Abstract
Background
Intravascular imaging can facilitate chronic total occlusion (CTO) percutaneous coronary intervention.
Methods and Results
We examined the frequency of use and outcomes of intravascular imaging among 619 CTO percutaneous coronary interventions performed between 2012 and 2015 at 7 US centers. Mean age was 65.4±10 years and 85% of the patients were men. Intravascular imaging was used in 38%: intravascular ultrasound in 36%, optical coherence tomography in 3%, and both in 1.45%. Intravascular imaging was used for stent sizing (26.3%), stent optimization (38.0%), and CTO crossing (35.7%, antegrade in 27.9%, and retrograde in 7.8%). Intravascular imaging to facilitate crossing was used more frequently in lesions with proximal cap ambiguity (49% versus 26%, P<0.0001) and with retrograde as compared with antegrade‐only cases (67% versus 31%, P<0.0001). Despite higher complexity (Japanese CTO score: 2.86±1.19 versus 2.43±1.19, P=0.001), cases in which imaging was used for crossing had similar technical and procedural success (92.8% versus 89.6%, P=0.302 and 90.1% versus 88.3%, P=0.588, respectively) and similar incidence of major cardiac adverse events (2.7% versus 3.2%, P=0.772). Use of intravascular imaging was associated with longer procedure (192 minutes [interquartile range 130, 255] versus 131 minutes [90, 192], P<0.0001) and fluoroscopy (71 minutes [44, 93] versus 39 minutes [25, 69], P<0.0001) time.
Conclusions
Intravascular imaging is frequently performed during CTO percutaneous coronary intervention both for crossing and for stent selection/optimization. Despite its use in more complex lesion subsets, intravascular imaging was associated with similar rates of technical and procedural success for CTO percutaneous coronary intervention.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02061436.
Keywords: chronic total occlusion, intravascular ultrasound, optical coherence tomography, percutaneous coronary intervention
Subject Categories: Percutaneous Coronary Intervention
Introduction
Use of intravascular ultrasound (IVUS) for stent optimization during chronic total occlusion percutaneous coronary intervention (CTO PCI) has been shown to improve long‐term outcomes,1, 2, 3 yet its impact on crossing has received limited study.1, 2, 3, 4, 5, 6, 7, 8, 9 Intravascular imaging can help resolve proximal cap ambiguity by identifying the position of the main branch10 and clarifying guidewire position during both antegrade and retrograde CTO crossing attempts.11 IVUS can determine optimal balloon sizing for the reverse controlled antegrade and retrograde tracking and dissection (reverse CART) technique.11, 12 Moreover, intravascular imaging can facilitate sizing of balloons and stents and optimize stent expansion and stent strut apposition.13 We examined a large multicenter contemporary CTO PCI registry to determine the frequency of intravascular imaging use during CTO PCI and the associated procedural outcomes.
Methods
Patient Population
We analyzed the frequency of use and outcomes of intravascular imaging among 619 chronic CTO PCIs performed between 2012 and 2015 at 7 US centers: Appleton Cardiology, Appleton Wisconsin; Henry Ford Hospital, Detroit, Michigan; Massachusetts General Hospital, Boston, Massachusetts; Medical Center of the Rockies, Loveland, Colorado; St. Luke's Health System's Mid‐America Heart Institute, Kansas City, Missouri; VA North Texas Health Care System, Dallas, Texas; and VA San Diego Healthcare System, San Diego, California.
Enrollment was performed during only part of the study period in some centers due to participation in other studies. Data collection was performed both prospectively and retrospectively and was recorded in a dedicated online database (PROGRESS CTO: Prospective Global Registry for the Study of Chronic Total Occlusion Intervention, Clinicaltrials.gov Identifier: NCT02061436).14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 The study was approved by the institutional review board of each site and a waiver of informed consent was obtained.
Definitions
Coronary CTOs were defined as coronary lesions with Thrombolysis In Myocardial Infarction grade 0 flow of at least 3‐month duration.25 Estimation of the occlusion duration was based on first onset of anginal symptoms, prior history of myocardial infarction in the target vessel territory, or comparison with a prior angiogram. Calcification was assessed by angiography as mild (spots), moderate (involving ≤50% of the reference lesion diameter), and severe (involving >50% of the reference lesion diameter). Moderate proximal vessel tortuosity was defined as the presence of at least 2 bends >70° or 1 bend >90° and severe tortuosity as 2 bends >90° or 1 bend >120° in the CTO vessel. The Japanese Chronic Total Occlusion score was calculated as described by Morino et al.26 The Progress CTO score was calculated as described by Christopoulos et al.22 Technical success was defined as successful CTO revascularization with achievement of <30% residual diameter stenosis within the treated segment and restoration of Thrombolysis In Myocardial Infarction grade 3 antegrade flow. Procedural success was defined as achievement of technical success with no in‐hospital major adverse cardiac events (MACE). In‐hospital MACE included any of the following adverse events prior to hospital discharge: death, myocardial infarction, urgent repeat target vessel revascularization with either PCI or coronary artery bypass graft surgery, tamponade requiring either pericardiocentesis or surgery, and stroke. Myocardial infarction was defined using the Third Universal Definition of Myocardial Infarction.27
Statistical Analysis
The primary comparison of the study was between procedures in which intravascular imaging (IVUS and/or optical coherence tomography [OCT]) was used versus those in which it was not used for crossing the occlusion (Figure 1). In a secondary analysis of cases that were successfully crossed with a guidewire, a comparison was made between use versus no use of intravascular imaging for stent sizing/optimization.
Figure 1.
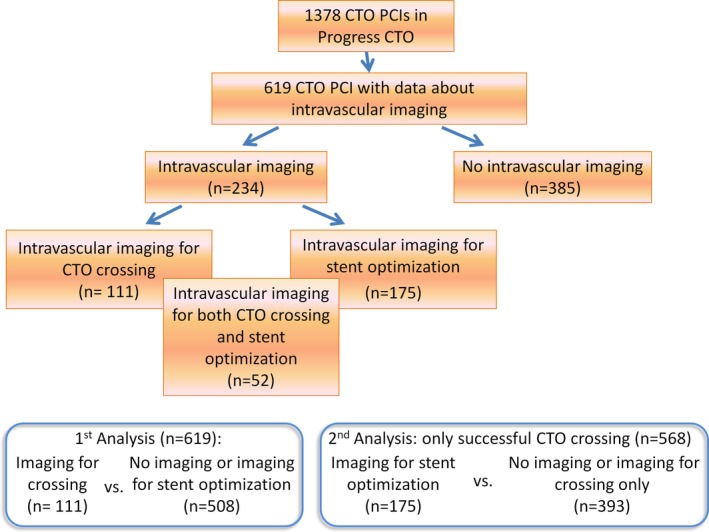
Flow chart of the study. CTO indicates chronic total occlusion; PCI, percutaneous coronary intervention.
Continuous variables were presented as mean±SD or median (interquartile range) and were compared using the t test, or Wilcoxon rank‐sum test, as appropriate. Categorical data were reported as frequencies or percentages and compared using the χ2 test or Fisher's exact test, as appropriate. The baseline clinical characteristics and the procedural outcomes were analyzed among the patients (606 patients), while the angiographic characteristics were analyzed among procedures (619 procedures). All statistical analyses were performed with JMP 11.0 (SAS Institute, Cary, NC). Two‐sided P<0.05 were considered statistically significant.
