Abstract
Background
Our dual aims were as follows: (1) to classify new or recurrent myocardial infarctions (MI) in patients with stable atherosclerosis using the Universal Definition of MI classification system; and (2) to characterize the effects of vorapaxar, a first‐in‐class platelet protease‐activated receptor ‐1 antagonist, on new or recurrent MI.
Methods and Results
We analyzed data from TRA 2°P‐TIMI 50, a multinational, randomized, double‐blind, placebo‐controlled trial of vorapaxar. This analysis included 20 770 patients with previous MI or peripheral arterial disease without a history of transient ischemic attack or stroke. Each new or recurrent MI after randomization that met the trial end point definition was further categorized according to the European Society of Cardiology, American College of Cardiology, American Heart Association, World Heart Federation Universal Definition classification of type and size. Of 1095 incident MIs, 77% were spontaneous (Type 1), with a smaller number (9.8%) of secondary MIs (Type 2). Vorapaxar reduced Type 1 MI (hazard ratio [HR] 0.84, CI 0.73–0.98, P=0.024), with a similar pattern for Type 2 MI (HR 0.74, CI 0.49–1.10, P=0.13). Notably, vorapaxar showed a consistent pattern of reduction across size of MIs, including MIs in the highest Universal MI size class (≥10× upper reference limit, HR 0.83, CI 0.70–0.98, P=0.025). As such, there was a significant reduction in larger, spontaneous MIs (Type 1, ≥10× upper reference limit, HR 0.81, CI 0.67–0.99, P=0.036), and a consistent pattern with respect to fatal MI (HR 0.66, CI 0.39–1.11, P=0.12).
Conclusions
Among stable patients with established atherosclerosis, the most common type of incident MI is spontaneous MI, and the reduction in MI with vorapaxar was consistent across MIs of varying type and size, including spontaneous infarctions ≥10× upper reference limit.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00526474.
Keywords: atherosclerosis, cardiovascular disease, myocardial infarction, platelet inhibitor
Subject Categories: Clinical Studies, Ischemia, Platelets
Introduction
Vorapaxar is a first‐in‐class protease‐activated receptor‐1 receptor antagonist that is effective for long‐term secondary prevention in patients with stable atherosclerosis without a history of stroke or transient ischemic attack.1, 2 Vorapaxar reduces the risk of recurrent thrombotic events in this population, decreasing the incidence of recurrent myocardial infarction (MI) in particular.1 Further characterization of the types, sizes, and consequences of MI that may be reduced with vorapaxar may be useful for informing clinicians weighing the ischemic benefits against increases in bleeding with additional, more potent antithrombotic therapy.3
Therefore, we performed an additional classification of the MI end points in the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P)‐TIMI 50 trial1 using the classification system from the European Society of Cardiology, American College of Cardiology, American Heart Association, World Heart Federation (ESC/ACC/AHA/WHF) Universal Definition of MI4, 5 with 2 objectives: (1) to characterize new or recurrent MI in patients with stable atherosclerosis, and delineate their type and size4, 5; and (2) to characterize more completely the effects of vorapaxar on new or recurrent MI using this classification system.
Methods
Study Population
The study design and primary results of the TRA 2°P‐TIMI 50 trial have been published previously.1, 6 TRA 2°P‐TIMI 50 was a multinational, randomized, double‐blind, placebo‐controlled trial of vorapaxar in 26 449 patients with prior MI, ischemic stroke, or peripheral arterial disease (PAD). The complete eligibility criteria for the trial have been described in detail.1, 6 Vorapaxar has been approved in the United States and Europe for clinical use in patients with prior MI, and in the United States also for use in patients with PAD, but is contraindicated in all existing labels in patients with a history of transient ischemic attack or stroke.2 Therefore, we performed the present analysis among the 20 770 patients without a known cerebrovascular event prior to randomization in the trial. Eligible patients had a history of stable atherosclerosis, defined as a spontaneous MI within the 2 weeks to 12 months preceding randomization or symptomatic PAD.1, 6 Patients were ineligible for the trial if they were clinically unstable, or planning to undergo a revascularization procedure that had yet to be performed. Sensitivity analyses designed to assess the impact of the timing of randomization relative to a qualifying MI were performed and are described in Statistical Methods. The study protocol was approved by all relevant institutional review boards, and written informed consent was obtained from all patients.
