Abstract
Background
Long‐term survival for persons born with congenital heart disease (CHD) is improved, but limited knowledge exists of this growing population's acquired cardiovascular risk profile. This study's purpose was to assess CHD survivors’ risk for type 2 diabetes mellitus (T2DM) with attention to the impact of cyanotic CHD.
Methods and Results
This population‐based cohort study included Danish subjects with CHD who were born between 1963 and 1980 and were alive at age 30 years. For each CHD case, we identified 10 individuals from the general population matched by sex and birth year, by using the Danish Civil Registration System. Complete follow‐up was obtained through Danish public registries for death, emigration, and T2DM (diagnosis and prescriptions record). We computed cumulative incidences and hazard ratios of developing T2DM after age 30 for 5149 CHD subjects compared with the general population. After adjusting for CHD severity, as well as age, sex, preterm birth, and extracardiac defects, we analyzed the impact of cyanotic compared with acyanotic CHD. By age 45 years, the cumulative incidence of T2DM after age 30 was 4% among subjects with CHD. Subjects with CHD were more likely to develop T2DM than the general population (hazard raio 1.4, 95% CI 1.1–1.6). Subjects CHD who had cyanotic defects were more likely to develop T2DM than were subjects with acyanotic CHD (hazard ratio 1.9, 95% CI 1.1–3.3).
Conclusions
CHD survivors had an increased risk of developing T2DM after age 30. Patients with cyanotic CHD are at particular risk. Given the cardiovascular health burden of T2DM, attention to its development in CHD survivors seems warranted.
Keywords: diabetes mellitus, epidemiology, heart defects (congenital), hypoxia
Subject Categories: Congenital Heart Disease; Diabetes, Type 2; Epidemiology
Introduction
Acongenital heart defect, or congenital heart disease (CHD), represents the most common congenital birth defect, affecting 1% of all live births (excluding bicuspid aortic valve).1 The overall survival for this population has improved dramatically over recent decades such that there are now more adults living with CHD than there are children.2 As a result of this success, it has become increasingly important to understand this population's potential for acquired morbidity and, in particular, to understand the risk of acquired diseases with cardiovascular health implications, such as diabetes mellitus (DM).3
The prevalence of type 2 diabetes mellitus (T2DM) in Western societies continues to climb; as of 2014, the Centers for Disease Control and Prevention estimates that 29 million persons in the United States (9.3% of the population) have DM.4 The estimated overall prevalence of DM in Denmark in 2012 was 5.7% with a steady incidence increase over recent decades and a lifetime risk of 30%.5 Onset of T2DM is often silent and proceeded by insulin resistance, decreases in insulin secretion capacity, and mild hyperglycemia (termed prediabetes); it is estimated that 86 million Americans have prediabetes.4, 6 Importantly, these initial physiologic derangements can lead to irreversible complications before treatment initiation. Data suggest that early glucose control (both medication and lifestyle) reduces the risk of long‐term microvascular complications by 10% to 25% and may also reduce long‐term macrovascular complications,6, 7 and in the case of CHD survivors, the capacity to reduce these complications may have long‐term benefits.
Limited population‐based data exist on long‐term acquired morbidity in the CHD population.3, 8, 9, 10 Given the escalating prevalence of both CHD survival and T2DM in the general population, we believe it is important to assess its incidence in the CHD population, as subjects with CHD may be both at particular risk of DM and uniquely vulnerable to its effects. For example, CHD survivors have several key risk factors for T2DM over their lifetime, such as activity restriction,11 sedentary lifestyle,12 and elevated risk of gestational exposures to glycemic dysregulation.13 Further, a growing body of evidence suggests hypoxia exposure is an independent risk factor of T2DM.14, 15, 16, 17, 18
The purpose of this study was to use Danish nationwide population‐based registries to examine whether patients with CHD are at increased risk of T2DM relative to the general population.19, 20 We aimed to separate T2DM from type 1 DM by focusing on DM development after age 30 years and by exclusion of patients with CHD or comparison cohort members with DM occurring before age 30.5 Further, we wished to examine whether T2DM risk is increased for patients with cyanotic CHD compared with those with acyanotic CHD, given the exposure to hypoxia.
Methods
Setting
Our nationwide population‐based cohort study was conducted in Denmark, with a current population of ≈5.6 million individuals. The Danish National Health Service provides tax‐supported health care, with free access to hospital‐based and primary medical care, including care for CHD and DM. This study was approved by the Danish Data Protection Agency (2013‐41‐1754), and as a result, informed consent of cohort subjects is waived.
