Abstract
Background
Bypass grafting remains the standard of care for coronary artery disease and severe lower extremity ischemia. Efficacy is limited by poor long‐term venous graft patency secondary to intimal hyperplasia (IH) caused by venous injury upon exposure to arterial pressure. We investigate whether photochemical tissue passivation (PTP) treatment of vein grafts modulates smooth muscle cell (SMC) proliferation and migration, and inhibits development of IH.
Methods and Results
PTP was performed at increasing fluences up to 120 J/cm2 on porcine veins. Tensiometry performed to assess vessel elasticity/stiffness showed increased stiffness with increasing fluence until plateauing at 90 J/cm2 (median, interquartile range [IQR]). At 90 J/cm2, PTP‐treated vessels had a 10‐fold greater Young's modulus than untreated controls (954 [IQR, 2217] vs 99 kPa [IQR, 63]; P=0.03). Each pig received a PTP‐treated and untreated carotid artery venous interposition graft. At 4‐weeks, intimal/medial areas were assessed. PTP reduced the degree of IH by 66% and medial hypertrophy by 49%. Intimal area was 3.91 (IQR, 1.2) and 1.3 mm2 (IQR, 0.97; P≤0.001) in untreated and PTP‐treated grafts, respectively. Medial area was 9.2 (IQR, 3.2) and 4.7 mm2 (IQR, 2.0; P≤0.001) in untreated and PTP‐treated grafts, respectively. Immunohistochemistry was performed to assess alpha‐smooth muscle actin (SMA) and proliferating cell nuclear antigen (PCNA). Objectively, there were less SMA‐positive cells within the intima/media of PTP‐treated vessels than controls. There was an increase in PCNA‐positive cells within control vein grafts (18% [IQR, 5.3]) versus PTP‐treated vein grafts (5% [IQR, 0.9]; P=0.02).
Conclusions
By strengthening vein grafts, PTP decreases SMC proliferation and migration, thereby reducing IH.
Keywords: cardiovascular disease, collagen, hyperplasia, intima‐media thickness, vein graft
Subject Categories: Smooth Muscle Proliferation and Differentiation, Vascular Disease, Stenosis
Introduction
Atherosclerosis is the underlying cause of coronary artery disease (CAD) and peripheral artery disease (PAD), two of the most prevalent pathological conditions in the United States. CAD affects ≈15.4 million people over age 20 and, in 2009, was responsible for one of every six deaths.1 Additionally, in 2010, an estimated 202 million people worldwide were affected by PAD.2 Though pharmacological and noninvasive therapies may improve atherosclerosis, the number of cardiovascular operations and procedures nevertheless increased by 28% in the decade between 2000 and 2010.1
Although percutaneous intervention with balloon angioplasty and/or stenting is performed for both CAD and PAD, surgical bypass grafting remains the standard of care for multivessel CAD and severe lower‐extremity ischemia. Indeed, coronary artery bypass grafting (CABG) has proven to be superior to percutaneous coronary interventions in patients with multivessel disease or diabetes mellitus, where it has significantly reduced patient morbidity and mortality.3, 4 Moreover, peripheral artery bypass surgery has demonstrated both improved amputation‐free survival and overall survival as compared to balloon angioplasty.5
For both CABG and peripheral artery bypass, autologous veins have proven to be the conduit of choice because of their availability, improved patency, and decreased risk of infection compared to synthetic grafts. However, despite the widespread use of vein grafts and the superiority of surgical bypass over percutaneous intervention, the effectiveness of autologous vein graft remains limited by poor long‐term patency. For example, the patency rate of saphenous vein grafts (SVGs) used in CABG procedures declines around 10% to 30% over the first year and 50% by 10 years.6, 7 Similarly, 5‐year patency rates as low as 25% to 55% have been reported when SVG is used for infragenicular bypass.8
Vein graft failure is ascribed to the luminal narrowing that results from intimal hyperplasia (IH), medial thickening, and subsequent concomitant accelerated atherosclerosis. In a recent analysis of vein graft failure, early denudation of endothelium attributed to surgical trauma, longitudinal wall (shear) stress, and venous exposure to increased arterial pressure were found to be the primary triggers toward the development of intimal hyperplasia.9 When a vein is exposed to arterial pressure, there is an increase in wall shear stress, triggering an adaptive response of vessel dilatation to accommodate the increased blood pressure and flow. This aggressive vessel dilatation increases wall tension, which leads to medial thickening, an additional adaptive response. The increased venous vessel wall tension that occurs after exposure to arterial pressure is analogous to that which occurs during a balloon angioplasty intervention. The shear stress and vessel stretch disrupts the vessel wall, in turn damaging the endothelial and medial smooth muscle cells (SMCs).10 This rapid change in the structural integrity of the vein is critically involved in the subsequent development of IH and SVG failure.
Although the pathway for IH remains complex with involvement of numerous cytokines, interleukins, and cellular interactions, few interventions have been employed to successfully impede its occurrence. However, one intervention currently under trial in humans is mechanical confinement through the use of external stents. Recent findings have shown a significant reduction in IH in externally stented SVG compared to control SVG in a multivessel CABG model.11 However, though external stents demonstrate the potential of preventing vessel dilatation and reducing IH, the clinical application of these stents may be limited secondary to the technical difficulty of insertion and the potential for foreign body response, infection, or erosion into adjacent structures.
With this in mind, we have developed photochemical tissue passivation (PTP) as a novel therapy that strengthens/stiffens venous adventitia and decreases vessel compliance. This is accomplished without the use of foreign bodies, thereby eliminating the risks associated with prosthetic stents. PTP is a technology that cross‐links extracellular tissue proteins (ie, collagen) by a light‐activated process. The tissue is stained with a photosensitizing dye, Rose Bengal (RB), which is activated with low‐energy visible light to generate collagen cross‐links that strengthen the venous conduit. The cross‐linking is highly selective and only occurs in the tissue volume where both light and RB are present (ie, the adventitia). PTP does not compromise the structural integrity of the endothelium, effectively limiting the subsequent injury cascade, which leads to IH and SVG failure.
Our laboratory previously demonstrated proof of principle of this approach for reducing IH in a rat arterialized vein graft model.12 Whereas PTP has been successful at inhibiting IH in a murine model, large animal models more closely mimic human condition and are superior to murine models for translational medicine.13 Pigs, in particular, have been found to have structurally similar vessels to humans, with equivalent response to vascular injury.14 Additionally, pigs are particularly prone to rapid development of IH.15, 16 Thus, the objective of this study is to investigate whether PTP treatment of vein grafts will modulate SMC proliferation and migration, and inhibit development of IH in a swine model.
