Abstract
Background
Obesity is a risk factor for various subtypes of cardiovascular disease (CVD), including coronary heart disease (CHD), heart failure (HF), and stroke. Nevertheless, there are limited comparisons of the associations of obesity with each of these CVD subtypes, particularly regarding the extent to which they are unexplained by traditional CVD mediators.
Methods and Results
We followed 13 730 participants in the Atherosclerosis Risk in Communities (ARIC) study who had a body mass index ≥18.5 and no CVD at baseline (visit 1, 1987–1989). We compared the association of higher body mass index with incident HF, CHD, and stroke before and after adjusting for traditional CVD mediators (including systolic blood pressure, diabetes mellitus, and lipid measures). Over a median follow‐up of 23 years, there were 2235 HF events, 1653 CHD events, and 986 strokes. After adjustment for demographics, smoking, physical activity, and alcohol intake, higher body mass index had the strongest association with incident HF among CVD subtypes, with hazard ratios for severe obesity (body mass index ≥35 versus normal weight) of 3.74 (95% CI 3.24–4.31) for HF, 2.00 (95% CI 1.67–2.40) for CHD, and 1.75 (95% CI 1.40–2.20) for stroke (P<0.0001 for comparisons of HF versus CHD or stroke). Further adjustment for traditional mediators fully explained the association of higher body mass index with CHD and stroke but not with HF (hazard ratio 2.27, 95% CI 1.94–2.64).
Conclusions
The link between obesity and HF was stronger than those for other CVD subtypes and was uniquely unexplained by traditional risk factors. Weight management is likely critical for optimal HF prevention, and nontraditional pathways linking obesity to HF need to be elucidated.
Keywords: coronary heart disease, epidemiology, heart failure, obesity, stroke
Subject Categories: Obesity, Epidemiology, Cardiovascular Disease
Introduction
Obesity is a common risk factor for several subtypes of cardiovascular disease (CVD), including coronary heart disease (CHD), stroke, and heart failure (HF)1, 2, 3, 4; however, increasing evidence suggests that obesity leads to various subtypes of CVD through multiple distinct pathways. Some traditional risk factors, including hypertension, diabetes mellitus, and dyslipidemia, are established as mediators between obesity and atherosclerotic vascular disease. Although weight management is a fundamental component of CVD prevention, the majority of persons with obesity in the general population do not achieve sufficient and sustained weight loss.5, 6, 7, 8 Consequently, there is great emphasis on controlling the traditional CVD risk factors resulting from obesity as a strategy for reducing cardiovascular risk. Nevertheless, uniform approaches to the control of cardiovascular risk factors may not have the same impact on the likelihood of developing different subtypes of CVD.
In this context, data conflict regarding whether the relationships of obesity with CHD and stroke are independent of established CVD mediators. Several prospective studies9, 10, 11, 12, 13, 14, 15, 16 and current scientific statements1 describe obesity as an independent risk factor for CHD and stroke, whereas other studies suggest that the associations are caused entirely by established mediators.17, 18, 19, 20, 21, 22, 23, 24 Nonetheless, a recent meta‐analysis indicated that most of the associations of obesity with CHD and stroke may be mediated by hypertension, dyslipidemia, and diabetes mellitus.25 Notably, this meta‐analysis did not include HF as an outcome, and indeed, data on an independent association of obesity with incident HF are relatively sparse.26, 27, 28, 29 Most important, very few prospective analyses have compared the association of obesity with each of these CVD subtypes within the same population after controlling for traditional CVD mediators.
It is possible that mechanisms other than hypertension, dyslipidemia, and diabetes mellitus, such as excess metabolic demand and direct adverse effects of adiposity on the myocardium, play especially important roles in the development of HF among persons with excess weight.30, 31 If this is the case, traditional CVD risk factor control alone may not lead to optimal prevention of HF. In this prospective analysis of white and black middle‐aged men and women without baseline CVD in the Atherosclerosis Risk in Communities (ARIC) study, we compared the associations of obesity with incident HF, CHD, and stroke before and after accounting for traditional CVD mediators.
Methods
The ARIC study is a prospective, predominantly biracial, community‐based cohort of 15 792 participants extensively characterized for cardiovascular risk factors and followed longitudinally for CVD events.32 Participants were recruited from 4 US population centers (Washington County, Maryland; Jackson, Mississippi; Forsyth County, North Carolina; and the suburbs of Minneapolis, Minnesota) and examined at a baseline visit in the period from 1987 to 1989. Participants were subsequently examined at 3 study visits spaced ≈3 years apart and at a fifth visit conducted recently from 2011 to 2013.
Given the focus on incident CVD events, we excluded those participants with a prior history of HF, CHD, stroke, or peripheral vascular disease at the first ARIC study visit in the period from 1987 to 1989 (n=1847). We also excluded those with missing data on body mass index (BMI; kg/m2; n=25) or with BMI <18.5 (n=142), along with the small number of participants who were not of either black or white race (n=48), leaving a study population of 13 730 participants. Informed consent was obtained from all study participants, and the institutional review boards affiliated with each ARIC field center and with the coordinating center approved the study protocol.