Data Linkage
Since 1968, a unique 10‐digit civil personal registration (CPR) number has been assigned to all residents of Denmark by the Central Office of Civil Registration. CPR numbers are used in all Danish registries, permitting unambiguous individual‐level linkage of data from all sources used in this study. This provided us with virtually complete follow‐up for death, emigration, and the outcome under study. The Civil Registration System also made it possible to identify a general population comparison cohort.
Congenital Heart Defect Cohort
We included Danish CHD survivors born between January 1, 1963, and December 31, 1980, who were alive at 30 years of age and had received a diagnosis of a CHD at any age. CHD survivors diagnosed between 1963 and 1974 were identified based on review of inpatient and outpatient medical records in all Danish pediatric and medical departments by an experienced physician, Henning Bækgaard Laursen.21 The review was done in 1970–1974, and the diagnoses were later translated from the International Society of Cardiology (1970) classification to the International Classification of Diseases, 10th revision (ICD‐10).22 Beginning in 1977, the Danish National Registry of Patients (DNRP) contains information on all hospital admissions in Denmark and includes subjects’ civil registration numbers,23 dates of admission and discharge, surgical procedures, and up to 20 discharge diagnoses coded by physicians according to the ICD. Since 1995, the DNRP also contains information on hospital outpatient clinic contacts.
There is a 2‐year gap between the CHD registry and the DNRP from 1975 to 1976. Those individuals with CHD diagnosed during these 2 years without any subsequent medical record data points in the DNRP after 1977 were not included in the study. As previously described, an algorithm developed by experienced cardiac surgeons, cardiologists, and epidemiologists and based on extensive medical record review enabled inclusion of all patients with valid CHD diagnoses.24
General Population Comparison Cohort
For each CHD subject, we identified 10 population‐comparison cohort members from the general population by using the Civil Registration System, matched by sex and birth year.
Diabetes Mellitus
DM was identified in all subjects by using 2 separate data sources: (1) the DNRP allowed identification of persons with any inpatient or outpatient hospital contact involving DM by using 8th and 10th edition ICD codes for DM (ICD‐10: DE10–DE14, DG632, DH360, DN083; ICD‐8: 249–250), and (2) the Danish National Prescription Registry allowed identification of DM prescriptions. The latter registry contains complete information on all prescriptions not only prescribed but actually filled at state‐controlled community pharmacies throughout Denmark since 1995. We used the following Anatomical Therapeutic Chemical Classification (ATC) codes to identify DM medications: A10A (insulin) and A10B (noninsulin glucose‐lowering drugs). The date of onset of DM was defined as the first record of a hospital contact involving DM or a first prescription for DM medications, whichever came first. The positive predictive value of the DM diagnosis using this methodology is 97%.25 To focus on T2DM, we defined the outcome as incident DM after age 30 years and excluded patients with CHD or comparison cohort members with DM occurring before age 30.5
It should be noted that the Department of Clinical Epidemiology is a member of the Danish Centre for Strategic Research in Type 2 Diabetes), supported by the Danish Agency for Science (grant nos. 09‐067009 and 09‐075724). The Danish Centre for Strategic Research in Type 2 Diabetes is also supported by the Danish Health and Medicines Authority, the Danish Diabetes Association, and an unrestricted donation from Novo Nordisk A/S. Partners in the Danish Centre for Strategic Research in Type 2 Diabetes project are listed on the project website at www.DD2.nu.
Covariates
We analyzed several additional factors by linkage to national and regional databases: birth history (The Danish Medical Birth Registry),26 presence of extracardiac defects (ECDs [DNRP]), and subject's prescription record (Danish National Prescription Registry).27
CHD survivors were categorized as cyanotic or acyanotic according to their ICD coding (8th and 10th edition). To decrease misclassification, the only lesions categorized as cyanotic were transposition of the great arteries, tetralogy of Fallot, and truncus arteriosus/common arterial trunk, and we included individuals identified as having univentricular physiology (post Glenn and/or Fontan per procedure code data). The confidence to classify these individuals as cyanotic is supported by their birth era (1963–1980). The representative isolated defects of the acyanotic group were similarly considered: ventricular septal defect, atrial septal defect, patent ductus arteriosus, and coarctation of the aorta. All individuals not identified as cyanotic or acyanotic CHD were termed “unclassified.” Those identified to have Eisenmenger physiology were excluded from all 3 subgroups.