End Points
All end points, including MI, were adjudicated according to the prespecified trial definitions by an independent Clinical Endpoints Committee that was blinded to treatment assignment. Local creatine kinase MB and cardiac troponin data were collected for suspected ischemic events throughout the study period. Potential myocardial ischemic events were identified through reporting by the investigator and programmatic checks of biomarker data.1 Each Clinical Endpoints Committee–determined MI underwent a supplemental classification according to the ESC/ACCF/AHA/WHF Universal Definition classification of MI type4, 5 into 1 of the following categories: spontaneous (Type 1); ischemic imbalance (Type 2); death without biomarker data available (Type 3); MI related to percutaneous coronary intervention (Type 4); and MI related to coronary artery bypass grafting (Type 5). Source narratives, progress notes, discharge summaries, and laboratory and electrocardiographic data were reviewed for clear evidence of a secondary cause of MI (eg, arrhythmia, profound anemia, or severe hypertension) prior to defining a nonprocedural MI as Type 1 or Type 2. Periprocedural (Type 4a) MI was defined as an increase of more than 3× the 99th percentile of cardiac biomarker. If the pre–percutaneous coronary intervention biomarker was greater than the upper reference limit (URL), both an increase by at least 50% over the previous value and documentation that the biomarker was decreasing prior to the suspected recurrent MI were required.1, 6 A sensitivity analysis was performed using a 5× URL threshold for Type 4a MI introduced in the 3rd Universal Definition after TRA 2°P‐TIMI 50 began. Type 4b MI was defined as a MI associated with stent thrombosis as documented by angiography or at autopsy.
In addition, each new or recurrent MI was categorized by size based on peak biomarker concentration, preferentially utilizing cardiac troponin over creatine kinase MB when both were available, in multiples of the 99th percentile URL according to the Universal Definition classification system.4, 5 Cardiac troponin was available in 94% of patients. Details of each local assay manufacturer and platform were not collected in this trial and, therefore, the URL was defined by the MI limit reported by the local laboratory. We have previously published the full details of incident stent thrombosis in this population7 and thus for the purpose of the current report, we present data focused on Type 1 and Type 2 MI. For the purpose of the present analysis, an MI was classified as fatal if the patient died within 30 days of the MI.
Statistical Methods
Efficacy analyses were performed using a Cox proportional‐hazards model, with the investigational treatment allocation, qualifying atherosclerosis (MI or PAD), and planned use of a thienopyridine as covariates. Cumulative event rates at 3 years were calculated by the Kaplan–Meier method. All patients contributed to the survival analyses through their final clinical event assessment, death, or withdrawal of consent, whichever occurred first. Efficacy data were analyzed on an intention‐to‐treat basis. Analyses of each MI type and size category were conducted using the time to the first MI in that category. In order to assess whether there was an impact on the timing of the qualifying MI relative to enrollment on the epidemiology of the types of recurrent MI, we performed 2 sensitivity analyses of the distribution of MI type over time: (1) We examined the distribution of the Universal Classification Type of new/recurrent MIs within groups defined by the time from the qualifying MI (<3, 3–6, >6 months); and (2) We assessed the distribution of MI type in a landmark analysis starting at 12 months after randomization in the trial. Safety analyses were performed among patients who received 1 or more doses of study drug and included events through 60 days after premature cessation of study therapy or 30 days after a final visit at the conclusion of the trial.
The risk of cardiovascular death was analyzed utilizing Cox regression with a landmark analysis starting at the time of a new or recurrent MI through 2 years after such an MI or starting at the time of randomization for those without an MI end point. This analysis was performed adjusting for covariates related to both incident MI and cardiovascular death, including age, hypertension, diabetes, hypercholesterolemia, smoking status, prior coronary revascularization, prior MI, peripheral arterial disease, congestive heart failure, chronic kidney disease (CrCl <60 mL/min), and planned thienopyridine at baseline. Analyses were performed using Stata v14.1 (Stata Corp., College Station, TX).
Results
Among the 20 770 patients included in this analysis, 1095 incident MIs of known type and size occurred during a median follow‐up of 30 months (Figure 1). There was 1 Type 3 and 1 Type 5 MI, which, given their rarity, are not characterized in further detail in this report. The majority of incident MIs (n=918, 84%) were in patients who qualified for the trial with a history of MI. Among patients with an incident MI after randomization, 132 patients had multiple MIs, accounting for an additional 203 of the 1095 MIs.