Methods
PTP Treatment
PTP is performed by complete coating of the external aspect of the vein (adventitia) with sterile 0.1% RB (w/v) in saline and then exposing the tissue to green light. RB was obtained from Sigma‐Aldrich (St. Louis, MO) and prepared as a 0.1% (w/v) solution in sterile normal saline (Owens & Minor, Mechanicsville, VA). The solution was then filtered through a 0.22‐μm Steriflip filter to ensure sterility of the applied dye solution. The treatment dose, also known as the fluence (J/cm2), was adjusted by varying either the distance of the light source from the tissue (and hence the irradiance [W/cm2]) or the duration of light exposure(s). Illumination was provided by a PhotoMedex Omnilux green LED diode array light source (PhotoMedex, Philadelphia, PA) emitting at a maximum wavelength of 550 nm with a full width at half maximum of ≈40 nm. All SVGs were harvested from the swine and immediately treated in a prototype illumination chamber (Figure 1A through 1C). The graft was attached to a rotor that spun the graft in front of the diode source at a frequency of 1 cycle/sec to ensure equal illumination of the full surface of the SVG.
Figure 1.
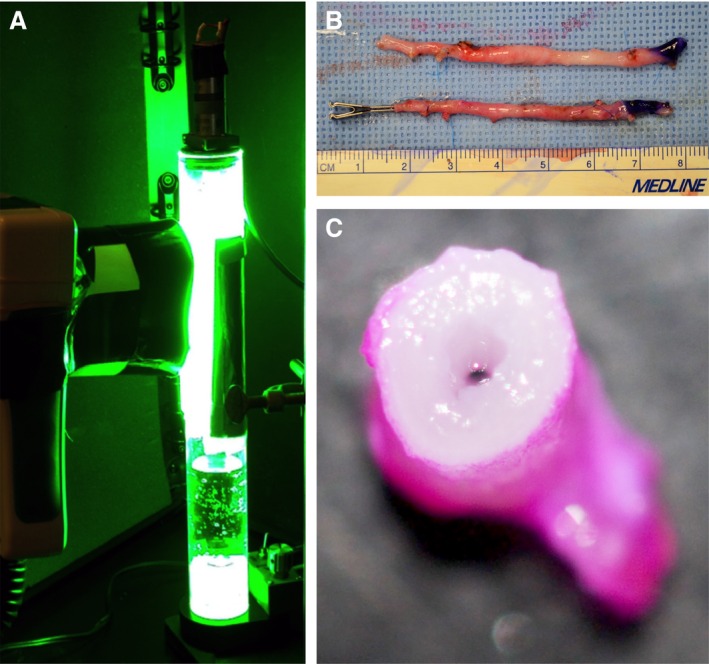
A, Photochemical tissue passivation (PTP) being performed on a porcine vein within a prototype illumination chamber. B, A PTP‐treated porcine saphenous vein (inferior) appears similar to an untreated porcine saphenous vein (superior) before installation as a vein graft. C, A porcine saphenous vein coated with Rose Bengal (RB) before exposure to green light‐emitting diode light. Note the minimal penetrance of RB within the vessel wall.
RB Vessel Penetration
To determine the depth of penetration of RB, the adventitial aspect of seven 1‐mm porcine saphenous vein specimens were coated with 0.1% (w/v) RB. Following RB application, specimens were visualized and imaged under a Nikon Eclipse E600 microscope (Nikon Corp., Tokyo, Japan) at ×40 magnification. Measurements of RB penetration were performed with the use of Adobe Photoshop (Adobe Systems, San Jose, CA).
Tensiometry
In order to determine an ideal fluence for PTP treatment of porcine vein grafts, we initially determined the dependence of vein mechanical properties on fluence of PTP in ex vivo porcine vein specimens. Specifically, six 2‐cm porcine lateral saphenous vein specimens were filleted open to create flat, noncylindrical specimens. These specimens were coated along their adventitial surfaces with 0.1% RB (w/v) and exposed to green light at an irradiance of 0.102 W/cm2 for different durations, resulting in treatment fluences of 0, 30, 60, 90, and 120 J/cm2. To quantitatively demonstrate the effect of PTP on vessel elasticity (stiffness), each specimen underwent tensiometry (MTESTQuattro; ADMET, Norwood, MA) before and after each increment of PTP fluence. Tensiometry involved one 0.5‐mm extension and relaxation cycle at a rate of 0.5 mm per minute. Peak load (N) at 0.5‐mm extension and Young's Modulus (kPa) were ascertained for each test. In order to preclude any effect of dehydration on vessel elasticity, veins were soaked in normal saline between each tensiometry cycle.
Compliance Testing
To quantitatively demonstrate the effect of PTP on vessel compliance, 3‐cm‐long porcine lateral saphenous vein samples from Yorkshire pigs were harvested utilizing a “no‐touch” surgical technique. All branches of these vessels were ligated at the time of harvest. Vessels were randomized to receive PTP treatment (n=6) or remain untreated (n=11). Carotid arteries (n=6) were harvested from Yorkshire pigs as well to serve as an arterial control. PTP was performed in the same manner as described above. Briefly, the external aspect of each vessel was coated with 0.1% RB (w/v) and exposed to green light for a fluence of 90 J/cm2. Experimental vessels were sealed at both ends and the pressure was recorded as a function of volume of saline injected into the vessel as measured by a force transducer. Specifically, the vessel lumen was securely applied over a 1‐cm‐long 24‐gauge angiocatheter attached to the manometer. The other end of the vessel was suture‐ligated to prevent fluid leak. Normal saline (1 cc) was instilled into each vessel in 0.1‐cc increments. For each 0.1 cc instilled, the corresponding pressure value was recorded. The slope of the force/volume plot is proportional to stiffness and inversely proportional to compliance.
