Abstract
Background
Gadolinium enhancement on high‐resolution magnetic resonance imaging (MRI) has been proposed as a marker of inflammation and instability in intracranial atherosclerotic plaque. We performed a systematic review and meta‐analysis to summarize the association between intracranial atherosclerotic plaque enhancement and acute ischemic stroke.
Methods and Results
We searched the medical literature to identify studies of patients undergoing intracranial vessel wall MRI for evaluation of intracranial atherosclerotic plaque. We recorded study data and assessed study quality, with disagreements in data extraction resolved by a third reader. A random‐effects odds ratio was used to assess whether, in any given patient, cerebral infarction was more likely in the vascular territory supplied by an artery with MRI‐detected plaque enhancement as compared to territory supplied by an artery without enhancement. We calculated between‐study heterogeneity using the Cochrane Q test and publication bias using the Begg‐Mazumdar test. Eight articles published between 2011 and 2015 met inclusion criteria. These studies provided information about plaque enhancement characteristics from 295 arteries in 330 patients. We found a significant positive relationship between MRI enhancement and cerebral infarction in the same vascular territory, with a random effects odds ratio of 10.8 (95% CI 4.1–28.1, P<0.001). No significant heterogeneity (Q=11.08, P=0.14) or publication bias (P=0.80) was present.
Conclusions
Intracranial plaque enhancement on high‐resolution vessel wall MRI is strongly associated with ischemic stroke. Evaluation for plaque enhancement on MRI may be a useful test to improve diagnostic yield in patients with ischemic strokes of undetermined etiology.
Keywords: cerebral infarction, culprit artery, enhancement gadolinium, ischemic stroke, magnetic resonance imaging, plaque, vessel wall imaging
Subject Categories: Ischemic Stroke, Magnetic Resonance Imaging (MRI), Atherosclerosis
Introduction
Intracranial atherosclerosis is one of the most common causes of ischemic stroke worldwide1, 2 and is associated with a high rate of recurrence.3 The most commonly used imaging techniques to assess intracranial atherosclerosis, such as computed tomographic angiography or magnetic resonance angiography, provide information on the degree of narrowing of the vascular lumen. Most classification schemes for ischemic stroke etiology require plaque to cause ≥50% stenosis for a given stroke to be attributable to large‐artery atherosclerosis.4 However, magnetic resonance imaging (MRI) studies of the extracranial carotid arteries suggest that many atherosclerotic plaques have high‐risk features despite the absence of significant luminal narrowing.5, 6 It is unknown to what extent similar nonstenosing intracranial atherosclerotic plaque might be responsible for a proportion of the approximately 1 in 3 ischemic strokes for which no clear etiology can be determined.7
Recent investigations have begun to address this problem by leveraging high‐resolution, multiplanar MRI to detect high‐risk abnormalities of the intracranial vessel walls. Previous studies in both the coronary and extracranial carotid arteries have shown that abnormal plaque enhancement after the administration of gadolinium contrast agent is a marker of inflammation, neovascularity, and plaque instability.8, 9 For this reason, plaque enhancement has been recently studied as a potential high‐risk plaque feature in the intracranial circulation (Figure 1). Plaque enhancement is a particularly attractive MRI biomarker because it can be rapidly detected, qualitatively assessed, and does not require significant image postprocessing to analyze. While research to date on the detection of nonstenosing intracranial atherosclerotic lesions based on enhancement characteristics has been promising, individual studies have been small, making it difficult to draw firm conclusions about this emerging technique. We therefore performed a systematic review and meta‐analysis to evaluate the association between abnormal plaque enhancement on high‐resolution MRI and acute ischemic stroke.
Figure 1.
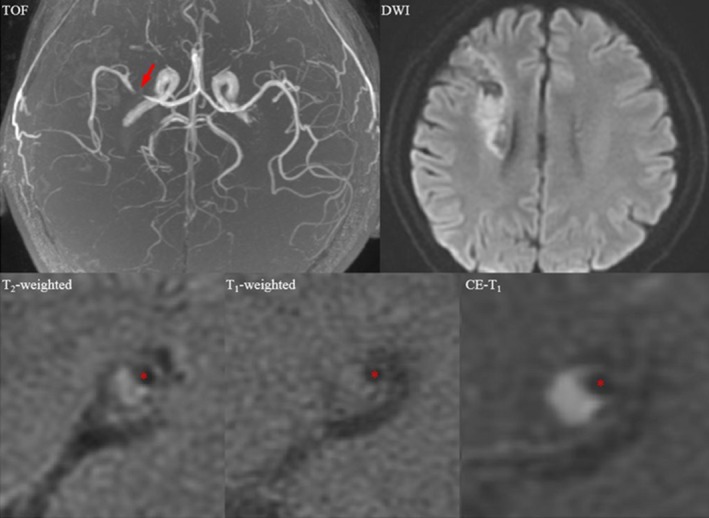
Magnetic resonance images showing an enhancing atherosclerotic plaque in the right middle cerebral artery in a symptomatic patient who had suffered a recent right cerebral hemispheric acute ischemic stroke. A mixture of acute and chronic infarction involving the right periventricular and frontal subcortical regions is seen in the diffusion‐weighted image (DWI); the plaque is shown by an arrow in the time‐of‐flight (TOF) image, and a cross section at the most stenotic site is shown in T2, T1, and contrast‐enhanced (CE) T1 images (red asterisks: lumen).