Information regarding covariates of interest was collected at visit 1. The primary exposure was BMI, calculated from measured weight and height (weight in kilograms divided by square meters). Smoking status was categorized as current, former, or never smoker. Self‐reported alcohol use was calculated in grams per week. Occupation was categorized into subtypes of employment. Exercise physical activity was self‐reported and assessed using a modified Baecke questionnaire, and each activity was subsequently converted into metabolic equivalents of task based on the Compendium of Physical Activities. Diabetes mellitus was defined as the presence of fasting blood sugar ≥126 mg/dL, nonfasting blood sugar ≥200 mg/dL, a self‐reported prior physician diagnosis of diabetes mellitus, or the use of hypoglycemic medications. Systolic blood pressure was measured 3 times during the same examination, and the average of the last 2 measurements was used for analysis. Enzymatic assays were used to measure levels of total cholesterol, high‐density lipoprotein cholesterol, and triglycerides; low‐density lipoprotein cholesterol was calculated for those with triglycerides ≤400 mg/dL, using the Friedewald's equation.33
The outcomes of interest were incident HF, CHD, and stroke, with follow‐up through December 31, 2012. After the baseline visit, ARIC participants were followed continuously for CVD events, receiving annual phone calls to obtain information regarding hospitalizations. For all deaths, vital records were examined, and in the majority of potential CHD cases occurring out of hospital, an interview with the decedent's next of kin and a questionnaire completed by the patient's physician were also reviewed. Incident HF was defined as the first hospitalization or death related to HF. HF events were identified by discharge codes from hospitalizations and death certificates (International Classification of Diseases, 9th Revision [ICD‐9] code 428 for hospitalizations and deaths in early years of follow‐up and ICD‐10 code I‐50 for deaths in later years of follow‐up). HF events from 2005 onward were also adjudicated by an expert panel.34 Incident CHD was defined as an adjudicated event of definite or probable nonfatal myocardial infarction or definite fatal CHD. Potential incident strokes were identified by the presence of related discharge codes (ICD‐9 codes code 430–437), the mention of a cerebrovascular condition or procedure in a discharge summary, or the presence of stroke findings on a computed tomography or magnetic resonance imaging report. If there was disagreement between automated and physician diagnoses, events were adjudicated from abstracted medical records by a third reviewer. Definite or probable strokes of ischemic or hemorrhagic etiology were included in this analysis.
Statistical Analysis
We performed univariate comparisons of baseline characteristics across BMI categories (normal weight 18.5 to <25.0, overweight 25.0 to <30.0, obese 30.0 to <35.0, and severely obese ≥35.0), using ANOVA for continuous variables and the chi‐square test for categorical variables. Using Poisson models, we calculated the adjusted incidence rates for each CVD subtype associated with higher BMI at mean levels of age, sex, race, smoking status, alcohol use, education level, occupation, and physical activity within the study population. In addition to the aforementioned categories, BMI was modeled continuously by constructing linear spline models with knots at the BMI values of 25, 30, 35, 40, and 45. We also assessed the median onset of incident events for each subtype of CVD.
For each CVD subtype, we constructed Cox proportional hazards models to estimate the hazard ratios (HRs) and 95% CIs associated with higher BMI, with 2 levels of adjustment. Model 1 was adjusted for the confounding variables of age sex, race, smoking status, alcohol use, physical activity, education level, and occupation. To estimate the associations between obesity and each CVD subtype after accounting for established mediators, model 2 was adjusted for the model 1 variables plus baseline values of traditional mediators of CVD in the setting of obesity: diabetes mellitus, systolic blood pressure, antihypertensive medication use, low‐ and high‐density lipoprotein cholesterol, triglycerides, and estimated glomerular filtration rate.
In sensitivity analyses, regression models were constructed for smoking status, hypertension, diabetes mellitus, and hypercholesterolemia as time‐varying covariates to account for potential changes in these variables after the baseline visit but prior to the onset of CVD events. In creating time‐varying covariates, measurements from ARIC visits 1, 2 (1990–1992), 3 (1993–1995), and 4 (1996–1999) were incorporated into regression analyses if the visit took place before an incident CVD event. Smoking status was categorized as current smoker versus current nonsmoker; hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications; diabetes mellitus was defined as described; and hypercholesterolemia was defined as low‐density lipoprotein cholesterol ≥160 mg/dL or the use of lipid‐lowering medications. For the latter 3 variables, after the onset of the mediator, participants were considered to have that mediator thereafter.
To formally compare the strength of associations for obesity with incident HF, CHD, and stroke, we used seemingly unrelated regression. This statistical approach accounts for correlations of the error terms in the risk equations for different outcomes within the same population by estimating the risk equations jointly, providing a means for formally comparing the magnitude of the different risk associations.35
We performed analyses stratified by race, sex, and age (≥60 or <60 years) and created product terms to assess for differences in associations across demographic subgroups. Sensitivity analyses were performed using only adjudicated events of definite or probable incident HF from 2005 onward. Additional sensitivity analyses were performed by adjusting for baseline lung function (forced expiratory volume in 1 second and forced vital capacity), by including coronary revascularization procedures (percutaneous intervention or coronary artery bypass grafting) in the definition of incident CHD, and by adding incident CHD to regression models as a time‐varying covariate to evaluate the contribution of ischemic events to the association observed between obesity and incident HF. To account for the possibility of death from other causes that may have prevented participants from experiencing one of the outcomes of interest, we also performed competing risks regression analyses. In addition, we performed analyses modeling obesity (BMI ≥30) from visits 1 through 4 as a time‐varying covariate to account for the impact of the development of obesity after the baseline ARIC visit. Additional analyses were performed using waist circumference, divided into sex‐specific quartiles, as a secondary metric of adiposity.