Animals and Operative Procedure
Three‐ to 4‐month‐old Yorkshire pigs (n=5; 40–50 kg) were purchased from Tufts University (Grafton, MA) and kept in standard animal care facilities at the Massachusetts General Hospital (Boston, MA). All experiments and animal care conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Anesthesia was induced with Telazol 4.4 mg/kg and Xylazine 2.2 mg/kg intramuscularly and maintained with isoflurane 1% to 5% (titrated to effect). The pig was intubated and received preoperative doses of cefazolin 40 mg/kg intravenously and buprenorphine 0.01 to 0.05 mg/kg intramuscularly. We chose to utilize a previously validated porcine vein graft model described by Mehta et al.17 The neck and bilateral hindlimbs of the animal were prepped and draped in sterile fashion. The operation began with bilateral lateral saphenous vein harvest. Incisions were made on the posterolateral aspect of each hindlimb between the animal's ankle and knee. The vein was dissected with suture ligation or bipolar electrocautery of all branches. A vein segment of at least 7‐cm was harvested from each lower extremity. Vessel diameter was recorded upon harvest of the vessel. One of the venous specimens was completely externally coated with 0.1% RB (w/v) and then illuminated with green light (λmax=550 nm) for a fluence of 90 J/cm2, whereas the other was left as an untreated control.
Following completion of vein dissection and PTP treatment, attention was turned to the recipient site on the animal. A midline anterior neck incision was made and the bilateral carotid arteries were carefully exposed. Before carotid artery clamping, intravenous heparin was administered (80 U/kg) as an initial bolus, followed by 2000 U after the first hour and 1000 U each hour after that until all anastomoses were completed. Following the initial heparin bolus, one carotid artery was clamped proximally and distally and a 2‐cm segment of common carotid artery was resected with the exposed ends beveled at 45 degrees. Then, a ≈4.5‐cm segment of reversed saphenous vein (either PTP‐treated or untreated) underwent proximal followed by distal anastomosis with interrupted 8‐0 Prolene sutures. Following completion of one graft, the next graft was performed in the same manner. Each graft was immersed in 1% lidocaine to decrease vasospasm. Immediate patency was confirmed with Doppler ultrasound. Fentanyl patches (50–100 μg/h) were applied for 72 hours of postoperative analgesia. The location (left or right) and order of performing the grafts were randomized between procedures.
Harvest
Grafts were recovered 1‐month after placement. The animal was placed under general anesthesia, as described above, and grafts were delicately exposed. Patency was confirmed with Doppler ultrasound at the time of harvest. The pig was then euthanized with 100 mg of intravenous euthasol and grafts (including proximal and distal common carotid artery) removed. Immediately after harvest, grafts were gently flushed with heparinized saline and then fixed in 10% formalin.
Histology and Immunohistochemistry
Formalin‐fixed grafts were divided into six segments and paraffin embedded. These segments spanned the proximal anastomosis, vein graft, and distal anastomosis. Five‐micron‐thick paraffin‐embedded sections were then cut on a microtome and used for histological, morphometric, and immunohistochemical analyses.
All sections from each specimen were prepared for light microscopy using hematoxylin and eosin stain and a Verhoeff Van Gieson elastin stain. Sections from each specimen demonstrating the greatest degree of IH were also assessed for cellular expression of α‐smooth muscle actin (SMA; 1:2000; Abcam, Cambridge, MA, 1:2000), an indicator of SMC or myofibroblast cells, and proliferating cell nuclear antigen (PCNA; 1:6000; Abcam), an indicator of cellular proliferation. Briefly, following deparaffinization and hydration, SMA slides were incubated with the primary antibody for 12 hours at 4°C and the secondary antibody (goat anti‐mouse immunoglobulin G [IgG]; 1:5000; Abcam) for 1‐hour at room temperature. PCNA slides were incubated with the primary antibody for 2‐hours at room temperature and the secondary antibody (goat anti‐mouse IgG, 1:500; Abcam) for 1‐hour at room temperature. Appropriate washes with 1% TBS or water were performed between each step. Slides were developed using 3,3′‐diaminobenzidine in a chromogen solution (ab64238; Abcam). Slides were then counterstained with hematoxylin and mounted using Permount medium. A brown color indicated a positive stain. Negative controls were performed for each run by substituting the primary antibody with 1% TBS.
All slides were imaged with a Nikon Eclipse E600 microscope (Nikon Corp.) and a Nanozoomer whole‐slide scanner (Hamamatsu Corp., Hamamatsu City, Japan). All sections of each specimen were evaluated for intimal area, medial area, maximal intimal thickness, and maximal medial thickness and reported as a median, interquartile (IQR). Intima was defined as the area between the endothelium and the internal elastic lamina, and media was defined as the area between the internal and external elastic laminae. Measurements were performed with the use of Adobe Photoshop (Adoobe Systems). Intimal/medial ratio (I/M) was also recorded to correct for possible artifactually increased intimal/medial thickness in nonpressure fixed vessels.
For quantification of PCNA staining, the number of PCNA‐positive cells and the total number of cells were counted in four quadrants of each slide at ×200 magnification. The degree of cellular proliferation was defined as a PCNA index (number of PCNA‐positive cells divided by the total number of cells).18
Statistical Analysis
Data are expressed as median, IQR. All data were treated as independent, without normal distribution, and group comparisons were made using the Mann–Whitney U rank‐sum test. A P value of <0.05 was considered statistically significant. Tensiometry results were compared using the Wilcoxon signed‐rank test as repeated measurements of increased fluence on each vessel were performed. Data analysis was performed using StatPlus statistical software (AnalystSoft, Inc., Vancouver, British Columbia, Canada).
Results
RB Penetration of Porcine Veins
We assessed the degree of vein RB penetration to determine the area of the vein that receives enhanced collagen cross‐linking with PTP treatment. On average, RB penetrance measured 91±27 μm (mean±SD). Grossly, the RB did not appear to penetrate into the medial layer of vessels. PTP‐mediated collagen cross‐linking is highly selective and only occurs in the tissue where both light and RB are present. Thus, PTP does not alter the medial or intimal architecture at the time of vessel treatment.
Tensiometry and Compliance Testing
The average peak load at 0.5‐mm extension and the average Young's modulus of elasticity for venous samples increased with increasing fluence, until plateauing by a fluence of 90 J/cm2 (Figure 2A and 2B). The peak load at 0.5‐mm extension for PTP‐treated vessels with a fluence of 90 J/cm2 was significantly greater than untreated controls (0.0469 [IQR, 0.0766] vs 0.0075 N [IQR, 0.0011]; P=0.028). Additionally, the Young's modulus was 10‐fold greater for PTP‐treated vessels with a fluence of 90 J/cm2 than for untreated controls (954 [IQR, 2217] vs 99 kPa [IQR, 63]; P=0.028). Because there was no statistically significant difference between treatment fluences of 90 and 120 J/cm2, we chose to utilize a fluence of 90 J/cm2 for vessel compliance testing.