Methods
We performed this study following the Meta‐Analysis of Observational Studies in Epidemiology (MOOSE) group guidelines10 and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement.11 Because this was an analysis of previously published data, this study did not undergo or require Institutional Review Board approval.
Data Sources and Searches
Two research librarians performed comprehensive searches of Ovid MEDLINE, Ovid Embase, and the Cochrane Library from database inception to December 21, 2015. We first conducted our search in Ovid MEDLINE. We then adapted headings and keywords for other databases and identified additional records by employing the “Cited by” and “View references” features in Scopus (see Data S1 for search methodology details).
Study Selection
We included studies evaluating the association of abnormal plaque enhancement on vessel wall MRI with recent ischemic stroke. Specific inclusion criteria were as follows: (1) studies of patients with acute cerebral infarction; (2) studies of patients who underwent MRI of the intracranial vessels with assessment of plaque signal abnormalities within 30 days of the ischemic stroke; (3) specific assessment of the presence or absence of plaque postgadolinium enhancement; (4) reporting of the prevalence of plaque enhancement in the arteries directly supplying blood to the territory of the infarction compared to the prevalence of enhancement in an unrelated vascular territory (such as contralateral hemisphere for an anterior circulation infarction); and (5) studies with ≥10 subjects to avoid the inclusion of case reports or small case series. We only included peer‐reviewed journal articles rather than conference proceedings or abstracts. We did this to ensure that the studies included in our analysis provided sufficient information to allow for the collection of patient characteristics, MRI study protocols, and MRI test results in a fashion that would allow us to perform a detailed systematic review and meta‐analysis. In otherwise eligible studies, we excluded patients with infarctions that were likely secondary to nonatherosclerotic etiologies such as vasculitis or reversible cerebral vasoconstriction syndrome. We also did not include patient data for chronic ischemic strokes or transient ischemic attacks. If based on review of study inclusion dates, authors appeared to have published data from a single cohort or medical center more than once, the single article with the largest sample size was included to minimize analysis of duplicate or overlapping samples. When necessary we attempted to contact the corresponding author for additional details to clarify our data extraction.
Data Extraction and Quality Assessment
A single investigator read the title and abstract of all references produced by our database search. After preliminary articles were shortlisted as potentially eligible, 2 readers read the articles in their entirety to determine eligibility, with disagreements resolved by consensus. We extracted data in duplicate using a prespecified data collection template. A third tie‐breaking reader resolved disagreements in data extraction. We extracted the following study characteristics: first author; study design (prospective or not); major study inclusion criteria; country of the study; total number of subjects; basic study demographics and the prevalence of stroke risk factors in the studied populations, including age, sex, hypertension, diabetes, atrial fibrillation, coronary artery disease, hyperlipidemia, and smoking history; definitions of ischemic stroke and delineation of vascular territories; and specific MRI techniques employed, including definitions of abnormal plaque enhancement. We classified strokes in each of 2 groups: (1) all strokes in the vascular territory of an artery with plaque enhancement and (2) all strokes in the vascular territory of arteries free of plaque enhancement. This allowed for the calculation of a pooled odds ratio (OR) comparing the prevalence of infarction in tissue supplied by an artery with enhancing plaque versus tissue supplied by unaffected arteries, with each patient serving as his or her own control; an OR >1 would indicate an association between plaque enhancement and infarction.
We adapted risk of bias assessments in previously published meta‐analyses of MRI biomarkers of stroke risk12, 13 and generated 8 specific questions to evaluate for potential selection, detection, reporting, and confounding bias (see Data S1). Two readers assessed for risks of bias using this questionnaire, with disagreements in assessment resolved by a third tie‐breaking evaluator.
Data Synthesis and Analysis
We performed a meta‐analysis of the individual study ORs (ie, odds of the strength of association between enhancement and ipsilateral infarction) using R package “meta” (version 4.3‐2). We also performed a sensitivity analysis limited to prospective studies. We pooled ORs using a random‐effects (DerSimonian‐Laird) model and generated a forest plot to display the individual study ORs and the pooled ORs. We used a random‐effects model because we conservatively assumed that individual studies did not have the same effect size given the high possibility of between‐study heterogeneity in terms of sample size, subject characteristics, and imaging methods. The random‐effects analysis allows for more variability in the individual study OR estimates when generating the pooled OR. A continuity correction of 0.5 was applied to studies with zero cell frequencies. To assess the combinability of the ORs, we calculated the P‐value from the Cochrane Q statistical heterogeneity test. For each meta‐analysis, the presence of publication bias was evaluated through a funnel plot. The Begg‐Mazumdar rank‐correlation test was used to statistically assess the presence of publication bias. All P<0.05 were considered statistically significant.
Results
Study Selection and Characteristics
We screened a total of 4437 titles and abstracts from which we identified 8 articles14, 15, 16, 17, 18, 19, 20, 21 that met all inclusion criteria for the systematic review. Study selection steps are summarized in Figure 2. In total, the 8 studies included a combined 330 individual subjects in whom data from 295 atherosclerotic plaques provided data eligible for meta‐analysis. Of the 8 articles meeting inclusion criteria (Table 1), 514, 18, 19, 20, 21 were prospective cross‐sectional studies and 315, 16, 17 were retrospective cross‐sectional studies. Three studies were conducted in China,19, 20, 21 2 in the United States,17, 18 and 1 each in Canada,16 the Netherlands,14 and South Korea.15 A preponderance of men were studied in 7 of the 8 included studies, with the range of percent of men in each study ranging from 46.9% to 88.9%. All studies had a mean age above 50 years (range 54.6–68.7 years). There were differences in the degree of intracranial luminal stenosis measurements required for patients to be included in the individual studies, with some studies17, 18, 20 requiring ≥50% or ≥70% stenosis while others focused on patients without significant stenosis (ie, <50%).19, 21 Though some studies included patients with transient ischemic attacks or nonacute ischemic strokes, all included studies provided adequate information to collect MRI enhancement data on the subset of relevant patients with acute ischemic stroke occurring within 30 days of imaging.