CHD survivors were also categorized according to defect complexity as follows: univentricular>biventricular severe complexity>biventricular moderate complexity (exclusively isolated or in combination: ventricular septal defect, atrial septal defect, and patent ductus arteriosus, as well as isolated coarctation of the aorta)>biventricular simple complexity (those without history of surgery or intervention). To optimize the accuracy of the defect categorization, we used a previously described hierarchical algorithm based on the medical facility of the provider issuing the diagnosis.27 Specifically, there are 4 separate academic medical centers in Denmark, and when a subject's CHD ICD codes were inconsistent, those issued by one of these centers were selected in place of those issued by community hospitals, birthing centers, and outpatient general providers. Additionally, when there was inconsistency within an academic center, we selected the diagnosis based on the hierarchy of physiologic complexity: univentricular>biventricular severe complexity>biventricular moderate complexity>biventricular simple complexity.
The study encompassed diagnoses of ECDs given at any time after birth. In accordance with a guideline from the European Surveillance of Congenital Anomalies (EUROCAT), we disregarded isolated minor defects such as subluxation or unstable hip, cryptorchidism, torticollis, or protuberant ears.28 We obtained data on gestational age from both the early era medical record review by Laursen21 and The Danish Medical Birth Registry26; preterm birth was defined as gestational age <37 weeks. Data in The Danish Medical Birth Registry begins in 1977 and therefore did not have readily available data on gestational age on individuals born before1977.
Statistical Analysis
Follow‐up of subjects with CHD and comparison cohort members began at 30 years of age and continued until death, emigration, onset of DM, or end of study on January 1, 2013, whichever came first. Data were analyzed with delayed entry on January 1, 1995, the earliest date that data from the Danish National Prescription Registry were available. Patients or comparison cohort members with DM before this date or before the date they turned 30 years old, if this date came later, were excluded as likely type 1 DM cases. In addition, we performed a sensitivity analysis to manage the risk of a left truncation bias by analyzing a 5‐year run‐in observation period from 1995 to 2000.
We computed curves of cumulative incidence of DM by age in years, while taking account of the competing risk of death. We made cumulative incidence curves for the CHD cohort and comparison cohort overall, as well as for subgroups of subjects with CHD according to presence of cyanotic defects and CHD severity. We examined specifically the cumulative incidence of DM at age 45 in order to determine if the CHD population demonstrated DM prematurely relative to matched controls (we chose 45 years to allow for adequate sample sizes to compare given the maximum of 50 years of individual follow‐up data). Using Cox proportional hazards regression, we computed hazard ratios (HRs) to compare the time to onset of DM after age 30 among subjects with CHD relative to the general population cohort. The assumption of proportional hazards was verified graphically using log–log plots. Comparisons with the general population were adjusted for sex and birth year categories only. We also compared time to onset of DM after age 30 for CHD survivors with a history of cyanotic CHD with that of survivors of acyanotic CHD. This comparison was adjusted for CHD complexity, as well as sex, birth year, presence of ECDs, and prematurity by using the Markov chain Monte Carlo method for multiple imputation to account for missing data on gestational age.
Results
We identified 5149 patients with CHD (47% male) who were born between January 1, 1963, and December 31, 1980, and were alive at 30 years of age (Table 1). The most frequent types of CHD diagnoses were ventricular septal defect and atrial septal defect. ECDs were present in 20% of the patients with CHD. Among patients with CHD, 469 (9%) were born preterm. After applying criteria for differentiation between cyanotic and acyanotic defects, the CHD cohort included 456 (9%) cyanotic lesions and 2052 (40%) acyanotic lesions by our study definition—those not meeting either definition were termed unclassified (2641; 48%). Within the cyanotic lesion category, 38 (8%) demonstrated moderate biventricular CHD complexity, while 361 (79%) demonstrated severe biventricular or univentricular complexity (remainder not able to be classified). In the acyanotic lesion category, 1086 (53%) demonstrated mild complexity, while 793 (39%) showed moderate biventricular complexity (remainder not able to be classified).
Table 1.
Baseline and Clinical Characteristics of Congenital Heart Disease (CHD) Cohort and Matched General Population Comparison Cohort
| CHD Survivors, n (%) | Comparison Cohort, n (%) | |
|---|---|---|
| Total | 5149 (100) | 49 968 (100) |
| Male | 2440 (47) | 23 361 (47) |
| Year of birth | ||
| 1963–1969 | 2181 (42) | 21 109 (42) |
| 1970–1974 | 1446 (28) | 13 998 (28) |
| 1975–1980 | 1522 (30) | 14 861 (30) |
| Extracardiac defecta | 1018 (20) | — |
| Preterm birtha | 469 (9) | — |
| Missing data on gestational age | 2666 (52) | — |
| CHD cyanosis category | ||
| Cyanotic CHD | 456 (9) | — |
| Acyanotic CHD | 2052 (40) | — |
| Unclassified | 2641 (48) | — |
| CHD severity | ||
| Mild biventricular | 1959 (38) | — |
| Moderate biventricular | 1204 (23) | — |
| Severe biventricular | 1057 (21) | — |
| Univentricular | 20 (0.5) | — |
| Unclassified | 909 (18) | — |
Complete data on extracardiac defects and preterm birth in comparison cohort are not readily available given that routine collection in the Danish National Registry of Patients began in 1977.
