Abstract
Background
Critical limb ischemia (CLI) is increasing in prevalence, and remains a significant source of mortality and limb loss. The decision to recommend surgical or endovascular revascularization for patients who are candidates for both varies significantly among providers and is driven more by individual preference than scientific evidence.
Methods and Results
The Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST‐CLI) Trial is a prospective, randomized, multidisciplinary, controlled, superiority trial designed to compare treatment efficacy, functional outcomes, quality of life, and cost in patients undergoing best endovascular or best open surgical revascularization. Approximately 140 clinical sites in the United States and Canada will enroll 2100 patients with CLI who are candidates for both treatment options. A pragmatic trial design requires consensus on patient eligibility by at least 2 investigators, but leaves the choice of specific procedural strategy within the assigned revascularization approach to the individual treating investigator. Patients with suitable single‐segment of saphenous vein available for potential bypass will be randomized within Cohort 1 (n=1620), while patients without will be randomized within Cohort 2 (n=480). The primary efficacy end point of the trial is Major Adverse Limb Event–Free Survival. Key secondary end points include Re‐intervention and Amputation‐Free‐Survival and Amputation Free‐Survival.
Conclusions
The BEST‐CLI trial is the first randomized controlled trial comparing endovascular therapy to open surgical bypass in patients with CLI to be carried out in North America. This landmark comparative effectiveness trial aims to provide Level I data to clarify the appropriate role for both treatment strategies and help define an evidence‐based standard of care for this challenging patient population.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT02060630.
Keywords: atherosclerosis, cost‐effectiveness, critical limb ischemia, endovascular, outcome, quality, stent treatment, surgery
Subject Categories: Clinical Studies, Peripheral Vascular Disease, Quality and Outcomes, Cardiovascular Surgery, Revascularization
Peripheral artery disease (PAD) affects 3% to 10% of all Americans and 15% to 20% of persons 70 years of age and older. PAD is particularly common among individuals who smoke or have diabetes mellitus.1, 2 A subset of patients with PAD have critical limb ischemia (CLI), which is characterized by varying degrees of foot or ankle pain at rest and/or the presence of ischemic ulcerations or necrotic tissue. The incidence of CLI in the United States is estimated to be between 500 and 1000 per million per year. Given the aging of the American population, the global increase in metabolic syndrome, and the ongoing impact of diabetes mellitus and tobacco use, the prevalence of both PAD and CLI is predicted to further increase.1 In addition, management of CLI has substantial healthcare and societal costs,3, 4 and these are expected to grow given current demographic, disease, and economic trends.1
CLI is associated with significant disability, morbidity, and mortality. In the absence of successful revascularization, 20% to 40% of patients will require amputation and over 20% will die within 6 months.1, 2, 5 In a recently published large German registry, CLI with tissue loss was associated with 4‐year amputation rates of 35% to 67% and mortality rates ranging from 52% to 64%.6
Because of the absence of medical therapy effective for salvage of threatened legs, CLI is typically treated with revascularization to improve limb perfusion distal to the zone of arterial stenosis or occlusion. Open surgical bypass has historically been the standard of care for patients with infrainguinal PAD and is associated with excellent limb salvage rates and clinical durability.7 Outcomes of surgical bypass are significantly affected by the quality of the conduit utilized,8, 9 the severity of ischemia at presentation,10 and the extent of infrainguinal arterial obstruction.10
Over the last 2 decades, the widespread adoption of endovascular techniques has led to a sharp increase in their application to patients with CLI11, 12 and there are numerous reports of excellent limb salvages rates.13, 14 In comparison to surgical bypass, endovascular therapy is associated with decreased periprocedural morbidity and mortality. However, questions about durability, cost, and appropriate case selection have not been rigorously answered.15, 16
At present, a variety of practitioners, including interventional cardiologists, vascular medicine specialists, interventional radiologists, and vascular surgeons, provide treatment for CLI.17 The decision to recommend surgical or endovascular revascularization varies significantly among providers and institutions and appears to be based on such factors as disease pattern, the availability of autogenous conduit, physician training and experience, surgical and endovascular skill sets, access to an appropriate procedural environment, and perhaps most importantly, treatment bias.15, 18, 19, 20 This lack of treatment uniformity is highlighted by the marked degree to which the primary treatment of CLI varies within the Society of Vascular Surgery (SVS) Vascular Quality Initiative, as illustrated in Figure 1.21 There is general agreement that some patients considered poor candidates for surgery benefit from endovascular revascularization.22, 23 What remains unknown is which therapy is most appropriate for patients who are candidates for both open and endovascular treatment. This uncertainty also has economic implications, potentially leading to suboptimal allocation of valuable healthcare resources.15
Figure 1.
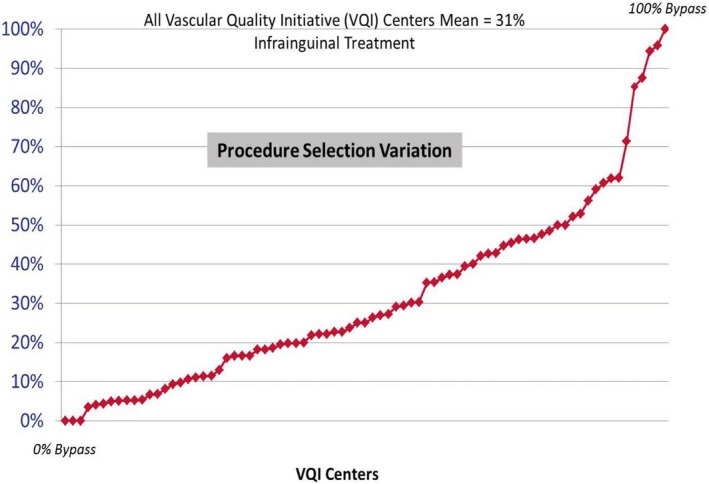
Critical limb ischemia: % treated by bypass (vs Endovascular).21
The lack of consensus underlying the current therapeutic approach to CLI directly stems from insufficient high‐quality data upon which to base treatment decisions. Although there are many studies evaluating management strategies for CLI, most have limitations arising from their use of retrospective data with incomplete control for potential confounders, sponsor and operator bias, inclusion of claudicants, and short or incomplete follow‐up.24, 25 To date, there is only 1 prospective randomized trial comparing endovascular techniques with surgical revascularization for limb ischemia, the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial.26, 27 Endovascular treatment in BASIL was limited to percutaneous transluminal angioplasty alone, as was standard practice in the United Kingdom where the trial was undertaken; as such, the applicability of the trial result to current practice in North America, which typically includes the use of stents, atherectomy, and drug‐coated balloons, has been questioned.15, 16 The choice of amputation‐free survival (AFS) as the primary end point in the BASIL trial has also been criticized, as it overemphasizes non‐treatment‐related mortality and underemphasizes limb‐related events specifically attributable to treatment modality.15
The BEST‐CLI (Best Endovascular versus Best Surgical Therapy in patients with Critical Limb Ischemia) trial was conceived to provide level I comparative‐effectiveness evidence to help guide treatment decisions in the management of CLI.28, 29 The trial will furnish contemporary information about therapeutic outcomes in patients with CLI who are candidates for either surgical bypass or endovascular therapy. It will also provide high‐quality cost‐effectiveness data about surgical and endovascular approaches to treating CLI, and the impact of those treatments on the quality of life (QoL) and overall function of the study population.
Materials and Methods
Study Plan and Patient Population
The BEST‐CLI Trial is a prospective, randomized, open label, 2‐arm, multicenter, multidisciplinary, controlled, superiority trial designed to compare treatment efficacy, functional outcomes, QoL, and cost in patients undergoing best endovascular or best open surgical revascularization for CLI. The choice of best therapy within the assigned treatment approach is left to the individual investigator. The planned enrollment is 2100 patients from ≈140 sites in the United States and Canada. Each site must have at least 1 investigator meeting BEST‐CLI credentialing criteria to perform open surgery, and at least 1 investigator meeting BEST‐CLI credentialing criteria to perform endovascular treatment. The BEST‐CLI trial began enrolling patients in August 2014 and has a planned total study duration of 50 months.
The trial encompasses 2 independently powered randomized cohorts. The first cohort (1620 subjects) consists of patients who are believed to have adequate single‐segment great saphenous vein available as a conduit for bypass; the second cohort (480 subjects) includes patients who do not have an adequate single‐segment great saphenous vein. Patients in the latter cohort who are randomized to surgical revascularization will be treated with surgical bypass using an arm vein, short saphenous vein, cryopreserved vein, prosthetic conduit, or composite conduit. Within each cohort, randomization will be stratified according to (1) clinical presentation, defined by presence of ischemic rest pain alone (Rutherford 4) versus tissue loss (Rutherford 5 and 6) with or without ischemic rest pain and (2) anatomic status, defined by the presence or absence of significant tibial disease. Randomization is accomplished using computer‐generated permuted blocks within each of the 8 combinations of cohort and stratum.
The trial is being conducted in accordance with the Declaration of Helsinki ethical standards30 and with adherence to the rules and regulations of the Institutional Review Board at each participating institution. All subjects are required to provide written informed consent using an Institutional Review Board–approved consent form. Research reported in this publication is supported by the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health under Award Numbers U01HL107352, U01HL107407, and U01HL115662 (clinicaltrials.gov NCT02060630). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. An Investigational Device Exemption was granted by the Food and Drug Administration to allow investigator use of Food and Drug Administration–approved devices in an off‐label fashion consistent with routine practice and current standards of care.
