Abstract
Background
Quercetin, the most abundant dietary flavonol, has antioxidant effects in cardiovascular disease, but the evidence regarding its effects on blood pressure (BP) has not been conclusive. We assessed the impact of quercetin on BP through a systematic review and meta‐analysis of available randomized controlled trials.
Methods and Results
We searched PUBMED, Cochrane Library, Scopus, and EMBASE up to January 31, 2015 to identify placebo‐controlled randomized controlled trials investigating the effect of quercetin on BP. Meta‐analysis was performed using either a fixed‐effects or random‐effect model according to I2 statistic. Effect size was expressed as weighted mean difference (WMD) and 95% CI. Overall, the impact of quercetin on BP was reported in 7 trials comprising 9 treatment arms (587 patients). The results of the meta‐analysis showed significant reductions both in systolic BP (WMD: −3.04 mm Hg, 95% CI: −5.75, −0.33, P=0.028) and diastolic BP (WMD: −2.63 mm Hg, 95% CI: −3.26, −2.01, P<0.001) following supplementation with quercetin. When the studies were categorized according to the quercetin dose, there was a significant systolic BP and diastolic BP‐reducing effect in randomized controlled trials with doses ≥500 mg/day (WMD: −4.45 mm Hg, 95% CI: −7.70, −1.21, P=0.007 and −2.98 mm Hg, 95% CI: −3.64, −2.31, P<0.001, respectively), and lack of a significant effect for doses <500 mg/day (WMD: −1.59 mm Hg, 95% CI: −4.44, 1.25, P=0.273 and −0.24 mm Hg, 95% CI: −2.00, 1.52, P=0.788, respectively), but indirect comparison tests failed to significant differences between doses.
Conclusions
The results of the meta‐analysis showed a statistically significant effect of quercetin supplementation in the reduction of BP, possibly limited to, or greater with dosages of >500 mg/day. Further studies are necessary to investigate the clinical relevance of these results and the possibility of quercetin application as an add‐on to antihypertensive therapy.
Keywords: blood pressure, flavonoids, high blood pressure, hypertension, lipids, meta‐analysis, nutrition, quercetin
Subject Categories: Hypertension, High Blood Pressure
Introduction
Nutraceuticals and flavonoid‐containing dietary supplements are becoming increasingly popular in the treatment and prevention of cardiovascular disease.1, 2, 3, 4 Flavonols, flavanols, and anthocyanidins are the main members of the group of natural phenolic compounds called flavonoids.5 The intervention‐based human studies performed as early as 1993 have shown a positive correlation between the dietary intake of flavonoids and reduced incidence and mortality from cardiovascular disease.6 The Zutphen Elderly Study has shown that flavonoids, including quercetin, reduced the risk of coronary death by 68% in men who consumed >29 mg flavonols/day compared with men who consumed <10 mg flavonols/day.6 Quercetin (3, 3′, 4′, 5, 7‐pentahydroxyflavone) has been singled out among flavonoids because of its ubiquitous presence and abundance in fruits and vegetables, as such or bound to sugar moieties in various glycosides.7 Quercetin can be found in apples, capers, cocoa powder, berries, red grapes, red wine, citrus fruits, broccoli, onions, bark roots, flowers, green tea, and black tea.8 This particular flavonol is the subject of about one third of the 35 000 studies on flavonoids.9 Prominent effects include antioxidant, anticarcinogenic,10, 11 antithrombotic,12 anti‐allergic,13 antidiabetic,14 antiobesity,15 immune and inflammation‐modulating activities,16 or different cell signaling effects.17 Anti‐atherosclerotic, antiproliferative, and anti‐inflammatory effects of quercetin have been documented in many human in vitro and in vivo models.18 Its positive effect on hypertension was documented for the first time on spontaneously hypertensive rats, in an experimental model that mimics human hypertension.19 Since then, many experimental and human studies showed that quercetin exerts vasodilator, antiplatelet, and antiproliferative effects, decreasing oxidative status, blood pressure (BP), and end‐organ damage.20, 21, 22, 23, 24 The BP‐lowering effect of quercetin is more evident in subjects with certain comorbidities such as metabolic syndrome or in smokers.25 Different studies tried to establish a connection between the antihypertensive effect of quercetin and certain phenotypes such as the apolipoproteins (apo) E3 and E4, but so far results are conflicting.26
However, evidence of the effects of quercetin on BP has not been conclusive. Therefore, we systematically reviewed all available randomized controlled trials (RCTs) investigating the impact of quercetin on BP.
Subjects and Methods
Design
This study was designed according to the guidelines of the 2009 preferred reporting items for systematic reviews and meta‐analysis (PRISMA) statement1) SCOPUS (http://www.scopus.com), Medline (http://www.ncbi.nlm.nih.gov/pubmed), Cochrane Library (www.cochranelibrary.com), and EMBASE (http://www.embase.com) databases were searched using the following search terms in titles and abstracts (also in combination with MESH terms): (quercetin) AND (blood pressure). The wild‐card term “*” was used to increase the sensitivity of the search strategy. No language restriction was used in the literature search. The search was limited to studies in humans. The literature was searched from inception to January 31, 2015.
Because of the study design (meta‐analysis of RCTs), no Institutional Review Board approval, as well as no patients’ informed consents were obtained.
Study Selection
Original studies were included if they met the following inclusion criteria: (1) randomized clinical trial in either parallel or crossover design versus placebo control, (2) investigated the impact of quercetin on BP, (3) presentation of sufficient information on baseline and at the end of study in both quercetin and control groups, and (4) administering quercetin for a period of at least 2 weeks. Exclusion criteria were the following: (1) nonclinical studies, (2) uncontrolled trials, (3) lack of sufficient information on baseline or follow‐up BP, and (4) administration of an active comparator in the control group.
Data Extraction
Eligible studies were reviewed and the following data were abstracted: (1) first author's name; (2) year of publication; (3) study location; (4) number of participants in the quercetin and control groups; (5) dose and duration of supplementation with quercetin; (6) age, sex, and body mass index of study participants; (7) circulating concentrations of total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, and glucose; (8) prevalence of smoking, type 2 diabetes, dyslipidemia, hypertension, and coronary heart disease; and (9) systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Access to Study
All authors had access to the study data and reviewed and approved the final manuscript.