Figure 2.
Study selection flow diagram.
Table 1.
Study Characteristics
| Study Number | Study First Author and Year | Study Design | Major Inclusion Criteria | Country | Total Number of Subjects | Mean Age | Men, no. (% male) | Hypertension (%) | Diabetes Mellitus (%) | Atrial Fibrillation (%) | Coronary Artery Disease (%) | Hyperlipidemia (%) | Smoking History (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | van der Kolk 201114 | Prospective | Patients admitted with ischemic infarct or TIA involving the anterior cerebral circulation; Healthy volunteers were also included | Netherlands | 32 | 59 years (range: 26–83 years) | 18 (51%) | N/A | N/A | N/A | N/A | N/A | N/A |
| 2 | Kim 201215 | Retrospective | Patients admitted with infarction involving the MCA territory or TIA due to MCA disease | South Korea | 34 | 64.9 years (SD: ±11.22) | 21 (62%) | 70.60% | 38.00% | N/A | 23.50% | 44.10% | N/A |
| 3 | Skarpathiotakis 201316 | Retrospective | Patients with documented prior ischemic stroke based on DWI and ≥1 intracranial atherosclerotic plaque on MRI | Canada | 29 | 61.2 years (range: 35–84) | 18 (62%) | N/A | N/A | N/A | N/A | N/A | N/A |
| 4 | Vakil 201317 | Retrospective | Patients with severe intracranial atherosclerosis causing ≥70% stenosis with detectable atherosclerotic plaque on MRI | United States (Illinois) | 19 | 68.7 years (SD: ±9.6) | 13 (68%) | 89.50% | 47.40% | 0% | N/A | 84.20% | 10.5% (active) |
| 5 | Qiao 201418 | Prospective | Patients with: (1) Intracranial stenosis ≥50% in a large intracranial artery; (2) TIA or stroke in the distribution of the narrowed vessel | United States (Baltimore, MD) | 27 | 56.8 years (SD: ±12.4) | 19 (70%) | 81% | 33% | N/A | N/A | 59% | 15% (active) |
| 6 | Teng 201619 | Prospective | (1) Absence of significant carotid arterial stenosis (<30%); (2) absence of atrial fibrillation; (3) absence of ascending aortic arch atheroma; and (4) ≥1 atherosclerotic risk‐factors | Shanghai, China | 139 | 57.1 years | 90 (64.7%) | 71.20% | 34.50% | N/A | 9.40% | Pre‐admission statin: 32.3% | 29% |
| 7 | Xu 201520 | Prospective | Unilateral MCA stenosis confirmed by MRI; age >50 years; ≥50% MCA stenosis; absence of nonatherosclerotic cerebrovascular disease | Xuzhou, China | 32 | 65.8 years (SD: ±13.3) | 15 (46.9%) | 78.10% | 28.10% | N/A | 37.50% | N/A | 34.40% |
| 8 | Zou 201521 | Prospective | (1) Recent single infarction confirmed by DWI; (2) no ipsilateral MCA stenosis based on MRI; (3) one or more risk factors for atherosclerosis | Beijing, China | 18 | 54.6 years (range: 40–70) | 16 (88.9%) | 66.70% | 22.20% | N/A | N/A | 55.60% | 72.20% |
DWI indicates diffusion‐weighted imaging; MCA, middle cerebral artery; N/A, data not available; MRI, magnetic resonance imaging; TIA, transient ischemic attack.
Definitions of Abnormal Plaque Enhancement and Delineation of Vascular Territories
Evaluation for abnormal plaque enhancement on all studies occurred on postcontrast T1‐weighted sequences, with most studies using a high‐resolution technique capable of submillimeter resolution (Table 2). Six studies15, 16, 18, 19, 20, 21 were performed on 3.0‐T MRI scanners, 114 on a 7.0‐T scanner, and 117 on a 1.5‐T scanner. All but 1 study16 involved more than 1 reader evaluating vessel wall MRI for abnormal vessel wall enhancement. Though there were some between‐study differences in the definition of abnormal plaque enhancement, abnormal plaque was most commonly defined as enhancement judged to be the same or greater than the degree of physiologic enhancement present in the adjacent pituitary gland. Similarly, though specific definitions varied, all studies explicitly differentiated acute neuroimaging‐confirmed infarctions occurring in the vascular territory of an artery with enhancing plaque, versus infarctions occurring in the vascular territory of an artery without enhancing plaque. All studies evaluated for enhancement in either the intracranial internal carotid artery or the middle cerebral artery.
Table 2.