For patients with CHD, the overall cumulative risk of developing DM by 45 years of age was ≈4% (Figure 1). Overall, the HR of development of DM after age 30 among CHD survivors compared with the general population cohort was 1.35 (95% CI 1.14–1.61) (Table 2). This result was unchanged after a sensitivity analysis to address the potential impact of a left truncation bias (HR 1.31, 95% CI 1.10–1.57). Compared with the general population cohort, the HR of developing DM for CHD survivors with cyanotic CHD was 2.85 (95% CI 1.77–4.57), and for those with acyanotic CHD, it was 1.35 (95% CI 1.02–1.77) (Table 2). When subjects with ECDs were excluded from the analysis, the estimates were not substantially changed.
Figure 1.
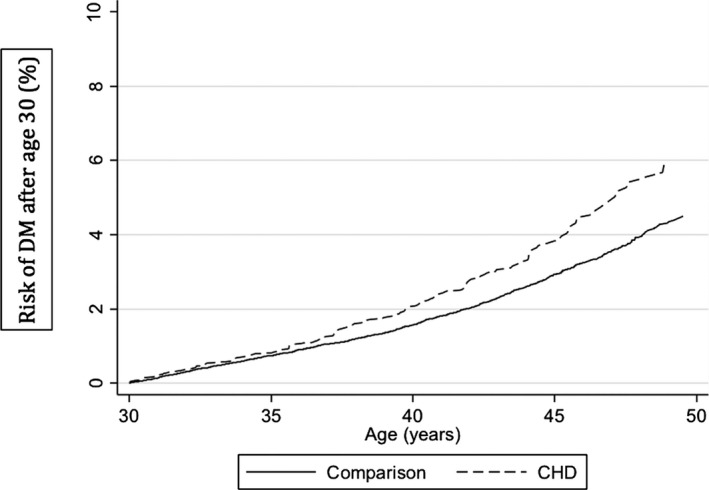
Cumulative incidence of diabetes mellitus (DM) after age 30 in the congenital heart disease (CHD) and general population comparison cohorts.
Table 2.
Cox Proportional Hazard Ratios—Diabetes Mellitus in the Congenital Heart Disease (CHD) Cohort Compared With General Population Cohort Adjusted for Sex and Birth Year
| Hazard Ratio | 95% CI | |
|---|---|---|
| All CHD | 1.35 | 1.14–1.61 |
| Among men | 1.45 | 1.12–1.87 |
| Among women | 1.29 | 1.02–1.63 |
| CHD | ||
| Cyanotic | 2.85 | 1.77–4.57 |
| Acyanotic | 1.35 | 1.02–1.77 |
| Unclassified | 1.15 | 0.89–1.49 |
| CHD complexity | ||
| Mild | 0.96 | 0.70–1.32 |
| Moderate | 1.57 | 1.11–2.21 |
| Severe | 1.55 | 1.07–2.26 |
| Univentricular | 2.53 | 0.28–22.7 |
| Unclassified | 1.73 | 1.20–2.51 |
| Extracardiac defects excluded | ||
| All CHD categories | 1.26 | 1.03–1.54 |
Compared with the acyanotic CHD cohort, the HR for the development of DM after age 30 when exposed to cyanotic CHD was 1.93 (95% CI 1.14–3.28) (Table 3). The relationship of cyanotic CHD versus acyanotic CHD exposure relative to the comparison cohort and unclassified CHD is shown in Figure 2. Starting at age 30, the cumulative incidence curve of the subjects with cyanotic CHD reveals an increased risk, with 8% of subjects demonstrating DM by 45 years of age. The acyanotic subjects with CHD demonstrate a cumulative incidence of DM that matches that of the unclassified subjects with CHD. Specifically, the cumulative incidence of DM by 45 years of age is 3.9% for the acyanotic subjects and 3.7% for the unclassified subjects. The general population comparison cohort demonstrates a cumulative incidence of DM by 45 years of age of 2.8%.
Table 3.
Cox Proportional Hazard Ratios—Diabetes Mellitus in the Cyanotic Compared With the Acyanotic Congenital Heart Disease (CHD) Cohorts
| Hazard Ratio | 95% CI | |
|---|---|---|
| Acyanotic CHD | Ref. | — |
| Cyanotic CHD | 1.93 | 1.14–3.28 |
Adjusted for sex, birth year, preterm birth, and CHD severity.