Figure 1.
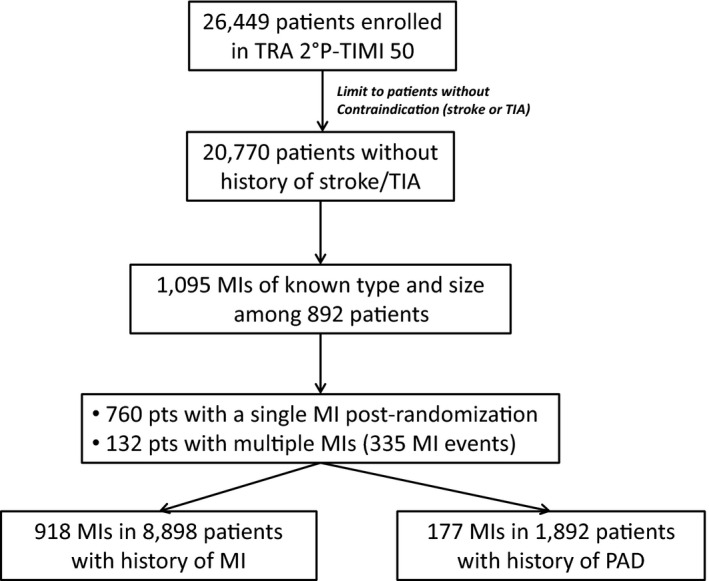
Flow diagram of myocardial infarctions included in the analysis. MI indicates myocardial infarction; PAD, peripheral arterial disease; TIA, transient ischemic attack.
The baseline characteristics for the population studied in this analysis are shown in the Table. Patients with incident MI were more likely to have a history of congestive heart failure and diabetes mellitus. Aspirin use was consistent between cohorts and there was a slightly higher rate of thienopyridine use in patients with incident MI.
Table 1.
Baseline Characteristics
| Characteristic | Incident MI (N=892) | No MI (N=19 131) |
|---|---|---|
| Demographics, n (%) | ||
| Age | ||
| Median, y (25th, 75th) | 62 (54, 70) | 60 (52, 67) |
| ≥75 y | 139 (15.6) | 1699 (8.9) |
| Female | 214 (24.0) | 4123 (21.6) |
| White race | 785 (88.0) | 16 951 (88.7) |
| Qualifying atherosclerosis, n (%) | ||
| Myocardial infarction | 749 (84.0) | 16 018 (83.7) |
| Peripheral arterial disease | 143 (16.0) | 3113 (16.3) |
| Clinical characteristics, n (%) | ||
| Diabetes mellitus | 324 (36.3) | 4392 (23.0) |
| Hypertension | 687 (77.1) | 12 291 (64.2) |
| Hyperlipidemia | 811 (90.9) | 16 192 (84.6) |
| Current smoker | 226 (25.3) | 4103 (21.4) |
| Previous coronary revascularization | 738 (82.7) | 15 049 (78.7) |
| Congestive heart failure | 162 (18.2) | 1547 (8.1) |
| Antiplatelet agents at randomization, n (%) | ||
| Thienopyridine | 684 (76.7) | 13 594 (71.1) |
| Aspirin | 853 (95.6) | 18 508 (96.7) |
MI indicates myocardial infarction.
Type and Size of New or Recurrent MI
The annualized rate of any new or recurrent MI after trial enrollment was 2% per year. The distribution of MIs according to MI type and size is shown in Figure 2. The majority of new or recurrent MIs were spontaneous (Type 1, n=842, 77%), with a smaller number of secondary MIs (Type 2, n=107, 9.8%) or procedurally related MIs (Type 4, n=144, 13%), of which only 15 (1.4%) were Type 4a MIs. Most MIs were non‐ST elevation MIs (NSTEMIs) (n=732, 81%) and were of the largest Universal MI size category ≥10× URL (n=645, 59%), followed by 5 to <10× URL (n=158, 14%). Among the 19% of MIs that were STEMIs, 84% were in the largest size category. Of the Type 4a MIs, only 1 had a peak concentration between <5× URL. In an analysis of MI size using only creatine kinase MB data, 29% of MIs were associated with peak creatine kinase MB ≥10× URL (n=128) and 57% were ≥3× URL. Among patients who had qualified for the trial with a prior MI, the distribution of type and size of incident MIs was remarkably consistent across groups defined by the time from a qualifying MI to study enrollment (Figure 3).