Inclusion and Exclusion Criteria
Patients appropriate for the BEST‐CLI trial must satisfy all of the inclusion and exclusion criteria and be considered suitable candidates for both endovascular and open surgical revascularization by at least 2 investigators at the participating trial site. At least 1 of the 2 investigators must be credentialed to perform endovascular revascularization and the other must be credentialed to perform open surgical bypass. One year after initiation of the trial, several modifications to the inclusion and exclusion criteria were approved by the Data and Safety Monitoring Board (DSMB). The changes incorporated investigator feedback and served either to clarify confusing elements of the criteria or to enhance enrollment while preserving the core objectives and aims of the trial. Table 1 compares the original and modified inclusion and exclusion criteria. Enrolled patients must have adequate aortoiliac inflow, as defined in Table 2, and appropriate proximal and distal anastomotic targets for a surgical bypass. The definition of CLI10 is listed in Table 3.
Table 1.
Inclusion and Exclusion Criteria (Prior to and After Modification of Protocol)
| Eligibility Criteria Prior to Modification | Eligibility Criteria After Modification |
|---|---|
| Inclusion criteria (all must be met for eligibility) | |
| Male or female, age 35 years or older | Male or female, age 18 years or older |
| Atherosclerotic, infrainguinal PAD (occlusive disease of the arteries below the inguinal ligament caused by atherosclerosis) | Infrainguinal PAD (occlusive disease of the arteries below the inguinal ligament) |
| CLI, defined as arterial insufficiency with gangrene, nonhealing ischemic ulcer, or rest pain consistent with Rutherford categories 4 to 6 | No change |
| Candidate for both endovascular and open infrainguinal revascularization as judged by the treating investigators (see MOO for guidelines on decision‐making) | No change |
| Adequate aortoiliac inflow | No change |
| Adequate popliteal, tibial, or pedal revascularization target defined as an infrainguinal arterial segment distal to the area of stenosis/occlusion that can support a distal anastomosis of a surgical bypass | No change |
| Willingness to comply with protocol, attend follow‐up appointments, complete all study assessments, and provide written informed consent | No change |
| Exclusion criteria (none can be met for eligibility) | |
| Disease limited to the femoropopliteal segment with TASC II A pattern | Deleted |
| Presence of severe (>50% stenosis) ipsilateral common femoral artery disease | Deleted |
| Presence of a popliteal aneurysm (>2 cm) in the index limb | No change |
| Life expectancy of less than 2 years due to reasons other than PAD | Life expectancy of less than 2 years due to reasons other than PAD |
| Considered to be at excessive risk for surgical bypass (as determined by the operating surgeon and the CLI Team following preoperative cardiac risk assessment) | Excessive risk for surgical bypass (as determined by the operating surgeon and the CLI Team) |
| Planned above‐ankle amputation on ipsilateral limb within 4 weeks of index procedure | No change |
| Known anti‐phospholipid antibodies or lupus anticoagulant; or known Protein C deficiency, Protein S deficiency, or Antithrombin III deficiency if treated or advised to be treated with long‐term anticoagulation on the basis of this diagnosis | Deleted |
| Nonatherosclerotic occlusive disease (eg, embolic disease, trauma, vasculitis, Buerger's disease) or acute limb‐threatening ischemia (defined as tissue loss or ischemic rest pain of less than 14 days duration) | Active vasculitis, Buerger's disease, or acute limb‐threatening ischemia |
| Any prior index limb infrainguinal stenting or stent grafting associated with significant restenosis | Any prior index limb infrainguinal stenting or stent grafting associated with significant restenosis within 1 cm of the stent or stent‐graft, unless the occlusion/restenosis site is outside the intended treatment zone (ie, a tibial vessel that is not currently intended to be revascularized as a part of the treatment for CLI) |
Any of the following procedures performed on the index limb within 6 months prior to enrollment:
|
Any of the following procedures performed on the index limb within 3 months prior to enrollment:
|
| Current immune‐suppressive medication, chemotherapy, or radiation therapy | Current chemotherapy or radiation therapy |
| Absolute contraindication to iodinated contrast due to prior near‐fatal anaphylactoid reaction (laryngospasm, bronchospasm, cardiorespiratory collapse, or equivalent) that would preclude patient participation in angiographic procedures | No change |
| Known allergy to stainless steel or nitinol | Deleted |
| Pregnancy or lactation | No change |
| Administration of an investigational drug for PAD within 30 days of randomization | No change |
| Participation in a clinical trial (except observational studies) within the previous 30 days | No change |
| Prior enrollment or randomization into BEST‐CLI | No change |
CLI indicates critical limb ischemia; MOO, manual of operations; PAD, peripheral artery disease; TASC, TransAtlantic Inter‐Society Consensus.
Table 2.
Definition of Adequate Aortoiliac Inflow
A patient will have adequate aortoiliac flow when the following criteria are met:
|
CLI indicates critical limb ischemia.
Table 3.
Definition of Critical Limb Ischemia (CLI)10
The target population is defined as patients with CLI, which is defined as arterial insufficiency with gangrene, a nonhealing ischemic ulcer, or rest pain, corroborated by at least 1 of the following hemodynamic criteria:
|
Randomization
The patient flow diagram for the BEST‐CLI trial is depicted in Figure 2. Subjects meeting all of the inclusion and none of the exclusion criteria are randomized within the outlined schema based on the results of a contrast angiogram of sufficient quality performed within the preceding 3 months. Randomization can alternatively be based on a magnetic resonance angiogram or computed tomographic angiogram of sufficient quality performed within the preceding 3 months, with the caveat that the infrapopliteal arteries are free of significant disease. If the infrapopliteal arteries are involved to such a degree that they would require treatment as part of an open or endovascular revascularization, then an additional contrast angiogram is required. If no recent and sufficient contrast angiogram, magnetic resonance angiogram, or computed tomographic angiogram is available, a diagnostic angiogram must be obtained prior to randomization to confirm trial eligibility.
Figure 2.
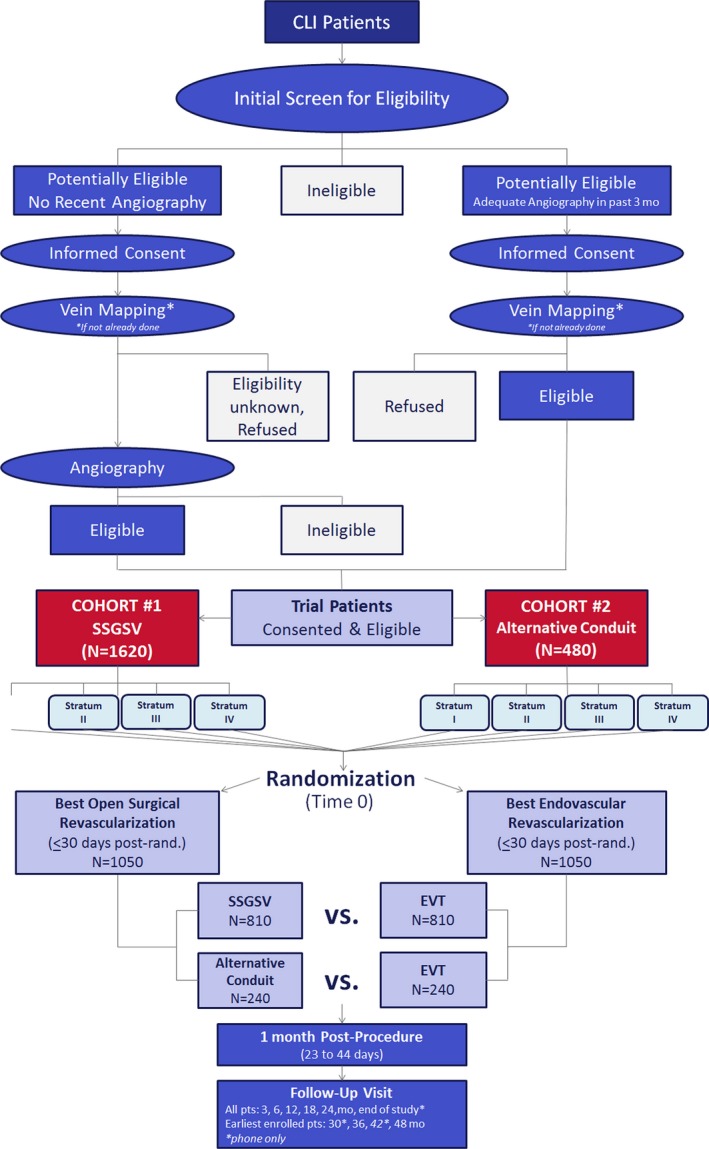
Patient flow chart. Stratum I: Ischemic Rest Pain (Rutherford Category 4) AND infrainguinal PAD without significant infrapopliteal occlusive disease; Stratum II: Tissue loss with or without ischemic rest pain (Rutherford category 5, 6) AND Infrainguinal PAD without significant infrapopliteal occlusive disease; Stratum III: Ischemic Rest Pain (Rutherford Category 4) AND Infrainguinal PAD with significant infrapopliteal occlusive disease; Stratum IV: Tissue loss with or without ischemic rest pain (Rutherford category 5, 6) AND Infrainguinal PAD with significant infrapopliteal occlusive disease. CLI indicates critical limb ischemia; EVT, endovascular therapy; PAD, peripheral artery disease; SSSGSV, single‐segment great saphenous vein.