MRI Protocols and Study Definitions
| Study Number | Study First Author and Year | MRI Field Strength | MRI Scanner Platform | Coil Type Used | T1‐Weighted Imaging Sequence Parameters | Field of View | Matrix | Acquired Resolution | Contrast Agent Administered | Number of MRI Readers | Definitions of Abnormal Vessel Wall Enhancement | Vessel Site Evaluated for Enhancement | Culprit and Non‐Culprit Plaque Definition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | van der Kolk 201114 | 7.0‐T | Philips Healthcare | 16‐channel receive coil | Magnetization preparation inversion recovery turbo spin‐echo sequence; TR/TE 6050/23, inversion time 1770 ms | 220×180×13 mm3 | N/A | 0.8×0.8×0.8 mm3 | 0.1 mL/kg of gadobutrol | 2 with consensus | After coregistering pre‐ and postcontrast scans, the images were subtracted and directly compared to determine enhancing areas and concomitant vessel wall anatomy. The infundibulum was used to assess whether normal contrast enhancement had taken place | Intracranial internal carotid artery | Culprit (defined during data extraction for meta‐analysis): Vessel immediately upstream of a stroke of undetermined etiology or stroke attributable to ipsilateral large vessel atherosclerosis; Non‐culprit plaque: vessels studied in the intracranial circulation but not meeting definition of culprit plaque |
| 2 | Kim 201215 | 3.0‐T | Conventional MRI: Signa, GE Medical Systems; High Resolution‐MRI: Veiro, Siemens Healthcare | 8‐channel head coil | T1‐weighted: TR/TE 600/12 | 120×120 mm | 384×269 | N/A | Gadolinium | 2 with a 3rd reader available to resolve disagreements | Atheromatous plaque: Eccentric or focal signal intensity within the vessel lumen on MRI. Abnormal enhancement: enhancement on T1‐enhanced images. Vulnerable symptomatic plaque: eccentric or focal signal intensity within vessel lumen on MRI accompanied by intraplaque heterogeneous signal intensity on T1 and T2 images and plaque | Middle cerebral artery | Vulnerable symptomatic plaque: eccentric or focal signal intensity within vessel lumen on MRI accompanied by intraplaque heterogeneous signal intensity on T1 and T2 images and plaque enhancement on T1‐enhanced images. Stable symptomatic plaque: eccentric or focal signal intensity within vessel lumen on MRI in the absence of the features described earlier in vulnerable symptomatic plaque. Culprit plaque defined as plaques upstream of ipsilateral ischemic infarctions |
| 3 | Skarpathiotakis 201316 | 3.0‐T | HDX platform, GE healthcare | 8‐channel head coil | T1 FLAIR TR/TE 2108/12, inversion time 860 ms | 16×22 cm | N/A | 384×384 | 7.5‐mL gadobutrol | 1 | Enhancement similar to pituitary parenchyma=strong; less than pituitary parenchyma=mild; no change from precontrast images=absent | Middle cerebral artery | Number of atherosclerotic plaques were tabulated and divided into plaques in stroke territory (culprit) and nonstroke territory (non‐culprit) vasculature |
| 4 | Vakil 201317 | 1.5‐T | Avanto/Espree, Siemens | Receive‐only head coil | T1‐weighted spin‐echo: TR/TE 663/15 | 220 to 240 mm | 256×256 | N/A | 0.1 mmol/kg of gadopentetate dimeglumine | 2 with a 3rd reader available to resolve disagreements | Qualitative assessment: 1=definite non‐enhancement, 2=suspected non‐enhancement, 3=uncertain, 4=suspected enhancement, 5=definite enhancement. A plaque with an average >3=enhancing, while plaque with an average <3=nonenhancing. Plaque enhancement was quantified as the relative increase in lesion T1‐signal postcontrast: mean T1 signal intensity in the outer wall area on postcontrast divided by its precontrast signal | Supraclinoid internal carotid artery | A symptomatic intracranial atherosclerotic plaque (culprit) was defined as presenting with acute neurologic symptoms and/or restricted diffusion weighted abnormalities corresponding to the vascular distribution of the intracranial stenosis. Non‐culprit plaques were asymptomatic plaques |
| 5 | Qiao 201418 | 3.0‐T | Achieva and Vista, Philips Healthcare | Body coil for transmission and 8‐channel head coil for reception | 3D black blood T1: TR/TE 2000/38 | 180×180×40 mm | 450×450×100 | 0.4×0.4×0.4 mm | 0.1 mmol/kg of gadopentetate dimeglumine | 1 reader for plaque identification and 2 for assessment of enhancement | Qualitative 3‐point scale: grade 0 enhancement similar to or less than normal vessels in the same individual; grade 1: enhancement was greater than that of grade 0 but less than that of the pituitary infundibulum; grade 2: enhancement was similar to or greater than that of the infundibulum | Cavernous and supraclinoid internal carotid artery | 1. Culprit: (a) the only lesion within the vascular territory of the stroke; (b) the most stenotic lesion when multiple plaques were present within the same vascular territory of the stroke. 2. Probably culprit: when the plaque was not the most stenotic lesion within the same vascular territory of the stroke. 3. Nonculprit: when the plaque was not within the vascular territory of the stroke |
| 6 | Teng 201519 | 3.0‐T | HDX, GE Healthcare | 8‐channel phased array brain coil | T1‐weighted: TR/TE 567/16 | 10×10 cm2 | 320×256 | N/A | 0.2 mmol/kg gadopentetic acid | 3 (2 residents, 1 neuroradiologist) with consensus | Average signal intensity enclosed between the lumen and outer wall normalized to adjacent gray matter. If normalized value >1.0=plaque enhancement | MCA | Culprit plaque: a lesion arising on the ipsilateral side to an ischemic stroke on neuro‐imaging with accompanying clinical symptoms; Non‐culprit plaque: either a plaque occurring in a contralateral artery of a symptomatic patient or one in asymptomatic controls |
| 7 | Xu 201520 | 3.0‐T | Discovery MR750, GE Healthcare | 16‐channel phase array coil | T1‐weighted: TR/TE 600/12 ms | 12×12 cm | 256×256 | N/A | 0.1 mmol/kg of gadopentetate dimeglumine | 2 with consensus | Enhancement between the outer wall boundary and vessel lumen on postcontrast images | Middle cerebral artery | Culprit: Atherosclerotic plaques upstream and ipsilateral to diffusion‐weighted imaging hyperintensity in the same vascular territory. All other plaques studied considered non‐culprit |
| 8 | Zou 201521 | 3.0‐T | Magnetom Verio, Siemens | 32‐channel head coil | T1‐weighted SPACE: TR/TE 938/24 ms | N/A | N/A | 0.5 to 0.7 mm3 | 0.1 mmol/kg of gadopentetate dimeglumine | 2 with consensus | Mean signal intensities of the MCA vessel wall on registered pre‐ and post‐contrast T1w‐SPACE were measured and normalized to gray matter. Signal intensity change ≥20% was defined as plaque enhancement | Middle cerebral artery | Culprit plaque: a lesion arising on the ipsilateral side to an ischemic stroke confirmed on brain MRI; Non‐culprit plaque: plaque occurring in a contralateral artery of a symptomatic patient |
MCA indicates middle cerebral artery; MRI, magnetic resonance imaging; N/A, data not available; SPACE, sampling perfection with application optimized contrasts using different flip‐angle evolution; T, Tesla; TE, time to echo; TR, time to repetition.