Figure 2.
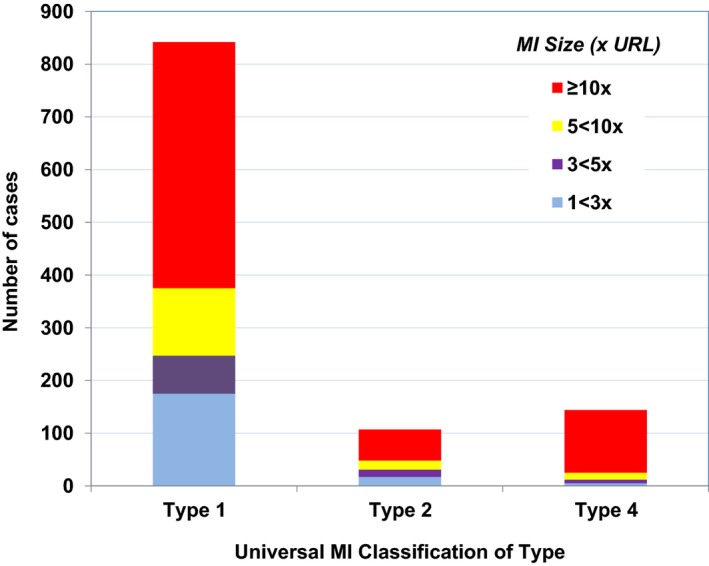
Distribution of incident MIs classified according to the universal MI classification system of type and size. There were a total of 190 STEMI and 732 NSTEMI. Type 4 MIs were dominated by Type 4b with only 15 Type 4a MIs. MI indicates myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction; URL, upper reference limit.
Figure 3.

Distribution of MI type and size by time from qualifying MI to study enrollment (first 3 bars). The 4th bar in each panel is from a landmark analysis starting at 1 year after enrollment for all subjects. MI indicates myocardial infarction.
Mortality After New or Recurrent MI
In an adjusted landmark analysis, compared to those without incident MI, patients with an incident MI had a significantly higher risk of cardiovascular death over the ensuing 2 years (hazard ratio [HR] 7.8, CI 6.0–10.1). This heightened risk of cardiovascular mortality was present for patients with a Type 1 MI (HR 7.4, CI 5.6–9.9), and for those with secondary (Type 2) MIs (Figure 4). In this population in which Type 4 MIs were predominantly due to stent thrombosis, Type 4 MI was associated with higher cardiovascular mortality. There were an insufficient number of Type 4a MIs to assess the relationship with mortality. In a sensitivity analysis using all‐cause mortality as the outcome, patients with an incident MI had a similarly increased risk of death from any cause (HR 6.62, CI 5.35–8.21) that was consistent across the types and sizes of MI. Interestingly, a higher risk of cardiovascular death was apparent for patients not only with MIs in the higher Universal MI size classes (≥10× URL, HR 9.1, CI 6.8–12.2; 5–<10× URL, HR 6.7, CI 3.6–12.3) but also those patients with a smaller incident MI (1≤3× URL; HR 9.6, CI 6.2–15.0).
Figure 4.
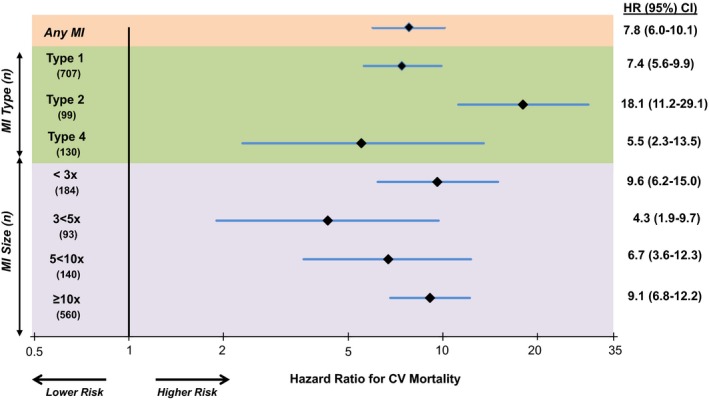
Risk of cardiovascular death following an incident MI compared with those without incident MI stratified by MI type and size. There was a consistent risk of CV death after both STEMI (HR 11.6, CI 6.7–20.1) and NSTEMI (HR 7.4, CI 5.6–9.8). CV indicates cardiovascular; HR, hazard ratio; MI, myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
Effect of Vorapaxar
Compared with placebo, vorapaxar significantly reduced the incidence of overall MI with a consistent pattern of reduction across MI of each type (Figure 5). Specifically, vorapaxar reduced spontaneous MI (Type 1, HR 0.84, CI 0.73–0.98, P=0.024; Figure 6A), with a similar pattern for secondary MI (Type 2, HR 0.74, CI 0.49–1.10, P=0.13). The reduction in incident MI with vorapaxar was also consistent across ST‐segment elevation MIs (HR 0.81, CI 0.61–1.07) and non‐ST‐segment elevation MIs (HR 0.83, CI 0.72–0.96).