Quality Assessment
A systematic assessment of bias in the included studies was carried out using the Cochrane criteria.27 The items used for the assessment of each study were as follows: adequacy of sequence generation, allocation concealment, blinding, addressing of dropouts (incomplete outcome data), selective outcome reporting, and other potential sources of bias. According to the recommendations of the Cochrane Handbook, a judgment of “yes” indicated low risk of bias, while “no” indicated high risk of bias. Labeling an item as “unclear” indicated an unclear or unknown risk of bias.
Statistical Analysis
Meta‐analysis was conducted using the Comprehensive Meta‐Analysis (CMA) V2 software (Biostat, NJ).28 SBP and DBP were collated in mm Hg. SDs of the mean difference were calculated using the following formula: SD=square root [(SDpretreatment)2+(SDposttreatment)2−(2R×SDpretreatment×SDposttreatment)], assuming a correlation coefficient (R)=0.5. In case of reporting SEM, SD was estimated using the following formula: SD=SEM×square root (n), where n is the number of subjects.
Net changes in measurements (change scores) were calculated for parallel and crossover trials, as follows: (measure at end of follow‐up in the treatment group−measure at baseline in the treatment group)−(measure at end of follow‐up in the control group−measure at baseline in the control group). Meta‐analysis was performed using either a fixed‐effects or random‐effect model according to I2 statistic. I2 values <50% and ≥50% suggested the use of fixed‐effects and random‐effects model, respectively. The generic inverse variance method was used to weight each individual study included in the meta‐analysis. Interstudy heterogeneity was assessed using Cochrane Q statistic and quantified by I2 statistic. Effect size was expressed as weighted mean difference (WMD) and 95% CI. In order to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using the 1‐study remove (leave‐1‐out) approach.
In the absence of trials making head‐to‐head comparison, the effects of different doses and supplementation durations of quercetin were compared using adjusted indirect comparison according to the method proposed by Song et al29 and Bucher et al.30 In this method, treatment effects estimated for each dose or administration duration in the random‐effects model could be compared indirectly through common controls.
Meta‐Regression
Meta‐regression was performed in order to evaluate the association between calculated WMD in SBP and DBP values with dose and duration of quercetin supplementation in the included studies. Meta‐regression was performed under a fixed‐effects or random‐effects (using unrestricted maximum likelihood method) model according to the results of heterogeneity analysis and I2 values. A covariance matrix was built to assess the covariance between regression coefficients of different confounders.
Publication Bias
Potential publication bias was explored using visual inspection of Begg's funnel plot asymmetry, and Begg's rank correlation and Egger's weighted regression tests. Duval and Tweedie “trim and fill” and “fail‐safe N” methods were used to adjust the analysis for the effects of publication bias.31
Results
Search Results
The preliminary screening ruled out articles whose titles and/or abstracts were obviously unimportant. After evaluation, 7 articles with 9 quercetin treatment arms met the inclusion criteria and were chosen for the final meta‐analysis. For crossover studies with a 2×2 crossover design, each of the study arms (placebo‐quercetin or quercetin‐placebo) was treated as a separate study. A listing of the study selection procedure is displayed in Figure 1.
Figure 1.
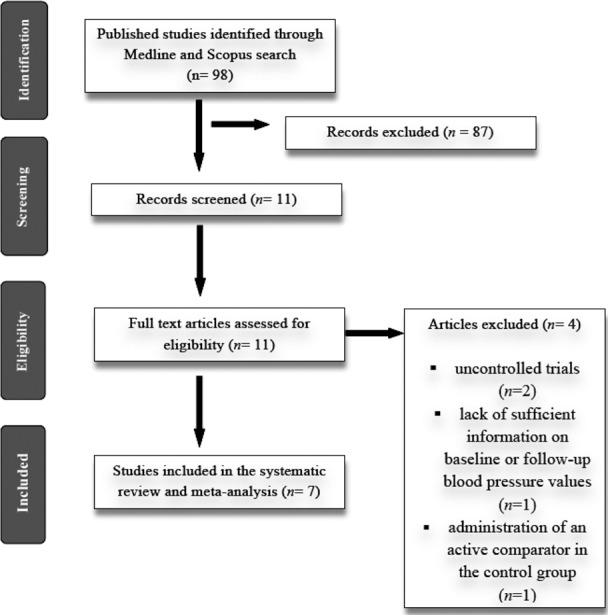
Flow chart of number of studies identified and included in the review.
Trial Characteristics
In total, 587 participants were randomized, of whom 299 were allocated to quercetin supplementation and 288 to the control group in the 7 selected studies with 9 treatment arms.32, 33, 34, 35, 36, 37, 38 The number of participants in these trials ranged from 13 to 93. Included studies were published between 1998 and 2014, and were conducted in the United States, Iran, Canada, Germany, and Korea. Ranges of doses from 100 to 1000 mg/day of quercetin were administered in the included trials. Duration of supplementation with quercetin ranged between 4 and 10 weeks. Five trials were designed as parallel‐group studies and 2 trials as 2×2 crossover design. Among all studies included in this meta‐analysis, only 1 study included men and women with prehypertension and stage 1 hypertension.35 All other studies included patients without hypertension. Demographic and baseline parameters of the included studies are shown in Table 1. Oral quercetin administration was safe and well tolerated in all of the RCTs included in this review, with no report of serious adverse events.
Table 1.