Results
Baseline Patient and Procedural Characteristics
A total of 619 CTO PCI procedures performed in 606 patients were included in the present analysis. The baseline patient and angiographic characteristics of the study population are summarized in Table 1. Mean age was 65.4±10 years and 85% of the patients were men with high prevalence of diabetes (50%), dyslipidemia (92%), and hypertension (88%). Approximately one third had congestive heart failure (33%) or prior coronary artery bypass graft surgery (32%).
Table 1.
Baseline Clinical Characteristics of the Study Patients, Classified According to Whether Intravascular Imaging Was Used to Guide CTO Crossing or Not
| Variable | Overall | Imaging for Crossing | No Imaging or Imaging for Stent Optimization | P‐Value |
|---|---|---|---|---|
| (n=606) | (n=111) | (n=495) | ||
| Age, ya | 65.4±10 | 65±10 | 66±10 | 0.466 |
| Men | 85% | 91% | 84% | 0.066 |
| BMI, kg/m2 a | 30.6±6 | 31.7±7 | 30.4±6 | 0.058 |
| Diabetes mellitus | 50% | 58% | 49% | 0.069 |
| Hypertension | 88% | 88% | 88% | 0.908 |
| Dyslipidemia | 92% | 94% | 92% | 0.631 |
| Smoking (current) | 23% | 77% | 77% | 0.908 |
| LVEF (%)a | 51±15 | 48±15 | 51±15 | 0.056 |
| Family history of CAD | 24% | 24% | 24% | 0.897 |
| Congestive heart failure | 33% | 37% | 32% | 0.29 |
| Prior myocardial infarction | 45% | 46% | 44% | 0.793 |
| Prior CABG | 32% | 41% | 30% | 0.024 |
| Prior CVD | 11% | 8% | 12% | 0.331 |
| Prior PVD | 15% | 12% | 15% | 0.403 |
| Baseline creatinine, mg/dLb | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.3) | 1.0 (0.8, 1.2) | 0.615 |
Imaging for crossing: cases in which intravascular imaging was used for crossing the chronic total occlusion. No imaging or imaging for stent optimization: cases in which intravascular imaging was not used or cases in which intravascular imaging was used for stent optimization. BMI indicates body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CTO, chronic total occlusion; CVD, cerebrovascular disease; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease.
Mean±SD.
Median (interquartile range).
Intravascular imaging was used in 38% of the procedures, as follows: IVUS in 36%, OCT in 3%, and both in 1.45%. The indications for intravascular imaging were to facilitate CTO crossing (overall 35.7%, antegrade in 27.9%, and retrograde in 7.8%) and stent sizing (26.3%) or optimization (38.0%) (Figure 2). Wide variability was observed in the frequency of intravascular imaging use among various centers (0–58%, Figure 3).
Figure 2.
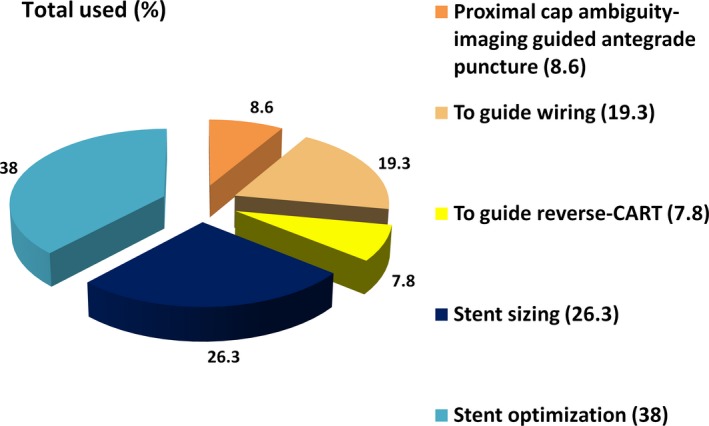
Use of intravascular imaging during chronic total occlusion percutaneous coronary intervention. CART indicates controlled antegrade and retrograde tracking and dissection.
Figure 3.
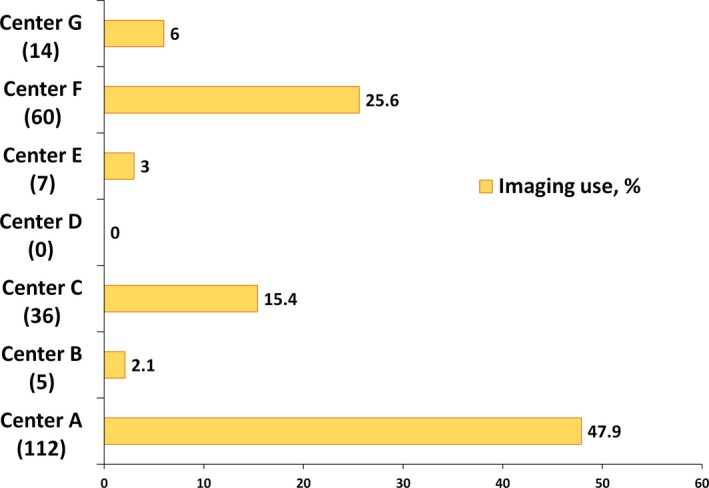
Frequency of intravascular imaging use at the study participating centers. The absolute number of the cases with intravascular imaging use is listed in parentheses.
Intravascular Imaging for Crossing
The baseline clinical and angiographic characteristics of patients who did and those who did not undergo intravascular imaging for crossing were similar (Tables 1 and 2). The most common CTO PCI target vessel was the right coronary artery (52%), followed by the left anterior descending artery (26%), and the left circumflex (22%). Moderate to severe calcification and moderate to severe tortuosity were present in 53% and 42%, respectively. Procedural outcomes are summarized in Table 3. Overall technical and procedural rates were 90.1% and 88.6%, respectively. Antegrade wiring was the successful crossing strategy in 48% of the cases, antegrade dissection and re‐entry in 23%, and the retrograde approach in 23%.
Table 2.