Duplex ultrasound‐based mapping of potential autogenous venous conduit is required prior to randomization to assign subjects into either Cohort 1 (adequate single‐segment greater saphenous vein) or Cohort 2 (absence of adequate‐single segment greater saphenous vein). Subjects entered into Cohort 1 are anticipated, based on preprocedure assessment of the saphenous vein status, to have a single segment of ipsilateral or contralateral greater saphenous vein of sufficient length to perform an open surgical bypass at the time of randomization. Conversely, subjects in Cohort 2 are believed not to have a single segment of saphenous vein sufficient for bypass surgery and are anticipated to require revascularization with either arm vein, short saphenous vein, spliced vein, cryopreserved vein, prosthetic conduit, or some combination of the above should they be randomized to surgical bypass. Subjects enrolled in Cohort 1 who are subsequently found to have saphenous vein that is insufficient in length or quality to perform a single‐segment bypass and who instead undergo bypass with an alternative conduit will be analyzed on an intention‐to‐treat basis in their originally designated cohort. Similarly, any subject enrolled into Cohort 2 and subsequently found at the time of bypass surgery to have a sufficient single segment of saphenous vein will remain in Cohort 2 for the intention‐to‐treat analysis.
Investigators have the option of randomizing and treating a subject immediately following confirmatory diagnostic angiography, or waiting until a later time to randomize. The assigned treatment must be undertaken within 30 days of randomization.
Study Procedures and Follow‐Up
For BEST‐CLI trial subjects with associated aortoiliac occlusive disease, concomitant treatment of the aortoiliac disease at the time of the index infrainguinal revascularization is allowed for patients with ischemic tissue loss. Patients presenting with ischemic rest pain as their sole manifestation of CLI, in contrast, must first undergo treatment of the aortoiliac segment to correct the suprainguinal inflow. Randomization of such subjects is subsequently allowed if they have persistent rest pain symptoms and continue to meet the hemodynamic definition of CLI on repeat assessment.
Since the BEST‐CLI trial aims to compare endovascular versus open approaches, hybrid procedures combining elements of both endovascular and open surgical treatment of infrainguinal disease were initially prohibited. Following modifications to the BEST‐CLI trial protocol approved by the DSMB in August 2015 (Table 1), investigators are allowed to combine surgical endarterectomy of the common femoral artery with postrandomization endovascular treatment of more distal disease. For patients with rest pain who undergo either open surgical or endovascular treatment of common femoral disease, a similar requirement to confirm persistence of rest pain symptoms and continued hemodynamic criteria of CLI after treatment of the common femoral artery and prior to randomization into the trial remains in place.
Although discouraged, unplanned staged revascularization following randomization to endovascular treatment is allowed if considered necessary (eg, in the event of patient intolerance of conscious sedation, concern for contrast dye load or radiation exposure) for up to 4 days following the initial endovascular effort, provided that the initial treatment rendered is well documented and the treatment plan for the subsequent stage is clearly delineated. Retreatment of lesions treated in the initial stage will be considered reinterventions or treatment failures.
The outline of postrevascularization follow‐up visits and the parameters to be measured is listed in Table 4.
Table 4.
Schedule of Measurements
| Base | Proc | 1M | 3M | 6M | 12M | 18M | 24M | 30M | 36M | 42M | 48M | EOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In‐person clinical evaluation | X | X | X | X | X | X | X | X | X | ||||
| Telephone clinical evaluation | X | X | X | ||||||||||
| Informed consent | X | ||||||||||||
| Angiogram; or CTA or MRA | X | ||||||||||||
| Medical history | X | ||||||||||||
| Physical examination | X | X | X | X | X | X | X | X | X | ||||
| Focused peripheral vascular history | X | X | X | X | X | X | X | X | X | ||||
| Duplex vein mapping | X | ||||||||||||
| Medications | X | X | X | X | X | X | X | X | X | X | X | X | |
| Assessment of CLI symptoms | X | X | X | X | X | X | X | X | X | X | X | X | |
| Pain scale (NRS) | X | X | X | X | X | X | X | ||||||
| Hemodynamic assessment | X | X | X | X | X | X | X | X | X | ||||
| Renal function | X | X | X | X | X | ||||||||
| Quality of Life (VascuQoL, SF‐12, EQ‐5D) | X | X | X | X | X | X | X | ||||||
| Health resources utilization | X | X | X | X | X | X | X | X | |||||
| Six‐minute Walk Test (subset) | X | X | |||||||||||
| Vital status | X | X | X | X | X | X | X | X | X | ||||
| Arterial duplex (if standard of care) | X | X | X | X | X | X | X | X | |||||
| AE/SAEs | Continuous | ||||||||||||
AE indicates adverse event; CLI, critical limb ischemia; CTA, computed tomographic angiogram; EOS, end of study; MRA, magnetic resonance angiogram; NRS, Numerical Rating Scale; Proc, procedure; SAEs, serious adverse events.
Primary and Secondary End Points
The primary efficacy end point in the BEST‐CLI trial is Major Adverse Limb Event (MALE)–free survival (Table 5). This aggregate measure best captures the therapeutic goals of treatment for CLI, which include preservation of a functional limb and avoidance of major reinterventions that significantly impact QoL. Accurately assessing limb‐related morbidity and procedure‐related need for reintervention is of paramount importance in a trial comparing revascularization strategies, particularly in light of remaining questions regarding treatment durability. The MALE end point was devised by the SVS Objective Performance Goals Working Group specifically for use in clinical trials involving CLI patients.10 This end point has been endorsed by both the SVS and the Food and Drug Administration.10, 15, 31 MALE captures all major repeat vascular procedures on the index limb, including above‐ankle amputation and major reinterventions, defined by the creation of a new bypass graft, a jump/interposition graft revision, surgical thrombectomy with or without surgical patch angioplasty, and thrombectomy of an occluded graft or arterial segment using pharmacologic or mechanical thrombolysis. Notably, MALE excludes minor reinterventions, defined as surgical patch angioplasty (without graft thrombectomy), percutaneous transluminal angioplasty, atherectomy, laser treatment and/or stenting, or stent/grafting via either an open surgical exposure or percutaneous approaches, which are presumed to have less clinical impact. MALE‐free survival is felt to be superior as a primary efficacy end point to the historic standard of AFS, given that the primary goal of limb‐directed therapies for CLI is not to prolong survival but rather to relieve ischemic rest pain and heal leg‐threatening tissue loss. The failure of AFS to capture major treatment‐related reinterventions, and their direct impact on QoL and cost, significantly limits its utility in a trial designed to compare effectiveness of revascularization strategies. Nonetheless, because it combines 2 penultimate events of greatest magnitude for the CLI patient, AFS is included as a key secondary end point. Reintervention and amputation‐free survival is an additional important secondary end point that is defined as survival free from above‐ankle amputation of the index limb or major or minor reintervention. The BEST‐CLI trial will also examine MALE‐Peri‐Operative Death, which includes 30‐day perioperative mortality but excludes longer‐term mortality that is less likely related to the treatment of the limb.
Table 5.
All End Points (Efficacy, Safety, Functional, Cost Effectiveness) With Definitions
| End Point | Definition |
|---|---|
| Primary efficacy end point | |
| Major adverse limb event (MALE)‐free survival | MALE is defined as above ankle amputation of the index limb or major reintervention (new bypass graft, jump/interposition graft revision or thrombectomy/thrombolysis) |
| Secondary end points | |
| Clinical | |
| Reintervention and amputation‐free survival (RAFS) | RAFS is defined as freedom from death, above‐ankle amputation and both major reintervention (eg, new bypass graft, jump/interposition graft revision, or thrombectomy/thrombolysis) and minor reintervention (surgical patch angioplasty [without graft thrombectomy], balloon angioplasty, atherectomy, laser treatment and/or stenting or stent/grafting via either an open surgical exposure [ie, a “hybrid” approach] or percutaneous approach) |
| Freedom from MALE‐POD | MALE‐POD is defined as composite (earliest occurring) of MALE and POD (death within 30 days of index open or endovascular revascularization) |
| Amputation‐free survival | Freedom from death or above‐ankle amputation |
| Freedom from POD | POD is defined as death within 30 days of index open or endovascular revascularization |
| Freedom from myocardial infarction (MI) | MI is defined as evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia |
| Freedom from stroke | Stroke is defined as a neurological deficit of cerebrovascular cause that persists beyond 24 h; is interrupted by thrombolytic therapy, stroke intervention, or death within 24 h; or is confirmed by imaging despite resolution of symptoms within 24 h |
| Freedom from reinterventions (major and minor) in index leg |
Reintervention is defined as:
|
| Number of reinterventions (major and minor) per limb salvaged | Not further defined |
| Freedom from hemodynamic failure |
Hemodynamic failure is defined as the occurrence of 1 or more of the following events:
|
| Freedom from clinical failure | Clinical failure is defined as death, MALE, nonhealing of index limb wound (either the original wound or the surgical wound from a minor index limb amputation to treat tissue loss), worsening of Rutherford category, recurrence of index limb wound, or recurrence of ischemic rest pain that resolved completely after initial revascularization |
| Freedom from CLI | Freedom from CLI is defined as survival to that time point without index limb major amputation, AND without wound or ischemic rest pain at the time of the visit |
| Freedom from all‐cause mortality | Not further defined |
| Functional | |
| Quality of life (QoL) and functional assessments using the VascuQoL and EuroQoL EQ‐5D. The SF‐12, Numerical Rating Scale (NRS) for pain, and at selected sites, the Six‐minute Walk Test will be performed to supplement the main QoL measurements | Not further defined |
| Costs and cost‐effectiveness | |
| Treatment‐associated costs (in‐ and outpatient) and incremental cost‐effectiveness measured in dollars per quality‐adjusted life years | Not further defined |
| Safety | |
| Serious Adverse Events (SAEs) from randomization through 30 days postprocedure | A SAE is defined by regulation as any adverse event (as defined above) that results in 1 or more of the following:
|
| SAEs from randomization throughout remaining time on study | See SAE definition above |
| Major Adverse Cardiovascular Events (MACE) from randomization through 30 days postprocedure | MACE is a composite of all‐cause death, myocardial infarction, and stroke |
| MACE from randomization throughout remaining time on study | See MACE definition above |
| Nonserious adverse events (AEs) from randomization through 30 days postprocedure | An AE is defined as any unfavorable medical occurrence experienced by a subject during participation in the trial. An adverse event may be a disease, a set of related symptoms or signs, or single symptom or sign (including an abnormal laboratory finding). Note: an AE is not a procedure (eg, PTA), action (eg, hospitalization), or outcome (eg, death). AEs are divided into 2 categories: (1) nonserious AEs and (2) serious AEs (SAEs) |
| Perioperative complications |
Surgical complications are inclusive of the following:
|
Endovascular complications are inclusive of the following:
| |
Systemic complications associated with surgical or endovascular revascularization are inclusive of the following:
| |
CLI indicates critical limb ischemia; PTA, percutaneous transluminal angioplasty.