Demographic Characteristics of the Included Studies
| Study | Javadi et al32 | Zahedi et al33 | Conquer et al34 | Edwards et al35 | Egert et al36 | Lee et al37 | Pfeuffer et al38 |
|---|---|---|---|---|---|---|---|
| Year | 2014 | 2013 | 1998 | 2007 | 2009 | 2011 | 2013 |
| Location | Iran | Iran | Canada | USA | Germany | Korea | Germany |
| Design | Randomized, double‐blind placebo‐controlled clinical trial | Randomized, double‐blind placebo‐controlled clinical trial | Randomized, double‐blind placebo‐controlled clinical trial | Randomized, double‐blind, placebo‐controlled, crossover trial | Randomized, double‐blind, placebo‐controlled crossover trial | Randomized, double‐blinded, placebo‐controlled clinical trial | Randomized, double‐blind, placebo‐controlled crossover trial |
| Duration of study | 8 weeks | 10 weeks | 4 weeks | 4 weeks | 6 weeks of treatment separated by a 5‐week washout period | 10 weeks | 8 weeks of treatment separated by a 3‐week washout period |
| Inclusion criteria | Women age 19 to 70 y old, unchanged type and dose of medications from previous month and no pregnancy or lactation | Women with a history of T2DM for at least 3 y, age between 35 to 55 y, not smoking and addiction, lack of insulin, lack of severe heart diseases, stroke, severe liver and renal diseases, gastrointestinal disorders, thyroid dysfunction, rheumatoid arthritis, and infectious diseases | Healthy men and women with cholesterol levels of 4.0 to 7.2 mmol/L | Men and women with prehypertension and stage 1 HTN | Patients with the following traits of the metabolic syndrome: central obesity (waist circumference ≥94 cm for men and ≥80 cm for women); serum concentration of TAG ≥1500 mg/L and/or serum concentration of hs‐CRP ≥2.0 mg/L | Healthy male smokers in the age range of 30 to 60 y | Healthy male patients with apolipoprotein E genotype |
| Route of administration | Oral | Oral | Oral | Oral | Oral | Oral | Oral |
| Quercetin dose | 500 mg/day | 500 mg/day | 1000 mg/day | 730 mg/day | 150 mg/day | 100 mg/day | 150 mg/day |
| Primary end point(s) | Changes in oxidant status, BP and C‐reactive protein | Changes in lipids, BP and inflammatory factors | Changes in plasma quercetin levels, cardiovascular and thrombotic factors | Changes in BP | Changes in BP | Changes in cardiometabolic risk | Changes in endothelial function |
| Participants | |||||||
| Case | 20 | 34 | 13 | 19a | 93 | 49 | 49 |
| 22b | |||||||
| Control | 20 | 28 | 14 | 19c | 93 | 43 | 49 |
| 22d | |||||||
| Age, y | |||||||
| Case | 46.5±9.9 | 46.4 (±4.5) | 42.0±2.7 | 47.8±3.5a | 45.1 (10.53) | 46.1±7.1 (32–62) | 59.4±0.9 |
| 49.2±2.9b | |||||||
| Control | 48.0±8.4 | 41.5±2.9 | 47.8±3.5c | 45.1 (10.53) | 42.4±8.2 (23–55) | 59.4±0.9 | |
| 49.2±2.9d | |||||||
| Male (%) | |||||||
| Case | 0 | 0 | NS | 68.4a | 45.2 | 100 | 100 |
| 59.1b | |||||||
| Control | 0 | 0 | NS | 68.4c | 45.12 | 100 | 100 |
| 59.1d | |||||||
| BMI, kg/m2 | |||||||
| Case | 27.99±4.4 | NS | 26.2±1.1 | 29.6±1.3a | 30.6 (3.23) | 24.7±3.0 | 26.3±0.3 |
| 29.3±1.3b | |||||||
| Control | 30.70±4.6 | NS | 26.0±1.3 | 29.8±1.3c | 30.6 (3.23) | 24.9±2.8 | 26.3±0.3 |
| 29.5±1.4d | |||||||
| TC, mg/dL | |||||||
| Case | NS | 189.2±7.5 | 196.83±10.05 | 198.37±8.89a | 221.19 (39.83) | 193.5±32.1 | 209.98±5.41 |
| 206.49±8.51b | |||||||
| Control | NS | 177.6±6.4 | 197.22±9.67 | 197.99±9.28c | 219.64 (40.60) | 194.3±36.4 | 209.98±5.41 |
| 205.72±8.12d | |||||||
| LDL‐C, mg/dL | |||||||
| Case | NS | 106.1±5.5 | 109.82±8.89 | 116.01±7.73a | 138.82 (37.51) | 113.2±20.3 | 135.73±4.64 |
| 124.90±9.28b | |||||||
| Control | NS | 103.6±4.5 | 111.37±6.57 | 117.17±6.57c | 137.27 (36.35) | 115.6±24.7 | 135.73±4.64 |
| 115.24±8.12d | |||||||
| HDL‐C, mg/dL | |||||||
| Case | NS | 45.2±1.7 | 58.00±4.25 | 47.95±5.03a | 52.20 (17.79) | 44.3±6.9 | 53.36±1.93 |
| 47.56±3.48b | |||||||
| Control | NS | 46.8±2.4 | 60.71±4.64 | 47.95±4.64c | 50.27 (16.63) | 45.0±5.3 | 53.36±1.93 |
| 49.11±3.09d | |||||||
| TG, mg/dL | |||||||
| Case | NS | 198.4±20.5 | 112.49±19.48 | 177.15±21.26a | 161.20 (86.80) | 163.5±87.7 | 106.29±6.20 |
| 205.49±34.54b | |||||||
| Control | NS | 151±9.4 | 124.89±20.37 | 161.20±21.26c | 172.72 (87.69) | 185.0±91.6 | 106.29±6.20 |
| 209.92±30.11d | |||||||
| Glucose, mg/dL | |||||||
| Case | NS | NS | NS | 107.82±4.5a | 98.82 (12.24) | 108.3±18.1 | 100.8±1.44 |
| 108.00±3.6b | |||||||
| Control | NS | NS | NS | 102.24±3.24c | 97.92 (12.6) | 109.9±28.5 | 100.8±1.44 |
| 114.66±5.04d | |||||||
| Smoking % | |||||||
| Case | 0 | 0 | NS | 0a | 0 | 100 | NS |
| 0b | |||||||
| Control | 0 | 0 | NS | 0c | 0 | 100 | NS |
| 0d | |||||||
| T2DM % | |||||||
| Case | NS | 100 | NS | 0a | 0 | 0 | 0 |
| 0b | |||||||
| Control | NS | 100 | NS | 0c | 0 | 0 | 0 |
| 0d | |||||||
| Dyslipidemia % | |||||||
| Case | NS | NS | NS | 0a | NS | NS | 0 |
| 0b | |||||||
| Control | NS | NS | NS | 0c | NS | NS | 0 |
| 0d | |||||||
| HTN % | |||||||
| Case | 0 | NS | NS | 0a | 0 | 0 | 0 |
| 53.65b | |||||||
| Control | 0 | NS | NS | 0c | 0 | 0 | 0 |
| 53.65d | |||||||
| CHD % | |||||||
| Case | NS | NS | NS | NSa | 0 | 0 | NS |
| NSb | |||||||
| Control | NS | NS | NS | NSc | 0 | 0 | NS |
| NSd | |||||||
| SBP, mm Hg | |||||||
| Case | 113.75±18.47 | 117.0±2.0 | 121.6±4.2 | 132±1a | 130.3 (16.4) | 132.9±14.9 | 132.9±2.2 |
| 145±2b | |||||||
| Control | 121.13±15.99 | 110±2.0 | 120.4±3.5 | 135±3c | 130.3 (16.4) | 135.5±11.3 | 132.9±2.2 |
| 141±2d | |||||||
| DBP, mm Hg | |||||||
| Case | 78.13±9.96 | 79±10 | 79.3±2.8 | 85±1a | 81.6 (9.3) | 88.7±9.9 | 80.8±1.3 |
| 97±1b | |||||||
| Control | 86.75±9.50 | 73±10 | 75.5±2.4 | 84±1c | 81.6 (9.3) | 86.9±10.4 | 80.8±1.3 |
| 94±2d | |||||||
Values are expressed as mean±SD or median (25–75 percentiles). BMI indicates body mass index; BP, blood pressure; CHD, coronary heart disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; NS, not stated; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TAG, triacylglycerol; TG, triglycerides.