Association Between Plaque Enhancement and Cerebral Infarction
We were able to obtain sufficient raw data to calculate a pooled OR expressing the strength of association between MRI enhancement and cerebral infarction in all of our included 8 studies (Table 3). In this analysis, information about plaque enhancement characteristics was available from 295 atherosclerotic lesions, 143 of which supplied a vascular territory containing an acute infarction and 152 of which supplied an infarct‐free vascular territory. We found a significant association between MRI enhancement of an artery and stroke within the vascular territory of that same artery, with a random effects OR of 10.8 (95% CI 4.1–28.1, P<0.001, Figure 3). These results were robust to a sensitivity analysis excluding the 2 retrospective studies (OR 8.4, 95% CI 3.1–22.8, P<0.001). There was neither statistically significant heterogeneity (Q=11.08, P=0.14) nor significant publication bias (Begg‐Mazumdar test for publication bias P=0.80, Figure 4) present in this analysis.
Table 3.
MRI Test Results
| Study Number | Study First Author and Year | Total Number of Suspected Culprit Plaques Causing Downstream Ischemic Stroke Only Imaged in Acute Phase | Number of Enhancing Suspected Culprit Plaques Causing Downstream Ischemic Stroke Imaged in Acute Phase | Total Number of Non‐Culprit Plaques | Number of Enhancing Non‐Culprit Plaques |
|---|---|---|---|---|---|
| 1 | van der Kolk 201114 | 5 | 3 | 12 | 1 |
| 2 | Kim 201215 | 21 | 14a | 8b | 0 |
| 3 | Skarpathiotakis 2013c, 16 | 13 | 13 | 2 | 0 |
| 4 | Vakil 201317 | 7 | 4 | 3 | 0 |
| 5 | Qiao 201418 | 21 | 19 | 45 | 10 |
| 6 | Teng 2015d, 19 | 82 | 41 | 27 | 7 |
| 7 | Xu 201520 | 15 | 12 | 17 | 4 |
| 8 | Zou 2015e, 21 | 12 | 11 | 5 | 4 |
MRI indicates magnetic resonance imaging.
Data obtained via direct author correspondence.
The nonculprit group had no evidence of any significant middle cerebral artery (MCA) plaque (and no abnormal wall or plaque enhancement).
Three patients had multiple plaques in the same territory for which individual plaque‐level data were not available.
The nonculprit group included plaques in separately recruited asymptomatic subjects.
Authors provided enhancement data specifically for superiorly situated MCA plaques ipsilateral and contralateral to infarctions.
Figure 3.
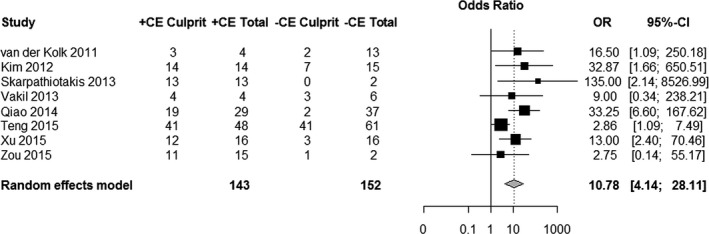
Forest plot of the association between magnetic resonance imaging–determined plaque contrast enhancement (CE) and acute ischemic stroke. The meta‐analysis was calculated using a random‐effects model, with the pooled odds ratio (OR) shown in the forest plot. Each square represents the point estimate of any given study's effect size. The size of the squares is proportional to the inverse of the variance of the estimate, while the horizontal lines represent each study's 95% CIs. The diamond represents the pooled estimate with the width of the diamond representing the pooled 95% CI.
Figure 4.