Table 5 lists the definitions of the major and key secondary end points, as well as those of additional secondary and safety end points. Freedom from clinical failure, freedom from CLI, and freedom from hemodynamic failure are particularly important end points that evaluate the end result of enhanced limb perfusion and the sustained hemodynamic impact of the treatment received in a way that has rarely been done in other PAD trials to date. Functional and QoL secondary end point assessments include the Numerical Rating Scale for pain,32 Vascular Quality of Life Questionnaire (VascuQoL),33 EuroQoL five dimensions questionnaire (EQ‐5D),34, 35 and 12‐Item Short‐Form Health Survey (SF‐12).36 At a subset of sites, the 6‐minute walk test will also be performed.37 Treatment‐associated costs (in‐ and outpatient) and incremental cost‐effectiveness measured in dollars per quality‐adjusted life year will also be measured.
Trial Organization and Oversight
Figure 3 details the overall structural organization of the BEST‐CLI trial and the specific interrelationships between the Clinical Coordinating Center, the Data Coordinating Center, the Cost‐Effectiveness Core, Data and Safety Monitoring Board (DSMB), the National Heart, Lung and Blood Institute study sponsor, and the participating trial sites. The Operations Committee comprises the Principal Investigators of the Clinical Coordinating Center, Data Coordinating Center and Cost‐Effectiveness Core, as well as the Program Officers of the National Heart, Lung and Blood Institute, and is responsible for the day‐to‐day conduct of the trial. The Executive Committee (EC) comprises members of the Operations Committee, in addition to leading experts in the disciplines of vascular medicine, interventional radiology, interventional cardiology, and vascular surgery (Table 6). The balance of subspecialty membership of the EC reflects the mix of clinical disciplines currently managing patients with CLI within the United States and Canada. The EC meets in person or by phone on a monthly basis and serves in both an advisory and an oversight role. An independent Protocol Review Committee of individuals with appropriate expertise was appointed by the National Heart, Lung and Blood Institute and was responsible for approval of the initial BEST‐CLI trial Protocol. Subsequently, a DSMB was constituted in part from members of the Protocol Review Committee. The DSMB meets semiannually over the course of the trial and has ongoing responsibility for the overall safe conduct of the trial, as well as approval of any protocol amendments and ancillary studies.
Figure 3.
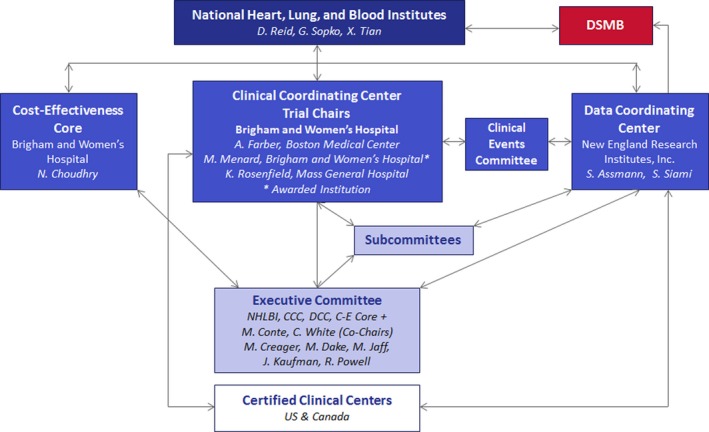
BEST‐CLI trial organizational chart. BEST‐CLI indicates Best Endovascular versus Best Surgical Therapy in patients with Critical Limb Ischemia trial.
Table 6.
Specialty Members of the Executive Committee (EC)
| EC Member | Specialty | Institution | City | State |
|---|---|---|---|---|
|
Michael Conte, MD Co‐Chair |
Vascular Surgery | University of California San Francisco Medical Center | San Francisco | CA |
|
Christopher White, MD Co‐Chair |
Interventional Cardiology | Ochsner Clinic Foundation | New Orleans | LA |
| Mark Creager, MD | Interventional Cardiology | Dartmouth‐Hitchcock Medical Center | Lebanon | NH |
| Michael Dake, MD | Interventional Radiology | Stanford Hospital | Palo Alto | CA |
| Michael Jaff, DO | Vascular Medicine | Massachusetts General Hospital | Boston | MA |
| John Kaufman, MD | Interventional Radiology | Oregon Health and Science University | Portland | OR |
| Richard Powell, MD | Vascular Surgery | Dartmouth‐Hitchcock Medical Center | Lebanon | NH |
The Executive Committee is constituted by the specialty members above, the trial Principal Investigators at the Clinical Coordinating Center, Data Coordinating Center and Cost‐Effectiveness Core and a Program Official of the National Heart, Lung and Blood Institute.
A multidisciplinary Clinical Events Classification Committee, comprised of board‐certified physicians with appropriate specialist training, will review and adjudicate all first major reinterventions on the index limb, as well as all myocardial infarctions and strokes. Identification of suspected events will arise from review of each patient's electronic case report forms and collected source documents. Clinical Events Classification Committee members will be blinded to study treatment assignment, and event adjudication will be undertaken in a manner independent of the BEST‐CLI trial EC, Operations Committee, and all participating site investigators. Two board‐certified physician specialists will serve as Independent Medical Reviewers, and review source documents for a random sample of site‐reported minor reinterventions, to confirm that they were not misidentified major reinterventions.
Trial sites currently participating in the BEST‐CLI trial are listed in Appendix A and the geographic distribution of sites is depicted in Figure 4.
Figure 4.
Site map.
Subcommittees
There are 7 BEST‐CLI Trial subcommittees (the chairs of which are listed in Appendix B). These include the following:
The Surgical and Interventional Management Committee is charged with credentialing each of the participating investigators, within the context of the trial, to perform endovascular revascularization, open surgical revascularization, or both. The predefined procedural credentialing criteria and conditional approval policy for investigators newly out of training are detailed in Appendix C. A minimum of 20% of participating sites have been or will be audited by the Surgical and Interventional Management Committee in an effort to ensure the integrity of the credentialing process.
The Ancillary Studies Committee is charged with reviewing potential ancillary studies with a view to granting or denying formal connection to and endorsement from the BEST‐CLI trial.
The Recruitment and Retention Committee is charged with optimizing subject enrollment and maintaining subject retention.
The Evolving Technology Committee is charged with critically evaluating new technologies as they become available and determining suitability for use in the trial.
The Optimal Medical Therapy Committee is charged with defining optimal medical therapy for patients with CLI and ensuring that patients enrolled in the trial are receiving this therapy.
The Conflict of Interest Committee is charged with adjudicating the potential conflicts of interest of the study leadership and all participating investigators.
The Publications and Presentation Committee is charged with reviewing all trial‐related content as it pertains to dissemination of scientific information emanating from the BEST‐CLI trial. This committee will prioritize queries of the database and act to ensure the scientific integrity of all trial‐related press, presentations, and publications.
Statistical Considerations
BEST‐CLI is designed as 2 separate concurrent trials (in Cohort 1 and in Cohort 2). There is no intent to pool the data between Cohorts, or to test whether the treatment group effects (if any) are similar between the 2 Cohorts. To avoid bias, particularly given that the BEST‐CLI trial by its nature cannot be a blinded study, all analyses will be on the basis of intention‐to‐treat unless otherwise specified. Per intention‐to‐treat, all participants will be analyzed in the Cohorts in which they were classified at the time of randomization, and all participants will be analyzed in the treatment groups to which they were randomly assigned, even if the other type of revascularization strategy was carried out instead or they did not undergo any revascularization procedure within the 30‐day window following randomization. There will also be an exploratory per‐protocol analysis, including only subjects who adhered to their randomly assigned treatment strategy. However, this analysis will be subject to bias, as the subjects who do not undergo their assigned procedure are unlikely to be a representative sample of all subjects assigned to that procedure. For each outcome, the null hypothesis is that there is no difference in that outcome between the 2 treatment groups. The alternative hypothesis is that there is a difference, in either direction, between the treatment groups; ie, the statistical tests will be 2‐sided.