Figure 2.
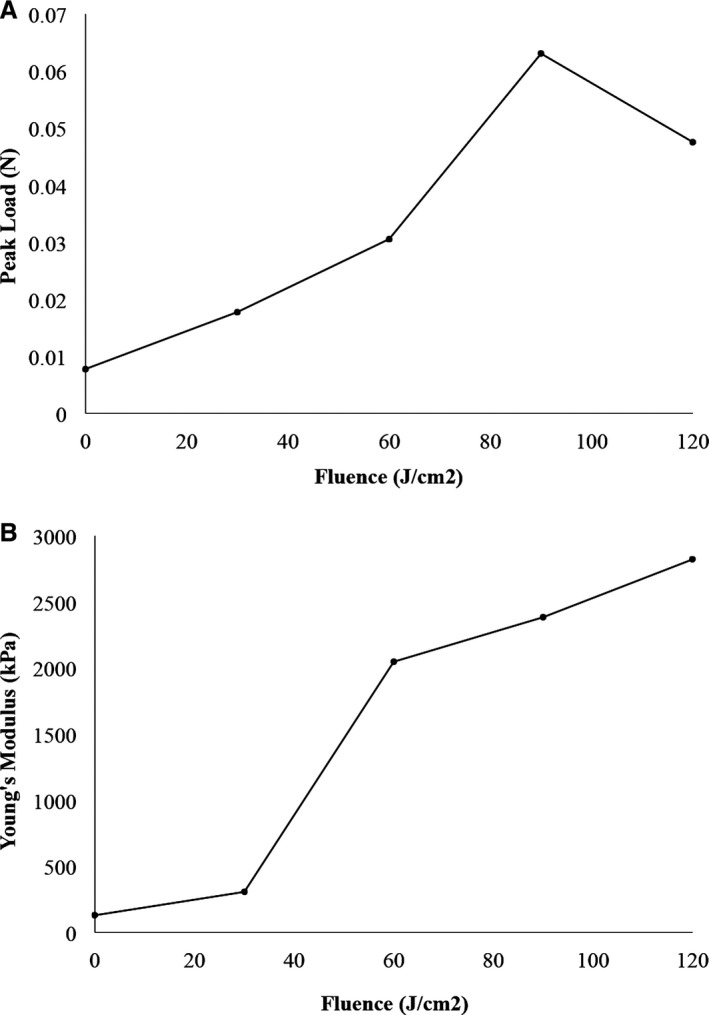
A, Average peak load at 0.5‐mm extension for venous samples with increasing fluence. Fluence zero represents the vein before photochemical tissue passivation treatment. B, Average Young's modulus of elasticity for venous samples with increasing fluence. Fluence zero represents the vein before PTP treatment.
Compliance testing demonstrated a significantly increased relative stiffness (or decreased compliance) of PTP‐treated porcine vein compared to untreated porcine vein (882 [IQR, 223] vs 133 mm Hg/mL [IQR, 151]; P=0.001; Figure 3). In fact, PTP treatment of porcine vein increased the degree of relative stiffness to that of a porcine artery (882 [IQR, 223] vs 1214 mm Hg/mL [IQR, 471]; P=0.2). These ex vivo studies confirmed that PTP significantly decreases vein graft compliance and decreases elasticity.
Figure 3.
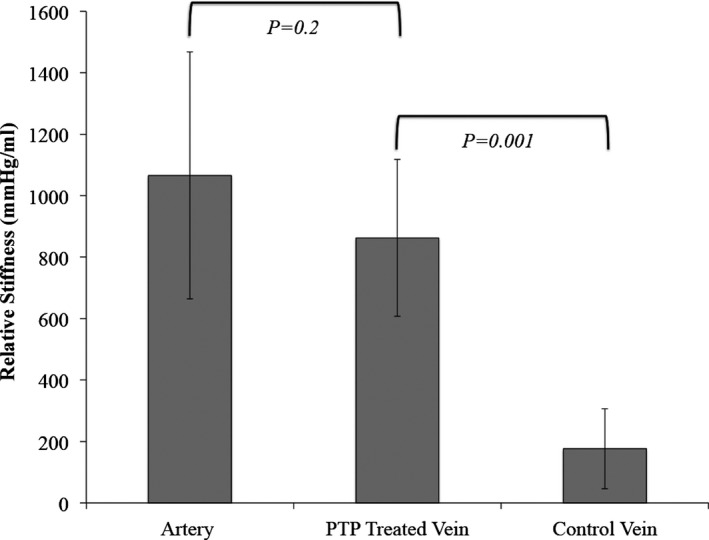
Bar graph comparing average relative stiffness of porcine carotid arteries, photochemical tissue passivation (PTP)‐treated porcine lateral saphenous vein, and untreated porcine lateral saphenous vein (data expressed as mean ± SD).
Arteriovenous Bypass Grafting
Four of the five swine operated on survived until the 1‐month harvest point and all grafts remained patent. One swine died 1‐week postoperatively, secondary to aneurysmal rupture of the control vein graft. At the time of 1‐month harvest, control vein grafts were more tortuous and possessed areas of large dilatation compared to PTP‐treated vein grafts (Figure 4).
Figure 4.