730 mg quercetin/day—prehypertension patients.
730 mg quercetin/day—stage 1 HTN patients.
Placebo—prehypertension patients.
Placebo—stage 1 HTN patients.
Risk of Bias Assessment
The assessment of risk of bias in the included studies using Cochrane criteria is shown in Table 2.
Table 2.
Risk of Bias Assessment in the Studies Included in This Meta‐Analysis
| Study | Reference | Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Potential Threats to Validity |
|---|---|---|---|---|---|---|---|---|
| Javadi et al | 32 | L | U | L | L | L | L | L |
| Zahedi et al | 33 | L | L | L | L | L | L | L |
| Conquer et al | 34 | U | U | L | L | L | L | L |
| Edwards et al | 35 | U | U | L | L | U | L | L |
| Egert et al | 36 | L | U | L | L | L | L | L |
| Lee et al | 37 | U | U | L | L | L | L | L |
| Pfeuffer et al | 38 | U | U | L | L | L | L | L |
H indicates high risk of bias; L, low risk of bias; U, unclear risk of bias.
Effect of Quercetin on SBP
Meta‐analysis of data from 9 treatment arms showed significant reductions in SBP (WMD: −3.04 mm Hg, 95% CI: −5.75, −0.33, P=0.028) following supplementation with quercetin (Figure 2 upper part). Removal of the study by Zahedi et al33 from the meta‐analysis yielded a nonsignificant effect size equivalent to −1.78 mm Hg; 95% CI: −4.07, 0.52, P=0.129. When the RCTs were stratified according to the duration of supplementation, there was no significant effect in the subsets of studies lasting <8 weeks (WMD: −2.18 mm Hg, 95% CI: −5.00, 0.64, P=0.130), while a marginally significant reducing effect was observed in trials with ≥8 weeks of follow‐up (WMD: −3.57 mm Hg, 95% CI: −7.51, 0.37, P=0.076). Likewise, a significant effect of quercetin was observed in the subset of trials administering doses ≥500 mg/day (WMD: −4.45 mm Hg, 95% CI: −7.70, −1.21, P=0.007) but not in the subset with <500 mg/day doses (WMD: −1.59 mm Hg, 95% CI: −4.44, 1.25, P=0.273) (Figure 3). When dose classification was set at ≤500 and >500 mg/day, no significant change was observed in either of the subgroups (P>0.05 for both). Adjusted indirect comparison did not suggest any significant difference between either of the dose (ΔWMD: −5.01 mm Hg, 95% CI: −9.19, −0.83, Δz‐score: −2.35, P>0.05) and supplementation duration (ΔWMD: −4.35 mm Hg, 95% CI: −8.46, −0.24, Δz‐score: −2.08, P>0.05) subgroup pairs.
Figure 2.
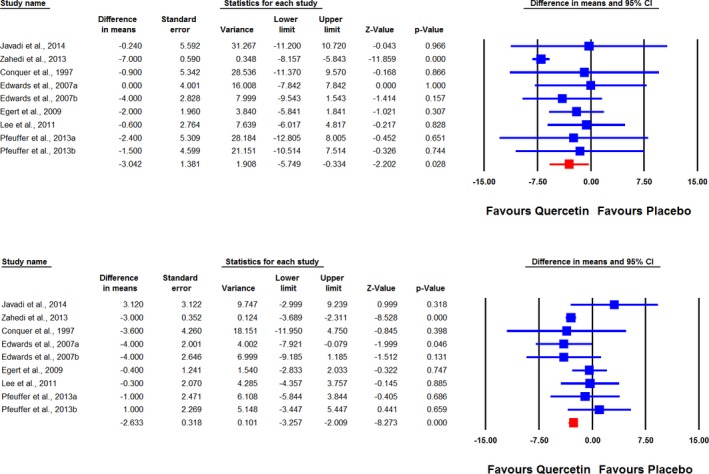
Forest plot displaying weighted mean difference and 95% CIs for the impact of quercetin on systolic (upper plot) and diastolic (lower plot) blood pressures.
Figure 3.
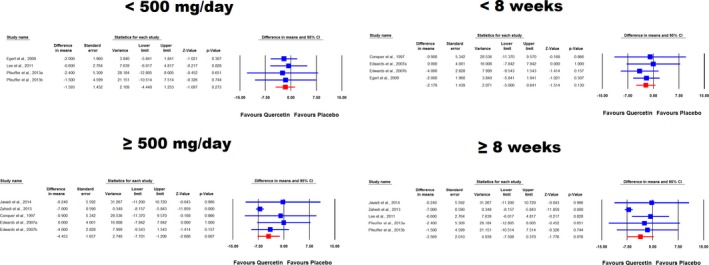
Forest plot displaying weighted mean difference and 95% CIs for the impact of quercetin on systolic blood pressure in different subgroups of trials stratified according to the administered quercetin dose and duration of supplementation.