Figure 2.
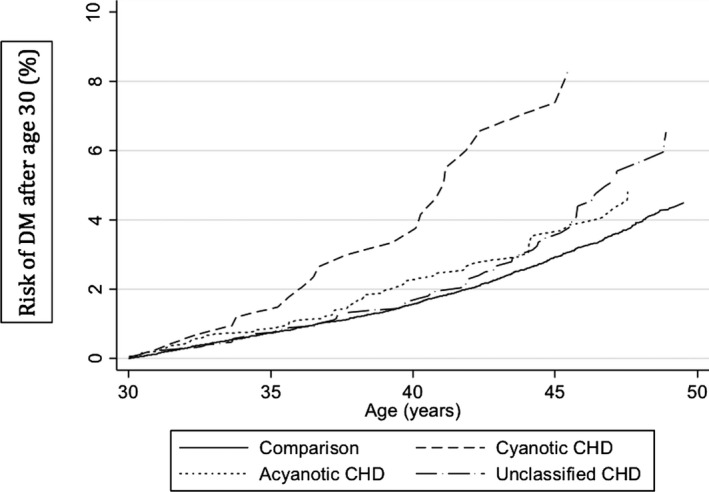
Cumulative incidence of diabetes mellitus (DM) after age 30 separated by cyanotic congenital heart disease (CHD), acyanotic CHD, unclassified CHD, and matched general population comparison cohorts.
The variation of DM risk according to the presence of cyanotic CHD and to increasing CHD complexity is represented in cumulative incidence curves (Figure 3A and 3B). If all forms of CHD are considered regardless of their cyanotic/acyanotic history, the risk of developing DM after age 30 for mild CHD tracks close to that of the general population comparison cohort. Those with moderate and severe biventricular forms of CHD and those with unclassified severity are at a similar increased risk. When considering only severe types of CHD (univentricular and biventricular severe complexity), cyanotic types of CHD continue to demonstrate a higher risk of developing DM after age 30 than acyanotic and unclassified types (Figure 3B).
Figure 3.
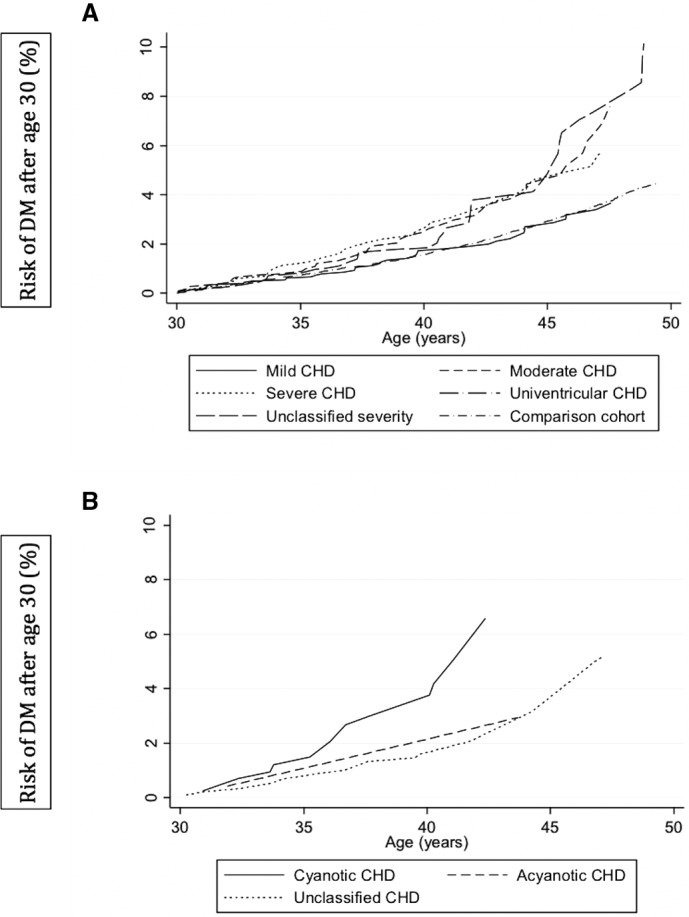
A and B, Cumulative incidence of diabetes mellitus (DM) after age 30 according to (A) congenital heart disease (CHD) complexity categories and (B) severe CHD complexity, including univentricular physiology, by cyanotic exposure.