All P values presented are 2‐sided. Statistical analyses were performed using Stata version 13.1 (StataCorp LP).
Results
Baseline characteristics of the study population are displayed in Table 1. Those with severe obesity were younger, were most likely to be female and black, were least likely to be current smokers, and had the lowest average levels of exercise physical activity. Higher BMI categories were associated with higher systolic blood pressure, triglycerides, and estimated glomerular filtration rate; lower high‐density lipoprotein cholesterol; and greater prevalence of antihypertensive medication use and diabetes mellitus.
Table 1.
Baseline Characteristics of Study Population, Stratified by BMI Category
| Normal Weight (n=4602) | Overweight (n=5480) | Obese (n=2471) | Severely Obese (n=1177) | P Value | |
|---|---|---|---|---|---|
| Age, y, mean (SD) | 53.8 (5.8) | 54.0 (5.7) | 54.0 (5.7) | 53.1 (5.6) | <0.001 |
| Female, % | 63.8 | 44.8 | 55.4 | 77.5 | <0.001 |
| Black, % | 16.9 | 25.3 | 35.1 | 48.5 | <0.001 |
| Current smoker, % | 31.7 | 23.6 | 20.3 | 14.9 | <0.001 |
| Exercise physical activity, (met×min)/week, mean (SD) | 736.6 (855.9) | 669.8 (777.7) | 511.9 (686.8) | 352.2 (567.8) | <0.001 |
| Systolic blood pressure, mm Hg, mean (SD) | 116.0 (18.1) | 121.1 (17.9) | 125.1 (17.8) | 129.9 (19.0) | <0.001 |
| Use of antihypertensive medication, % | 12.2 | 20.8 | 30.7 | 42.1 | <0.001 |
| Diabetes mellitus, % | 4.2 | 9.0 | 17.3 | 26.6 | <0.001 |
| LDL‐C, mg/dL, mean (SD) | 130.7 (38.6) | 140.3 (38.9) | 142.3 (39.1) | 136.0 (36.6) | <0.001 |
| HDL‐C, mg/dL, mean (SD) | 58.9 (18.4) | 49.6 (15.6) | 47.0 (14.5) | 48.2 (13.7) | <0.001 |
| Triglycerides, mg/dL, median (IQR) | 91 (68–126) | 112 (80–160) | 128 (92–179) | 124 (90–170) | <0.001 |
| eGFR, mL/min/1.73 m2, median (IQR) | 104.0 (96.5–111.1) | 102.3 (94.4–110.5) | 103.0 (93.8–112.7) | 108.0 (98.4–118.5) | <0.001 |
| Waist circumference, cm, mean (SD) | 84.5 (7.5) | 96.8 (7.0) | 107.1 (7.6) | 122.0 (11.6) | <0.001 |
BMI indicates body mass index; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol.
Over ≈23 years of follow‐up, there were 2235 HF events, 1653 CHD events, and 986 strokes. For each subtype of CVD, higher BMI was associated with a greater adjusted incidence of events at mean levels of demographic variables, smoking, alcohol use, and physical activity (Figure 1). The incidence rate difference for severe obesity versus normal weight (per 1000 person‐years) was 12.1 for HF, 4.0 for CHD, and 2.7 for stroke. The median time to incident events for each CVD subtype was 15.7 years for HF, 12.7 years for CHD, and 13.7 years for stroke.
Figure 1.
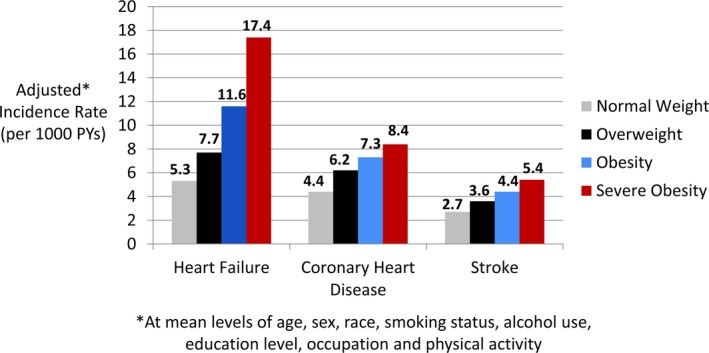
Association of BMI categories with adjusted incidence rates for different CVD subtypes. Incidence rates were calculated at mean levels of age, sex, race, smoking status, alcohol use, education level, occupation, and physical activity within the study population. BMI indicates body mass index; CVD, cardiovascular disease; PY, person‐year.
In regression models adjusted for confounding variables (model 1), overweight status and obesity were significantly associated with increased HRs for each CVD subtype; however, the strongest associations with higher BMI were seen for incident HF (Table 2). Severe obesity, for example, was associated with a nearly 4‐fold higher risk of incident HF (HR 3.74, 95% CI 3.24–4.31) and a ≈2‐fold higher risk of CHD (HR 2.00, 95% CI 1.67–2.40) and stroke (HR 1.75, 95% CI 1.40–2.20) compared with normal weight. In seemingly unrelated regression analyses, the risk coefficient between severe obesity and incident HF was significantly greater than those for incident CHD and stroke (P<0.0001 for both comparisons). In the confounder‐adjusted model, with BMI modeled linearly, each 5‐U increase in BMI was associated with a 46% higher risk of incident HF compared with 22% and 16% higher risks for incident CHD and stroke, respectively (P<0.0001 for comparisons of risk coefficients).