Figure 5.
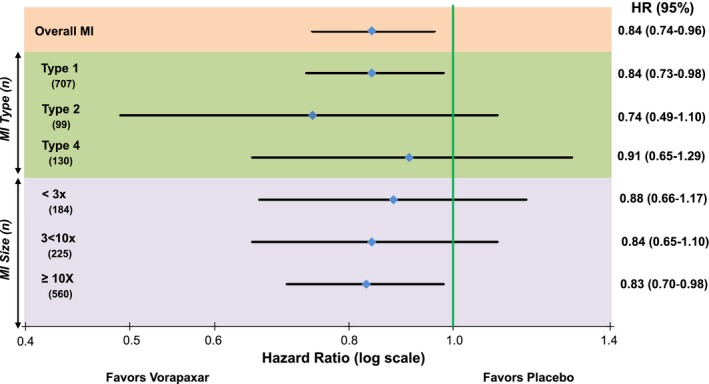
Effect of vorapaxar on incident MI stratified by universal MI type and size. This analysis is based on the first MI of each given type or size. HR indicates hazard ratio; MI, myocardial infarction.
Figure 6.
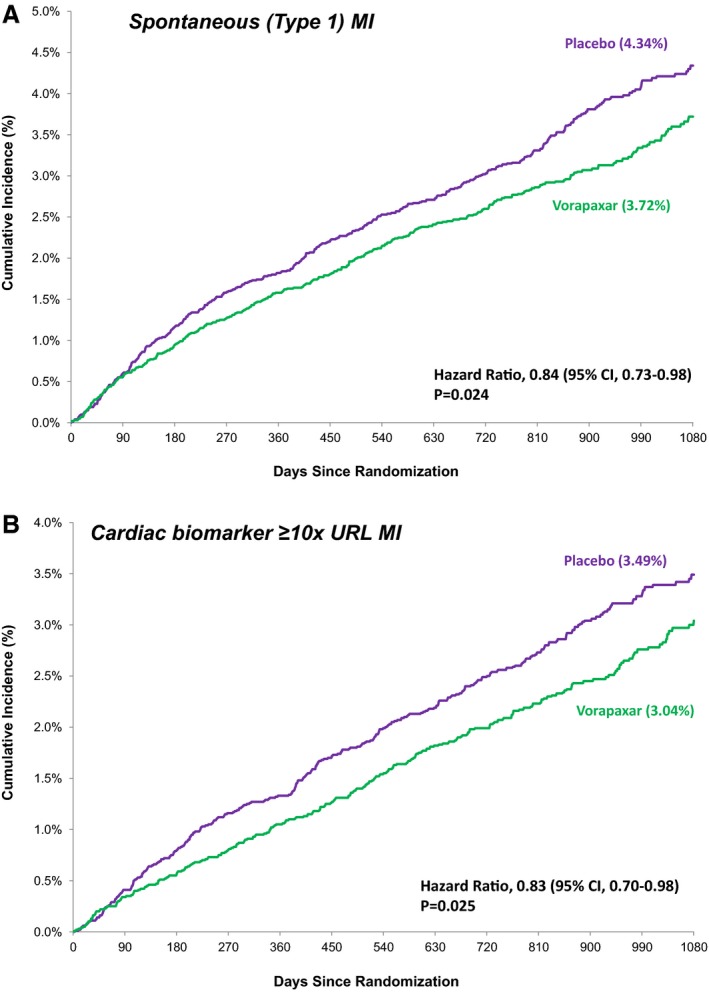
Kaplan–Meier estimated rates of MIs that were spontaneous (A) or in the highest Universal classification system size class (≥10× URL) (B) with vorapaxar vs placebo. MI indicates myocardial infarction; URL, upper reference limit.