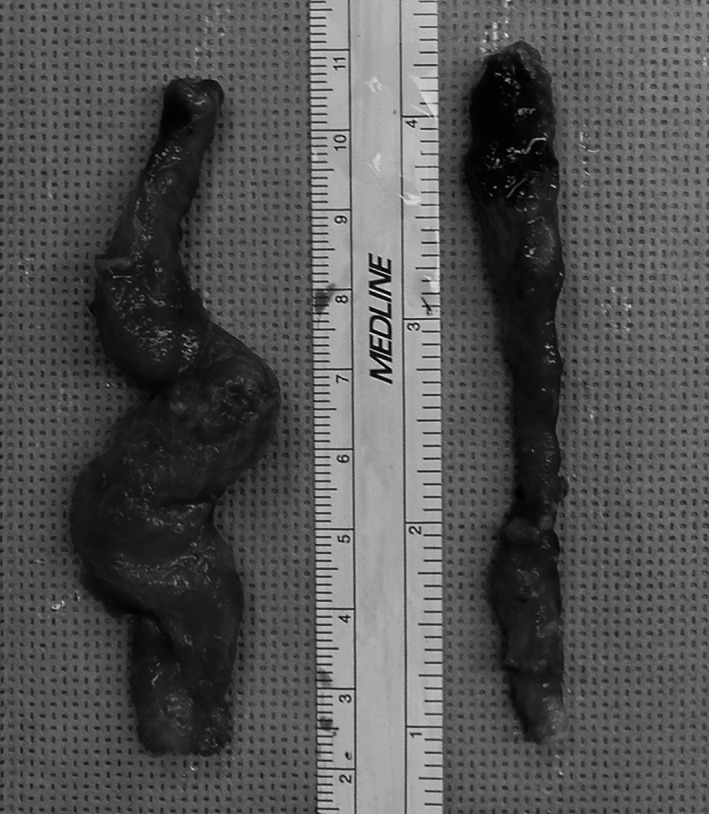
Comparison of control (left) and photochemical tissue passivation–treated (right) vein grafts at 1‐month harvest.
Histomorphometric Analysis
PTP‐treated venous grafts had a statistically significant decrease in intimal area and intimal thickness compared to the untreated control venous grafts (Figure 5A and 5B). Specifically, intimal area was 3.9 (IQR, 1.2) and 1.3 mm2 (IQR, 0.97; P≤0.001) in untreated and PTP‐treated grafts, respectively, and intimal thickness was 0.74 (IQR, 0.14) and 0.38 mm (IQR, 0.20; P≤0.001) in untreated and PTP‐treated grafts, respectively (Figure 6). Medial area and thickness also exhibited a statistically significantly decrease in PTP‐treated grafts compared to controls. Medial area was 9.2 (IQR, 3.2) and 4.7 mm2 (IQR, 2.0; P≤0.001), and medial thickness was 1.08 (IQR, 0.16) and 0.80 mm (IQR, 0.23; P≤0.01) in untreated and PTP‐treated grafts, respectively (Figure 5A and 5B). In order to control for possible increased intimal thickness attributed to nonpressure fixed vessels, I/M area ratio was performed for each vessel. The I/M of PTP‐treated grafts (0.26 ±mm2 [IQR, 0.25]) remained significantly less than that of controls (0.43 mm2 [IQR, 0.41]; P=0.03). In total, PTP reduced the degree of IH by 66% and medial hypertrophy by 49%.
Figure 5.
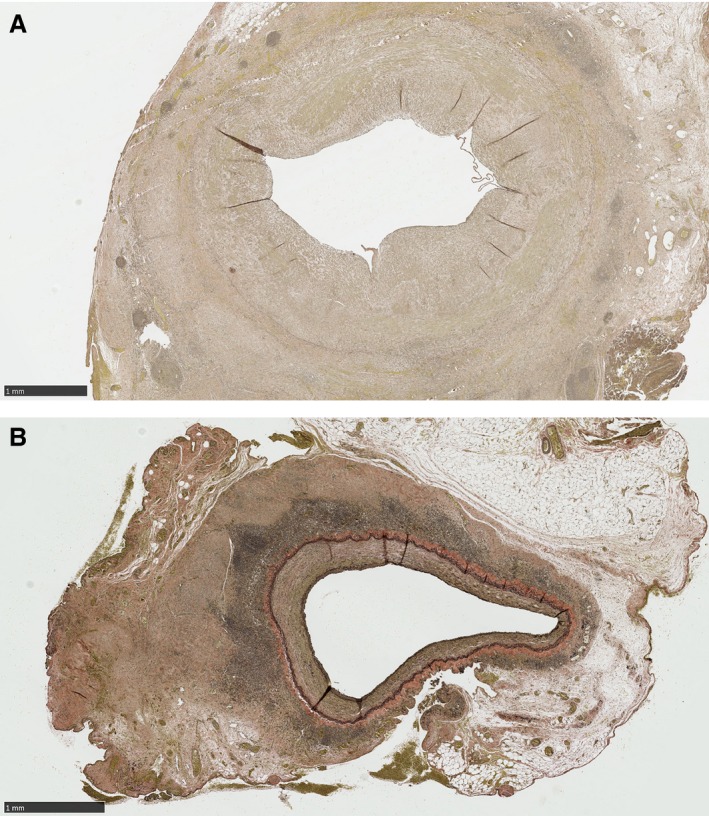
Verhoeff Van Gieson elastin stain slides demonstrating greater intimal area and thickness in control vein grafts (A) compared to photochemical tissue passivation–treated vein grafts (B). Scale bar=1 mm.
Figure 6.
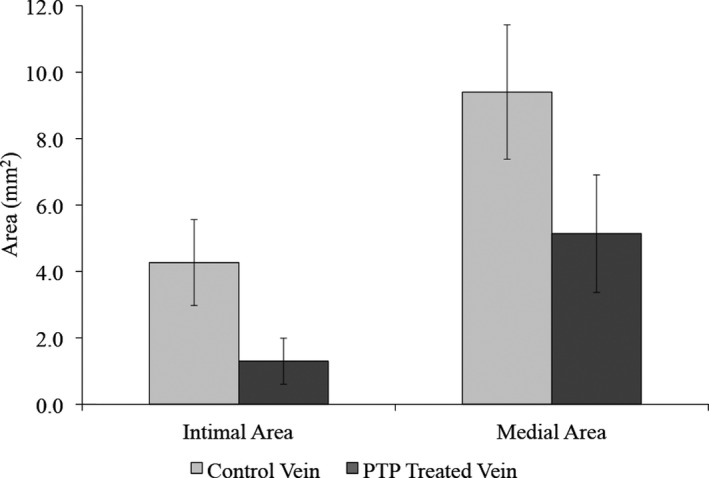
Comparison of control (grey) and photochemical tissue passivation (PTP)‐treated (black) vein graft intimal and medial areas at 1‐month. PTP‐treated vein grafts had significantly smaller intimal and medial areas than control vein grafts (data expressed as mean±SD).
In general, control venous grafts had complete loss of the internal elastic lamina and large disruption of the external elastic lamina. PTP‐treated venous grafts, on the other hand, had significant preservation of both internal and external elastic lamina at 1‐month.