In each cohort, the primary analysis of the primary outcome will be a Cox regression model of time to major revascularization of the index limb, above‐ankle amputation of the index limb, or death from any cause, whichever occurs first. Participants who do not experience a primary outcome event will be considered censored on the last date of study contact. The Cox model will be stratified by randomization stratum, which allows the baseline hazard function to differ between the 4 randomization strata, but assumes a common hazard ratio between endovascular versus open revascularization in all 4 strata. The Cox model will also be adjusted for the following prespecified list of baseline covariates, all of which are expected to be strong predictors of the primary outcome: end‐stage renal disease, diabetes mellitus, prior revascularization of the index leg, and smoking history.
Other time‐to‐event outcomes will be analyzed in a similar way, using covariate‐adjusted Cox models stratified for randomization stratum. Table 7 shows which covariates will be adjusted for in the models for each time‐to‐event outcome. Mixed‐model linear regression will be used to examine treatment effects for continuous outcomes such as the various QoL measures, both longitudinally and with contrasts of treatment differences at specific time points. A Wilcoxon rank sum test will be used to compare 6‐minute walk times from the Month 6 visit. Rates of procedure complications in the first 30 days after the index procedure will be compared using Poisson regression, and Fisher's exact test will be used to compare the proportion of subjects with at least 1 procedure complication. Rates of adverse events will also be compared using Poisson regression.
Table 7.
Covariates for Time‐to‐Event Analyses
| Outcome | Baseline Covariates for Cox Modelsa | |||
|---|---|---|---|---|
| End‐stage Renal Disease | Prior Revascularization of the Index Limb | Diabetes Mellitus | Smoking Status | |
| Primary outcome (death, above‐ankle amputation of the index limb, or major reintervention of the index limb, whichever occurs first) | X | X | X | X |
| All‐cause mortality | X | X | X | |
| Above‐ankle amputation of the index limb | X | X | X | X |
| Major reintervention of the index limb | X | X | X | |
| Minor reintervention of the index limb | X | X | X | |
| Myocardial infarction | X | X | X | |
| Stroke | X | X | ||
| Hemodynamic failure | X | X | X | X |
| Clinical failure | X | X | X | X |
| CLI | X | X | X | X |
CLI indicates critical limb ischemia.
These Cox models will also be stratified by randomization stratum.
The primary cost‐effectiveness analysis will use measures of costs and outcomes occurring during the trial period to estimate differences in incremental dollars per incremental quality‐adjusted life‐year. In addition, a Markov state‐transition model will be developed to estimate the cost‐effectiveness of each treatment strategy over a lifetime horizon. Costs will be estimated by multiplying the magnitude of resources each patient consumes by unit prices obtained from nationally and regionally representative data sources.38, 39
Subgroup analyses of the primary outcome will be carried out using Cox models, including an interaction term between treatment group and a participant characteristic known at baseline. In each Cohort, the following subgroup analyses have been prespecified:
Isolated femoral disease versus tibial disease
Rutherford category 4 (ischemic rest pain only) versus categories 5 and 6 (tissue loss)
Sex
Race (white versus black versus Asian versus all others)
Ethnicity (Hispanic versus non‐Hispanic)
Race/ethnicity (Hispanic versus black non‐Hispanic versus all others)
Age <80 years versus ≥80 years
Presence versus absence of diabetes mellitus
Presence versus absence of Wound, Ischemia, and foot Infection Grade 3 wound on index limb40
Presence versus absence of renal dysfunction
Absence of renal dysfunction versus non‐dialysis‐dependent renal dysfunction versus dialysis‐dependent renal dysfunction
The DSMB will monitor the trial to enhance participant safety and the scientific integrity of the study. Interim analyses of the primary outcome and all‐cause mortality will be performed, using stopping guidelines based on an α‐spending approach approximating O'Brien‐Fleming boundaries.41 The DSMB may recommend stopping the trial early in a particular cohort (or in both cohorts), based on the totality of evidence, including results of these analyses, adverse event rates in each treatment group, new information from outside the BEST‐CLI trial, and other considerations.
Table 8 shows the statistical power available in Cohort 1 to detect various true differences in several of the study outcomes at the 0.05 significance level, assuming the specified true event rates for each treatment group before any crossovers (treatment violations). Additional assumptions include the following: 5% premature cessation of study follow‐up, 2% of participants assigned to endovascular therapy receiving open surgery instead, 10% of participants assigned to open surgery receiving endovascular therapy instead, 24 months of accrual with a slow ramp‐up of enrollment rates at the start of the trial, 26 months of additional follow‐up after the end of the enrollment period, and 2% sample‐size inflation to take into account interim monitoring. Table 9 shows similar information for Cohort 2.
Table 8.
Power for Cohort 1
| Endpoint | 2.95‐Year Event Rate if All Subjects Receive the Assigned Treatment | Hazard Ratio | Δ | Powera | |
|---|---|---|---|---|---|
| Endovascular | Open Surgery | ||||
| MALE/death | 61.1% | 53.0% | 1.25 | 8.1% | 83% |
| MALE/death | 45.3% | 53.0% | 0.80 | 7.7% | 77% |
| MALE/death | 61.3% | 53.0% | 1.26 | 8.3% | 85% |
| MALE/death | 45.1% | 53.0% | 0.79 | 7.9% | 79% |
| MALE+POD | 50.4% | 41.0% | 1.33 | 9.4% | 92% |
| MALE+POD | 32.7% | 41.0% | 0.75 | 8.3% | 85% |
| Amputation/death | 44.8% | 36.0% | 1.33 | 8.8% | 88% |
| Amputation/death | 28.4% | 36.0% | 0.75 | 7.6% | 80% |
MALE indicates major adverse limb event; POD, death within 30 days of index open or endovascular revascularization.
Power calculations are based on 1,588 subjects, allowing for 2% inflation to account for interim looks; Assumed accrual fractions for 8 quarters (24 months) are 0.01905, 0.03810, 0.07619, 0.17333 x5.
Table 9.
Power for Cohort 2
| Endpoint | 2.95‐Year Event Rate if All Subjects Receive the Assigned Treatment | Hazard Ratio | Δ | Powera | |
|---|---|---|---|---|---|
| Endovascular | Open Surgery | ||||
| MALE/death | 61.1% | 74.0% | 0.70 | 12.9% | 78% |
| MALE/death | 85.4% | 74.0% | 1.43 | 10.4% | 86% |
| MALE/death | 60.7% | 74.0% | 0.69 | 13.3% | 80% |
| MALE/death | 85.3% | 74.0% | 1.42 | 11.3% | 85% |
| MALE+POD or amputation/death | 35.4% | 49.0% | 0.65 | 13.6% | 75% |
| 64.5% | 49.0% | 1.54 | 15.5% | 86% | |
Power calculations are based on 470 subjects, allowing for 2% inflation to account for interim looks. Assumed accrual fractions for 8 quarters (24 months) are: 0.01905, 0.03810, 0.07619, 0.17333 x5.
Results and Discussion
The BEST‐CLI trial aims to provide Level I evidence that will significantly enhance therapeutic decision‐making and help establish a much‐needed standard of care for patients with CLI. Recent data from the SVS Vascular Quality Initiative highlight the remarkably high degree of equipoise currently associated with the treatment of CLI in North America (Figure 1).21 The widely disparate and often tightly held treatment biases regarding the relative role of open surgical and endovascular therapy for CLI serve as the central rationale and ongoing motivation for a trial such as BEST‐CLI.
Several unique features of the BEST‐CLI trial bear further mention, among them the trial design, comprehensive cost‐effectiveness and quality of life analyses, use of novel end points, and collaborative approach. The trial focuses on patients with CLI who are candidates for both infrainguinal open surgical and endovascular revascularization and was purposely designed as a pragmatic trial. The most significant feature of such a design is that the definition of what constitutes “best” therapy within the assigned revascularization approach is left to each individual investigator. Accordingly, all commercially available endovascular therapies (with the exception of cryoplasty) are allowed, as are all surgical bypass techniques and types of conduit. This pragmatic element is of particular importance in the effort to avoid the common pitfall of conducting a trial that is of limited relevance to current clinical practice by the time of final analysis.42
The BEST‐CLI trial has a robust cost‐effectiveness component that will serve to quantify the accumulated financial costs in each intervention arm. In addition, the trial will analyze a wide spectrum of relevant functional and QoL outcomes. Because endovascular and open revascularization may be associated with either similar or different rates of treatment success, assessing the additional safety, cost‐effectiveness, and QoL end points will be critical to accurately capturing the entirety of the clinical and economic benefit of each therapy. Cost‐effectiveness analysis will be based on longevity, QoL, and the economic value of any observed differences in these outcomes. This analysis will rely on prospectively collected information to characterize the resources consumed during each subject's initial revascularization hospitalization and all related subsequent inpatient and outpatient contacts with the medical system, including repeat hospitalization, outpatient physician visits, outpatient tests and procedures, emergency department visits, and medication use. The main measures of functional outcome will be EQ‐5D and VascuQoL, which are standardized, well‐validated instruments. The VascuQoL, a disease‐specific questionnaire that makes it possible to detect subtle changes in disease severity, will be used as the main CLI‐specific QoL outcome tool.33 EQ‐5D comprehensively gauges global health‐related QoL and utilities, and will be the primary measure for cost‐utility analysis.35
Given the limitations of the widely used Rutherford classification system, the SVS has recently developed a novel classification scheme for lower‐extremity threatened limbs, known as WIfI (Wound, Ischemia, and foot Infection), that is based on the extent and depth of wounds, the degree of ischemia, and the presence and extent of infection.40 In addition to capturing the TransAtlantic Inter‐Society Consensus anatomic status of the index extremity, the BEST‐CLI trial has incorporated and aims to prospectively validate this novel Wound, Ischemia, and foot Infection classification system. The trial also utilizes a number of novel end points intended to capture all outcome parameters of interest. Some of these end points will focus on the implications of reinterventions, both major and minor, while others will directly measure the clinical and hemodynamic consequences of the CLI treatments under investigation.