Angiographic Characteristics Classified According to Whether Intravascular Imaging was Used to Guide CTO Crossing or Not
| Variable | Overall | Imaging for Crossing | No Imaging or Imaging for Stent Optimization | P‐Value |
|---|---|---|---|---|
| (n=619) | (n=111) | (n=508) | ||
| CTO target vessel | 0.861 | |||
| RCA | 52% | 51% | 52% | |
| LAD | 26% | 28% | 26% | |
| LCX | 22% | 21% | 22% | |
| Successful crossing strategy | <0.0001 | |||
| Antegrade wiring | 48% | 23% | 53% | |
| Retrograde | 23% | 47% | 17% | |
| Antegrade dissection and re‐entry | 23% | 25% | 23% | |
| None | 6% | 5% | 7% | |
| First crossing strategy | 0.889 | |||
| Antegrade wiring | 78% | 78% | 78% | |
| Retrograde | 14% | 15% | 14% | |
| Antegrade dissection and re‐entry | 8% | 6% | 8% | |
| Retrograde crossing attempt | 37% | 67% | 31% | <0.0001 |
| J‐CTO scorea | 2.51±1.20 | 2.86±1.19 | 2.43±1.19 | 0.001 |
| P‐CTO scorea | 1.37±1.01 | 1.64±1.00 | 1.18±1.02 | <0.0001 |
| Calcification (moderate/severe) | 53% | 60% | 51% | 0.103 |
| Tortuosity (moderate/severe) | 42% | 48% | 40% | 0.126 |
| Proximal cap ambiguity | 31% | 49% | 26% | <0.0001 |
| In‐stent restenosis | 17% | 20% | 16% | 0.334 |
| Prior failure to open CTO | 16% | 21% | 15% | 0.147 |
| Interventional collaterals | 53% | 52% | 53% | 0.794 |
| Side branch at the proximal cap | 50% | 61% | 47% | 0.009 |
| Blunt/no stump, % | 57% | 68% | 55% | 0.009 |
| Vessel diameter, mmb | 2.6 (2.5, 3.0) | 2.5 (2.5, 3.0) | 2.7 (2.5, 3.0) | 0.684 |
| Occlusion length, mmb | 30 (19, 45) | 30 (22, 50) | 30 (18, 40) | 0.093 |
| Number of stents used | 2.53±1.2 | 2.78±1.4 | 2.48±1.19 | 0.047 |
Imaging for crossing: cases in which intravascular imaging was used for crossing the chronic total occlusion. No imaging or imaging for stent optimization: cases in which intravascular imaging was not used or cases in which intravascular imaging was used for stent optimization. CTO indicates chronic total occlusion; J‐CTO score, Japanese chronic total occlusion score; LAD, left anterior descending artery; LCX, left circumflex artery; P‐CTO score, Progress chronic total occlusion score; RCA, right coronary artery.
Mean±SD.
Median (interquartile range).
Table 3.
Procedural Outcomes of the Study Patients, Classified According to Whether Intravascular Imaging was Used to Guide CTO Crossing or Not
| Variable | Overall | Imaging for Crossing | No Imaging or Imaging for Stent Optimization | P‐Value |
|---|---|---|---|---|
| Technical success | 90.1% | 92.8% | 89.6% | 0.302 |
| Procedural success | 88.6% | 90.1% | 88.3% | 0.588 |
| Procedural time, minutea | 142 (96, 210) | 192 (130, 255) | 131 (90, 192) | <0.0001 |
| Fluoroscopy time, minutea | 45 (27, 75) | 71 (44, 93) | 39 (25, 69) | <0.0001 |
| Air kerma radiation dose (Gray)a | 3.59 (2.27, 5.40) | 4.98 (3.11, 6.04) | 3.42 (2.09, 5.09) | <0.0001 |
| Contrast volumea | 280 (205, 367) | 310 (240, 400) | 270 (200, 360) | 0.004 |
| MACE | 3.1% | 2.7% | 3.2% | 0.772 |
| Death | 0.5% | 0.0% | 0.6% | 0.411 |
| Acute Q wave MI | 0% | 0% | 0.0% | — |
| Acute MI | 1.3% | 1.8% | 1.2% | 0.623 |
| Re‐PCI | 0.3% | 0.0% | 0.4% | 0.502 |
| Stroke | 0.4% | 0.0% | 0.6% | 0.411 |
| Emergency CABG | 0% | 0% | 0.0% | — |
| Pericardiocentesis | 0.9% | 0.9% | 1.0% | 0.916 |
Imaging for crossing: cases in which intravascular imaging was used for crossing the chronic total occlusion; No imaging or imaging for stent optimization: cases in which intravascular imaging was not used or cases in which intravascular imaging was used for stent optimization. CABG indicates coronary artery bypass grafting; CTO, chronic total occlusion; MACE, major adverse cardiac events; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Median (interquartile range).
Intravascular imaging for crossing was used more commonly in lesions with proximal cap ambiguity (49% versus 26%, P<0.0001), side branch at the proximal cap (61% versus 47%, P=0.035), longer occlusion length (30 mm [interquartile range: 22, 50] versus 28 mm [15, 44], P=0.009), and higher Japanese Chronic Total Occlusion (2.86±1.19 versus 2.43±1.19, P=0.001) and Progress CTO (1.64±1.00 versus 1.18±1.02, P<0.0001) score. Cases in which intravascular imaging was used for crossing were more likely to succeed using the retrograde approach or antegrade dissection and reentry (47% versus 17% and 25% versus 23%), as compared with antegrade wiring (23% versus 53%, P<0.0001).
Procedural outcomes are summarized in Table 3 and Figure 4. Technical and procedural success were similar in cases in which intravascular imaging was used for crossing (92.8% versus 89.6%, P=0.302 and 90.1% versus 88.3%, P=0.588, respectively), whereas the incidence of MACE was similarly low in both groups (2.7% versus 3.2%, P=0.772). Success and complication rates were similar among centers with high versus low intravascular imaging use (data not shown). There was no significant difference in the incidence of death, myocardial infarction, repeated PCI, stroke, and pericardiocentesis. Mean procedure duration was significantly longer among procedures in which intravascular imaging was used for crossing (192 minutes [130, 255] versus 131 minutes [90, 192], P<0.0001), as was median fluoroscopy time (71 minutes [44, 93] versus 39 minutes [25, 69], P<0.0001), mean air kerma radiation dose (4.98 Gray [3.11, 6.04] versus 3.42 Gray [2.09, 5.09], P<0.0001), and median contrast volume (310 mL [240, 400] versus 270 mL [200, 360], P=0.004) as compared with cases in which intravascular imaging was not used.
Figure 4.
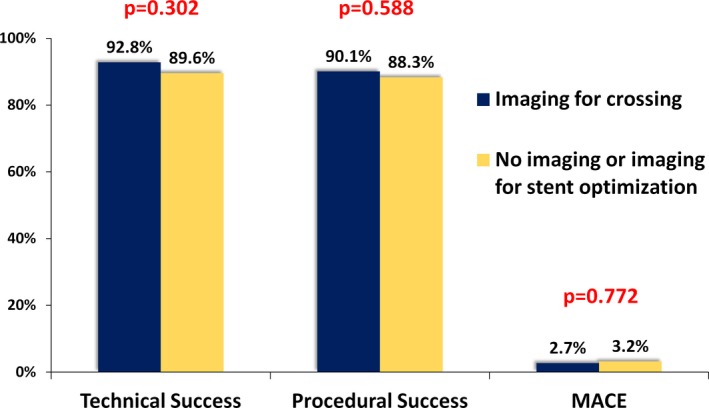
Technical, procedural success and MACE among study procedures classified according to use of intravascular imaging for crossing. MACE indicates major cardiac adverse event.
