Abstract
Background
Prior studies show an increased risk of ischemic stroke (IS) after myocardial infarction; however, there is limited evidence on long‐term risk and whether it is directly related to cardiac injury. We hypothesized that the risk of IS after acute coronary syndrome is significantly higher if there is evidence of cardiac injury, such as ST‐segment elevation myocardial infarction (STEMI) or non‐STEMI, than when there is no evidence of cardiac injury, such as in unstable angina.
Methods and Results
Administrative claims data were obtained from all emergency department encounters and hospitalizations at California's nonfederal acute care hospitals between 2008 and 2011. Patients with STEMI, non‐STEMI, and unstable angina were identified using appropriate International Classification of Diseases, Ninth Revision, Clinical Modification codes. The primary outcome was IS during 2 years of follow‐up. Unadjusted and adjusted Cox proportional hazards models were used to determine the association between acute coronary syndrome subtype and IS risk. We identified 73 059 patients with a diagnosis of STEMI (n=26 427), non‐STEMI (n=39 833), or unstable angina (n=6819) during the study period. In the fully adjusted models that included potential confounders such as atrial fibrillation and congestive heart failure, the risk of IS was higher with STEMI (hazard ratio 4.17, 95% CI 3.00–5.83; P<0.001) and non‐STEMI (hazard ratio 3.73, 95% CI 2.68–5.19, P<0.001) compared with unstable angina.
Conclusions
Non‐STEMI and STEMI confer an equally increased risk of IS. Studies exploring IS mechanisms in cardiac patients are needed to improve and tailor stroke prevention strategies.
Keywords: angina, cardiac biomarkers, coronary artery disease, embolism, ischemic stroke, myocardial infarction
Subject Categories: Cerebrovascular Disease/Stroke, Acute Coronary Syndromes
Introduction
Prior studies have shown an increased risk of ischemic stroke (IS) after myocardial infarction (MI) that is highest in the first few days after the event.1 The early IS risk after ST‐segment elevation MI (STEMI) has been shown to be related to left ventricular thrombi, which tend to develop within the first 2 weeks,2 and has been reduced with reperfusion therapy.3 In addition, several factors, such as the use of antiplatelets,4 statins,4 and anticoagulants,5, 6 have contributed to a reduction in the early risk of IS after MI. The presence of cardiac injury in acute coronary syndrome (ACS) may induce cardiac arrhythmias or cause cardiac dysfunction, which in turn may increase the long‐term stroke risk. There is limited evidence, however, on the duration of the elevated IS risk in patients with ACS and whether it is directly related to cardiac injury. We hypothesized that the risk of IS after ACS is significantly higher when there is evidence of cardiac injury, such as in STEMI or non‐STEMI (NSTEMI), than when there is no evidence of cardiac injury, such as in unstable angina (UA).
Methods
Design
We performed a retrospective analysis using administrative claims data on all hospitalizations and emergency department visits at nonfederal health care facilities in California. Data are publicly available and deidentified with a unique linkage number that allows each patient to be followed up for several years across emergency department encounters and hospitalizations. Each encounter included up to 25 discharge diagnoses that were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9), with labels indicating whether the diagnosis was present before hospital admission or developed during the hospitalization. The ICD‐9 codes, including those for STEMI, NSTEMI, and UA, that we used have been used in prior studies of the same data set7, 8, 9 and have been validated previously.10 Because the data are publicly available and deidentified, no institutional review board approval was required to perform the analysis.
Population
The study cohort consisted of all adult patients hospitalized with a primary diagnosis of STEMI (ICD‐9 codes 410.XX‐ 410.6X, 410.8X, 410.9X), NSTEMI (ICD‐9 code 410.7X), or UA (ICD‐9 code 411.1)11 during the years 2009 and 2010. These dates were chosen to ensure each patient had up to 2 years of follow‐up from their coronary event because the latest data were available up to the year 2011. Because we were interested in the long‐term IS risk after a coronary event, we excluded patients with known cerebrovascular disease before the index hospitalization. As an additional safeguard against missed or faulty claims data, patients with any cerebrovascular disease in 2008 were also excluded.