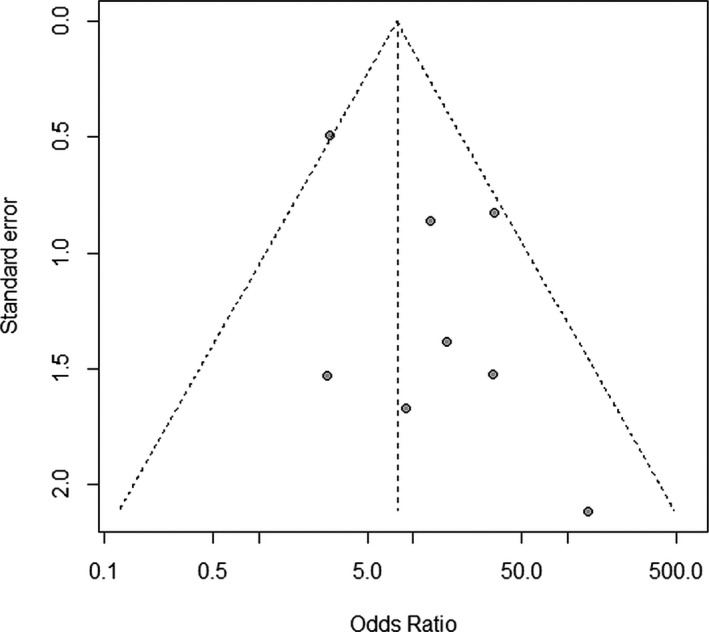
Funnel plot to evaluate for publication bias. Individual study effect sizes expressed as odds ratios are shown on the x‐axis and each study's standard error is shown on the y‐axis. Larger and more precise studies are plotted at the top, near the combined (pooled) odds ratio, whereas smaller and less precise studies will show a wider distribution below. If there is no publication bias, the studies would be expected to be symmetrically distributed on both sides of the pooled odds ratio line. In the case of publication bias, the funnel plot may be asymmetrical, since the absence of studies would distort the distribution on the scatter plot.
Assessment of the Quality of the Included Studies
The results from the quality assessment questionnaire are shown in Table 4. All studies provided detailed description of study inclusion/exclusion criteria and 6 of 8 studies14, 17, 18, 19, 20, 21 were prospectively conducted. Seven15, 16, 17, 18, 19, 20, 21 of 8 studies had as a primary objective to determine whether enhancement was associated with acute ischemic change in the brain. Similarly, 715, 16, 17, 18, 19, 20, 21 of 8 studies had investigators blinded to the location of infarction during vessel wall enhancement detection and utilized more than 1 reader to evaluate for plaque enhancement, though only 2 studies17, 18 reported interreader reproducibility measures. The classification of strokes as occurring in the territory of enhancing arterial plaque versus in territory without enhancing plaque occurred by an adjudicated panel of more than 1 investigator in only 1 study.18 Finally, no studies consistently matched enhancing plaques leading to downstream infarction versus plaque in infarct‐free territory in regard to stenosis severity.
Table 4.
Risk of Bias Question Results
| Question | Answers | van der Kolk 201114 | Kim 201215 | Skarpathiotakis 201316 | Vakil 201317 | Qiao 201418 | Teng 201519 | Xu 201520 | Zou 201521 |
|---|---|---|---|---|---|---|---|---|---|
| Was the study sample prospectively selected to minimize the risk of selection bias? | Yes (+) or no (−) | + | + | − | − | + | + | + | + |
| Were the inclusion and exclusion criteria adequately described? | Yes (+) or no (−) | + | + | + | + | + | + | + | + |
| Was the study's primary objective to assess whether enhancement was associated with ischemic presentations? | Yes (+) or no (−) | − | + | + | + | + | + | + | + |
| Were the investigators blinded to the location of infarction during vessel wall enhancement detection? | Yes (+) or no (−) | − | + | + | + | + | + | + | + |
| Did more than 1 investigator assess for the presence of vessel enhancement? | Yes (+) or no (−) | + | + | − | + | + | + | + | + |
| Was a measure of interreader reproducibility for enhancement detection reported? | Yes (+) or no (−) | − | − | − | + | + | − | − | − |
| Did more than 1 investigator adjudicate culprit lesion detection? | Yes (+) or no (−) | − | − | − | − | + | − | − | − |
| Were culprit and nonculprit lesions matched in terms of vessel stenosis severity? | Yes (+) or no (−) | − | − | − | − | − | − | − | − |
Note: If data not provided or not specified, recorded as no (−).
Discussion
Increasing the diagnostic confidence that a specific atherosclerotic lesion is the cause of a patient's ischemic stroke is important because it can guide optimal secondary stroke prevention measures. By characterizing plaque characteristics beyond luminal stenosis, MRI of the vessel wall is a promising technique to detect specific plaque features associated with acute ischemic infarction. In this systematic review and meta‐analysis we studied nearly 300 plaques imaged with vessel wall MRI pooled together from 8 individual studies, and focused on 1 such high‐risk atherosclerotic plaque feature, enhancement after gadolinium administration. We found that plaque enhancement is strongly associated with downstream acute infarction. Infarction was 10 times more likely in tissue supplied by an enhancing artery than in tissue supplied by nonenhancing arteries.