Intravascular Imaging for Stent Sizing and/or Optimization
Among CTOs successfully crossed with a guidewire, cases in which imaging was used for stent sizing and optimization were more complex, as reflected by higher Japanese Chronic Total Occlusion (2.65±1.17 versus 2.38±1.22, P=0.013) and Progress CTO (1.39±1.09 versus 1.19±0.98, P=0.035) scores (Tables 4 and 5). They were also more likely to have moderate/severe calcification (63% versus 47%, P=0.001), longer occlusion length (30 mm [20, 50] versus 28 mm [15, 40], P=0.030) or be due to in‐stent restenosis (23% versus 14%, P=0.015) and required longer procedure (162 minutes [113, 216] versus 133 minutes [91, 201], P=0.001) and fluoroscopy (52 minutes [33, 81] versus 40 minutes [26, 73], P=0.014) time with a trend for higher air kerma radiation dose (3.90 Gray [2.48, 5.46] versus 3.48 Gray [2.13, 5.34], P=0.249) and contrast volume (300 mL [228, 368] versus 277 mL [200, 370], P=0.106). Use of intravascular imaging was associated with similar technical (97.7% versus 97.5%, P=0.854) and procedural (97.1% versus 95.4%, P=0.347) success rates and similarly low MACE rates (2.3% versus 3.1%, P=0.622) (Figure 5). There was a trend toward larger number of stents in procedures where intravascular imaging was used for stent sizing/and/or optimization (2.7±1.3 versus 2.5±1.2, P=0.07).
Table 4.
Angiographic Characteristics Classified According to Whether or Not Intravascular Imaging Technique Was Used for Stent Optimization
| Variable | Overall | Imaging for Stent Optimization | No Imaging or Imaging for Crossing Only | P‐Value |
|---|---|---|---|---|
| (n=568) | (n=175) | (n=393) | ||
| CTO target vessel | 0.137 | |||
| RCA | 51% | 49% | 52% | |
| LAD | 27% | 32% | 25% | |
| LCX | 22% | 19% | 23% | |
| Successful crossing strategy | 0.001 | |||
| Antegrade wiring | 51% | 42% | 55% | |
| Retrograde | 24% | 27% | 23% | |
| Antegrade dissection and re‐entry | 25% | 31% | 22% | |
| First crossing strategy | 0.321 | |||
| Antegrade wiring | 78% | 77% | 79% | |
| Retrograde | 14% | 13% | 14% | |
| Antegrade dissection and re‐entry | 8% | 10% | 7% | |
| Retrograde crossing attempt | 36% | 45% | 32% | 0.003 |
| J‐CTO scorea | 2.47±1.21 | 2.65±1.17 | 2.38±1.22 | 0.013 |
| Progress CTO scorea | 1.25±1.02 | 1.39±1.09 | 1.19±0.98 | 0.035 |
| Calcification (moderate/severe) | 52% | 63% | 47% | 0.001 |
| Tortuosity (moderate/severe) | 41% | 42% | 40% | 0.742 |
| Proximal cap ambiguity | 30% | 34% | 28% | 0.155 |
| In‐stent restenosis | 17% | 23% | 14% | 0.015 |
| Prior failure to open CTO | 16% | 18% | 15% | 0.429 |
| Interventional Collaterals | 53% | 52% | 54% | 0.648 |
| Side branch at the proximal cap | 49% | 50% | 48% | 0.753 |
| Blunt/no stump | 55% | 50% | 57% | 0.123 |
| Vessel diameter, mmb | 2.5 (2.5, 3.0) | 2.8 (2.5, 3) | 2.5 (2.5, 3) | 0.257 |
| Occlusion length, mmb | 30 (18, 45) | 30 (20, 50) | 28 (15, 40) | 0.03 |
| Number of stents used | 2.5±1.2 | 2.7±1.3 | 2.5±1.2 | 0.076 |
Imaging for stent optimization: cases in which intravascular imaging was used for stent optimization. No imaging or imaging for crossing only: cases in which intravascular imaging was not used or cases in which intravascular imaging was used only for crossing the chronic total occlusion. CTO indicates chronic total occlusion; J‐CTO score, Japanese chronic total occlusion score; LAD, left anterior descending artery; LCX, left circumflex artery; P‐CTO score, Progress chronic total occlusion score; RCA, right coronary artery.
Mean±SD.
Median (interquartile range).
Table 5.
Procedural Outcomes of the Study Patients, Classified According to Whether Intravascular Imaging was Used for Stent Optimization or Not
| Variable | Overall | Imaging for Stent Optimization | No Imaging or Imaging for Crossing Only | P‐Value |
|---|---|---|---|---|
| Technical success | 97.5% | 97.7% | 97.5% | 0.854 |
| Procedural success | 95.9% | 97.1% | 95.4% | 0.347 |
| Procedural time, mina | 143 (97, 205) | 162 (113, 216) | 133 (91, 201) | 0.001 |
| Fluoroscopy time, mina | 44 (27, 75) | 52 (33, 81) | 40 (26, 73) | 0.014 |
| Air kerma radiation dose (Gray)a | 3.60 (2.24, 5.37) | 3.90 (2.48, 5.46) | 3.48 (2.13, 5.34) | 0.249 |
| Contrast volumea | 282 (205, 369) | 300 (228, 368) | 277 (200, 370) | 0.106 |
| MACE | 2.9% | 2.3% | 3.1% | 0.622 |
| Death | 0.4% | 0.0% | 0.5% | 0.347 |
| Acute Q wave MI | 0% | 0% | 0.0% | |
| Acute MI | 1.3% | 0.6% | 1.6% | 0.346 |
| Re‐PCI | 0.4% | 0.0% | 0.5% | 0.347 |
| Stroke | 0.7% | 0.6% | 0.8% | 0.808 |
| Emergency CABG | 0% | 0% | 0.0% | |
| Pericardiocentesis | 0.7% | 1.2% | 0.5% | 0.398 |
Imaging for stent optimization: cases in which intravascular imaging was used for stent optimization. No imaging or imaging for crossing only: cases in which intravascular imaging was not used or cases in which intravascular imaging was used only for crossing the chronic total occlusion. CABG, coronary artery bypass graft; MACE, major adverse cardiac events; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Median (interquartile range).
Figure 5.
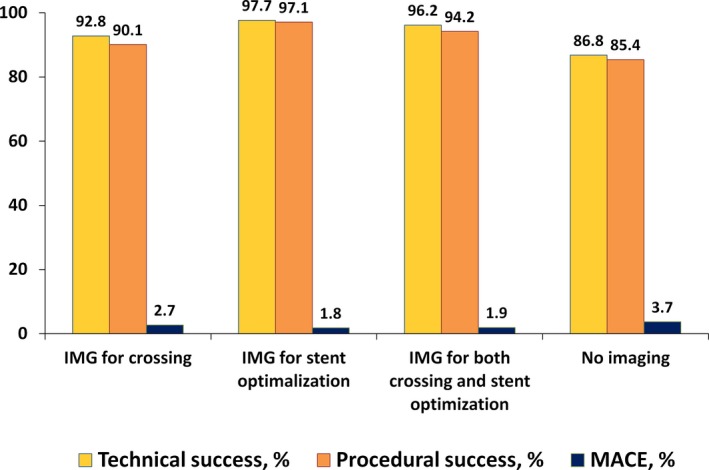
Technical, procedural success and major cardiac adverse events according to purpose of intravascular imaging techniques. IMG indicates imaging; MACE, major cardiac adverse events.