Main Predictors
The primary predictors were STEMI and NSTEMI versus UA. Patients were stratified based on their highest achieved diagnosis during follow‐up, considering the ranking of STEMI to be the highest, followed by NSTEMI and then UA.
Outcomes
The primary outcome during 2‐year follow‐up was IS, defined as ICD‐9 codes 433.x1, 434.x1, or 436.x in any hospital discharge diagnosis without an accompanying diagnosis of intracerebral hemorrhage (ICD‐9 code 431) or subarachnoid hemorrhage (ICD‐9 code 430). This validated algorithm has sensitivity of 86% and specificity of 95% for IS.12 The secondary outcome was IS or death during 2‐year follow‐up.
Covariates
We adjusted for baseline demographics and several covariates that could potentially influence the long‐term risk of IS. Baseline demographics included age, sex, race and ethnicity, and insurance status. Covariates included history of hypertension (ICD‐9 codes 401.00–405.90), history of diabetes (ICD‐9 code 250.XX), history of hyperlipidemia (ICD‐9 code 272.4), smoking history (ICD‐9 code 305.1), history of atrial fibrillation (AF; ICD‐9 code 427.3), history of chronic kidney disease (ICD‐9 code 585.XX), and history of congestive heart failure (CHF; ICD‐9 code 428.0X).
Analytic Plan
The study population was characterized using descriptive statistics; categorical variables are presented with counts and frequencies, and continuous variables are presented with means and standard deviations. Time‐to‐event data (eg, IS, mortality) were compared using log‐rank tests and presented as Kaplan–Meier curves. Kaplan–Meier survival analysis was performed to compare the associations between the highest type of ACS and the probability of IS and IS or death over the study period, with log rank to detect significant differences. To account for any patient‐specific and disease‐related factors, a Cox proportional hazards regression model was used with adjustment for age; sex; race and ethnicity; insurance status; and baseline hypertension, diabetes, hyperlipidemia, smoking, CHF, chronic kidney disease, and AF at baseline and during follow‐up. The assumption of proportionality was tested using time‐varying effects. Because the expected IS risk after ACS varies with time based on previous studies of ACS, models accounting for nonproportionality were used.
Unadjusted and adjusted Cox proportional hazards models were used to estimate the associations between the type of ACS and IS risk during 2‐year follow‐up after adjusting for potential confounders. Time‐dependent variables were used to eliminate immortal time bias for patients with changes in ACS status, and baseline values were used for covariates. The models were predefined as follows: Model 1 adjusted for age, sex, and race and ethnicity; model 2 adjusted for age, sex, race and ethnicity, insurance status, history of hypertension, history of diabetes, history of hyperlipidemia, smoking history, history of AF, chronic kidney disease, and history of CHF; model 3 adjusted for age, sex, race and ethnicity, insurance status, history of hypertension, history of diabetes, history of hyperlipidemia, smoking history, history of CHF, chronic kidney disease, and history of AF or subsequent AF during follow‐up, which was represented with a time‐dependent covariate. Statistical analysis was performed using SAS 9.3 (SAS Institute). P<0.05 was considered statistically significant.
Results
Baseline Demographics
We identified 73 079 patients with a diagnosis of STEMI (n=26 427), NSTEMI (n=39 833), or UA (n=6819) during the study period. The mean age was 66.6±14.4 years, and 61.8% of the patients were male. IS during 2‐year follow‐up occurred in 1956 patients (2.7%); 2.43% (n=641) had STEMI, 3.12% (n=1243) had NSTEMI, and 1.06% (n=72) had UA. Other baseline demographics and clinical characteristics of the study population are listed in Table 1.
Table 1.