Table 2.
Association of Higher BMI Categories With CVD Subtypes
| Normal Weight (BMI 18.5–24.9), n=4602 | Overweight (BMI 25–29.9), n=5480 | Obese (BMI 30–34.9), n=2471 | Severely Obese (BMI >35), n=1177 | |
|---|---|---|---|---|
| Model 1a | ||||
| Incident HF | Reference | 1.38 (1.23–1.54) | 2.10 (1.85–2.38) | 3.74 (3.24–4.31) |
| Incident CHD | Reference | 1.26 (1.11–1.43) | 1.53 (1.33–1.77) | 2.00 (1.67–2.40) |
| Incident stroke | Reference | 1.17 (1.00–1.37) | 1.32 (1.10–1.60) | 1.75 (1.40–2.20) |
| Model 2b | ||||
| Incident HF | Reference | 1.12 (0.99–1.26) | 1.50 (1.32–1.72) | 2.27 (1.94–2.64) |
| Incident CHD | Reference | 0.96 (0.84–1.09) | 0.95 (0.81–1.11) | 1.06 (0.87–1.29) |
| Incident stroke | Reference | 0.99 (0.84–1.17) | 0.97 (0.80–1.19) | 1.13 (0.88–1.44) |
Data are shown as adjusted hazard ratio (95% CI). BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure.
Model 1: Adjusted for age, race, sex, alcohol use, smoking status, physical activity, occupation, and education level.
Model 2: Adjusted for model 1 variables plus diabetes mellitus, systolic blood pressure, antihypertensive medication use, high‐ and low‐density lipoprotein cholesterol, triglycerides, and estimated glomerular filtration rate.
After further adjustment for traditional CVD mediators (model 2), a statistically significant association remained between higher BMI and incident HF (HR for severe obesity 2.27, 95% CI 1.94–2.64), but no significant associations were seen for incident CHD and stroke. Similarly, in the fully adjusted model, every 5‐U increase in BMI was associated with a 29% higher risk of incident HF, whereas no significant associations were seen for CHD and stroke. Our findings were analogous when BMI was modeled continuously in linear spline models (Figure 2).
Figure 2.
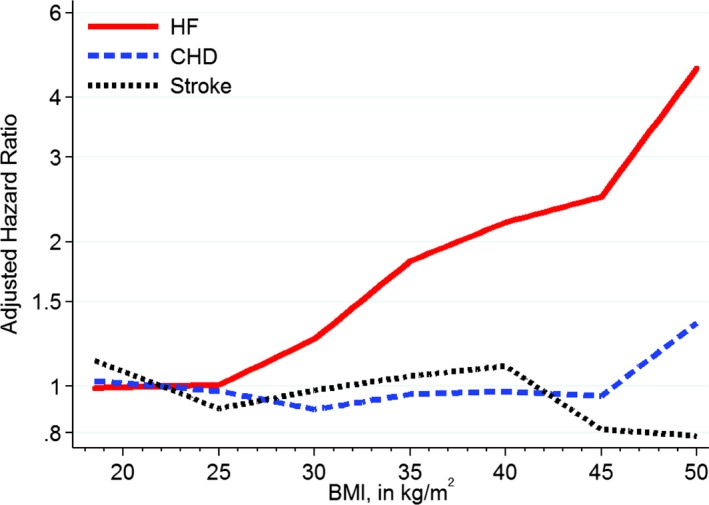
Relationship of continuous BMI with incident cardiovascular disease subtypes in linear spline models after multivariable adjustment. Linear spline with knots at the BMI values of 25, 30, 35, 40, and 45 and reference at BMI of 22. Adjusted for age, race, sex, alcohol use, smoking status, occupation, education level, physical activity, diabetes mellitus, systolic blood pressure, antihypertensive medication use, HDL‐C and LDL‐C, and estimated glomerular filtration rate. BMI indicates body mass index; CHD, coronary heart disease; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; LDL‐C, low‐density lipoprotein cholesterol.
In regression analyses stratified by demographic subgroups using the fully adjusted model, significant associations between higher BMI category and incident HF were seen within each of the prespecified demographic subgroups (Figure 3). Notably, a trend toward weaker associations between higher BMI and incident HF was seen among black participants in comparison to white participants (P interaction=0.4), although significant associations were seen for both races. In contrast, no significant associations between higher BMI and either incident CHD or stroke were seen in any of the demographic subgroups after adjustment for both confounders and traditional CVD mediators (data not shown).
Figure 3.