The mechanisms of plaque enhancement in vulnerable intracranial atherosclerotic plaques are likely complex and multifactorial. Much of our understanding of the pathophysiology of gadolinium enhancement in the cerebral arterial vessel wall arises from literature on the extracranial carotid arteries where endarterectomy specimens are more readily accessible for pathological correlation. In these studies, carotid plaque gadolinium enhancement has been shown to spatially correlate with histological markers of vessel wall neovascularization and inflammation, both well‐known markers of unstable atherosclerotic plaque.9 Enhancement detectable on MRI may be related to endothelial dysfunction present in the diseased intraplaque microvasculature of atherosclerotic vessels.22 Such compromised microvascular endothelium likely results in the vascular leakage needed for gadolinium to accumulate in the perivascular spaces and become detectable on T1‐weighted MRI sequences. However, it is important to note that unlike the extracranial carotid arteries, most intracranial vessels do not have a vasa vasorum, though there is evidence that arteriosclerosis can promote the development of vasa vasorum in the proximal intracranial vasculature.23, 24 As a result, though it is likely that a leaky endothelial barrier due to inflammation is responsible for the gadolinium enhancement seen in the studies included in this meta‐analysis, the specific contribution of vasa vasorum to this enhancement requires further histopathological evaluation.
Understanding the time course of intracranial plaque enhancement after an acute ischemic event would help optimize the timing and diagnostic yield of intracranial vessel wall MRI in patients presenting with ischemic stroke. One study16 in this meta‐analysis also focused on the longitudinal pattern of plaque enhancement after ischemic stroke and found that the strength of enhancement was its highest in the acute stroke phase (within 4 weeks), and that enhancement steadily decreased as the infarct became chronic in age (after 12 weeks). Though there are limited data suggesting the plaque enhancement may also predict future stroke recurrence,25 further work is necessary to more fully elucidate the temporal relationships between MRI plaque enhancement, prior ischemic stroke, and future ischemic stroke risk.
We focused our analysis on gadolinium enhancement rather than other specific proposed high‐risk plaque elements such as intraplaque hemorrhage, fibrous cap abnormalities, or lipid‐rich necrotic core because of the paucity of studies evaluating these specific elements in the intracranial circulation. The lack of studies evaluating these features may be because these specific elements are much more difficult to detect in the intracranial vasculature due to its smaller size and deeper location compared to the extracranial carotid arteries where they have been more well characterized, including in studies with histopathological confirmation.13 Similarly, though some recent studies have proposed quantitative biomarkers of high‐risk plaque such as arterial remodeling ratios or surface area measurements of plaque,19, 20, 21 such measures require intensive and precise measurements of plaque that may be challenging to implement into clinical practice. Evaluating for the presence or absence of gadolinium enhancement, on the other hand, may offer a relatively simple and rapid method of detecting high‐risk plaque that could be used routinely in MRI examinations of patients presenting with acute ischemic stroke.
Our study has revealed some limitations about the existing literature evaluating the relationship between plaque MRI enhancement and acute ischemic stroke. First, though there was general agreement on the MRI features of plaque enhancement, including a greater degree of signal intensity than normal vessel wall enhancement, the reference standard by which abnormal enhancement is determined varied by study. The pituitary gland may offer an easily identifiable reference standard by which plaque enhancement can be subjectively assessed since it is located in close proximity to the circle of Willis vessels, thereby ensuring its presence in the MRI field‐of‐view. Indeed, good interobserver consistency was shown using the pituitary gland as a reference to judge enhancement in the 1 study18 reporting interpretive reproducibility. Future studies will be strengthened if authors report interreader reproducibility measures more consistently in their interpretation of intracranial vessel wall MRI studies. Second, we found that studies did not routinely control for or match enhancing plaques leading to downstream infarction versus plaque in infarct‐free territory in regard to stenosis severity. Indeed, stenosis severity was not systematically reported in all included studies. This makes it unclear to what extent stenosis severity and plaque volume may have acted as confounding factors in the strong association we found between plaque enhancement and ipsilateral ischemic stroke. Third, we found variability in how rigorously patients with alternative causes of ischemic stroke such as cardioembolism or more proximal extracranial artery‐to‐artery embolism were excluded from individual studies. Therefore, it is possible that infarcts attributed to an enhancing plaque supplying that vascular territory may have actually been caused by another mechanism. We believe that this risk is relatively low given our within‐subjects design, in which infarcts in the territory of enhancement were compared to infarcts in other territories within the same patient. In other words, if an infarct were due to cardioembolism, that infarct should not be more likely to occur in the territory of an enhancing plaque than in the vascular territory of an artery without enhancing plaque. However, we believe that future studies should be conducted in patients with strictly defined cryptogenic stroke in whom nonstenosing intracranial atherosclerotic plaques are present. Fourth, existing data only provided a crude OR and not covariate‐adjusted OR. However, because most studies utilized a within‐subject design, the risk of confounding systemic vascular risk factors should be relatively low. Fifth, as an emerging diagnostic technique, there are relatively few studies addressing the topic of intracranial plaque enhancement and its association with ischemic stroke. As such, though our study showed no statistical evidence of publication bias, this possibility should be re‐evaluated as more studies are published in this field since an increasing study sample size will allow for a more valid and reliable estimate of publication bias. Sixth, there were differences in the MRI protocols used to evaluate for enhancing intracranial plaques, including differences in magnet field strength and specific pulse sequences used by investigators. We believe that for intracranial vessel wall MRI to be a useful tool in both clinical trial and patient care settings, future efforts to further standardize MRI acquisition parameters are warranted.
In summary, our systematic review and meta‐analysis suggests that MRI‐detected intracranial plaque enhancement is strongly associated with ipsilateral acute ischemic stroke. Plaque enhancement may offer a relatively simple means to detect a high‐risk vessel wall feature that may play a complementary role to luminal stenosis measurements in diagnosing stroke etiology. Since conventional stroke etiology classification schemes attribute the cause of a stroke to large vessel intracranial atherosclerosis only when luminal stenosis is ≥50%, MRI for plaque enhancement may be most useful to evaluate the etiology of strokes currently considered cryptogenic. Future prospective studies in patients with ischemic strokes of undetermined etiology should be undertaken in which patients demonstrating vessel wall enhancement are followed for recurrent ischemic events. Such studies are necessary to test whether proven medical therapies for secondary stroke reduction are beneficial in patients with an enhancing intracranial plaque in the vascular territory of an acute ischemic stroke.