Discussion
In this population‐based follow‐up study, we found an elevated risk for the development of T2DM in the CHD survivor population by using DM after age 30 as a surrogate for T2DM. In particular, we found a markedly elevated risk of T2DM in those CHD survivors born with cyanotic CHD relative to those with acyanotic CHD, and notably, the strength of this association remained after adjustment for complexity of CHD subtype.
Our study findings expand on the limited previous research regarding the risk of T2DM in CHD survivors.3, 8, 29 Previous studies have explored this association but have been restricted in their capacity for longitudinal follow‐up data or have been unable to compare to a general population cohort. Using the Danish population‐based registries with the capacity for unambiguous data linkage, we were able to overcome many of these limitations with the benefit of decades of follow‐up data. Dellborg and colleagues8 examined the prevalence of adult patients with CHD and DM in Sweden but did not provide data on the risk of DM in the CHD population, nor were they able to examination the interaction of cyanosis and disease complexity.
The notion of abnormal glucose regulation in adults with CHD was previously suggested by Ohuchi and colleagues.29 They performed a 75‐g oral glucose tolerance test on 205 consecutive inpatient CHD adults with various levels of complexity and concluded that those subjects with higher complexity, regardless of hemodynamics, had a prevalence of abnormal glucose metabolism 10 times that of their normative peers. However, their findings are not immediately generalizable as all subjects were inpatients at the time of study.
Although those authors did not analyze or speculate on the mechanism of elevated risk, several traditional risk factors for T2DM such as obesity30 and sedentary lifestyle12 have been described in the aging CHD population. In a cross‐sectional study, Moons and colleagues collected data on 1976 adults with CHD (median age 26 years)3 and demonstrated a higher rate of hypertension, obesity, and T2DM relative to the national population, with only 20% of subjects with CHD abiding by a “heart‐healthy” lifestyle. Barbiero and colleagues10 demonstrated in 316 CHD outpatients a rate of dyslipidemia, excess weight, and family history of T2DM that equaled the rate of the general population and called for additional attention to the aim for healthy cardiovascular behaviors in the CHD community.
Beyond traditional risk factors for T2DM, our study demonstrates that exposure to cyanotic CHD is associated with the development of DM after age 30, beyond the effect of CHD severity. This is consistent with the collective body of experimental and observational evidence, both animal model based14, 15 and in human subjects,16, 17, 18 that suggests that hypoxia has an independent negative impact on glucose metabolism. A considerable portion of this literature discusses the impact of acute intermittent hypoxia as experienced during periods of obstructive sleep apnea as critical to the risk of abnormal glucose metabolism by impairment of insulin sensitivity, glucose effectiveness, and insulin secretion.16, 17 However, chronic hypoxia associated with respiratory conditions has also demonstrated similar associations with glucose intolerance.18 While this mechanism appears plausible, the observed association between cyanotic CHD and increased risk of DM after age 30 may also indicate the presence of a common genetic or environmental risk factor.
A specific strength of this study is its population‐based design and the complete long‐term follow‐up, substantially reducing selection bias. However, some limitations should also be acknowledged. Our findings are reliant on the accuracy of the coding of both our exposure and outcome. Importantly, the positive predictive value of CHD diagnoses in the DNRP is high and any misclassification of overall CHD status is small and independent of future development of DM.31 Additionally, the inclusion of data from the Danish CHD registry benefits from the validation previously performed.21 To minimize misclassification of the CHD severity, we took the additional steps to develop a hierarchical approach to define the CHD subtype for each participant, drawing on the opportunity to identify the qualifications of the medical center at which each diagnosis was determined. In addition, it should be noted that the univentricular group is small in sample size as a result of the era of birth under study. As a consequence, when data from this group are presented, such as in Table 2, it is advisable to not draw any firm conclusions based on the wide statistical variation inherent when analyzing a small sample size.
In terms of misclassification of the T2DM outcome, previous work on DM in the Danish registries supports the accuracy and high positive predictive value of the methods used,5 in particular when including the data from the prescription record. We excluded those with DM before age 30 years for 2 reasons: (1) to increase the potential of excluding those more likely to have T1DM and (2) to avoid a potential surveillance bias given the absence of restrictions on age at CHD diagnosis (other than before age 30), which may have increased the potential that a patient with mild CHD could have been discovered during clinical workup related to the DM.
We do not believe that CHD survivors were more likely to receive a diagnosis of T2DM relative to the population‐based controls. Although CHD survivors, in particular those with complex CHD, may have more contact with the medical establishment than controls as a result of their chronic condition, it is first important to consider that in Denmark all persons benefit from a universal healthcare system that enables equal and timely access for all citizens. Second, given that our study is one of the first to report on the association between CHD and T2DM, there is not at present a screening recommendation for the CHD population that might alert the provider to the presence of T2DM sooner than population controls. Thus, we believe that most misclassification of T2DM status would be nondifferential between the CHD and comparison cohorts and would therefore lead us to underestimate associations between CHD and T2DM.