When considering MI size, vorapaxar reduced MIs in the highest Universal MI size class (≥10× URL, HR 0.83, CI 0.70–0.98, P=0.025; Figure 6B). Importantly, there was a significant reduction in these larger, spontaneous MIs (Type 1, ≥10× URL, HR 0.81, CI 0.67–0.99, P=0.036), and a consistent pattern with respect to fatal MI (HR 0.66, CI 0.39–1.11, P=0.12).
An exploratory analysis of net clinical outcomes limiting incident MI to those that were spontaneous or >10× URL revealed a more favorable outcome with vorapaxar; cardiovascular death, Type 1 MI, stroke, or Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) severe bleeding: HR 0.83, CI 0.74–0.92, P=0.001; and cardiovascular death, MI with peak biomarker ≥10× URL, stroke, GUSTO severe bleed: HR 0.83, 0.74–0.93, P=0.001.
Discussion
In this analysis of an international trial conducted at 1032 sites including 20 770 stable patients with prior MI or symptomatic PAD and more than 50 000 patient‐years of follow‐up, we found that Type 1 MI was the predominant type of incident MI and that most such MIs were associated with >10‐fold elevation of cardiac biomarkers of necrosis. Although other types of MI were less frequent, Type 2 MI accounted for almost 10% of incident MIs. Type 4a MIs were very uncommonly detected in this stable population with established atherosclerosis. Moreover, incident MIs of Type 1, Type 2, or Type 4b were associated with a higher mortality risk in this population. These findings are relevant to strategies for secondary prevention of atherothrombosis in these high‐risk patients and establish a rationale for consideration of additional long‐term antithrombotic therapy directed at reducing thrombotic events. We also found a lower risk of new or recurrent MI with vorapaxar in this population with a consistent pattern of effect across MIs of varying type and size.
Epidemiology of MI in Stable Atherosclerosis
We and others have previously described the epidemiology of MI using the Universal MI Classification system in patients presenting with unstable chest symptoms or after presentation with an acute coronary syndrome.8, 9, 10, 11 In these studies, Type 1 (spontaneous) and Type 4 (procedurally related) MIs have predominated. For example, in a trial of patients with ACS undergoing percutaneous coronary intervention, 33% of new or recurrent MIs were spontaneous and 63% were procedurally related (Type 4).8 Subsequent analyses revealed that spontaneous MIs contributed the majority of events after the initial hospitalization. Type 2 (secondary/demand‐related) MIs accounted for less than 4% of the incident MIs over a 15‐month period. However, with the increasing sensitivity of assays for cardiac troponin, experts have speculated that small Type 2 or Type 4a (peri‐percutaneous coronary intervention) MI might dominate the epidemiology of incident MIs.12 Estimates of the incidence of Type 2 MI from previously published studies are heterogeneous, likely owing to differences across settings as well as variable definitions of MI and adjudication processes.13, 14
The current study adds to this growing experience. We found that in this population with symptomatic atherosclerosis, most MIs were spontaneous. Notably, in our population of stable patients who were at least 2 weeks after a prior MI, the distribution of the types of recurrent MI was very similar irrespective of whether the patient was <3, 3 to 6, or >6 months after the preceding MI. In this trial of a medical therapy among patients with stable atherosclerosis, there were very few Type 4a MIs detected. Moreover, it is likely that the clinical criteria incorporated into the 3rd Universal Definition of Type 4a MI would have only increased the relative proportion of Type 1 MIs in our cohort. These findings substantiate spontaneous atherothrombotic events as a target for secondary prevention in patients with known coronary and other atherosclerotic vascular disease, who are well treated with guideline‐directed secondary preventive therapies, with both recent and more remote MI.1, 9 However, at the same time ≈10% were Type 2, a proportion that is greater than some previous studies applying the Universal Definition of MI.13, 14 Additionally, the risk of cardiovascular death was higher among patients with both Type 1 and Type 2 MI. The varying estimates of both incidence and outcomes associated with Type 2 MI point to the emerging need for well‐designed prospective studies of this population.9
Effect of Vorapaxar
Our findings expand the evidence that vorapaxar is effective in reducing the risk of new or recurrent MI when used for long‐term secondary prevention in stable patients with established atherosclerosis. This reduction in MI was observed for both ST‐segment elevation MIs and non‐ST‐segment elevation MIs, and was consistent across all sizes of spontaneous MI, including directional consistency for fatal MI. It is intriguing that we also observed a consistent pattern of efficacy with vorapaxar when considering Type 2 MI, a type of myocardial injury considered to result primarily from a mismatch in myocardial oxygen delivery (anemia, hypoxemia, etc) and demand (eg, tachycardia, heart failure) without plaque instability.5, 8, 15 In addition to its action on human platelets, activation of protease activated receptor‐1 on other cell types stimulates recruitment of inflammatory cells, and proliferation of vascular smooth muscle cells within atherosclerotic arteries.16 It is possible that the observed reduction in MI with vorapaxar is mediated, in part, through mechanisms unrelated to platelet activation. Alternatively, platelet activation may have a more substantial role in the pathophysiology of a subset of patients with Type 2 MI than previously thought.