Sources of Funding
This study was funded by grant K23NS082367 (Kamel) from National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) and by grant KL2TR000458 (Gupta) from National Institutes of Health/National Center for Advancing Translational Sciences (NIH/NCATS).
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Acknowledgments
The authors wish to thank Dr Jeong‐Min Kim, MD, PhD for providing unpublished data used in this meta‐analysis.
(J Am Heart Assoc. 2016;5:e003816 doi: 10.1161/JAHA.116.003816)
References
- 1. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. [DOI] [PubMed] [Google Scholar]
- 2. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. [DOI] [PubMed] [Google Scholar]
- 3. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL Jr, Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 5. Gupta A, Gialdini G, Lerario MP, Baradaran H, Giambrone A, Navi BB, Marshall RS, Iadecola C, Kamel H. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015;4:e002012 doi: 10.1161/JAHA.115.002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freilinger TM, Schindler A, Schmidt C, Grimm J, Cyran C, Schwarz F, Bamberg F, Linn J, Reiser M, Yuan C, Nikolaou K, Dichgans M, Saam T. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. 2012;5:397–405. [DOI] [PubMed] [Google Scholar]
- 7. Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, Harris D, Williams EB, Horgan G, Kyne L, McCormack PM, Duggan J, Moore A, Crispino‐O'Connell G, Kelly PJ. Stroke subtype classification to mechanism‐specific and undetermined categories by TOAST, A‐S‐C‐O, and causative classification system: direct comparison in the North Dublin population stroke study. Stroke. 2010;41:1579–1586. [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim T, Makowski MR, Jankauskas A, Maintz D, Karch M, Schachoff S, Manning WJ, Schomig A, Schwaiger M, Botnar RM. Serial contrast‐enhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC Cardiovasc Imaging. 2009;2:580–588. [DOI] [PubMed] [Google Scholar]
- 9. Millon A, Boussel L, Brevet M, Mathevet JL, Canet‐Soulas E, Mory C, Scoazec JY, Douek P. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke. 2012;43:3023–3028. [DOI] [PubMed] [Google Scholar]
- 10. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 12. Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, Wright C, Beiser AS, Seshadri S, Pandya A, Kamel H. Silent brain infarction and risk of future stroke: a systematic review and meta‐analysis. Stroke. 2016;47:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta‐analysis. Stroke. 2013;44:3071–3077. [DOI] [PubMed] [Google Scholar]
- 14. van der Kolk AG, Zwanenburg JJ, Brundel M, Biessels GJ, Visser F, Luijten PR, Hendrikse J. Intracranial vessel wall imaging at 7.0‐T MRI. Stroke. 2011;42:2478–2484. [DOI] [PubMed] [Google Scholar]
- 15. Kim JM, Jung KH, Sohn CH, Moon J, Han MH, Roh JK. Middle cerebral artery plaque and prediction of the infarction pattern. Arch Neurol. 2012;69:1470–1475. [DOI] [PubMed] [Google Scholar]
- 16. Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. 2013;34:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vakil P, Vranic J, Hurley MC, Bernstein RA, Korutz AW, Habib A, Shaibani A, Dehkordi FH, Carroll TJ, Ansari SA. T1 gadolinium enhancement of intracranial atherosclerotic plaques associated with symptomatic ischemic presentations. AJNR Am J Neuroradiol. 2013;34:2252–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, Wasserman BA. Intracranial plaque enhancement in patients with cerebrovascular events on high‐spatial‐resolution MR images. Radiology. 2014;271:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teng Z, Peng W, Zhan Q, Zhang X, Liu Q, Chen S, Tian X, Chen L, Brown AJ, Graves MJ, Gillard JH, Lu J. An assessment on the incremental value of high‐resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol. 2016;26:2206–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu P, Lv L, Li S, Ge H, Rong Y, Hu C, Xu K. Use of high‐resolution 3.0‐T magnetic resonance imaging to characterize atherosclerotic plaques in patients with cerebral infarction. Exp Ther Med. 2015;10:2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zou XD, Chung YC, Zhang L, Han Y, Yang Q, Jia J. Middle cerebral artery atherosclerotic plaques in recent small subcortical infarction: a three‐dimensional high‐resolution MR study. Biomed Res Int. 2015;2015:540217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, Duimel H, Frederik PM, van Hinsbergh VW, Virmani R, Daemen MJ. Thin‐walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Portanova A, Hakakian N, Mikulis DJ, Virmani R, Abdalla WM, Wasserman BA. Intracranial vasa vasorum: insights and implications for imaging. Radiology. 2013;267:667–679. [DOI] [PubMed] [Google Scholar]
- 24. Aydin F. Do human intracranial arteries lack vasa vasorum? A comparative immunohistochemical study of intracranial and systemic arteries. Acta Neuropathol. 1998;96:22–28. [DOI] [PubMed] [Google Scholar]
- 25. Kim JM, Jung KH, Sohn CH, Moon J, Shin JH, Park J, Lee SH, Hee Han M, Roh JK. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke. 2016;11:171–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.