Baseline Characteristics of Patients in the Cohort (n=73 079)
| Clinical Characteristic | Value |
|---|---|
| Age, y, mean±SD | 66.6±14.4 |
| Race and ethnicity (n=71 128) | |
| White | 64.3 (45 740) |
| Black | 7.2 (5139) |
| Hispanic | 19.8 (14 050) |
| Asian | 8.7 (6199) |
| Sex (% male) | 61.8 (45 117) |
| Insurance status (n=73 071) | |
| Medicare | 51.2 (37 443) |
| Medicaid | 8.6 (6310) |
| Private or other | 35.0 (25 587) |
| Self‐pay | 5.1 (3731) |
| Hypertension | 71.5 (52 229) |
| Diabetes | 34.2 (25 028) |
| Hyperlipidemia | 48.2 (35 243) |
| Congestive heart failure | 25.9 (18 920) |
| AF | 15.0 (10 990) |
| Smoking | 19.1 (13 981) |
| Chronic kidney disease | 22.0 (13 460) |
| Unstable angina | 9.3 (6819) |
| NSTEMI | 54.5 (39 833) |
| STEMI | 36.2 (26 427) |
| Ischemic stroke at follow‐up | 2.68 (1956) |
| Death during follow‐up | 8.83 (6450) |
Data are shown as percentage (number) except as indicated. AF indicates atrial fibrillation; NSTEMI, non–ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
Risk of IS With Respect to Type of Initial Event (STEMI, NSTEMI, or UA)
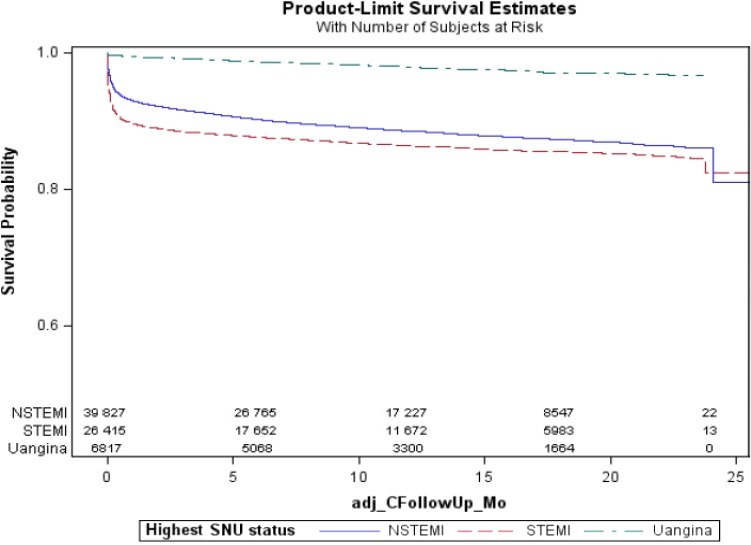
The unadjusted risk of IS over 2 years was higher in patients with NSTEMI (hazard ratio [HR] 4.86, 95% CI 3.51–6.72; P<0.001) and STEMI (HR 4.23, 95% CI 3.04–5.90; P<0.001) compared with UA (Figure 1). In the fully adjusted models that included potential confounders such as AF and CHF, the risk of IS remained elevated with STEMI (HR 4.17, 95% CI 3.00–5.83; P<0.001) and NSTEMI (HR 3.73, 95% CI 2.68–5.19, P<0.001) compared with UA (Table 2), and there was no difference in risk between STEMI and NSTEMI.
Figure 1.

Kaplan–Meier curves for ischemic stroke events in different types of acute coronary syndrome (NSTEMI, STEMI, UA), log‐rank test P<0.001. NSTEMI indicates non–ST‐segment elevation myocardial infarction; SNU, STEMI/NSTEMI/Unstable Angina; STEMI, ST‐segment elevation myocardial infarction; UA, unstable angina.
Table 2.