Discussion
The main findings of our study are that intravascular imaging is frequently performed during CTO PCI both for crossing and for stent selection/optimization. Intravascular imaging was used in more complex occlusions and was associated with similarly high success rates, but longer procedure time and higher radiation dose.
Frequency of Intravascular Imaging Use in CTO PCI
In our study, intravascular imaging was used in 38% of CTO PCI cases, which is similar to 39% utilization in the Multicenter Korean CTO Registry.1 Okamura et al reported use of IVUS in 47.5% of patients in their study about complications during retrograde approach in the Japanese Multicenter CTO Registry IVUS.28 In contrast, in the European Registry of Chronic Total Occlusion, IVUS use was significantly lower (2.9% overall29 and 9.2% in retrograde cases30), suggesting that imaging use may be low even among experienced operators and centers. Habara et al31 compared CTO PCI outcomes according to operator experience: when using the antegrade approach after retrograde failure, the success rate of IVUS‐guided techniques was significantly higher in higher‐volume centers than lower‐volume centers (13.3% versus 3.3%; P=0.018). Therefore, IVUS guidance for antegrade crossing requires high operator skill and experience.32 Moreover, the cost of catheters and the additional time required for obtaining and interpreting the images can affect the use of intravascular imaging and may explain the wide variability in its use for CTO (and non‐CTO) PCI.
Selection of Intravascular Imaging Modality for CTO PCI
IVUS was the intravascular imaging modality used in most CTO PCIs, and in contrast to OCT, does not require flushing of the blood column within the arterial lumen and has higher penetration depth. OCT performed before stenting could also cause subintimal hematoma due to the need for contrast administration for image acquisition. OCT, however, offers superior resolution compared to IVUS and has been used in CTO PCI to determine guidewire position and stent optimization after deployment. The ALSTER OCT‐CTO (AskLepios ST. GEoRg's Hospital‐Optical Coherence Tomography for follow‐up of Chronic Total Occlusions) registry reported a significantly higher rate of uncovered and malapposed stent struts in CTOs as compared to nonocclusive lesions.33 These findings may favor prolonged administration of dual antiplatelet therapy, in an attempt to reduce the risk of stent thrombosis.34
Solid‐state, phased‐array catheters (Eagle‐Eye, Volcano) are preferred over rotational IVUS systems, because the imaging transducer is closer to the tip of the IVUS catheter. A short‐tip solid‐state IVUS catheter (Eagle Eye Short Tip, Volcano) is advantageous for imaging in CTO PCI, as it minimizes the extent of distal advancement required for distal imaging and may be more deliverable.10
Imaging for CTO Crossing
Intravascular imaging can assist CTO crossing by (1) identifying the proximal cap in cases with proximal cap ambiguity (for example, by imaging through a side branch adjacent to the occlusion)4; (2) confirming whether the antegrade guidewire has engaged the occlusion and navigating the antegrade guidewire to the true lumen in case of dissection5, 35; (3) confirming that the retrograde guidewire has entered the proximal true lumen before externalization; and (4) determining the appropriate balloon size for the CART and reverse CART techniques.10, 11 Moreover, use of IVUS could assist re‐entry into the distal true lumen after subintimal crossing35 and reduce the need for fluoroscopy and contrast injection.5 In our study, 3 characteristics of CTOs were associated with IVUS utilization during crossing: side branch at proximal cap (61% versus 47%, P=0.009), proximal cap ambiguity (49% versus 26%, P<0.001), and blunt/no stump (68% versus 55%, 0.009).
Park et al reported that the IVUS‐guided wiring technique was useful and safe for antegrade recanalization of 31 stumpless CTOs (Table 6). The IVUS catheter was advanced into the side branch to identify the CTO entry point, while another stiffer guidewire was directed under IVUS guidance to the occlusion entry point and penetrated the proximal cap. In case of subintimal position of the guidewire, IVUS was also used to redirect the wire into the true lumen. However, this technique has 2 potential limitations: first, IVUS cannot provide information on the course of the vessel distal to the occlusion (dual injection can be used to visualize the entire course of the vessel distal to the occlusion); second, IVUS‐guided wiring cannot be applied in cases without appropriate side branches (for example, with smaller vessel diameter than the IVUS catheters).4
Table 6.
Summary of Published Reports of Intravascular Ultrasound Use in CTO PCI
| Author | Year | No. of Patients | No. of Lesions | Imaging Frequency | Comments | |
|---|---|---|---|---|---|---|
| Intravascular imaging for crossing | ||||||
| Antegrade crossing | Park et al4 | 2011 | 31 | 32 | 100% | IVUS‐guided wiring is technically feasible and safe for recanalization of stumpless CTO lesions |
| Ito et al35 | 2004 | 2 | 2 | 100% | Case report: (1) IVUS advanced into a side branch to identify the entry point of the major branch (2) IVUS‐guided penetration of the guidewire from the false lumen to the true lumen after dissection | |
| Matsubara et al5 | 2004 | 2 | 2 | 100% | Case report: (1) IVUS catheter in the subintima was used to guide the wire into the true lumen. (2) The orifice of the LAD was identified by imaging with IVUS in a diagonal branch | |
| Retrograde crossing | Dai et al11 | 2013 | 49 | 49 | 100% | IVUS‐guided reverse CART approach is efficient and safe for revascularization of complex CTOs |
| Intravascular imaging for stent optimization | ||||||
| Retrospective studies | Kang et al6 | 2015 | 126 | 126 | 100% | Among patients in whom IVUS was used post CTO PCI, post CTO‐PCI angiographic minimum luminal diameter ≤2.4 mm and stent expansion ratio ≤70% as assessed by IVUS were both independent predictors of in‐stent restenosis |
| Hong et al1 | 2014 | 534 | 534 | 50% | IVUS was used in 39% of CTO PCI and was associated with lower risk for stent thrombosis and a trend for lower incidence of myocardial infarction as compared with angiography‐guided CTO PCI | |
| Tsujita et al32 | 2009 | 48 | 48 | 100% | Compared antegrade and retrograde approaches with IVUS after crossing; IVUS can be a useful tool for the detection of procedure‐related vessel damage and subintimal wire tracking | |
| Prospective randomized‐controlled clinical trials | Kim et al3 | 2015 | 402 | 402 | 50% | Randomized‐controlled trial of IVUS guidance in CTO PCI demonstrating lower 12‐month incidence of MACE in the IVUS‐guidance group |
| Tian et al2 | 2015 | 230 | 230 | 50% | Randomized‐controlled trial of IVUS guidance in CTO PCI demonstrating that IVUS guidance was associated with less late lumen loss and a lower incidence of 12‐month in‐stent restenosis | |
CART indicates controlled antegrade and retrograde tracking and dissection; CTO, chronic total occlusion; IVUS, intravascular ultrasound; LAD, left anterior descending artery; MACE, major adverse cardiac events; PCI, percutaneous coronary intervention.