Effect of Quercetin on DBP
Combined analysis of 9 RCT arms revealed a significant reduction of DBP (WMD: −2.63 mm Hg, 95% CI: −3.26, −2.01, P<0.001) following supplementation with quercetin (Figure 2 lower part). Removal of the study by Zahedi et al33 yielded an effect size equivalent to −0.98 mm Hg (95% CI: −2.44, 0.49, P=0.191). In subgroup analysis, a marginally significant effect was found in the subset of trials with <8 weeks of follow‐up (WMD: −1.85 mm Hg, 95% CI: −3.72, 0.02, P=0.053) but not in the subset lasting ≥8 weeks (WMD: −0.88 mm Hg, 95% CI: −3.23, 1.47, P=0.464) (Figure 3). When the studies were categorized according to administered quercetin dose, there was a greater DBP‐reducing effect in trials with ≥500 mg/day (WMD: −2.98 mm Hg, 95% CI: −3.64, −2.31, P<0.001) versus those with <500 mg/day dosage (WMD: −0.24 mm Hg, 95% CI: −2.00, 1.52, P=0.788) (Figure 4). This result was also consistent when the dose classification was set at ≤500 and >500 mg/day (P>0.05 and <0.05, respectively). However, adjusted indirect comparison did not suggest any significant difference between either of the dose (ΔWMD: −2.74 mm Hg, 95% CI: −4.34, −1.14, Δz‐score: −3.36, P>0.05) and supplementation duration (ΔWMD: −0.88 mm Hg, 95% CI: −2.68, 0.92, Δz‐score: −0.96, P>0.05) subgroup pairs.
Figure 4.
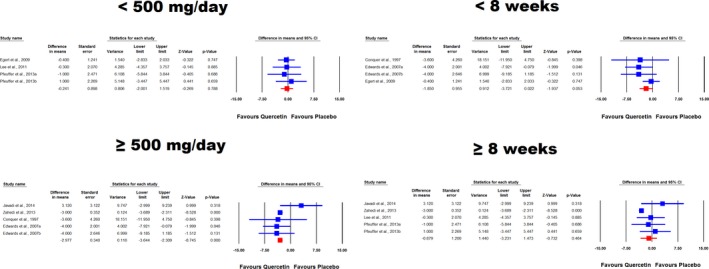
Forest plot displaying weighted mean difference and 95% CIs for the impact of quercetin on diastolic blood pressure in different subgroups of trials stratified according to the administered quercetin dose and duration of supplementation.
Meta‐Regression Analysis
Potential associations between the antihypertensive effects of quercetin with dose and duration of supplementation were evaluated using meta‐regression analysis. SBP‐lowering effect of quercetin was associated with duration of supplementation (slope: −0.92; 95% CI: −1.52, −0.32; P=0.003) but not the administered dose (slope: −0.003; 95% CI: −0.01, 0.07; P=0.548). With respect to DBP, there was a dose–response association (slope: −0.07; 95% CI: −0.01, −0.002; P=0.005) but the observed effect size was independent of supplementation duration (slope: −0.22; 95% CI: −0.62, 0.18; P=0.276) (Figure 5). Using a covariance matrix analysis of regression coefficients, the covariance of treatment dose and duration was 0 for SBP analysis and −0.0001 for DBP analysis.
Figure 5.
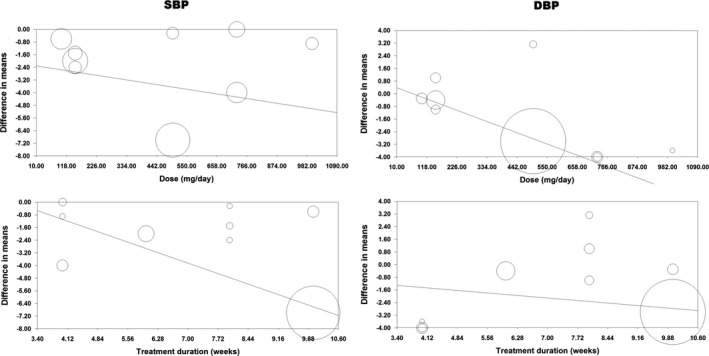
Meta‐regression bubble plots of the association between mean changes in systolic and diastolic blood pressure values after quercetin supplementation with quercetin dose and duration of supplementation. The size of each circle is inversely proportional to the variance of change.
Publication Bias
Visual inspection of the funnel plot of SE versus effect size (mean difference) suggested a potential publication bias for the impact of quercetin on both SBP and DBP. Using trim‐and‐fill correction, 4 and 3 potentially missing studies were imputed for the analysis of SBP and DBP, respectively. The imputed effect sizes of quercetin on SBP and DBP were −4.51 mm Hg (95% CI: −6.55, −2.47; P<0.05) and −2.83 mm Hg (95% CI: −3.44, −2.22; P<0.05), respectively (Figure 6).
Figure 6.
Funnel plot displaying publication bias in the studies reporting the impact of quercetin on systolic (upper plot) and diastolic (lower plot) blood pressure. The size of each circle is inversely proportional to the variance of change.
In addition to visual inspection of funnel plots, presence of publication bias was explored using Begg's rank correlation test, Egger's linear regression test, and “fail‐safe N” test. The results of these tests are summarized in Table 3.
Table 3.
Assessment of Publication Bias in the Impact of Quercetin on Blood Pressure
| Begg's Rank Correlation Test | Egger's Linear Regression Test | Fail‐Safe N Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kendall's Taua | z‐Value | P Value | Intercept | 95% CI | t | df | P Value | nb | |
| SBP, mm Hg | −0.08 | 0.31 | 0.754 | 1.69 | 0.91, 2.47 | 5.12 | 7 | 0.001 | 54 |
| DBP, mm Hg | 0.08 | 0.31 | 0.754 | 0.94 | −0.24, 2.13 | 1.88 | 7 | 0.102 | 31 |
DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
With continuity correction.
Number of theoretically missing studies.