HRs for IS and Probability for Patients Diagnosed With ACS Between 2009 and 2010
| Risk of IS | Risk of IS or Death | |||
|---|---|---|---|---|
| NSTEMI, HR (95% CI); P value | STEMI, HR (95% CI); P value | NSTEMI, HR (95% CI); P value | STEMI, HR (95% CI); P value | |
| Unadjusted | 4.86 (3.51–6.72); P<0.001 | 4.23 (3.04–5.90); P<0.001 | 8.62 (6.89–10.79); P<0.001 | 12.75 (10.18–15.96); P<0.001 |
| Model 1 | 4.11 (2.96–5.71); P<0.001 | 4.27 (3.06–5.95); P<0.001 | 5.95 (4.74–7.47); P<0.001 | 11.06 (8.8–13.89); P<0.001 |
| Model 2 | 3.68 (2.65–5.12); P<0.001 | 4.11 (2.94–5.75); P<0.001 | 4.88 (3.88–6.13); P<0.001 | 9.99 (7.96–12.57); P<0.001 |
| Model 3 | 3.73 (2.68–5.19); P<0.001 | 4.17 (3.00–5.83); P<0.001 | 4.95 (3.94–6.22); P<0.001 | 10.06 (8.0–12.64); P<0.001 |
Unstable angina was the reference for all models. Model 1 adjusted for age, sex, race and ethnicity. Model 2 adjusted for age; sex; race and ethnicity; insurance status; and baseline hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, chronic kidney disease, and congestive heart failure. Model 3 adjusted for age; sex; race and ethnicity; insurance status; and baseline hypertension, diabetes, hyperlipidemia, smoking, congestive heart failure, chronic kidney disease, and atrial fibrillation at baseline and during follow‐up. ACS indicates acute coronary syndrome; HR, hazard ratio; IS, ischemic stroke; NSTEMI, non–ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
Risk of IS or Death With Respect to Type of Highest Event (STEMI, NSTEMI, or UA)
The unadjusted risk of IS or death over 2 years was higher in patients with STEMI (HR 12.75, 95% CI 10.18–15.96; P<0.001) and NSTEMI (HR 8.62, 95% CI 6.89–10.79; P<0.001) compared with UA (Figure 2). In the fully adjusted models that included potential confounders such as AF and CHF, the risk of IS or death remained elevated with STEMI (HR 10.06, 95% CI 8.01–12.64; P<0.001) and NSTEMI (HR 4.95, 95% CI 3.94–6.22; P<0.001) compared with UA (Table 2).
Figure 2.
Kaplan–Meier curves for ischemic stroke events or death in different types of acute coronary syndrome (NSTEMI, STEMI, UA), log‐rank test P<0.001. NSTEMI indicates non–ST‐segment elevation myocardial infarction; SNU, STEMI/NSTEMI/Unstable Angina; STEMI, ST‐segment elevation myocardial infarction; UA, unstable angina.
Risk of IS With Time
There was a time‐dependent decrease in the risk of IS after STEMI and NSTEMI. In fully adjusted models, for patients with STEMI, the HR for IS dropped from 3.68 at 1 month to 0.93 at 12 months after the event. Similarly, in fully adjusted models, there was a drop in the HR of IS after NSTEMI from 3.34 at 1 month to 0.99 at 12 months following the event (Figure 3). The elevated risk of IS after STEMI or NSTEMI was during the first 6 months after the event.
Figure 3.

A, Hazard ratios of ischemic stroke as a function of time after NT (A) and as a function of time after ST (B). LCL, lower confidence level; NT, non–ST‐segment elevation myocardial infarction; ST, ST‐segment elevation myocardial infarction; UCL, upper confidence level.