IVUS may be particularly useful for the retrograde approach to CTO crossing, as retrograde cases are often more complex than antegrade‐only cases due to difficulties crossing the collateral and/or crossing the occlusion and externalizing the guidewire. Indeed, IVUS was used in 67% of retrograde versus 31% of antegrade‐only cases in our study (P<0.0001). IVUS can clarify the location of guidewires and guide balloon size selection when performing reverse CART. Dai et al showed that the IVUS‐guided reverse CART approach is efficient and safe for revascularization of complex CTOs. They overlapped an antegrade and a retrograde guidewire within the occlusion and inflated a small balloon (1.2–1.5 mm) to create an antegrade subintimal or intimal dissection. The IVUS catheter was then advanced into the dissection plane to guide crossing of the occlusion.11
Imaging for Stent Optimization
Intravascular imaging can assist with optimizing stent diameter and length selection, and further ensure that optimal expansion has occurred.10 Two randomized‐controlled trials have compared IVUS guidance versus angiographic guidance for stent optimization after CTO PCI. Kim et al randomized 402 patients to IVUS guidance versus angiographic guidance and found that IVUS guidance reduced the subsequent incidence of MACE.3 Similarly, Tian et al in the AIR‐CTO (Angiographic and clinical comparisons of intravascular ultrasound‐ versus angiography‐guided drug‐eluting stent implantation for patients with Chronic Total Occlusion lesions) study randomized 230 patients to IVUS or angiographic guidance and found that IVUS guidance was associated with lower in‐stent late lumen loss at 1‐year angiographic and IVUS follow‐up, leading to less frequent restenosis and lower rates of stent thrombosis.2 These findings are in agreement with the findings of the IVUS‐XPL (The Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions) study that randomized 1400 patients to undergo IVUS‐guided or angiography‐guided everolimus‐eluting stent implantation in non‐CTO long lesions and resulted in a significantly lower rate of 12‐month MACE, primarily driven by lower risk for target lesion revascularization.36 Use of intravascular imaging (either IVUS or OCT) can help identify and treat stent underexpansion, which is an important risk factor for both restenosis6 and stent thrombosis. Use of intravascular imaging may be of particular importance in long and calcified CTOs. In our study we observed a trend toward a higher number of stents in procedures guided by IVUS. This could be related to higher lesion complexity among imaged lesions, but could also indicate increased detection of dissection flaps, gaps between stents, or untreated residual coronary disease that might have not been apparent during diagnostic angiography.
Intravascular Imaging and Contrast Use
Mariani et al in the MOZART (Minimizing cOntrast utilization With IVUS Guidance in CoRonary angioplasTy) trial found that IVUS as a primary imaging tool to guide PCI was safe and markedly reduced the volume of iodine contrast as compared with angiography‐guided PCI.37 Dai et al suggested that the IVUS‐guided reverse CART technique could reduce the contrast volume.11 However, in our study contrast volume was higher among cases in which intravascular imaging was used for crossing, likely reflecting the higher complexity of such cases.
Study Limitations
Our study has potential limitations. First, PROGRESS CTO is an observational registry without adjudication of clinical events by an independent event committee. Second, quantitative coronary angiographic analysis was not performed and therefore assessment of angiographic characteristics was subject to operator‐related bias. Third, procedures were performed by experienced operators in CTO PCI, limiting extrapolation of the study results to less experienced centers and operators. Fourth, use of intravascular imaging was performed at the discretion of the operator, with high variability between centers. Fifth, few patients (n=13) had more than 1 CTO PCI.
Conclusion
In summary, intravascular imaging is frequently performed during CTO PCI both for crossing and for stent selection/optimization. Even though intravascular imaging was used in more complex lesions, it was associated with similar rates of technical and procedural success, but higher use of radiation and longer procedure time.
Sources of Funding
This was work was supported by Clinical and Translational Science Awards National Institutes of Health Grant UL1‐RR024982.
Disclosures
Dr Alaswad receives consulting fees from Terumo and Boston Scientific; and is an uncompensated consultant to Abbott Laboratories. Dr Jaffer is a consultant to Boston Scientific, Siemens, and Merck, receives nonfinancial research support from Abbott Vascular, and research grant from National Institutes of Health (HL‐R01‐108229). Dr Yeh receives Career Development Award (1K23HL118138) from the National Heart, Lung, and Blood Institute. Dr Grantham receives speaking fees, consulting, and honoraria from Boston Scientific and Asahi Intecc; and Research grants from Boston Scientific, Asahi Intecc, Abbott Vascular, and Medtronic. Dr Karmpaliotis is a member of the speaker bureau for Abbott Vascular, Medtronic, and Boston Scientific. Dr Kirtane receives Institutional research grants to Columbia University from Boston Scientific, Medtronic, Abbott Vascular, Abiomed, St. Jude Medical, Vascular Dynamics, Glaxo SmithKline, and Eli Lilly. Dr Parikh is a member of the speaker bureau for Abbot Vascular, Medtronic, CSI, BSc; and a member of the advisory boards of Medtronic, Abbott Vascular, and Philips. Dr Ali receives grant support and is a consultant for St Jude Medical and InfraReDx. Dr Lombardi has equity with Bridgepoint Medical. Dr Kandzari receives research/grant support and consulting honoraria from Boston Scientific and Medtronic Cardiovascular, and research/grant support from Abbott. Dr Lembo is a member of the speaker bureau of Medtronic and advisory board for Abbott Vascular and Medtronic. Dr Garcia receives consulting fees from Medtronic. Dr Wyman receives Honoraria/consulting/speaking fees from Boston Scientific, Abbott Vascular, and Asahi. Dr Rangan receives Research grants from InfraReDx, Inc., and The Spectranetics Corporation. Dr Thompson is an employee of Boston Scientific. Dr Banerjee receives research grants from Gilead and the Medicines Company; consultant/speaker honoraria from Covidien and Medtronic; has ownership in MDCARE Global (spouse) and intellectual property in HygeiaTel. Dr Brilakis receives consulting/speaker honoraria from Abbott Vascular, Asahi, Cardinal Health, GE Healthcare, Elsevier, and St Jude Medical; and research support from Boston Scientific and InfraRedx. His spouse is an employee of Medtronic. The remaining authors have no disclosures to report.