When interpreting the results regarding cyanotic and acyanotic defects, it should be noted that we were not able to directly identify cyanosis or its duration or severity. To minimize the risk of misclassifying cyanosis and acyanosis, we restricted our analysis to defects that could with highest certainty be placed in each classification. For example, we purposely excluded, from either category, atrioventricular canal defects and pulmonary stenosis because we were limited in our ability to correctly categorize the cyanosis potential of these defects. It is also important to recognize the birth era of this cohort (1963–1980); this marks an era when the opportunities for newborn and infant surgical interventions were limited, and as a consequence, the natural history of each CHD lesion is more completely expressed. In further consideration of natural history and birth era, we also took the extra step to exclude those subjects with documentation of Eisenmenger physiology. Last, when comparing individuals with cyanotic and those with acyanotic CHD, we were able to adjust for potential confounders such as CHD severity, ECDs, and prematurity by using multiple imputation to account for missing data on gestational age. We did not adjust for prematurity when comparing risk of DM after age 30 of the CHD cohort with that of the general population cohort. Preterm birth is physiologically linked to CHD,32 and any adjustment would have inappropriately attenuated the relative risk estimates.
We were not able to assess the impact of obesity on either cohort as a limitation of the registry‐based data (height and weight not available). While obesity is a clearly described risk factor for T2DM, we are not able to determine to what extent the elevated rate of DM after age 30 in the CHD population is driven by body habitus. However, we can draw from previous literature on the rate of overweight/obesity in the CHD population from 2 large American centers,31 as well as data from Belgium3 and Brazil,10 that demonstrate a rate of overweight and obesity that is not substantially worse in CHD survivors relative to their general population.
In conclusion, CHD survivors were at substantially increased risk of developing DM after age 30 compared with the general population, especially those with a history of cyanotic CHD. Unfortunately, reports suggest that promoting cardiovascular health is not typical of most clinical interactions with the aging CHD population.3 Given the cardiovascular health burden of T2DM, attention to its development in CHD survivors seems warranted.
Sources of Funding
All funding support was internal as provided by Cincinnati Children's Hospital Medical Center and the Department of Clinical Epidemiology, Aarhus, Denmark.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003076 doi: 10.1161/JAHA.115.003076)
References
- 1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2. Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL; American Heart Association Council on Cardiovascular Disease in the Young . Noninherited risk factors and congenital cardiovascular defects: current knowledge. A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. [DOI] [PubMed] [Google Scholar]
- 3. Moons P, Van Deyk K, Dedroog D, Troost E, Budts W. Eur J Cardiovasc Prev Rehabil. 2006;13:612–616. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . 2014 National Diabetes Statistics Report. Available at: http://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html. Accessed March 21, 2015.
- 5. Thomsen RW, Sørensen HT. Using registries to identify type 2 diabetes patients. Clin Epidemiol. 2014;7:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 7. Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;11:CD008143. [DOI] [PubMed] [Google Scholar]
- 8. Dellborg M, Björk A, Pirouzi Fard MN, Ambring A, Eriksson P, Svensson AM, Gudbjörnsdottir S. High mortality and morbidity among adults with congenital heart disease and type 2 diabetes. Scand Cardiovasc J. 2015;49:344–350. [DOI] [PubMed] [Google Scholar]
- 9. Chung ST, Hong B, Patterson L, Petit CJ, Ham JN. High overweight and obesity in Fontan patients: a 20‐year history. Pediatr Cardiol. 2016;37:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbiero SM, D'Azevedo Sica C, Schuh DS, Cesa CC, de Oliveira Petkowicz R, Pellanda LC. Overweight and obesity in children with congenital heart disease: combination of risks for the future? BMC Pediatr. 2014;14:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stone N, Obeid J, Dillenburg R, Milenkovic J, Macdonald MJ, Timmons BW. Objectively measured physical activity levels of young children with congenital heart disease. Cardiol Young. 2015;25:520–525. [DOI] [PubMed] [Google Scholar]
