, Miller VM, Mulvagh SL. Endothelial function in women of the kronos early estrogen prevention study. Climacteric. 2015;18:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR, Miller VM, Neal‐Perry G, Santoro N, Taylor HS, Vittinghoff E, Yan M, Hodis HN. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–260. [DOI] [PubMed] [Google Scholar]
- 50. Appiah D, Schreiner PJ, Bower JK, Sternfeld B, Lewis CE, Wellons MF. Is surgical menopause associated with future levels of cardiovascular risk factor independent of antecedent levels? The CARDIA study. Am J Epidemiol. 2015;182:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perry JR, Corre T, Esko T, Chasman DI, Fischer K, Franceschini N, He C, Kutalik Z, Mangino M, Rose LM, Vernon Smith A, Stolk L, Sulem P, Weedon MN, Zhuang WV, Arnold A, Ashworth A, Bergmann S, Buring JE, Burri A, Chen C, Cornelis MC, Couper DJ, Goodarzi MO, Gudnason V, Harris T, Hofman A, Jones M, Kraft P, Launer L, Laven JS, Li G, McKnight B, Masciullo C, Milani L, Orr N, Psaty BM, ReproGen C, Ridker PM, Rivadeneira F, Sala C, Salumets A, Schoemaker M, Traglia M, Waeber G, Chanock SJ, Demerath EW, Garcia M, Hankinson SE, Hu FB, Hunter DJ, Lunetta KL, Metspalu A, Montgomery GW, Murabito JM, Newman AB, Ong KK, Spector TD, Stefansson K, Swerdlow AJ, Thorsteinsdottir U, Van Dam RM, Uitterlinden AG, Visser JA, Vollenweider P, Toniolo D, Murray A. A genome‐wide association study of early menopause and the combined impact of identified variants. Hum Mol Genet. 2013;22:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Appiah D, Winters SJ, Allison MA, Baumgartner RN, Groves FD, Myers JA, Hornung CA. Cardiovascular disease among women with and without diabetes mellitus and bilateral oophorectomy. Diabetes Res Clin Pract. 2015;108:473–481. [DOI] [PubMed] [Google Scholar]
- 53. Hahn RA, Eaker E, Rolka H. Reliability of reported age at menopause. Am J Epidemiol. 1997;146:771–775. [DOI] [PubMed] [Google Scholar]
- 54. Phipps AI, Buist DS. Validation of self‐reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause. 2009;16:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA. 2004;292:344–350. [DOI] [PubMed] [Google Scholar]
- 56. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circulation. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]