Immunohistochemistry Analysis
In both PTP‐treated and control vein grafts, the majority of cells within the media and the areas of IH were SMA‐positive staining cells. SMA‐positive cells represent either SMCs or myofibroblasts. The presence of great quantities of SMA‐positive cells within the neointima correlates well with the theory that IH occurs secondary to SMC or myofibroblast migration into vessel intima. Objectively, there were less SMA‐positive cells within the intima/media of PTP‐treated vessels than control vessels (Figure 7A and 7B).
Figure 7.
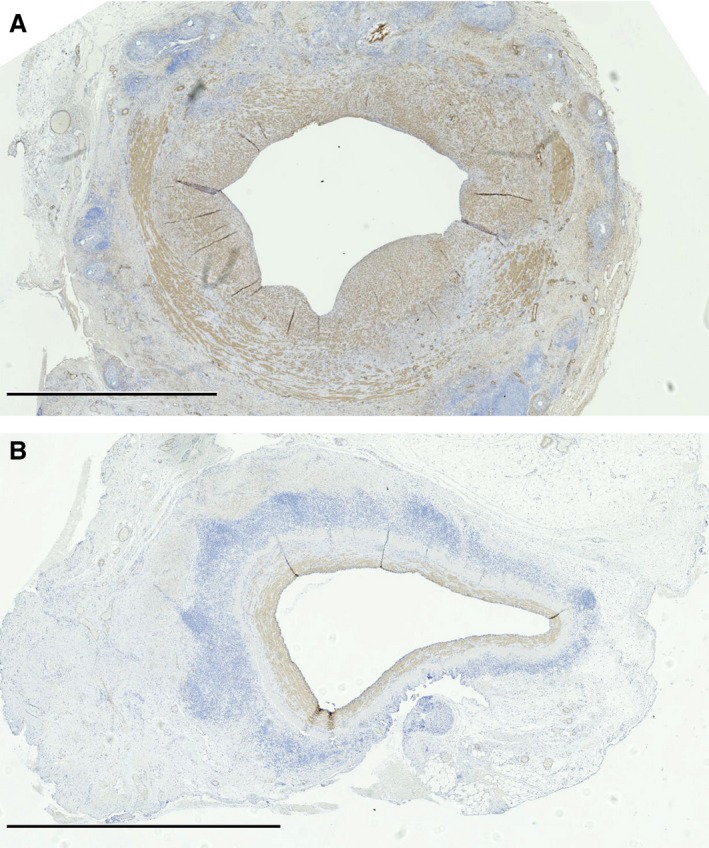
Smooth‐muscle actin stain demonstrating a greater concentration of smooth‐muscle cells within the intima/media of control (A) versus photochemical tissue passivation–treated (B) vein grafts. Positive cells show a brown stain. Scale bar=2.5 mm.
PCNA‐positive cells were prominent within the intima and media of control vein grafts, but limited in PTP‐treated vein grafts (Figure 8A and 8B). There was a statistically significant greater PCNA index within the control vein grafts 18% (IQR, 5.3) versus the PTP‐treated vein grafts 5% (IQR, 0.9; P=0.02).
Figure 8.
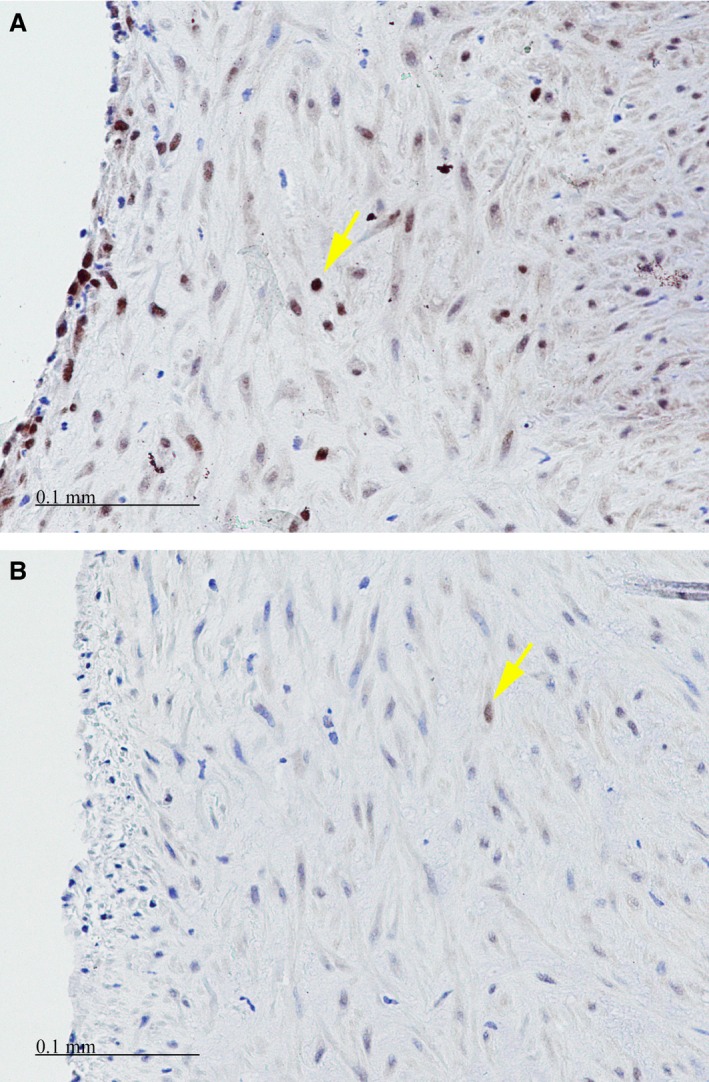
Proliferating cell nuclear antigen stain demonstrating a greater number of proliferating cells within the intima of control (A) versus photochemical tissue passivation–treated (B) vein grafts. Positive cells (yellow arrows) show a brown nuclear staining. Scale bar=0.1 mm.
Discussion
This study represents the first investigation of the effect of PTP on the modulation of SMC proliferation and migration, and the inhibition of the development of IH in a porcine arteriovenous bypass model. Our results clearly demonstrate that PTP treatment of porcine vein decreases vessel elasticity and compliance to more resemble that of a porcine artery. These structural modifications significantly limit the development of vein graft IH by 66%.