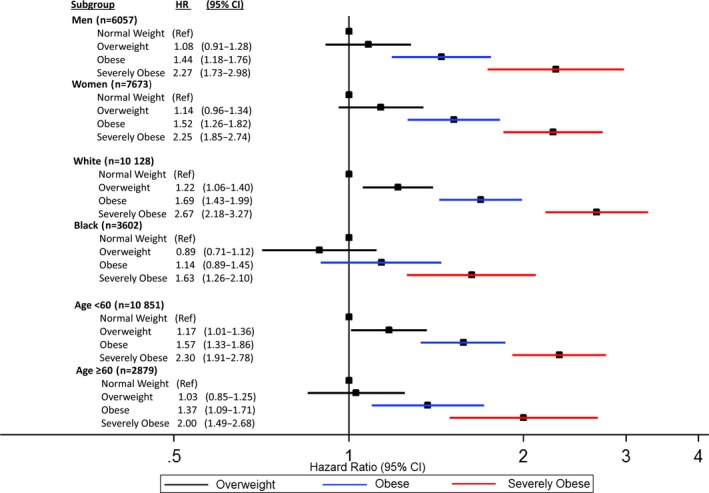
Association of higher BMI categories with incident heart failure after multivariable adjustment within demographic subgroups. Adjusted for age, race, sex, alcohol use, smoking status, occupation, education level, physical activity, diabetes mellitus, systolic blood pressure, antihypertensive medication use, HDL‐C and LDL‐C, triglycerides, and estimated glomerular filtration rate. BMI indicates body mass index; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; ; LDL‐C, low‐density lipoprotein cholesterol; Ref, reference.
Similar findings were seen when smoking status, hypertension, diabetes mellitus, and hypercholesterolemia from ARIC visits 1 through 4 were modeled as time‐varying covariates (Table 3), with severe obesity having a mildly positive association with incident CHD (HR 1.20, 95% CI 1.02–1.41) and no significant association with stroke (HR 1.15, 95% CI 0.90–1.47) compared with a stronger association with incident HF (HR 2.18, 95% CI 1.87–2.54). Notably, in this time‐varying analysis, HF was the only outcome for which significant associations remained for the obese category (BMI 30.0–34.9).
Table 3.
Association of Higher BMI Categories With Incident CVD Subtypes With Adjustment for Major Risk Factorsa as Time‐Varying Covariates
| Normal Weight (BMI 18.5–24.9), n=4602 | Overweight (BMI 25–29.9), n=5480 | Obese (BMI 30–34.9), n=2471 | Severely Obese (BMI >35), n=1177 | |
|---|---|---|---|---|
| Incident HF | 1.0 (Reference) | 1.11 (0.99–1.25) | 1.40 (1.22–1.60) | 2.18 (1.87–2.54) |
| Incident CHD | 1.0 (Reference) | 0.98 (0.88–1.09) | 0.97 (0.86–1.11) | 1.20 (1.02–1.41) |
| Incident stroke | 1.0 (Reference) | 0.98 (0.83–1.16) | 0.94 (0.77–1.15) | 1.15 (0.90–1.47) |
Data are shown as adjusted hazard ratio (95% CI). BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure.
The following risk factors were modeled as time varying covariates from ARIC visits 1 through 4 to account for their potential onset after the baseline visit: smoking status (current smoker versus current nonsmoker); hypertension (defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications); diabetes mellitus (defined as fasting blood sugar ≥126 mg/dL, nonfasting blood sugar ≥200 mg/dL, self‐reported prior physician diagnosis, or use of hypoglycemic medications); and hypercholesterolemia (low‐density lipoprotein cholesterol ≥160 mg/dL or use of lipid‐lowering medications). Measurements from study visits occurring before incident CVD events were incorporated in regression analyses. For hypertension, diabetes mellitus, and hypercholesterolemia, risk factors were considered to be present after onset. Regression models were additionally adjusted for age, race, sex, alcohol use, occupation, education level, physical activity, high‐density lipoprotein cholesterol, triglycerides, and estimated glomerular filtration rate.
Our findings were similar in sensitivity analyses using only adjudicated HF events (n=663), with severe obesity associated with an HR of 2.22 (95% CI 1.74–2.85) for incident HF compared with normal weight in the fully adjusted model. Findings were also not appreciably different when lung function was included in regression models, when revascularization procedures were included in the definition of incident CHD, or when incident CHD was modeled as a time‐varying covariate on the outcome of incident HF. Similar findings were also seen in competing risks regression analyses to account for the possibility of non‐CVD death precluding the development of the outcomes of interest. Analogous findings were seen when obesity (defined as BMI ≥30) was modeled as a time‐varying covariate to account for the onset of obesity after the baseline ARIC visit, with significant associations seen only for incident HF after adjustment for traditional CVD mediators (Table 4). In analyses using waist circumference divided into quartiles as a secondary measure of adiposity, higher waist circumference quartiles were significantly associated with only incident HF (HR 1.96, 95% CI 1.70–2.27, for quartile 4 versus 1) among CVD subtypes in the fully adjusted model (Table 5).
Table 4.
Association of Obesity (BMI ≥30), Modeled as a Time‐Varying Covariatea, With Incident CVD Subtypes
| Nonobese (BMI 18.5–29.9) | Obese (BMI ≥30.0) | |
|---|---|---|
| Incident HF | 1.0 (Reference) | 1.44 (1.32–1.58) |
| Incident CHD | 1.0 (Reference) | 1.00 (0.92–1.10) |
| Incident stroke | 1.0 (Reference) | 1.05 (0.91–1.20) |
Data are shown as adjusted hazard ratio (95% CI). BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure.
Obesity, defined as a BMI ≥30, was modeled as a time‐varying covariate from ARIC visits 1 through 4 to account for its potential onset after the baseline visit. The reference group was nonobese (BMI <30). Measurements from study visits occurring before incident CVD events were incorporated in regression analyses. Obesity was considered to be present after onset. Regression models were adjusted for age, race, sex, alcohol use, smoking status, occupation, education level, physical activity, diabetes mellitus, systolic blood pressure, antihypertensive medication use, high‐ and low‐density lipoprotein cholesterol, triglycerides, and estimated glomerular filtration rate.