Other Risk Factors for Stroke After ACS
Other risk factors for IS after ACS in the fully adjusted models (model 3) were age (per 10 years: HR 1.25, 95% CI 1.20–1.32), female sex (HR 1.24, 95% CI 1.13–1.36), black race (compared with white: HR 1.70, 95% CI 1.45–1.99), Asian race (compared with white: HR 1.48, 95% CI 1.29–1.75), Hispanic ethnicity (compared with white non‐Hispanic: HR 1.25, 95% CI 1.10–1.41), chronic kidney disease (HR 1.15, 95% CI 1.02–1.31), diabetes (HR 1.30, 95% CI 1.18–1.43), CHF (HR 1.38, 95% CI 1.25–1.53), and AF (HR 1.58, 95% CI 1.39–1.80) (Table 3). In addition, hyperlipidemia (HR 0.72, 95% CI 0.62–0.84) and private insurance (compared with Medicare: HR 0.74, 95% CI 0.64–0.85) were associated with a reduced risk of IS. Other risk factors including history of hypertension, history of hyperlipidemia, and history of smoking were not associated with increased risk of IS.
Table 3.
Fully Adjusted (Model 3) HR for IS for Patients Diagnosed With ACS Between 2009 and 2010
| Other Risk Factors for IS After ACS | Adjusted HR (95% CI); P Value |
|---|---|
| Age (per 10 years) | 1.25 (1.20–1.32); P<0.001 |
| Female | 1.24 (1.13–1.36); P<0.001 |
| Race and ethnicity | |
| Black (compared with non‐Hispanic white) | 1.70 (1.45–1.99); P<0.001 |
| Hispanic (compared with non‐Hispanic white) | 1.48 (1.29–1.75); P<0.001 |
| Asian (compared with non‐Hispanic white) | 1.25 (1.10–1.41); P<0.001 |
| Insurance status | |
| Private insurance (compared with Medicare) | 0.74 (0.64–0.85); P<0.001 |
| Chronic kidney disease | 1.15 (1.02–1.31); P=0.0248 |
| Diabetes | 1.30 (1.18–1.43); P<0.001 |
| Hyperlipidemia | 0.72 (0.62–0.84); P<0.001 |
| Atrial fibrillation | 1.58 (1.39–1.80); P<0.001 |
| Congestive heart failure | 1.38 (1.25–1.53); P<0.001 |
ACS indicates acute coronary syndrome; HR, hazard ratio; IS, ischemic stroke.
Discussion
The long‐term risk of IS after MI in our cohort was relatively low (2.7% at 2 years), which is likely related to the aggressive use of antiplatelet agents, reperfusion therapies, and statins after an acute coronary event.4 This is consistent with what has been reported in prior studies.1, 4 This risk, however, is ≈4‐fold higher in the presence of cardiac injury, such as STEMI and NSTEMI, as opposed to the absence of cardiac injury, such as in UA. In addition, unlike prior studies, our study demonstrated that NSTEMI conferred a similarly increased risk of IS as STEMI. The fact that the risk for STEMI is less affected by adjusting for other risk factors may suggest that direct cardiac mechanisms may be more likely after STEMI, whereas residual confounding or other mechanisms may play a role in at least some of the NSTEMI strokes. When stroke or death was used as a combined outcome, patients with STEMI had the highest risk, followed by NSTEMI and UA. This was not an unexpected finding, given the higher mortality rate after STEMI versus NSTEMI.13, 14 As in patients with STEMI, in our study, most IS in patients with NSTEMI occurred in the early period after the coronary event. In fact, our results suggest that the time period in which the risk of IS was elevated was in the first 6 months after the event. After 1 year, the risk of IS after UA becomes significantly higher than that of STEMI and NSTEMI. The reason for this is unclear; however, it is likely that patients with STEMI and NSTEMI who remain stroke free after 1 year may be at an inherently lower risk of IS.
The risk factors for IS after ACS in our study were age, female sex, CHF, diabetes, and AF, which were similar to those reported in prior studies.1, 4 Interestingly, these risk factors are also risk factors for embolism in patients with AF15; therefore, they either may increase the risk of formation of cardiac thrombi after MI or may lead to cerebrovascular atherosclerosis and IS risk.