Discussion
To our knowledge, the present meta‐analysis is the first to assess the effects of quercetin supplementation on BP based on the results from RCTs. Meta‐analysis of data from 9 treatment arms showed significant reductions in SBP and DBP following supplementation with quercetin. The estimated effect sizes for the impact of quercetin on BP were sensitive only to the study of Zahedi et al.33 The distinctive feature of this study in comparison to other studies included in the meta‐analysis is recruitment of diabetic subjects. Hence, the greater effect size observed by Zahedi et al33 might be attributed to the higher activity of quercetin in diabetic subjects attributable to the reported hypoglycemic and insulin‐sensitizing activities of this phytochemical in diabetes, which can eventually lead to attenuation of diabetes‐induced vasoconstriction.7, 39 In addition, the heightened state of oxidative stress in diabetes might justify a more sizable effect of quercetin as an efficient antioxidant,25 providing another potential mechanism for the antihypertensive effect of quercetin.
The calculated BP‐lowering effect of quercetin is substantial (−3.04, −2.63 mm Hg SBP/DBP), particularly considering that the cohorts of the studies included were largely made up of normotensive individuals. When the RCTs were stratified according to the duration of supplementation, there was no significant effect in the subsets of studies lasting <8 weeks, while a marginally significant reducing effect was observed in trials with ≥8 weeks of follow‐up. Likewise, a significant effect of quercetin was observed in the subset of trials administering doses ≥500 mg/day, but not in the subset with <500 mg/day doses. The results indicated a significant antihypertensive effect of quercetin supplementation on both SBP and DBP. In interpreting the results of the subanalyses based on length of administration and dose, caution should be used because of loss of statistical power attributable to RCT stratification, and because indirect comparison tests failed to confirm statistically significant differences. Clearly, studies directly comparing different doses or treatment duration are necessary.
The mechanisms accountable for these effects of quercetin are not completely understood, with multiple modulation in cell signaling and gene expression being the most probable. Some attempts to clarify the mechanism of action of quercetin in hypertension were performed.40, 41 Hypotheses tested in different experimental and clinical trials included the following: lowering of oxidative stress,19 interference with the renin–angiotensin system,42 improvement of endothelial function,43 downregulation of endothelin‐1 expression,44 downregulation of nicotinamide adenine dinucleotide phosphate‐oxidase,45 increasing of endothelial nitric oxide synthase activity,45 downregulation of angiotensin II 1a receptor expression in the kidney, or improving the balance between circulating endothelin‐1 and NO.22 Also, the exact contribution of quercetin metabolites to the overall antihypertensive effect must be clarified: about 90% of dietary quercetin is not absorbed and undergoes extensive metabolization by colic microbiota, resulting in phenolic acids, compounds that have not been investigated yet in the context of hypertension.46 Furthermore, the antihypertensive effects of quercetin and captopril were similar in an experimental study on Dahl salt‐sensitive rats.22
A challenge to the explanation of quercetin bioactivity was represented, until recently, by the contradiction between its extremely low plasma concentration after oral administration and the demonstrable systemic effects.47 The resolution of this inconsistency, termed the “flavonoid paradox” came with the full comprehension of the conjugation–deconjugation steps of these compounds in humans.9 It has been proven that after oral absorption, quercetin is rapidly converted to circulating conjugates through glucuronidation, sulfatation, or methylation, as a measure of classical xenobiotic detoxification.46 This accounts for the very low aglycone concentrations in human plasma (in the nanomolar range).48 At a vascular level, quercetin glucuronides, but not sulfoconjugates, are freed of their sugar moiety by a deconjugation process performed by β‐glucuronidases, and the free aglycone is delivered to tissues.9 Given the importance of quercetin glucuronide deconjugation, β‐glucuronidase activity is crucial for the therapeutic effectiveness of this flavonol.9 Furthermore, correlations between β‐glucuronidase activity and apo E phenotype49 may explain the efficacy of quercetin in patients with apo E3 phenotype as opposed to those expressing apo E4 phenotype.50 On the other hand, an increased activity of β‐glucuronidase under inflammatory conditions has also been pointed out, raising the hypothesis that quercetin may be more effective under inflammatory conditions—a valuable aspect since hypertension may be associated with comorbidities having an inflammatory component.51
Our findings that higher doses (>500 mg) of quercetin yield greater effect size on BP are to a certain extent opposite to those obtained in the Cancer Prevention Study II Nutrition Cohort that reported that even relatively small quantities of flavonoid‐rich foods may be beneficial in reducing the risk of cardiovascular disease.52 This could be explained by the fact that the amounts used in most dietary intervention studies are higher than those used in the general public. Indeed, a recent trial showed that the habitual intake of flavonoids in Europe is much below the amounts found to have a significant health effect.53
Quercetin has a generally recognized as safe status according to the U.S. Food and Drug Administration; only some minor side‐effects such as headache, nausea, and tingling of the extremities were observed in long‐term quercetin supplementation at 1000 mg/day.54 In 2011, the European Food Safety Authority released a variety of health claims underlying the protective effects of quercetin against oxidative damage.55 Considering the seasonality of food extracts of flavonoids, the Recommended Dietary Allowance of total flavonoids might be between 250 and 400 mg/day.56
This meta‐analysis has several limitations. Most importantly, eligible RCTs involved in this meta‐analysis had small populations and short durations of follow‐up. The number of included studies also was not large enough to allow robust subgroup analyses. Moreover, there is considerable heterogeneity in the groups studied: females with rheumatoid arthritis, females with diabetes, healthy people, overweight high‐risk subjects, and male smokers. Finally, most of the individuals included were normotensive or prehypertensive. It will be necessary to evaluate the effects of quercetin supplementation on long‐term control of hypertension and its complications.
In conclusion, the results of this meta‐analysis showed a significant effect of quercetin supplementation in the reduction of BP, which suggest that this nutraceutical might be considered as an add‐on to antihypertensive therapy. Further well‐designed trials are necessary to elucidate the clinical value of quercetin supplementation in hypertension therapy, to adjust the dosage, and to explore possible drug interactions between quercetin and antihypertensive drugs, as this flavonol is metabolized by the cytochrome P450 system.57, 58 Additional long‐term studies on quercetin safety at pharmacological doses are warranted as well, since quercetin supplementation as an antihypertensive implies a 10‐ to 60‐fold increase in its average dietary intake. Exploration of possibly greater benefits of quercetin supplementation in RCTs among hypertensive and/or diabetic populations merits further investigations.