Neointimal hyperplasia is a complex process and the leading cause of vein graft failure. Although many different physiological and molecular elements are involved in the formation of IH, the initiating event is venous injury, which occurs during both surgical resection and upon exposure to the intense pulsatile stretch forces when incorporated into the arterial environment.19, 20 Surgical injury results from vessel ischemia attributed to disruption of the vaso vasorum within the vessel adventitia,21 as well as injury from overaggressive manipulation at the time of harvest and anastomosis. Procedures to reduce this injury include in situ vein grafts and use of the no‐touch surgical technique.22, 23
Venous injury upon inclusion within the arterial environment is caused by exposure to significant pulsatile stretch force, turbulent flow, and wall shear stress. Under normal physiologic conditions, veins exist as thin‐walled, tubular structures permitting low flow in a low‐pressure environment. The vein is composed of endothelial cells, SMCs, elastin fibers, and extracellular matrix. Endothelial cells are responsible for vessel relaxation by synthesis of nitric oxide, and prevention of platelet and inflammatory cell adhesion and aggregation. SMCs are responsible for vessel contraction and remain in a quiescent and nonmigratory state secondary to inhibitory cytokines and a stable extracellular matrix.19, 20, 24
Upon exposure to arterial pressure, the compliant vein undergoes significant distension, inducing endothelial cell injury and denudation. Endothelial injury triggers platelet aggregation, inflammatory cell recruitment, and vessel wall invasion. This process stimulates extra‐ and intracellular signaling cascades, with the eventual induction of SMC proliferation and migration.20
Within these complex pathways, one variable of significant interest is venous exposure to pulsatile stretch force and turbulent flow. Mechanical stretch has been shown to induce upregulation of insulin like growth factor‐1 (IGF‐1) and IGF‐1 receptors, leading to vascular SMC proliferation.25 Thus, reducing the mechanical stretch imposed on venous grafts should reduce SMC proliferation and thereby reduce IH.
In our study, we utilized PTP to strengthen/stiffen venous adventitia, decreasing its compliance to more resemble that of an artery without altering the endothelial architecture. PTP strengthened and reinforced the external aspect of the vein by collagen cross‐linking only within the vessel adventitia. Our in vitro tensiometry and compliance testing demonstrate that PTP therapy significantly increases Young's modulus of elasticity by 10‐fold and decreases saphenous vein compliance to more resemble that of a porcine carotid artery. These structural modifications of the vein prepare the vein graft to better tolerate arterial pressure, limiting shear stress, vessel stretch, and resulting injury.
Our porcine arteriovenous bypass graft study further demonstrates the efficacy of PTP therapy to reduce IH. The intimal and medial areas of PTP‐treated grafts were significantly less than that of controls. In fact, PTP reduced IH by 66% and medial hypertrophy by 49%.
Immunohistochemistry revealed that the neointimal hyperplasia observed in both our PTP‐treated and control venous grafts consisted mostly of SMA cells. These results are consistent with many other studies that demonstrate that IH occurs secondary to SMC proliferation and migration.19, 20, 25 Our immunohistochemistry analyses confirm partial inhibition of this pathway. We demonstrate far fewer SMCs within the PTP‐treated vessel IH than the control vessel IH. Moreover, a significantly decreased PCNA index was observed in the PTP‐treated vessels than the control vessels, indicating lower degrees of cellular proliferation. Thus, PTP treatment likely reduces IH by reducing SMC proliferation and migration.
Mechanistically, SMC migration is mitigated by extracellular matrix degradation.20 Review of our porcine vein graft histology revealed large preservation of the internal and external elastic lamina of PTP‐treated vessels as compared to controls. This suggests that PTP conservation of venous graft structural architecture is partially responsible for the reduction of SMC migration.
Our results compare favorably to those of Angelini et al. in evaluation of the use of external stenting in a swine model of arteriovenous bypass grafting.26 Angelini et al. demonstrated a similar reduction in intimal area from 3.84 mm2 in unstented grafts to 1.06 mm2 in stented grafts. The similarity in results is not unexpected given that both external stenting and PTP therapy work to reduce vein graft mechanical trauma from overdilatation. Although similar, PTP presents many potential benefits over external stenting. First, PTP does not include the risks associated with foreign bodies, including infection and erosion into adjacent tissue. Second, PTP does not require the technical challenge of appropriate sizing and application of the external stent to the vessel. Instead, PTP is a simple and rapid procedure that involves simple external application of RB dye and short‐term exposure to green light. This photochemically induced cross‐linking does not cause thermal damage to treated tissue. In fact, photo‐cross‐linking has been successfully used for sutureless skin closure, sutureless microvascular anastomoses, and sutureless bonding of human amniotic membrane to corneal defects.27, 28, 29
Our study does possess a few limitations. First, the sample size is small with inclusion of only four pigs for a total of four PTP‐treated and four control vein grafts. Even with a limited sample size, it was possible to achieve statistically significant reductions in intimal and medial hyperplasia, the primary endpoint of our study. A second limitation is that we did not pressure fix the vein grafts at the time of harvest. However, all vessels were nonpressure fixed and our results of decreased IH in PTP‐treated, as compared to untreated, vein grafts remain significant when calculating I/M ratio.
In conclusion, this study demonstrates proof of concept of a novel treatment modality for the prevention of vein graft IH in a large animal model. These data support progression toward a first‐in‐human clinical trial for evaluation of PTP as a prophylactic therapy to improve vein graft patency. We estimate the barriers to clinical adoption to be rather low, considering the negligible effect of a few minutes of illumination on the course of the surgical procedure and the compelling nature of the results to clinicians, patients, and insurers.
Sources of Funding
This project was funded by the American College of Surgeons Resident Research Scholarship and a Partners HealthCare Innovation Development Grant.
Disclosures
Austen, Redmond, and McCormack have a patent “Methods for Tissue Passivation” pending and a patent “Method and Apparatus for Improving Vein Graft” pending.
Acknowledgments
The authors thank Bill Farinelli for building the custom prototype and supplying the Photomedix Omnilux green LED diode array.