- 12. Longmuir PE, Brothers JA, de Ferranti SD, Hayman LL, Van Hare GF, Matherne GP, Davis CK, Joy EA, McCrindle BW; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young . Promotion of physical activity for children and adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;127:2147–2159. [DOI] [PubMed] [Google Scholar]
- 13. Madsen NL, Schwartz SM, Lewin MB, Mueller BA. Prepregnancy body mass index and congenital heart defects among offspring: a population‐based study. Congenit Heart Dis. 2013;8:131–141. [DOI] [PubMed] [Google Scholar]
- 14. Sherwani SI, Aldana C, Usmani S, Adin C, Kotha S, Khan M, Eubank T, Scherer PE, Parinandi N, Magalang UJ. Intermittent hypoxia exacerbates pancreatic β‐cell dysfunction in a mouse model of diabetes mellitus. Sleep. 2013;36:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato Y, Inoue M, Yoshizawa T, Yamagata K. Moderate hypoxia induces β‐cell dysfunction with HIF‐1‐independent gene expression changes. PLoS One. 2014;9:e114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, Born J, Fehm HL, Peters A. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med. 2004;169:1231–1237. [DOI] [PubMed] [Google Scholar]
- 18. Hjalmarsen A, Aasebo U, Birkeland K, Sager G, Jorde R. Impaired glucose tolerance in patients with chronic hypoxic pulmonary disease. Diabetes Metab. 1996;22:37–42. [PubMed] [Google Scholar]
- 19. Cohen MS. Clinical practice: the effect of obesity in children with congenital heart disease. Eur J Pediatr. 2012;171:1145–1150. [DOI] [PubMed] [Google Scholar]
- 20. Ohuchi H, Miyamoto Y, Yamamoto M, Ishihara H, Takata H, Miyazaki A, Yamada O, Yagihara T. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30–39. [DOI] [PubMed] [Google Scholar]
- 21. Laursen HB. Some epidemiological aspects of congenital heart disease in Denmark. Acta Paediatr Scand. 1980;69:619–624. [DOI] [PubMed] [Google Scholar]
- 22. Olsen M, Videbæk J, Johnsen SP; Danish Register of Congenital Heart Disease . The Danish register of congenital heart disease. Scand J Public Health. 2011;39:50–53. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 24. Olsen M, Garne E, Sværke C, Søndergaard L, Nissen H, Andersen HØ, Hjortdal VE, Johnsen SP, Videbæk J. Cancer risk among patients with congenital heart defects: a nationwide follow‐up study. Cardiol Young. 2014;24:40–46. [DOI] [PubMed] [Google Scholar]
- 25. Thomsen RW, Hundborg HH, Lervang HH, Johnsen SP, Schønheyder HC, Sørensen HT. Risk of community‐acquired pneumococcal bacteremia in patients with diabetes: a population‐based case‐control study. Diabetes Care. 2004;27:1143–1147. [DOI] [PubMed] [Google Scholar]
- 26. Knudsen LB, Olsen J. The Danish medical birth registry. Dan Med Bull. 1998;45:320–323. [PubMed] [Google Scholar]
- 27. Olsen M, Sørensen HT, Hjortdal VE, Christensen TD, Pedersen L. Congenital heart defects and developmental and other psychiatric disorders: a Danish nationwide cohort study. Circulation. 2011;124:1706–1712. [DOI] [PubMed] [Google Scholar]
- 28. European Surveillance of Congenital Anomalies . Guide 1.3: instructions for the registration and surveillance of congenital anomalies. 2009. Available at: http://www.eurocat-network.eu/content/EUROCAT-Guide-1.3.pdf. Accessed September 10, 2014.
- 29. Ohuchi H, Yasuda K, Ono S, Hayama Y, Negishi J, Noritake K, Mizuno M, Iwasa T, Miyazaki A, Yamada O. Low fasting plasma glucose level predicts morbidity and mortality in symptomatic adults with congenital heart disease. Int J Cardiol. 2014;174:306–312. [DOI] [PubMed] [Google Scholar]
- 30. Pinto NM, Marino BS, Wernovsky G, de Ferranti SD, Walsh AZ, Laronde M, Hyland K, Dunn SO Jr, Cohen MS. Obesity is a common comorbidity in children with congenital and acquired heart disease. Pediatrics. 2007;120:e1157–e1164. [DOI] [PubMed] [Google Scholar]
- 31. Jepsen B, Jepsen P, Johnsen SP, Espersen GT, Sorensen HT. Validity of diagnoses of cardiac malformations in a Danish population‐based hospital‐discharge registry. Int J Risk Saf. 2006;18:77–81. [Google Scholar]
- 32. Lass E, Lelong N, Thieulin AC, Houyel L, Bonnet D, Ancel PY, Kayem G, Goffinet F, Khoshnood B; on behalf of the EPICARD Study Group . Preterm birth and congenital heart defects: a population‐based study. Pediatrics. 2012;130:e829–e837. [DOI] [PubMed] [Google Scholar]