Table 5.
Association of Sex‐Specific Waist Circumference Quartilesa With CVD Subtypes
| Quartile 1, n=3286 | Quartile 2, n=3299 | Quartile 3, n=3534 | Quartile 4, n=3605 | |
|---|---|---|---|---|
| Model 1b | ||||
| Incident HF | 1.0 (Ref) | 1.43 (1.23–1.67) | 1.74 (1.50–2.00) | 3.01 (2.63–3.44) |
| Incident CHD | 1.0 (Ref) | 1.25 (1.06–1.46) | 1.42 (1.22–1.66) | 1.87 (1.61–2.16) |
| Incident stroke | 1.0 (Ref) | 1.13 (0.92–1.38) | 1.33 (1.09–1.61) | 1.67 (1.38–2.01) |
| Model 2c | ||||
| Incident HF | Ref | 1.25 (1.07–1.46) | 1.33 (1.15–1.55) | 1.96 (1.70–2.27) |
| Incident CHD | Ref | 1.01 (0.86–1.18) | 0.97 (0.83–1.14) | 1.04 (0.89–1.22) |
| Incident stroke | Ref | 0.99 (0.80–1.22) | 1.04 (0.85–1.28) | 1.14 (0.92–1.39) |
Data are shown as adjusted hazard ratio (95% CI). BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; Ref, reference.
Waist circumference quartile ranges: quartile 1: women 52–83 cm, men 52–91 cm; quartile 2: women 84–92 cm, men 92–97 cm; quartile 3: women 93–103 cm, men 98–104 cm; quartile 4: women 105–178 cm, men 104–178 cm.
Model 1: adjusted for age, race, sex, alcohol use, smoking status, physical activity, occupation, and education level.
Model 2: adjusted for model 1 variables plus diabetes mellitus, systolic blood pressure, antihypertensive medication use, high‐ and low‐density lipoprotein cholesterol, triglycerides, and estimated glomerular filtration rate.
Discussion
In this prospective analysis of a biracial community‐based cohort of 13 730 adult men and women without baseline CVD, we compared the relationship of higher BMI with incident HF, CHD, and stroke. After controlling for confounding variables, we found that overweight status and obesity were most strongly associated with incident HF among CVD subtypes. Severe obesity was linked to a nearly 4‐fold higher risk of incident HF compared with ≈2‐fold higher risks for incident CHD and stroke.
In regression analyses adjusting for both confounders and traditional CVD mediators, we found that traditional mediators explained all association of higher BMI with incident CHD and stroke. In contrast, the relationship of obesity with incident HF was largely unexplained by traditional mediators. These findings were qualitatively consistent across demographic subgroups. Similar findings were seen when major risk factors and obesity were modeled as time‐varying covariates to account for their potential onset after the baseline visit. Our results were also largely unchanged when the analysis was restricted to adjudicated HF events and when CHD was included in regression models as a time‐varying covariate to account for antecedent myocardial infarction as a precipitant of HF.
The 2006 American Heart Association Scientific Statement on obesity and CVD describes obesity as a risk factor for CHD and stroke independent of established mediators,1 but prior data on this subject have been inconsistent. Some prospective data suggested an association between obesity and CHD and stroke beyond that explained by traditional CVD mediators,10, 11, 12, 14 whereas other longitudinal studies indicated that extensive adjustment for these mediators fully explains these associations.17, 18, 21 A meta‐analysis indicated that at least half of the association between obesity and CHD and three‐quarters of the association between obesity and stroke are likely explained by hypertension, dyslipidemia, and hyperglycemia. The relatively limited prospective data regarding an independent link between obesity and incident HF, however, suggest a persistent risk association after accounting for traditional CVD mediators.26, 27, 28, 29
Most notably, there are very limited analyses comparing the relationship of obesity with HF, CHD, and stroke within the same population. A recent analysis of a Norwegian cohort found that “metabolically healthy” obese participants were at increased risk for HF but not for acute myocardial infarction.28 Our study, using contemporary follow‐up data in a biracial community‐based cohort, extended prior research by demonstrating a much stronger relationship between obesity and incident HF than with CHD and stroke within the same population. Furthermore, among major CVD subtypes, the obesity–HF relationship was the only one unexplained by traditional risk factors.
The mechanisms underlying the association between obesity and HF remain incompletely understood. Increased fat mass is associated with expanded blood volume and increased myocardial workload.31, 36 Obesity is also associated with adverse cardiac remodeling and abnormalities of myocardial structure and function, changes that are known to precede the development of clinical HF.30, 31, 37 Several adipokines, which are associated in particular with abdominal obesity, are linked to structural and functional myocardial abnormalities and increased HF risk.29, 38, 39, 40, 41 Increasing laboratory and clinical data suggest a direct link between obesity and myocardial injury that may predispose to fibrosis, myocardial dysfunction, and future HF.42, 43, 44 Several processes are hypothesized to contribute to myocardial injury and dysfunction among persons with obesity, including increased metabolic demand, the paracrine effects of adipose tissue, and increased myocardial triglyceride accumulation leading to myocardial damage and potential apoptosis44, 45, 46; however, the pathophysiological links between obesity and HF have not yet been fully elucidated.