Recognizing the degree to which CLI is currently managed by a range of subspecialists in the United States and Canada, principally vascular surgeons, interventional cardiologists, interventional radiologists, and vascular medicine specialists, every effort has been made to make the BEST‐CLI trial a fully multidisciplinary endeavor. To have the trial most accurately reflect contemporary practice and, to the extent possible, have the results accepted by the entire spectrum of CLI caregivers, it was felt important to have all those providing CLI care at participating BEST‐CLI trial sites involved. Towards this end, the creation of “CLI teams” has been promoted at each institution. The goals of the CLI team include supporting the enrollment of patients, creating and fostering an environment conducive to constructive communication and physician collaboration, and ensuring standard‐of‐care treatment within the strategy to which each subject has been randomized. A hallmark of the CLI team is the requirement that each patient be reviewed by a minimum of 2 members of the CLI team: 1 credentialed in endovascular treatment and the other credentialed for open surgical revascularization. This same requirement also applies to any randomized subject being considered for reintervention. Further underscoring the collaborative design and intent of the BEST‐CLI trial, the EC, the Clinical Events Classification Committee, the DSMB, and each of the 7 subcommittees detailed above has well‐balanced representation from all participating disciplines. Finally, and as an additional reflection of the efforts to integrate the contributions of all interested parties, the BEST‐CLI trial has received a broad level of support from numerous relevant professional societies and organizations. The trial has been formally endorsed by the SVS, the Society of Interventional Radiology, the Society for Cardiovascular Angiography and Intervention, the Society of Vascular Medicine, the Vascular Disease Foundation, Vascular InterVentional Advances, and the Food and Drug Administration.
Limitations
The pragmatic design of the BEST‐CLI trial accommodates the spectrum of revascularization techniques in current use by participating specialists. In so doing, it optimizes investigator engagement and subject enrollment and maximizes the generalizability and long‐term relevance of the trial. Inclusion of such a broad array of open and endovascular therapies, however, introduces significant heterogeneity into the data set and may limit the ability to ascertain the relative effectiveness of specific treatment techniques. The generalizability of the trial could also be compromised if practice patterns during the trial do not mimic those outside of trial conditions. Additionally, although certain strata have been prespecified according to anticipated differences in outcome, additional factors may prove to be equally or more relevant and the statistical power may be insufficient to detect the importance of such parameters.
Conclusions
CLI continues to represent a formidable healthcare challenge. Over and above its major impact on the morbidity, mortality, and QoL of a growing number of patients with PAD, the associated financial burden on our healthcare economy is substantial and growing. The BEST‐CLI trial is a timely and much‐needed study that will help to define best practice and provide a foundation for thoughtful application of current and future treatment options. More information on the BEST‐CLI trial can be found at www.BESTCLI.com and through clinicaltrials.gov: NCT02060630.
Sources of Funding
This trial is funded by National Heart, Lung, and Blood Institute through grant numbers U01HL107407, U01HL107352, and U01HL115662.
Disclosures
Menard is an advisor for Proteon (minor) and AnGes (minor, Clinical Events Classification Committee Agility Trial). Farber is a consultant for Bard. Conte is an advisor for Cook (minor), Bard (DSMB; minor), and reports speaking for Gore (honorarium). Dake is a compensated advisor for Cardinal Health, Cook Medical, and W. L. Gore. He is a board member at Vascular InterVentional Advances Physicians, a 501(c)(3) not‐for‐profit education and research organization. Jaff is a noncompensated advisor for Abbott Vascular, Boston Scientific, Cordis, and Medtronic Vascular. He is a compensated advisor for Cardinal Health, Volcano Medical, American Orthotics and Prosthetics Association, and an Equity Investor in PQ Bypass; Embolitech; and Vascular Therapies. Dake is also a board member at Vascular InterVentional Advances Physicians, a 501(c)(3) not‐for‐profit education and research organization; Society for Cardiovascular Angiography and Intervention; and Intersocietal Accreditation Commission. Kaufman is on the Advisory Board for MarrowStim, a Consultant (clinical trial committee member) for Spectranetics, and board member at Vascular InterVentional Advances Physicians. Powell is a trial principal investigator for AnGes. White is a Research Investigator for Bard (Lutonix) and Surmodics. He is on the Steering Committee for AstraZeneca (Euclid Trial). Rosenfield reports relationships with Abbott Vascular (Consultant/Scientific Advisory Board, Research Support), Cardinal Health (Consultant/Scientific Advisory Board), Inari Medical (Consultant/Scientific Advisory Board), InspireMD (Consultant/Scientific Advisory Board), Surmodics (Consultant/Scientific Advisory Board), Volcano/Philips (Consultant/Scientific Advisory Board), Proteon (Consultant/Scientific Advisory Board), Access Vascular (Personal Equity), CardioMEMs (Personal Equity), Contego (Personal Equity, Consultant/Scientific Advisory Board with Equity or Stock Options), CRUZAR Systems (Personal Equity, Consultant/Scientific Advisory Board with Equity or Stock Options), Embolitech (Personal Equity), Icon (Personal Equity), Janacare (Personal Equity), MD Insider (Personal Equity, Consultant/Scientific Advisory Board with Equity or Stock Options), Primacea (Personal Equity), PQ Bypass (Personal Equity), Vortex (Personal Equity), Capture Vascular (Consultant/Scientific Advisory Board with Equity or Stock Options), Endospan (Consultant/Scientific Advisory Board with Equity or Stock Options), Eximo (Consultant/Scientific Advisory Board with Equity or Stock Options), Micell (Consultant/Scientific Advisory Board with Equity or Stock Options), Shockwave (Consultant/Scientific Advisory Board with Equity or Stock Options), Silk Road Medical (Consultant/Scientific Advisory Board with Equity or Stock Options), Valcare (Consultant/Scientific Advisory Board with Equity or Stock Options), Atrium (Research Support), Lutonix‐Bard (Research Support), and Vascular InterVentional Advances Physicians (Board Member). The remaining authors have no disclosures to report.
Appendix A. List of Trial Sites
| Site # | Site Name | Contact PI Name | City | State |
|---|---|---|---|---|
| 1003 | Allegheny General Hospital | Satish Muluk | Pittsburgh | PA |
| 1005 | Brigham and Women's Hospital | Michael Belkin | Boston | MA |
| 1007 | Cleveland Clinic Foundation | Mehdi Shishehbor | Cleveland | OH |
| 1008 | Columbia University Medical Center | Danielle Bajakian | New York | NY |
| 1009 | Dartmouth Hitchcock Medical Center | Philip Goodney | Lebanon | NH |
| 1010 | Emory University | Khusrow Niazi | Atlanta | GA |
| 1013 | Harbor‐UCLA Medical Center | Rodney White | Torrance | CA |
| 1019 | Jewish General Hospital | Daniel Obrand | Montreal | Canada |
| 1023 | Massachusetts General Hospital | Glenn LaMuraglia | Boston | MA |
| 1024 | Mayo Clinic (Rochester) | Manju Kalra | Rochester | MN |
| 1026 | Medstar Washington Hospital Center | Nelson Bernardo | Washington | DC |
| 1029 | Michael E. DeBakey VA Med Center | Neal Barshes | Houston | TX |
| 1030 | Montefiore Medical Center | Evan Lipsitz | Bronx | NY |
| 1034 | Ochsner Medical Center | J. Stephen Jenkins | New Orleans | LA |
| 1041 | San Francisco Veterans Affairs Medical Center | Christopher Owens | San Francisco | CA |
| 1046 | Steward St. Elizabeth's Medical Center | Frank Pomposelli | Brighton | MA |
| 1054 | University of Colorado Hospital | Kevin Rogers | Aurora | CO |
| 1055 | Mount Sinai Medical Center | Ageliki Vouyouka | New York | NY |
| 1059 | The University of Alabama at Birmingham | Marc Passman | Birmingham | AL |
| 1061 | Baptist Hospital of Miami | James Benenati | Miami | FL |
| 1066 | Arizona Heart Hospital | Venkatesh Ramaiah | Phoenix | AZ |
| 1072 | University of Wisconsin ‐ Madison | John Hoch | Madison | WI |
| 1075 | Swedish Medical Center | Robert Bersin | Seattle | WA |