Appendix
The Lipid and Blood Pressure Meta‐analysis Collaboration (LBPMC) Group members are the following: Maciej Banach, MD, PhD, FAHA, Maria‐Corina Serban, MD, PhD, Amirhossein Sahebkar, PharmD, Alberto Zanchetti, MD, PhD, Dimitri P. Mikhailidis, MD, PhD, George Howard, DrPH, Diana Antal, PharmD, Florina Andrica, PhDs, Ali Ahmed, MD, MPH, Wilbert S. Aronow, MD, Paul Muntner, PhD, Gregory Y.H. Lip, MD, Ian Graham, MD, PhD, Nathan Wong, MD, PhD, and Jacek Rysz, MD, PhD.
Disclosures
None. This meta‐analysis was written independently; no company or institution supported it financially. Some of the authors have given talks, attended conferences, and participated in trials and advisory boards sponsored by various pharmaceutical companies. No professional writer was involved in the preparation of this meta‐analysis.
(J Am Heart Assoc. 2016;5:e002713 doi: 10.1161/JAHA.115.002713)
References
- 1. Balentine DA, Dwyer JT, Erdman JW, Ferruzzi MG, Gaine PC, Harnly JM, Kwik‐Uribe CL. Recommendations on reporting requirements for flavonoids in research. Am J Clin Nutr. 2015;101:1113–1125. [DOI] [PubMed] [Google Scholar]
- 2. Serban C, Sahebkar A, Ursoniu S, Andrica F, Banach M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta‐analysis of randomized controlled trials. J Hypertens. 2015;33:1119–1127. [DOI] [PubMed] [Google Scholar]
- 3. Ursoniu S, Sahebkar A, Andrica F, Serban C, Banach M. Effects of flaxseed supplements on blood pressure: a systematic review and meta‐analysis of controlled clinical trial. Clin Nutr. 2016;35:615–625. [DOI] [PubMed] [Google Scholar]
- 4. Banach M, Aronow WS, Serban MC, Sahebkar A, Rysz J, Voroneanu L, Covic A. Lipids, blood pressure and kidney update 2014. Pharmacol Res. 2015;95‐96c:111–125. [DOI] [PubMed] [Google Scholar]
- 5. Lairon D, Amiot MJ. Flavonoids in food and natural antioxidants in wine. Curr Opin Lipidol. 1999;10:23–28. [DOI] [PubMed] [Google Scholar]
- 6. Hertog MG, Feskens EJ, Kromhout D, Hollman P, Katan M. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. [DOI] [PubMed] [Google Scholar]
- 7. Kelly GS. Quercetin. Monograph. Altern Med Rev. 2011;16:172–194. [PubMed] [Google Scholar]
- 8. Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res. 2004;24:851–874. [Google Scholar]
- 9. Perez‐Vizcaino F, Duarte J, Santos‐Buelga C. The flavonoid paradox: conjugation and deconjugation as key steps for the biological activity of flavonoids. J Sci Food Agric. 2012;92:1822–1825. [DOI] [PubMed] [Google Scholar]
- 10. Men K, Duan X, Wei XW, Gou ML, Huang MJ, Chen LJ, Qian ZY, Wei YQ. Nanoparticle‐delivered quercetin for cancer therapy. Anticancer Agents Med Chem. 2014;14:826–832. [DOI] [PubMed] [Google Scholar]
- 11. Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, Palumbo R. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res. 2014;159:185–205. [DOI] [PubMed] [Google Scholar]
- 12. Hubbard G, Stevens J, Cicmil M, Sage T, Jordan P, Williams C, Lovegrove J, Gibbins J. Quercetin inhibits collagen‐stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signaling pathway. J Thromb Haemost. 2003;1:1079–1088. [DOI] [PubMed] [Google Scholar]
- 13. Chirumbolo S. Quercetin as a potential anti‐allergic drug: which perspectives? Iran J Allergy Asthma Immunol. 2011;10:139–140. [PubMed] [Google Scholar]
- 14. Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014;109:8–14. [DOI] [PubMed] [Google Scholar]
- 15. Nabavi SF, Russo GL, Daglia M, Nabavi SM. Role of quercetin as an alternative for obesity treatment: you are what you eat! Food Chem. 2015;179C:305–310. [DOI] [PubMed] [Google Scholar]
- 16. Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010;9:263–285. [DOI] [PubMed] [Google Scholar]
- 17. Pan H‐C, Jiang Q, Yu Y, Mei J‐P, Cui Y‐K, Zhao W‐J. Quercetin promotes cell apoptosis and inhibits the expression of MMP‐9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem Int. 2015;80:60–71. [DOI] [PubMed] [Google Scholar]
- 18. Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T. Anti‐inflammatory, anti‐proliferative and anti‐atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44–52. [DOI] [PubMed] [Google Scholar]
- 19. Duarte J, Pérez‐Palencia R, Vargas F, Angeles Ocete M, Pérez‐Vizcaino F, Zarzuelo A, Tamargo J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol. 2001;133:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao H, Chen C, Huang S, Li B. Quercetin attenuates the progression of monocrotaline‐induced pulmonary hypertension in rats. J Biomed Res. 2012;26:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garcia‐Saura MF, Galisteo M, Villar IC, Bermejo A, Zarzuelo A, Vargas F, Duarte J. Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol Cell Biochem. 2005;270:147–155. [DOI] [PubMed] [Google Scholar]
- 22. Mackraj I, Govender T, Ramesar S. The antihypertensive effects of quercetin in a salt‐sensitive model of hypertension. J Cardiovasc Pharmacol. 2008;51:239–245. [DOI] [PubMed] [Google Scholar]
- 23. Montenegro MF, Neto‐Neves EM, Dias‐Junior CA, Ceron CS, Castro MM, Gomes VA, Kanashiro A, Tanus‐Santos JE. Quercetin restores plasma nitrite and nitroso species levels in renovascular hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:293–301. [DOI] [PubMed] [Google Scholar]
- 24. Morales‐Cano D, Menendez C, Moreno E, Moral‐Sanz J, Barreira B, Galindo P, Pandolfi R, Jimenez R, Moreno L, Cogolludo A, Duarte J, Perez‐Vizcaino F. The flavonoid quercetin reverses pulmonary hypertension in rats. PLoS One. 2014;9:e114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. [DOI] [PubMed] [Google Scholar]
- 26. Islam MA, Schmidt RW, Gunaseelan S, Sanchez A. An update on the cardiovascular effects of quercetin, a plant flavonoid. Curr Nutr Food Sci. 2014;10:36–48. [Google Scholar]
- 27. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. 2 [updated September 2009]. The Cochrane collaboration, 2009. 2013. Available at: www.cochrane-handbook.org/. Accessed May 18, 2009. [Google Scholar]