Mechanism of Risk and Clinical Implications
The relationship between cardiac injury in ACS and long‐term IS risk may be related to several potential mechanisms. Cardiac injury may lead to cardiac dysfunction and hypokinesis of cardiac chambers, which in turn may predispose the patient to thrombus formation16, 17, 18 and embolism. In addition, cardiac injury may cause atrial dysfunction or cardiopathy, which portends an increased risk of IS even in the absence of AF,19, 20, 21, 22, 23, 24, 25 or may induce atrial arrhythmias such as AF, which in turn may lead to an increased risk of IS. Last, cardiac injury in ACS may be a marker of severe systemic and cerebrovascular atherosclerotic disease that in turn is associated with IS risk.
Our study has several clinical implications. Because both NSTEMI and STEMI confer a similarly elevated risk of stroke compared with that of patients with UA, studies to better understand stroke mechanisms in these patients are necessary to potentially improve stroke prevention strategies. The use of long‐term outpatient monitoring in these patients, for example, may improve detection of AF and lead to improved stroke prevention strategies. Furthermore, because cardiac injury may be a marker of cerebrovascular disease, evaluating these patients for cerebrovascular disease may improve stroke prevention strategies in these patients.
Strengths and Limitations
Our study has several limitations including lack of data on the location of MI, echocardiographic findings, relatively short‐term follow‐up, and use of ICD‐9 codes that may be subject to error. These codes, however, have been used and validated in prior studies using the same data set.7, 8, 9 In addition, we lacked data on medications used and stroke mechanisms after ACS that may limit both our understanding of the stroke risk and our ability to recommend stroke prevention strategies. Furthermore, this study included patients who were hospitalized at nonfederal hospitals in the state of California; therefore, hospitalizations outside the state of California were not captured in the database. Although this may potentially underestimate the risk of IS after ACS, it would not necessarily lead to a differential increase in ischemic risk in one category of ACS over the others. Moreover, our study has several strengths, including a large sample size with a wide variety of hospital settings, making our results more generalizable.
Conclusion
NSTEMI and STEMI conferred equally increased risks of IS compared with UA. Studies exploring IS mechanisms in these patient groups are needed to improve and tailor stroke prevention strategies.
Author Contributions
Yaghi was involved in manuscript preparation and analytical plan. Pilot was involved in analytical plan and data analysis. Song was involved in manuscript revision. Blum was involved in manuscript revision and methodology. Yakhkind was involved in manuscript preparation and revision. Silver was involved in manuscript revision. Furie was involved in manuscript revision. Elkind was involved in manuscript revision and methodology. D. Sherzai was involved in analytical plan and manuscript revision. A. Sherzai was involved in analytical plan and manuscript revision.
Sources of Funding
This work was funded by the NIH.
Disclosures
Dr Yaghi received funding from the New York Stroke Trials Network of Columbia and Cornell (NYCCSTN, NINDS U10NS086728), Dr Elkind discloses receiving personal compensation for serving on advisory boards and consulting from Boehringer‐Ingelheim, Inc., BMS‐Pfizer Partnership, Daiichi‐Sankyo, Janssen Pharmaceuticals, and BioTelemetry/Cardionet, Dr A. Sherzai received funding from NIH T32 NS07153.