Overweight status and obesity were associated with higher risks for all forms of CVD, a finding of considerable public health importance, given that the majority of the US adult population falls into one of these weight categories. This analysis, however, has particularly important implications for HF prevention. The clinical importance of refining strategies for HF prevention in obesity is reinforced by the finding that the obesity–HF relationship was strongest among the incident CVD subtypes. Although weight reduction remains advisable as a first‐line strategy for CHD and stroke prevention in the setting of obesity, this analysis indicates that controlling the traditional risk factors associated with obesity may address much of the excess risk for these outcomes in this population. In contrast, our findings suggest that controlling traditional risk factors alone will not be sufficient to address the excess risk of HF in association with obesity.
This study further indicates that weight management, including the avoidance of weight gain and weight reduction among those who are overweight or obese, is likely critical for optimal HF prevention. Several prior analyses, however, have documented the challenges of achieving significant and sustained weight loss in the population setting,5, 6, 7, 8 indicating the importance of developing additional approaches to reduce the risk of HF associated with obesity. These findings underscore the need for further investigation to elucidate the nontraditional pathways linking obesity to HF to inform novel preventive strategies. In addition, the later onset of HF relative to CHD and stroke among those with obesity may indicate that the processes leading to myocardial dysfunction and HF in this population generally require a cumulative effect over an extended time period prior to the development of clinical disease, and that period may provide an important window for preventive interventions.
Although this analysis highlights the markedly increased risk of incident HF associated with obesity, an “obesity paradox” has been noted among those with prevalent HF, among whom higher BMI is associated with increased survival.47, 48 Further investigation is needed to elucidate the mechanisms underlying the obesity paradox and to understand how the processes leading from obesity to incident HF affect myocardial function, structure, and overall prognosis among those who have already developed clinical HF.
This analysis has certain limitations. Although the ARIC population was rigorously assessed regarding traditional risk factors, the observational nature of this analysis makes the presence of some residual confounding still possible. In addition, the use of discharge codes for the diagnosis of HF may have resulted in some misclassification, although analyses restricted to only adjudicated HF events yielded largely unchanged results. This analysis also does not account for therapies administered during follow‐up that may have had variable effects on the risk of developing different subtypes of CVD. Nonetheless, this prospective analysis from an extremely well‐characterized cohort provides important insights regarding the relative associations of higher BMI with different subtypes of incident CVD and the extent to which the associations are unexplained by traditional CVD mediators. The community‐based design and the inclusion of a biracial population of middle‐aged men and women makes the results broadly generalizable. The large number of events allowed for direct statistical comparisons of the risk associations among obesity and different CVD subtypes and permitted assessments of the robustness of the findings across demographic subgroups.
In conclusion, this analysis demonstrates that the associations of overweight status and obesity with incident HF are stronger than those for CHD and stroke. Although traditional CVD mediators explained the associations of obesity with CHD and stroke, the association between higher BMI and incident HF was largely unexplained by adjustment for traditional CVD risk factors and mediators. These findings highlight the importance of weight management for optimal HF prevention and the need to identify nontraditional pathways linking obesity to incident HF to inform the development of additional preventive strategies.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was supported by the Robert E. Meyerhoff Professorship, a Robert Wood Johnson Amos Medical Faculty Development Award and an NIH/NHLBI grant (K23HL12247) awarded to Dr Ndumele. Dr Selvin was supported by NIH/NIDDK grant K24DK106414.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The views expressed here are those of the authors and do not necessarily reflect those of the Department of Veterans Affairs.
(J Am Heart Assoc. 2016;5:e003921 doi: 10.1161/JAHA.116.003921)
References
- 1. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi‐Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. [DOI] [PubMed] [Google Scholar]
- 2. Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Med Clin North Am. 2011;95:919–937. [DOI] [PubMed] [Google Scholar]
- 3. Pandey A, Berry JD, Lavie CJ. Cardiometabolic disease leading to heart failure: better fat and fit than lean and lazy. Curr Heart Fail Rep. 2015;12:302–308. [DOI] [PubMed] [Google Scholar]
- 4. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose‐response meta‐analysis of prospective studies. Circulation. 2016;133:639–649. [DOI] [PubMed] [Google Scholar]
- 5. Davis MP, Rhode PC, Dutton GR, Redmann SM, Ryan DH, Brantley PJ. A primary care weight management intervention for low‐income African‐American women. Obesity (Silver Spring). 2006;14:1412–1420. [DOI] [PubMed] [Google Scholar]
- 6. Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, Sciamanna CN. Long‐term weight loss maintenance in the United States. Int J Obes (Lond). 2010;34:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low‐income minority women. Obesity (Silver Spring). 2008;16:2462–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ockene IS, Hebert JR, Ockene JK, Saperia GM, Stanek E, Nicolosi R, Merriam PA, Hurley TG. Effect of physician‐delivered nutrition counseling training and an office‐support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia (WATCH). Arch Intern Med. 1999;159:725–731. [DOI] [PubMed] [Google Scholar]