- 28. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta‐Analysis Version 2. Englewood, NJ: Biostat; 2005:104. [Google Scholar]
- 29. Song F, Altman DG, Glenny A‐M, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ. 2003;326:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. [DOI] [PubMed] [Google Scholar]
- 31. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 32. Javadi F, Eghtesadi S, Ahmadzadeh A, Aryaeian N, Zabihiyeganeh M, Foroushani AR, Jazayeri S. The effect of quercetin on plasma oxidative status, C‐reactive protein and blood pressure in women with rheumatoid arthritis. Int J Prev Med. 2014;5:293. [PMC free article] [PubMed] [Google Scholar]
- 33. Zahedi M, Ghiasvand R, Feizi A, Asgari G, Darvish L. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: a double‐blind randomized controlled clinical trial. Int J Prev Med. 2013;4:777. [PMC free article] [PubMed] [Google Scholar]
- 34. Conquer J, Maiani G, Azzini E, Raguzzini A, Holub B. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998;128:593–597. [DOI] [PubMed] [Google Scholar]
- 35. Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405–2411. [DOI] [PubMed] [Google Scholar]
- 36. Egert S, Bosy‐Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta‐Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G. Quercetin reduces systolic blood pressure and plasma oxidised low‐density lipoprotein concentrations in overweight subjects with a high‐cardiovascular disease risk phenotype: a double‐blinded, placebo‐controlled cross‐over study. Br J Nutr. 2009;102:1065–1074. [DOI] [PubMed] [Google Scholar]
- 37. Lee K‐H, Park E, Lee H‐J, Kim M‐O, Cha Y‐J, Kim J‐M, Lee H, Shin M‐J. Effects of daily quercetin‐rich supplementation on cardiometabolic risks in male smokers. Nutr Res Pract. 2011;5:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfeuffer M, Auinger A, Bley U, Kraus‐Stojanowic I, Laue C, Winkler P, Rüfer C, Frank J, Bösch‐Saadatmandi C, Rimbach G. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr Metab Cardiovasc Dis. 2013;23:403–409. [DOI] [PubMed] [Google Scholar]
- 39. Mahmoud MF, Hassan NA, El Bassossy HM, Fahmy A. Quercetin protects against diabetes‐induced exaggerated vasoconstriction in rats: effect on low grade inflammation. PLoS One. 2013;8:e63784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larson AJ, Symons JD, Jalili T. Quercetin: a treatment for hypertension?—a review of efficacy and mechanisms. Pharmaceuticals. 2010;3:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larson AJ, Symons JD, Jalili T. Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv Nutr. 2012;3:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Häckl L, Cuttle G, Dovichi S, Lima‐Landman M, Nicolau M. Inhibition of angiotensin‐converting enzyme by quercetin alters the vascular response to bradykinin and angiotensin I. Pharmacology. 2002;65:182–186. [DOI] [PubMed] [Google Scholar]
- 43. Yamamoto Y, Oue E. Antihypertensive effect of quercetin in rats fed with a high‐fat high‐sucrose diet. Biosci Biotechnol Biochem. 2006;70:933–939. [DOI] [PubMed] [Google Scholar]
- 44. Nicholson SK, Tucker GA, Brameld JM. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc. 2008;67:42–47. [DOI] [PubMed] [Google Scholar]
- 45. Sanchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, Pérez‐Vizcaíno F, Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. [DOI] [PubMed] [Google Scholar]
- 46. Sahebkar A, Serban MC, Gluba‐Brzózka A, Mikhailidis DP, Cicero AF, Rysz J, Banach M. Lipid‐modifying effects of nutraceuticals: An evidence‐based approach. Nutrition 2016; doi:10.1016/j.nut.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 47. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. [DOI] [PubMed] [Google Scholar]
- 48. Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr. 2010;103:249–255. [DOI] [PubMed] [Google Scholar]
- 49. Lee‐Hilz YY, Stolaki M, van Berkel WJ, Aarts JM, Rietjens IM. Activation of EpRE‐mediated gene transcription by quercetin glucuronides depends on their deconjugation. Food Chem Toxicol. 2008;46:2128–2134. [DOI] [PubMed] [Google Scholar]
- 50. Egert S, Boesch‐Saadatmandi C, Wolffram S, Rimbach G, Müller MJ. Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J Nutr. 2010;140:278–284. [DOI] [PubMed] [Google Scholar]
- 51. Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos. 2001;29:1521–1524. [PubMed] [Google Scholar]
- 52. McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vogiatzoglou A, Mulligan AA, Lentjes MA, Luben RN, Spencer JP, Schroeter H, Khaw KT, Kuhnle GG. Flavonoid intake in European adults (18 to 64 years). PLoS One. 2015;10:e0128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harwood M, Danielewska‐Nikiel B, Borzelleca J, Flamm G, Williams G, Lines T. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. [DOI] [PubMed] [Google Scholar]
- 55. EFSA panel on dietetic products, nutrition and allergies (NDA). Scientific opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage (ID 1647), “cardiovascular system” (ID 1844), “mental state and performance” (ID 1845), and “liver, kidneys” (ID 1846) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA J. 2011;9:2067 [2015 pp.] [Google Scholar]
- 56. Peluso I, Palmery M. Flavonoids at the pharma‐nutrition interface: is a therapeutic index in demand? Biomed Pharmacother. 2015;71:102–107. [DOI] [PubMed] [Google Scholar]
- 57. Michalska M, Gluba A, Mikhailidis DP, Nowak P, Bielecka‐Dabrowa A, Rysz J, Banach M. The role of polyphenols in cardiovascular disease. Med Sci Monit. 2010;16:RA110–RA119. [PubMed] [Google Scholar]
- 58. Hodek P, Trefil P, Stiborová M. Flavonoids—potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact. 2002;139:1–21. [DOI] [PubMed] [Google Scholar]