(J Am Heart Assoc. 2016;5:e003856 doi: 10.1161/JAHA.116.003856)
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UKA, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 3. Sipahi I, Akay MH, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long‐term mortality and morbidity in multivessel disease: meta‐analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med. 2014;174:223–230. [DOI] [PubMed] [Google Scholar]
- 4. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S III, Bertrand M, Fuster V; Investigators FT . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 5. Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, Raab G, Ruckley CV. Multicentre randomised controlled trial of the clinical and cost‐effectiveness of a bypass‐surgery‐first versus a balloon‐angioplasty‐first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial. Health Technol Assess. 2010;14:1–210, iii‐iv. [DOI] [PubMed] [Google Scholar]
- 6. Fitzgibbon G, Kafka H, Leach A, Keon W, Hooper D, Burton J. Coronary bypass graft fate and patient outcome‐ angiographic follow‐up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. [DOI] [PubMed] [Google Scholar]
- 7. Sabik JF III, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544–551. [DOI] [PubMed] [Google Scholar]
- 8. Belkin M, Knox J, Donaldson MC, Mannick JA, Whittemore AD. Infrainguinal arterial reconstruction with nonreversed greater saphenous vein. J Vasc Surg. 1996;24:957–962. [DOI] [PubMed] [Google Scholar]
- 9. Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. 2013;257:824–833. [DOI] [PubMed] [Google Scholar]
- 10. Portugaller RH, Kalmar PI, Deutschmann H. The eternal tale of dialysis access vessels and restenosis: are drug‐eluting balloons the solution? J Vasc Access. 2014;15:439–447. [DOI] [PubMed] [Google Scholar]
- 11. Taggart DP, Ben Gal Y, Lees B, Patel N, Webb C, Rehman SM, Desouza A, Yadav R, De Robertis F, Dalby M, Banning A, Channon KM, Di Mario C, Orion E. A randomized trial of external stenting for saphenous vein grafts in coronary artery bypass grafting. Ann Thorac Surg. 2015;99:2039–2045. [DOI] [PubMed] [Google Scholar]
- 12. Salinas HM, Khan SI, McCormack MC, Fernandes JR, Gfrerer L, Watkins MT, Redmond RW, Austen WG Jr. Prevention of vein graft intimal hyperplasia with photochemical tissue passivation. J Vasc Surg. April 8, 2016. doi: 10.1016/j.jvs.2015.11.049. Available at: http://www.jvascsurg.org/article/S0741-5214(15)02451-9/abstract. Accessed July 20, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Loveland‐Jones CE, Jayarajan S, Fang J, Monroy A, Zhang HM, Holt‐Bright L, Choi ET. A new model of arteriovenous fistula to study hemodialysis access complications. J Vasc Access. 2014;15:351–357. [DOI] [PubMed] [Google Scholar]
- 14. Sims FH. A comparison of structural features of the walls of coronary arteries from 10 different species. Pathology. 1989;21:115–124. [DOI] [PubMed] [Google Scholar]
- 15. Wang XQ, Mill J, Kravchuk O, Kimble RM. Ultrasound assessed thickness of burn scars in association with laser Doppler imaging determined depth of burns in paediatric patients. Burns. 2010;36:1254–1262. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz RSE, Edwards WD, Bailey KR, Camrud ARJ, Jorgenson MA, Holmes DR Jr. Differential neointimal response to coronary artery injury in pigs and dogs. Arterioscler Thromb. 1994;14:395–400. [DOI] [PubMed] [Google Scholar]
- 17. Mehta D, George S, Jeremy J, Izzat M, Southgate K, Bryan A, Newby A, Angelini G. External stenting reduces long‐term medial and neointimal thickening and platelet derived growth factor expression in a pig model of arteriovenous bypass grafting. Nat Med. 1998;4:235–239. [DOI] [PubMed] [Google Scholar]
- 18. Izzat MB, Mehta D, Bryan AJ, Reeves B, Newby AC, Angelini GD. Influence of external stent size on early medial and neointimal thickening in a pig model of saphenous vein bypass grafting. Circulation. 1996;94:1741–1745. [DOI] [PubMed] [Google Scholar]
- 19. Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. [DOI] [PubMed] [Google Scholar]
- 20. Muto A, Model L, Ziegler K, Eghbalieh SDD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation. Circ J. 2010;74:1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGeachie J, Meagher S, Prendergast F. Vein‐to‐artery grafts: the long‐term development of neo‐intimal hyperplasia and its relationship to vasa vasorum and sympathetic innervation. Aust N Z J Surg. 1989;59:59–65. [DOI] [PubMed] [Google Scholar]
- 22. Conte MS. Technical factors in lower‐extremity vein bypass surgery: how can we improve outcomes? Semin Vasc Surg. 2009;22:227–233. [DOI] [PubMed] [Google Scholar]
- 23. Souza D, Bomfim V, Skoglund H, Dashwood M, Borowiec J, Bodin L, Filbey D. High early patency of saphenous vein graft for coronary artery bypass harvested with surrounding tissue. Ann Thorac Surg. 2001;71:797–800. [DOI] [PubMed] [Google Scholar]
- 24. Reilly C, McFall R. Platelet‐derived growth factor and transforming growth factor‐beta regulate plasminogen activator inhibitor‐1 synthesis in vascular smooth muscle cells. J Biol Chem. 1991;266:9419–9527. [PubMed] [Google Scholar]
- 25. Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin‐like growth factor‐1 receptor. Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. [DOI] [PubMed] [Google Scholar]
- 26. Angelini GD, Izzat MB, Bryan AJ, Newby AC. External stenting reduces early medial and neointimal thickening in a pig model of arteriovenous bypass grafting. J Thorac Cardiovasc Surg. 1996;112:79–84. [DOI] [PubMed] [Google Scholar]
- 27. Kamegaya Y, Farinelli WA, Vila Echague AV, Akita H, Gallagher J, Flotte TJ, Anderson RR, Redmond RW, Kochevar IE. Evaluation of photochemical tissue bonding for closure of skin incisions and excisions. Lasers Surg Med. 2005;37:264–270. [DOI] [PubMed] [Google Scholar]
- 28. O'Neill AC, Winograd JM, Zeballos JL, Johnson TS, Randolph MA, Bujold KE, Kochevar IE, Redmond RW. Microvascular anastomosis using a photochemical tissue bonding technique. Lasers Surg Med. 2007;39:716–722. [DOI] [PubMed] [Google Scholar]
- 29. Verter EE, Gisel TE, Yang P, Johnson AJ, Redmond RW, Kochevar IE. Light‐initiated bonding of amniotic membrane to cornea. Invest Ophthalmol Vis Sci. 2011;52:9470–9477. [DOI] [PubMed] [Google Scholar]