| 1076 | Northwestern Memorial Hospital | Mark Eskandari | Chicago | IL |
| 1085 | Cedars‐Sinai Heart Institute | Aamir Shah | Los Angeles | CA |
| 1095 | Johns Hopkins Hospital | Ying Wei Lum | Baltimore | MD |
| 1101 | Albany Medical Center | R. Clement Darling III | Albany | NY |
| 1104 | Palo Alto VA | Wei Zhou | Palo Alto | CA |
| 1105 | Medical College of Wisconsin | Parag Patel | Milwaukee | WI |
| 1108 | Michigan Heart/St Joseph Mercy Ann Arbor Hospital | Brian Halloran | Ann Arbor | MI |
| 1113 | Oregon Health and Science University | Erica Mitchell | Portland | OR |
| 1116 | Rush University Medical Center | Ulku Cenk Turba | Chicago | IL |
| 1121 | Temple University | Eric Choi | Philadelphia | PA |
| 1125 | University of California San Francisco Medical Center | Jade Hiramoto | San Francisco | CA |
| 1126 | University of Chicago Medicine | Ross Milner | Chicago | IL |
| 1131 | University of Maryland Medical System | Robert Crawford | Baltimore | MD |
| 1134 | University of Michigan Health System | Peter Henke | Ann Arbor | MI |
| 1135 | University of Pittsburgh Medical Center | Rabih Chaer | Pittsburgh | PA |
| 1137 | University of Vermont Medical Center | Julie Adams | Burlington | VT |
| 1140 | VA Greater Los Angeles Healthcare System/West LA Medical Center | Hugh Gelabert | Los Angeles | CA |
| 1151 | William Beaumont Hospital | Robert Safian | Royal Oak | MI |
| 1154 | Yale New Haven Hospital | Carlos Mena‐Hurtado | New Haven | CT |
| 1160 | Keck Medical Center of USC | Vincent Rowe | Los Angeles | CA |
| 1169 | University Hospitals of Cleveland/Case Western Reserve University | Vikram Kashyap | Cleveland | OH |
| 1173 | SUNY Upstate Medical University | Palma Shaw | Syracuse | NY |
| 1182 | Providence Heart and Vascular Institute | Ethan Korngold | Portland | OR |
| 1188 | Toronto General Hospital | Thomas Lindsay | Toronto | Canada |
| 1217 | University of California Davis Medical Center | William Pevec | Sacramento | CA |
| 1229 | Penn State Milton S. Hershey Medical Center | Faisal Aziz | Hershey | PA |
| 1234 | University of Toledo Medical Center | Munier Nazzal | Toledo | OH |
| 1238 | University of Massachusetts Medical School | Andres Schanzer | Worcester | MA |
| 1256 | Beth Israel Deaconess Medical Center | Allen Hamdan | Boston | MA |
| 1257 | University of Arkansas for Medical Services | Matthew Smeds | Little Rock | AR |
| 1258 | Boston Medical Center | Jeffrey Kalish | Boston | MA |
| 1259 | Rhode Island Hospital | Jeffrey Slaiby | Providence | RI |
| 1260 | Greenville Memorial Hospital | Tod Hanover | Greenville | SC |
| 1261 | Indiana University Medical School | Raghu Motaganahalli | Indianapolis | IN |
| 1263 | Kaiser Permanente (San Diego) | Robert Hye | San Diego | CA |
| 1264 | Minneapolis Heart Hospital/Abbott Northwestern Hospital | Jason Alexander | Minneapolis | MN |
| 1269 | Ohio Health Research Institute | Gary Ansel | Columbus | OH |
| 1270 | Scott and White ‐ Temple | Todd Bohannon | Temple | TX |
| 1271 | Southern Illinois University School of Medicine | Sapan Desai | Springfield | IL |
| 1272 | St. Boniface General Hospital | Randolph Guzman | Winnipeg | Canada |
| 1273 | University of Florida (Gainesville) | Thomas Huber | Gainesville | FL |
| 1274 | University of Oklahoma Health Sciences Ctr. | Beau Hawkins | Oklahoma City | OK |
| 1275 | Medical University of South Carolina | Thomas Edward Brothers | Charleston | SC |
| 1276 | Memorial Hermann Hospital TMC | Ali Azizzadeh | Houston | TX |
| 1277 | The University of Utah | Benjamin S. Brooke | Salt Lake City | UT |
| 1278 | University of California‐Irvine | Nii‐Kabu Kabutey | Orange | CA |
| 1279 | North Carolina Heart and Vascular Research | Ravish Sachar | Raleigh | NC |
| 1281 | Western NY VA Healthcare System | Hasan Dosluoglu | Buffalo | NY |
| 1282 | Carondelet Heart & Vascular Institute | Scott Berman | Tucson | AZ |
| 1283 | University of Oklahoma College of Medicine | John Blebea | Oklahoma City | OK |
| 1284 | Chu de Quebec/St‐Francois d' Assise Hospital | Yvan Douville | Quebec City | QC |
| 1285 | Duke University | Cynthia Shortell | Durham | NC |
| 1287 | Providence Sacred Heart Medical Center | Joseph Davis | Spokane | WA |
| 1288 | Kaiser Foundation Hospital | Peter Schneider | Honolulu | HI |
| 1290 | Loma Linda University Medical Center |
Ahmed Abou‐Zamzam |
Loma Linda | CA |
| 1293 | LSU Health Sciences/University Health System | Tze‐Woei Tan | Shreveport | LA |
| 1294 | North Central Heart Institute | Michael Bacharach | Sioux | SD |
| 1296 | Sacred Heart Hospital River Bend | Craig Seidman | Springfield | OR |
| 1298 | Tufts Medical Center | Mark Iafrati | Boston | MA |
| 1299 | University of Tennessee Medical Center | Laura Findeiss | Knoxville | TN |
| 1300 | Tampa General Hospital | Martin Back | Tampe | FL |
| 1301 | UCSD‐Sulpizio Cardiovascular Center | John Lane | La Jolla | CA |
| 1302 | UCLA‐Gonda Vascular Surgery | Peter Lawrence | Los Angeles | CA |
| 1304 | CAMC Clinical Trials Center | Patrick Stone | Charleston | WV |
| 1305 | University of Virginia | Margaret Tracci | Charlottesville | VA |
| 1306 | McGill University | Kent Mackenzie | Montreal | Canada |
| 1307 | Univ. of Rochester | Michael Stoner | Rochester | NY |
| 1308 | The Ohio State University | Jean Starr | Columbus | OH |
| 1309 | Mercy Hospital Medical Center | David McAllister | West Des Moines | IA |
| 1310 | Harborview Medical Center | Niten Singh | Seattle | WA |
| 1311 | Dallas VA Medical Center | J. Gregory Modrall | Dallas | TX |
| 1314 | VA Boston Healthcare System | Scott Kinlay | West Roxbury | MA |
| 1315 | GW Medical Faculty Associates, Inc. | Richard Neville | Washington | DC |
| 1316 | Holy Name Medical Center | John Rundback | Teaneck | NJ |
| 1318 | University of North Carolina Hospitals (Chapel Hill) | Raghu Vallabhaneni | Chapel Hill | NC |
| 1319 | Hunterdon Medical Center | Andrey Espinoza | Flemington | NJ |
| 1320 | Portland VA Medical Center | Amir Azarbal | Portland | OR |
| 1323 | University of Nebraska Medical Center | G. Matthew Longo | Omaha | NE |
| 1325 | Deborah Heart and Lung Center | Richard Kovach | Brown Mills | NJ |
| 1326 | The Miriam Hospital/Brown Medical School | Peter Soukas | Providence | RI |
| 1327 | Wellmont Holston Valley Medical Center | Chris Metzger | Kingsport | TN |
| 1330 | The Heart Center of Lake County | Andre Artis | Merriville | IN |
| 1331 | Pinnacle Health System | William Bachinsky | Wormleysburg | PA |
| 1332 | Denver VA Medical Center | Ehrin Armstrong | Denver | CO |
| 1334 | Stanford Hospital | Venita Chandra | Stanford | CA |
| 1336 | Staten Island University Hospital | Jonathan Schor | Staten Island | NY |
| 1337 | Loma Linda VA Medical Center | Christian Bianchi | Loma Linda | CA |
| 1338 | Piedmont Healthcare | Eyal Ben‐Arie | Atlanta | GA |
| 1339 | Cadence Health | Michael Verta | Winfield | IL |
| 1340 | Wake Forest Baptist Health | Justin Hurie | Winston Salem | NC |
| 1341 | Meriter Wisconsin Heart | Victor Weiss | Madison | WI |
| 1342 | Regina Qu'Appelle | David Kopriva | Regina | Canada |
| 1344 | Michigan Vascular Center | Robert Molnar | Flint | MI |
| 1345 | Los Angeles Medical Center, Kaiser Permanente | Kaushal Patel | Los Angeles | CA |
| 1346 | Gundersen Health System | Ezana Azene | La Crosse | WI |
| 1347 | Maine Medical Center | Elizabeth Blazick | Portland | ME |
Appendix B. Best‐CLI Trial Subcommittee Chairs
The Surgical and Interventional Management Committee (SIMC) – Richard Powell, M.D.
The Ancillary Studies Committee (ASC) – Mark Creager, M.D.
The Recruitment and Retention Committee (RRC) – Matthew Menard, M.D.
The Evolving Technology Committee (ETC) – John Kaufman, M.D.
The Optimal Medical Therapy Committee (OMTC) – Michael Jaff, M.D.
The Conflict of Interest Committee (COIC) – co‐chaired by Michael Conte, M.D. and Christopher White, M.D.
The Publications and Presentations Committee (PPC) – Alik Farber, M.D.
Appendix C. Surgical and Interventional Management Committee Credentialing Criteria
BEST‐CLI Credentialing Criteria
Open Surgical Revascularization
The investigator must be a board‐certified Vascular Surgeon, Cardiothoracic Surgeon or
General Surgeon. The investigator must also have performed either
A minimum of 50 infrainguinal open surgical bypasses over preceding 5 years or
A minimum of 10 infrainguinal open surgical bypasses over preceding 2 years.
Regardless of the categories above, at least 5 of the lower extremity bypasses performed must have involved an artery below the knee joint, and at least 5 must have been performed with a venous conduit.