Acknowledgments
Study data were collected and managed using REDCap electronic data capture tools hosted at University of Texas Southwestern Medical Center.38 REDCap (Research Electronic Data Capture) is a secure, web‐based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
(J Am Heart Assoc. 2016;5:e003890 doi: 10.1161/JAHA.116.003890)
References
- 1. Hong SJ, Kim BK, Shin DH, Kim JS, Hong MK, Gwon HC, Kim HS, Yu CW, Park HS, Chae IH, Rha SW, Lee SH, Kim MH, Hur SH, Jang Y. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second‐generation drug‐eluting stents for chronic total occlusions (from the Multicenter Korean‐Chronic Total Occlusion Registry). Am J Cardiol. 2014;114:534–540. [DOI] [PubMed] [Google Scholar]
- 2. Tian NL, Gami SK, Ye F, Zhang JJ, Liu ZZ, Lin S, Ge Z, Shan SJ, You W, Chen L, Zhang YJ, Mintz G, Chen SL. Angiographic and clinical comparisons of intravascular ultrasound‐ versus angiography‐guided drug‐eluting stent implantation for patients with chronic total occlusion lesions: two‐year results from a randomised AIR‐CTO study. EuroIntervention. 2015;10:1409–1417. [DOI] [PubMed] [Google Scholar]
- 3. Kim BK, Shin DH, Hong MK, Park HS, Rha SW, Mintz GS, Kim JS, Lee SJ, Kim HY, Hong BK, Kang WC, Choi JH, Jang Y. Clinical impact of intravascular ultrasound‐guided chronic total occlusion intervention with zotarolimus‐eluting versus biolimus‐eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8:e002592. [DOI] [PubMed] [Google Scholar]
- 4. Park Y, Park HS, Jang GL, Lee DY, Lee H, Lee JH, Kang HJ, Yang DH, Cho Y, Chae SC, Jun JE, Park WH. Intravascular ultrasound guided recanalization of stumpless chronic total occlusion. Int J Cardiol. 2011;148:174–178. [DOI] [PubMed] [Google Scholar]
- 5. Matsubara T, Murata A, Kanyama H, Ogino A. Ivus‐guided wiring technique: promising approach for the chronic total occlusion. Catheter Cardiovasc Interv. 2004;61:381–386. [DOI] [PubMed] [Google Scholar]
- 6. Kang J, Cho YS, Kim SW, Park JJ, Yoon YE, Oh IY, Yoon CH, Suh JW, Youn TJ, Chae IH, Choi DJ. Intravascular ultrasound and angiographic predictors of in‐stent restenosis of chronic total occlusion lesions. PLoS One. 2015;10:e0140421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujii K, Ochiai M, Mintz GS, Kan Y, Awano K, Masutani M, Ashida K, Ohyanagi M, Ichikawa S, Ura S, Araki H, Stone GW, Moses JW, Leon MB, Carlier SG. Procedural implications of intravascular ultrasound morphologic features of chronic total coronary occlusions. Am J Cardiol. 2006;97:1455–1462. [DOI] [PubMed] [Google Scholar]
- 8. Wallace EL, Ziada KM. Intravascular‐ultrasound assisted localization and revascularization of an ostial chronic total occlusion: utility of near‐field and far‐field imaging. J Invasive Cardiol. 2015;27:E37–E39. [PubMed] [Google Scholar]
- 9. Nakashima M, Ikari Y, Aoki J, Tanabe K, Tanimoto S, Hara K. Intravascular ultrasound‐guided chronic total occlusion wiring technique using 6 Fr catheters via bilateral transradial approach. Cardiovasc Interv Ther. 2015;30:68–71. [DOI] [PubMed] [Google Scholar]
- 10. Brilakis ES. Manual of Chronic Total Occlusion Interventions. A Step‐by‐Step Approach. Waltham, MA: Elsevier; 2013. [Google Scholar]
- 11. Dai J, Katoh O, Kyo E, Tsuji T, Watanabe S, Ohya H. Approach for chronic total occlusion with intravascular ultrasound‐guided reverse controlled antegrade and retrograde tracking technique: single center experience. J Interv Cardiol. 2013;26:434–443. [DOI] [PubMed] [Google Scholar]
- 12. Rathore S, Katoh O, Tuschikane E, Oida A, Suzuki T, Takase S. A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound‐guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc Interv. 2010;3:155–164. [DOI] [PubMed] [Google Scholar]
- 13. Estevez‐Loureiro R, Ghione M, Kilickesmez K, Agudo P, Lindsay A, Di Mario C. The role for adjunctive image in pre‐procedural assessment and peri‐procedural management in chronic total occlusion recanalisation. Curr Cardiol Rev. 2014;10:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christopoulos G, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman RM, Lombardi WL, Menon RV, Grantham JA, Kandzari DE, Lembo N, Moses JW, Kirtane AJ, Parikh M, Green P, Finn M, Garcia S, Doing A, Patel M, Bahadorani J, Tarar MN, Christakopoulos GE, Thompson CA, Banerjee S, Brilakis ES. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christopoulos G, Karmpaliotis D, Wyman MR, Alaswad K, McCabe J, Lombardi WL, Grantham JA, Marso SP, Kotsia AP, Rangan BV, Garcia SA, Lembo N, Kandzari D, Lee J, Kalynych A, Carlson H, Thompson CA, Banerjee S, Brilakis ES. Percutaneous intervention of circumflex chronic total occlusions is associated with worse procedural outcomes: insights from a multicentre US registry. Can J Cardiol. 2014;30:1588–1594. [DOI] [PubMed] [Google Scholar]
- 16. Christopoulos G, Menon RV, Karmpaliotis D, Alaswad K, Lombardi W, Grantham A, Patel VG, Rangan BV, Kotsia AP, Lembo N, Kandzari D, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. The efficacy and safety of the “hybrid” approach to coronary chronic total occlusions: insights from a contemporary multicenter US registry and comparison with prior studies. J Invasive Cardiol. 2014;26:427–432. [PMC free article] [PubMed] [Google Scholar]
- 17. Sapontis J, Christopoulos G, Grantham JA, Wyman RM, Alaswad K, Karmpaliotis D, Lombardi WL, McCabe JM, Marso SP, Kotsia AP, Rangan BV, Christakopoulos GE, Garcia S, Thompson CA, Banerjee S, Brilakis ES. Procedural failure of chronic total occlusion percutaneous coronary intervention: insights from a multicenter US registry. Catheter Cardiovasc Interv. 2015;85:1115–1122. [DOI] [PubMed] [Google Scholar]
- 18. Alaswad K, Menon RV, Christopoulos G, Lombardi WL, Karmpaliotis D, Grantham JA, Marso SP, Wyman MR, Pokala NR, Patel SM, Kotsia AP, Rangan BV, Lembo N, Kandzari D, Lee J, Kalynych A, Carlson H, Garcia SA, Thompson CA, Banerjee S, Brilakis ES. Transradial approach for coronary chronic total occlusion interventions: insights from a contemporary multicenter registry. Catheter Cardiovasc Interv. 2015;85:1123–1129. [DOI] [PubMed] [Google Scholar]
- 19. Christopoulos G, Wyman RM, Alaswad K, Karmpaliotis D, Lombardi W, Grantham JA, Yeh RW, Jaffer FA, Cipher DJ, Rangan BV, Christakopoulos GE, Kypreos MA, Lembo N, Kandzari D, Garcia S, Thompson CA, Banerjee S, Brilakis ES. Clinical utility of the Japan‐chronic total occlusion score in coronary chronic total occlusion interventions: results from a multicenter registry. Circ Cardiovasc Interv. 2015;8:e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christopoulos G, Menon RV, Karmpaliotis D, Alaswad K, Lombardi W, Grantham JA, Michael TT, Patel VG, Rangan BV, Kotsia AP, Lembo N, Kandzari DE, Lee J, Kalynych A, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. Application of the “hybrid approach” to chronic total occlusions in patients with previous coronary artery bypass graft surgery (from a contemporary multicenter US registry). Am J Cardiol. 2014;113:1990–1994. [DOI] [PubMed] [Google Scholar]