- 9. Bodenant M, Kuulasmaa K, Wagner A, Kee F, Palmieri L, Ferrario MM, Montaye M, Amouyel P, Dallongeville J. Measures of abdominal adiposity and the risk of stroke: the MOnica Risk, Genetics, Archiving and Monograph (MORGAM) study. Stroke. 2011;42:2872–2877. [DOI] [PubMed] [Google Scholar]
- 10. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body‐mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 11. Empana JP, Ducimetiere P, Charles MA, Jouven X. Sagittal abdominal diameter and risk of sudden death in asymptomatic middle‐aged men: the Paris Prospective Study I. Circulation. 2004;110:2781–2785. [DOI] [PubMed] [Google Scholar]
- 12. Garrison RJ, Castelli WP. Weight and thirty‐year mortality of men in the Framingham Study. Ann Intern Med. 1985;103:1006–1009. [DOI] [PubMed] [Google Scholar]
- 13. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26‐year follow‐up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 14. Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. [DOI] [PubMed] [Google Scholar]
- 15. Suk SH, Sacco RL, Boden‐Albala B, Cheun JF, Pittman JG, Elkind MS, Paik MC. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study. Stroke. 2003;34:1586–1592. [DOI] [PubMed] [Google Scholar]
- 16. Folsom AR, Stevens J, Schreiner PJ, McGovern PG. Body mass index, waist/hip ratio, and coronary heart disease incidence in African Americans and whites. Atherosclerosis Risk in Communities Study Investigators. Am J Epidemiol. 1998;148:1187–1194. [DOI] [PubMed] [Google Scholar]
- 17. Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15‐year follow‐up of middle‐aged men and women in eastern Finland. Circulation. 1996;93:1372–1379. [DOI] [PubMed] [Google Scholar]
- 18. Schulte H, Cullen P, Assmann G. Obesity, mortality and cardiovascular disease in the Munster Heart Study (PROCAM). Atherosclerosis. 1999;144:199–209. [DOI] [PubMed] [Google Scholar]
- 19. Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N, Arima H, Tanaka K, Ibayashi S, Fujishima M. Incidence and risk factors for subtypes of cerebral infarction in a general population: the Hisayama study. Stroke. 2000;31:2616–2622. [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Liu Y, Yang Q, Dai X, Wu S, Wang W, Ji X, Li L, Fang X. Body mass index and risk of total and type‐specific stroke in Chinese adults: results from a longitudinal study in China. Int J Stroke. 2013;8:245–250. [DOI] [PubMed] [Google Scholar]
- 21. Wormser D, Kaptoge S, Di AE, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body‐mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yatsuya H, Yamagishi K, North KE, Brancati FL, Stevens J, Folsom AR. Associations of obesity measures with subtypes of ischemic stroke in the ARIC Study. J Epidemiol. 2010;20:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markus HS, Khan U, Birns J, Evans A, Kalra L, Rudd AG, Wolfe CD, Jerrard‐Dunne P. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circulation. 2007;116:2157–2164. [DOI] [PubMed] [Google Scholar]
- 24. Kurth T, Gaziano JM, Rexrode KM, Kase CS, Cook NR, Manson JE, Buring JE. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111:1992–1998. [DOI] [PubMed] [Google Scholar]
- 25. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body‐mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Del Gobbo LC, Kalantarian S, Imamura F, Lemaitre R, Siscovick DS, Psaty BM, Mozaffarian D. Contribution of major lifestyle risk factors for incident heart failure in older adults: the Cardiovascular Health Study. JACC Heart Fail. 2015;3:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 28. Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord‐Trondelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–1078. [DOI] [PubMed] [Google Scholar]
- 29. Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Obesity and risk of incident heart failure in older men with and without pre‐existing coronary heart disease: does leptin have a role? J Am Coll Cardiol. 2011;58:1870–1877. [DOI] [PubMed] [Google Scholar]
- 30. Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400. [DOI] [PubMed] [Google Scholar]
- 32. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 33. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 34. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc. 1962;57:348–368. [Google Scholar]
- 36. Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–1354. [DOI] [PubMed] [Google Scholar]
- 37. Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi‐Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010;3:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frankel DS, Vasan RS, D'Agostino RB Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham Offspring Study. J Am Coll Cardiol. 2009;53:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karas MG, Benkeser D, Arnold AM, Bartz TM, Djousse L, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Tracy RP, Mantzoros CS, Gottdiener JS, deFilippi CR, Kizer JR. Relations of plasma total and high‐molecular‐weight adiponectin to new‐onset heart failure in adults >/=65 years of age (from the Cardiovascular Health Study). Am J Cardiol. 2014;113:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McManus DD, Lyass A, Ingelsson E, Massaro JM, Meigs JB, Aragam J, Benjamin EJ, Vasan RS. Relations of circulating resistin and adiponectin and cardiac structure and function: the Framingham Offspring Study. Obesity (Silver Spring). 2012;20:1882–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Negi SI, Jeong EM, Shukrullah I, Raicu M, Dudley SC Jr. Association of low plasma adiponectin with early diastolic dysfunction. Congest Heart Fail. 2012;18:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garg S, Narula J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol. 2005;38:73–79. [DOI] [PubMed] [Google Scholar]
- 43. Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, Selvin E, Ballantyne CM, Nambi V. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. [DOI] [PubMed] [Google Scholar]
- 46. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 47. Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56:409–414. [DOI] [PubMed] [Google Scholar]
- 48. Lavie CJ, Sharma A, Alpert MA, De SA, Lopez‐Jimenez F, Milani RV, Ventura HO. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58:393–400. [DOI] [PubMed] [Google Scholar]