(J Am Heart Assoc. 2016;5:e002590 doi: 10.1161/JAHA.115.002590)
References
- 1. Mooe T, Eriksson P, Stegmayr B. Ischemic stroke after acute myocardial infarction. A population‐based study. Stroke. 1997;28:762–767. [DOI] [PubMed] [Google Scholar]
- 2. Kupper AJ, Verheugt FW, Peels CH, Galema TW, Roos JP. Left ventricular thrombus incidence and behavior studied by serial two‐dimensional echocardiography in acute anterior myocardial infarction: left ventricular wall motion, systemic embolism and oral anticoagulation. J Am Coll Cardiol. 1989;13:1514–1520. [DOI] [PubMed] [Google Scholar]
- 3. Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta‐analysis. J Am Coll Cardiol. 1993;22:1004–1009. [DOI] [PubMed] [Google Scholar]
- 4. Ulvenstam A, Kajermo U, Modica A, Jernberg T, Soderstrom L, Mooe T. Incidence, trends, and predictors of ischemic stroke 1 year after an acute myocardial infarction. Stroke. 2014;45:3263–3268. [DOI] [PubMed] [Google Scholar]
- 5. Kontny F, Dale J, Abildgaard U, Pedersen TR. Randomized trial of low molecular weight heparin (dalteparin) in prevention of left ventricular thrombus formation and arterial embolism after acute anterior myocardial infarction: the Fragmin in Acute Myocardial Infarction (FRAMI) Study. J Am Coll Cardiol. 1997;30:962–969. [DOI] [PubMed] [Google Scholar]
- 6. Turpie AG, Robinson JG, Doyle DJ, Mulji AS, Mishkel GJ, Sealey BJ, Cairns JA, Skingley L, Hirsh J, Gent M. Comparison of high‐dose with low‐dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med. 1989;320:352–357. [DOI] [PubMed] [Google Scholar]
- 7. Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general Medicare population: a 10‐year perspective (1992 to 2002). Stroke. 2006;37:1969–1974. [DOI] [PubMed] [Google Scholar]
- 8. Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;8:884–890. [DOI] [PubMed] [Google Scholar]
- 9. Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long‐term risk of ischemic stroke. JAMA. 2014;312:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dudas K, Bjorck L, Jernberg T, Lappas G, Wallentin L, Rosengren A. Differences between acute myocardial infarction and unstable angina: a longitudinal cohort study reporting findings from the Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (RIKS‐HIA). BMJ Open. 2013;3:pii: e002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, Pollack CV Jr, Gore JM, Chandra‐Strobos N, Peterson ED, French WJ. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non‐ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1026–1034. [DOI] [PubMed] [Google Scholar]
- 12. Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 13. Invasive compared with non‐invasive treatment in unstable coronary‐artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet. 1999;354:708–715. [PubMed] [Google Scholar]
- 14. Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. [DOI] [PubMed] [Google Scholar]
- 15. Coppens M, Eikelboom JW, Hart RG, Yusuf S, Lip GY, Dorian P, Shestakovska O, Connolly SJ. The CHA2DS2‐VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34:170–176. [DOI] [PubMed] [Google Scholar]
- 16. Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two‐dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI‐3 study. Am J Cardiol. 1998;81:822–827. [DOI] [PubMed] [Google Scholar]
- 17. Greaves SC, Zhi G, Lee RT, Solomon SD, MacFadyen J, Rapaport E, Menapace FJ, Rouleau JL, Pfeffer MA. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol. 1997;80:442–448. [DOI] [PubMed] [Google Scholar]
- 18. Neskovic AN, Marinkovic J, Bojic M, Popovic AD. Predictors of left ventricular thrombus formation and disappearance after anterior wall myocardial infarction. Eur Heart J. 1998;19:908–916. [DOI] [PubMed] [Google Scholar]
- 19. Yaghi S, Song C, Gray WA, Furie KL, Elkind MS, Kamel H. Left atrial appendage function and stroke risk. Stroke. 2015;46:3554–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamel H, O'Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the Atherosclerosis Risk in Communities study. Ann Neurol. 2015;78:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, Devereux RB, Fink ME. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT Jr, Nazarian S, Okin PM. P‐wave morphology and the risk of incident ischemic stroke in the Multi‐Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, Huxley RR, Ballantyne CM. Troponin T, N‐terminal pro‐B‐type natriuretic peptide, and incidence of stroke: the Atherosclerosis Risk in Communities study. Stroke. 2013;44:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, Ahmed A, Thacker EL, Zakai NA. N‐terminal pro‐B‐type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45:1646–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yaghi S, Moon YP, Mora‐McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, Homma S, Kamel H, Sacco RL, Elkind MS. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]