Endovascular Revascularization
The investigator must have either
Board certification in Interventional Cardiology, Vascular Surgery, Interventional Radiology, or Vascular Medicine (interventional) or
Proof of completion of training in endovascular therapy that meets the Society for Vascular Surgery (SVS) endovascular training guidelines.
Reference: Calligaro KD, Toursarkissian B, Clagett PG, et al. Guidelines for hospital privileges in vascular and endovascular surgery: Recommendations of the Society for vascular surgery. J Vasc Surg. 2008; 47:1–5.
The investigator must also have performed at least 12 infrainguinal endovascular procedures in patients with CLI involving an artery below the knee joint over the preceding 2 years.
BEST‐CLI Trial Policy for Conditional Approval of Investigators
In an effort to ensure the highest level of quality and safety for patients enrolled in the BEST‐CLI trial, the Surgical and Interventional Management Committee (SIMC) has decided to recognize junior investigators who are within 24 months of graduation from their specialty training program, and who otherwise are felt appropriate for participation as BEST‐CLI investigators, as “Conditionally Approved” to provide revascularization services as part of the trial. The SIMC strongly encourages Conditionally Approved investigators to enroll any eligible critical limb ischemia patients in their practice into the BEST‐CLI trial.
Conditionally Approved investigators should perform revascularization procedures with a proctor/collaborator who is a fully approved investigator for revascularization procedures in the BEST‐CLI trial. The status of such investigators will be reviewed on a yearly basis to determine if sufficient experience has been gained to warrant a change to full credentialing as an “Approved” investigator.
(J Am Heart Assoc. 2016;5:e003219 doi: 10.1161/JAHA.116.003219)
References
- 1. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;45:S5–S67. [DOI] [PubMed] [Google Scholar]
- 2. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 3. Hunink MGM, Wong JB, Donaldson MC, Meyerovitz MF, Vries J, Harrington DP. Revascularization for femoropopliteal disease: a decision and cost‐effectiveness analysis. JAMA. 1995;274:165–171. [PubMed] [Google Scholar]
- 4. Singh S, Evans L, Datta D, Gaines P, Beard JD. The costs of managing lower limb‐threatening ischaemia. Eur J Vasc Endovasc Surg. 1996;12:359–362. [DOI] [PubMed] [Google Scholar]
- 5. Benoit E, O'Donnell TF Jr, Kitsios GD, Iafrati MD. Improved amputation‐free survival in unreconstructable critical limb ischemia and its implications for clinical trial design and quality measurement. J Vasc Surg. 2012;55:781–789. [DOI] [PubMed] [Google Scholar]
- 6. Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, Gebauer K, Roeder N, Berger K, Malyar NM. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36:932–938. [DOI] [PubMed] [Google Scholar]
- 7. Shah DM, Darling RC III, Chang BB, Fitzgerald KM, Paty PS, Leather RP. Long‐term results of in situ saphenous vein bypass. Analysis of 2058 cases. Ann Surg. 1995;222:438–446; discussion 446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, Moneta GL, Conte MS. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46:1180–1190; discussion 1190. [DOI] [PubMed] [Google Scholar]
- 9. Veith FJ, Gupta SK, Ascer E, White‐Flores S, Samson RH, Scher LA, Towne JB, Bernhard VM, Bonier P, Flinn WR, Astelford P, Yao JST, Bergan JJ. Six‐year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104–114. [DOI] [PubMed] [Google Scholar]
- 10. Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, Nehler MR, Powell RJ, Sidawy AN. Suggested objective performance goals and clinical trial design for evaluating catheter‐based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–1473.e1‐3. [DOI] [PubMed] [Google Scholar]
- 11. Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54:1021–1031.e1. [DOI] [PubMed] [Google Scholar]
- 12. Goodney PP, Tarulli M, Faerber AE, Schanzer A, Zwolak RM. Fifteen‐year trends in lower limb amputation, revascularization, and preventive measures among Medicare patients. JAMA Surg. 2015;150:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rocha‐Singh KJ, Jaff M, Joye J, Laird J, Ansel G, Schneider P. Major adverse limb events and wound healing following infrapopliteal artery stent implantation in patients with critical limb ischemia: the XCELL trial. Catheter Cardiovasc Interv. 2012;80:1042–1051. [DOI] [PubMed] [Google Scholar]
- 14. Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, Schmidt A, Tessarek J, Vinck E, Schwartz LB. Randomized comparison of everolimus‐eluting versus bare‐metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390–398. [DOI] [PubMed] [Google Scholar]
- 15. Conte MS. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) and the (hoped for) dawn of evidence‐based treatment for advanced limb ischemia. J Vasc Surg. 2010;51:69S–75S. [DOI] [PubMed] [Google Scholar]
- 16. Setacci C, de Donato G, Teraa M, Moll FL, Ricco J‐B, Becker F, Robert‐Ebadi H, Cao P, Eckstein HH, De Rango P, Diehm N, Schmidli J, Dick F, Davies AH, Lepäntalo M, Apelqvist J. Chapter IV: treatment of critical limb ischaemia. Eur J Vasc Endovasc Surg. 2011;42:S43–S59. [DOI] [PubMed] [Google Scholar]
- 17. Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. [DOI] [PubMed] [Google Scholar]
- 18. Bradbury A, Wilmink T, Lee AJ, Bell J, Prescott R, Gillespie I, Stansby G, Fowkes FG. Bypass versus angioplasty to treat severe limb ischemia: factors that affect treatment preferences of UK surgeons and interventional radiologists. J Vasc Surg. 2004;39:1026–1032. [DOI] [PubMed] [Google Scholar]
- 19. White CJ, Gray WA. Endovascular therapies for peripheral arterial disease: an evidence‐based review. Circulation. 2007;116:2203–2215. [DOI] [PubMed] [Google Scholar]
- 20. Goodney PP, Travis LL, Nallamothu BK, Holman K, Suckow B, Henke PK, Lucas FL, Goodman DC, Birkmeyer JD, Fisher ES. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2012;5:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cronenwett JL. “SVS vascular quality initiative annual report: national data analyses”. Presented at the Vascular Annual Meeting of the Society for Vascular Surgery, San Francisco, CA, June 1, 2013. [Google Scholar]
- 22. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary: a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). J Am Coll Cardiol. 2006;47:1239–1312. [DOI] [PubMed] [Google Scholar]
- 23. Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beard JD. The Robert B. Rutherford Lecture: which is the best revascularization for critical limb ischemia: endovascular or open surgery? J Vasc Surg. 2008;48:11S–16S. [DOI] [PubMed] [Google Scholar]
- 25. Meier GH. Current literature for evidence‐based infrainguinal endovascular treatment. Semin Vasc Surg. 2008;21:210–216. [DOI] [PubMed] [Google Scholar]
- 26. Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. [DOI] [PubMed] [Google Scholar]
- 27. Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, Ruckley CV, Raab GM. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: an intention‐to‐treat analysis of amputation‐free and overall survival in patients randomized to a bypass surgery‐first or a balloon angioplasty‐first revascularization strategy. J Vasc Surg. 2010;51:5S–17S. [DOI] [PubMed] [Google Scholar]
- 28. Menard MT, Farber A. The BEST‐CLI trial: a multidisciplinary effort to assess whether surgical or endovascular therapy is better for patients with critical limb ischemia. Semin Vasc Surg. 2014;27:82–84. [DOI] [PubMed] [Google Scholar]
- 29. Farber A, Rosenfield K, Menard M. The BEST‐CLI trial: a multidisciplinary effort to assess what therapy is best for patients with critical limb ischemia. Tech Vasc Interv Radiol. 2014;17:221–224. [DOI] [PubMed] [Google Scholar]
- 30. World Medical Association declaration of Helsinki recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 31. Kumar A, Brooks SS, Cavanaugh K, Zuckerman B. FDA perspective on objective performance goals and clinical trial design for evaluating catheter‐based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1474–1476. [DOI] [PubMed] [Google Scholar]
- 32. Ferreira‐Valente MA, Pais‐Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. [DOI] [PubMed] [Google Scholar]
- 33. Conway K, Uzun V, Girod I, Morgan M, Koch P. Linguistic validation of the Vascular Quality of Life Questionnaire (VASCUQOL) in 8 languages. Value Health. 2000;3:113. [Google Scholar]
- 34. The EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 35. Chetter IC, Spark JI, Dolan P, Scott DJ, Kester RC. Quality of life analysis in patients with lower limb ischaemia: suggestions for European standardisation. Eur J Vasc Endovasc Surg. 1997;13:597–604. [DOI] [PubMed] [Google Scholar]
- 36. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 37. McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six‐minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forbes JF, Adam DJ, Bell J, Fowkes FG, Gillespie I, Raab GM, Ruckley CV, Bradbury AW; BASIL Trial Participants . Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: health‐related quality of life outcomes, resource utilization, and cost‐effectiveness analysis. J Vasc Surg. 2010;51:43S–51S. [DOI] [PubMed] [Google Scholar]
- 39. Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, Reed S. Good research practices for cost‐effectiveness analysis alongside clinical trials: the ISPOR RCT‐CEA Task Force report. Value Health. 2005;8:521–533. [DOI] [PubMed] [Google Scholar]
- 40. Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220–234. [DOI] [PubMed] [Google Scholar]
- 41. DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–1352; discussion 1353–1356. [DOI] [PubMed] [Google Scholar]
- 42. Mcpherson H. Pragmatic clinical trials. Complement Ther Med. 2004;12:136–140. [DOI] [PubMed] [Google Scholar]