- 21. Christopoulos G, Karmpaliotis D, Alaswad K, Lombardi WL, Grantham JA, Rangan BV, Kotsia AP, Lembo N, Kandzari DE, Lee J, Kalynych A, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. The efficacy of “hybrid” percutaneous coronary intervention in chronic total occlusions caused by in‐stent restenosis: insights from a US multicenter registry. Catheter Cardiovasc Interv. 2014;84:646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, Alaswad K, Lombardi W, Grantham JA, Moses J, Christakopoulos G, Tarar MN, Rangan BV, Lembo N, Garcia S, Cipher D, Thompson CA, Banerjee S, Brilakis ES. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (prospective global registry for the study of chronic total occlusion intervention) score. JACC Cardiovasc Interv. 2016;9:1–9. [DOI] [PubMed] [Google Scholar]
- 23. Karacsonyi J, Karatasakis A, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman MR, Lombardi WL, Grantham JA, Kandzari DE, Lembo N, Moses JW, Kirtane AJ, Parikh MA, Green P, Finn M, Garcia S, Doing A, Patel M, Bahadorani J, Martinez Parachini JR, Resendes E, Rangan BV, Ungi I, Thompson CA, Banerjee S, Brilakis ES. Effect of previous failure on subsequent procedural outcomes of chronic total occlusion percutaneous coronary intervention (from a contemporary multicenter registry). Am J Cardiol. 2016;117:1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danek BA, Karatasakis A, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Patel M, Bahadorani J, Lombardi WL, Wyman MR, Grantham JA, Doing A, Moses JW, Kirtane A, Parikh M, Ali ZA, Kalra S, Kandzari DE, Lembo N, Garcia S, Rangan BV, Thompson CA, Banerjee S, Brilakis ES. Use of antegrade dissection re‐entry in coronary chronic total occlusion percutaneous coronary intervention in a contemporary multicenter registry. Int J Cardiol. 2016;214:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stone GW, Reifart NJ, Moussa I, Hoye A, Cox DA, Colombo A, Baim DS, Teirstein PS, Strauss BH, Selmon M, Mintz GS, Katoh O, Mitsudo K, Suzuki T, Tamai H, Grube E, Cannon LA, Kandzari DE, Reisman M, Schwartz RS, Bailey S, Dangas G, Mehran R, Abizaid A, Moses JW, Leon MB, Serruys PW. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation. 2005;112:2530–2537. [DOI] [PubMed] [Google Scholar]
- 26. Morino Y, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Morimoto T, Abe M, Hinohara T, Mitsudo K. In‐hospital outcomes of contemporary percutaneous coronary intervention in patients with chronic total occlusion insights from the J‐CTO Registry (Multicenter CTO Registry in Japan). JACC Cardiovasc Interv. 2010;3:143–151. [DOI] [PubMed] [Google Scholar]
- 27. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 28. Okamura A, Yamane M, Muto M, Matsubara T, Igarashi Y, Nakamura S, Muramatsu T, Fujita T, Oida A, Tsuchikane E. Complications during retrograde approach for chronic coronary total occlusion: Sub‐analysis of japanese multicenter registry. Catheter Cardiovasc Interv. 2016;88:7–14. [DOI] [PubMed] [Google Scholar]
- 29. Galassi AR, Tomasello SD, Reifart N, Werner GS, Sianos G, Bonnier H, Sievert H, Ehladad S, Bufe A, Shofer J, Gershlick A, Hildick‐Smith D, Escaned J, Erglis A, Sheiban I, Thuesen L, Serra A, Christiansen E, Buettner A, Costanzo L, Barrano G, Di Mario C. In‐hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) Registry. EuroIntervention. 2011;7:472–479. [DOI] [PubMed] [Google Scholar]
- 30. Galassi AR, Sianos G, Werner GS, Escaned J, Tomasello SD, Boukhris M, Castaing M, Buttner JH, Bufe A, Kalnins A, Spratt JC, Garbo R, Hildick‐Smith D, Elhadad S, Gagnor A, Lauer B, Bryniarski L, Christiansen EH, Thuesen L, Meyer‐Gessner M, Goktekin O, Carlino M, Louvard Y, Lefevre T, Lismanis A, Gelev VL, Serra A, Marza F, Di Mario C, Reifart N. Retrograde recanalization of chronic total occlusions in europe: procedural, in‐hospital, and long‐term outcomes from the multicenter ERCTO registry. J Am Coll Cardiol. 2015;65:2388–2400. [DOI] [PubMed] [Google Scholar]
- 31. Habara M, Tsuchikane E, Muramatsu T, Kashima Y, Okamura A, Mutoh M, Yamane M, Oida A, Oikawa Y, Hasegawa K. Comparison of percutaneous coronary intervention for chronic total occlusion outcome according to operator experience from the Japanese retrograde summit registry. Catheter Cardiovasc Interv. 2016;87:1027–1035. [DOI] [PubMed] [Google Scholar]
- 32. Tsujita K, Maehara A, Mintz GS, Kubo T, Doi H, Lansky AJ, Stone GW, Moses JW, Leon MB, Ochiai M. Intravascular ultrasound comparison of the retrograde versus antegrade approach to percutaneous intervention for chronic total coronary occlusions. JACC Cardiovasc Interv. 2009;2:846–854. [DOI] [PubMed] [Google Scholar]
- 33. Heeger CH, Busjahn A, Hildebrand L, Fenski M, Lesche F, Meincke F, Kuck KH, Bergmann MW. Delayed coverage of drug‐eluting stents after interventional revascularisation of chronic total occlusions assessed by optical coherence tomography: the ALSTER‐OCT‐CTO registry. EuroIntervention. 2016;11:1004–1012. [DOI] [PubMed] [Google Scholar]
- 34. Niccoli G, Ferrante G, Galassi AR, Montone RA, Crea F. Optical coherence tomography follow‐up of the subintimal tracking and re‐entry technique for chronic total occlusion. EuroIntervention. 2010;6:662–663. [DOI] [PubMed] [Google Scholar]
- 35. Ito S, Suzuki T, Ito T, Katoh O, Ojio S, Sato H, Ehara M, Kawase Y, Myoishi M, Kurokawa R, Ishihara Y, Suzuki Y, Sato K, Toyama J, Fukutomi T, Itoh M. Novel technique using intravascular ultrasound‐guided guidewire cross in coronary intervention for uncrossable chronic total occlusions. Circ J. 2004;68:1088–1092. [DOI] [PubMed] [Google Scholar]
- 36. Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Kang WC, Her AY, Kim YH, Hur SH, Hong BK, Kwon H, Jang Y, Hong MK. Effect of intravascular ultrasound‐guided vs angiography‐guided everolimus‐eluting stent implantation: the IVUS‐XPL randomized clinical trial. JAMA. 2015;314:2155–2163. [DOI] [PubMed] [Google Scholar]
- 37. Mariani J Jr, Guedes C, Soares P, Zalc S, Campos CM, Lopes AC, Spadaro AG, Perin MA, Filho AE, Takimura CK, Ribeiro E, Kalil‐Filho R, Edelman ER, Serruys PW, Lemos PA. Intravascular ultrasound guidance to minimize the use of iodine contrast in percutaneous coronary intervention: the MOZART (minimizing contrast utilization with IVUS guidance in coronary angioplasty) randomized controlled trial. JACC Cardiovasc Interv. 2014;7:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]