These effects of vorapaxar on thrombotic events must be clinically weighed against the risk of bleeding as we have described previously1, 2 in developing risk:benefit decisions for the individual patient.
Limitations
First, the analyses presented in the present report are based on a subset of the overall cardiovascular end points for which the trial was powered. Therefore, we have limited power within smaller subsets of events for individual hypothesis testing (eg, among Type 2 MIs). Second, although our Clinical Endpoints Committee is highly experienced with the adjudication of Type 2 MI from previous studies by our group, the adjudication of Type 2 MI is, at present, inherently dependent on judgment by blinded adjudicators regarding the plausible contribution of increased myocardial oxygen demand and the absence of acute atherothrombosis based on the complete constellation of diagnostic information available. Third, the worldwide pattern of use of assays for cardiac troponin is changing as high sensitivity assays are now used commonly outside of the United States. Our results reflect the general use of troponin assays and cut points in practice at the time of the trial. Growing use of high‐sensitivity assays and lower cut points may increase the proportion of smaller infarctions as well as Type 2 MIs that are detected. These limitations would not influence our randomized assessment of the effect of vorapaxar versus placebo. Future studies will be useful to characterize the distribution of MI size in the era of high‐sensitivity assays. The trial was not designed with the intent to provide sufficient power to perform hypothesis testing with respect to each subtype of MI but rather to examine the consistency of the estimates of the effect of vorapaxar compared with the overall result. The sample size for some individual MI types is therefore small and resulting confidence limits are wide. In particular, we cannot conclude that there is a definite effect of vorapaxar on Type 2 MI. Fourth, in contrast to the comparison between vorapaxar and placebo, the assessment of the risk of cardiovascular death between patients with and without incident MI after randomization (Figure 4) was a nonrandomized analysis and thus we cannot definitively infer a causal relationship. Despite adjusting for multiple known potential confounders, it is possible that unmeasured confounders remain and that the relative risk associated with MI is smaller in magnitude than estimated in this study.
Conclusions
Among stable patients with established atherosclerosis, the most common type of incident MI is spontaneous (Type 1) MI with peak biomarkers ≥10× URL. Type 2 (demand‐related) MIs appear more common than previously appreciated. The reduction in MI with vorapaxar was consistent across MIs of varying type and size, including larger spontaneous infarctions.
Sources of Funding
The TRA 2°P‐TIMI 50 trial was funded by Merck and Co.
Disclosures
Dr Kidd has no disclosures. The TIMI Study Group has received significant research grant support from Abbott Laboratories, Amgen, AstraZeneca, Beckman Coulter, Bristol‐Myers Squibb, Daiichi Sankyo Co Ltd, Eli Lilly and Co, Gilead, GlaxoSmithKline, Merck and Co, Nanosphere, Novartis Pharmaceuticals, Nuvelo, Ortho‐Clinical Diagnostics, Pfizer, Roche Diagnostics, Sanofi‐Aventis, Sanofi‐Synthelabo, Siemens Medical Solutions, and Singulex. Dr Bonaca is a member of the TIMI Study Group and has received consulting fees from Merck and Co, AstraZeneca, Bayer, and Roche Diagnostics. Dr Braunwald is a member of the TIMI Study Group and reports consulting fees/honoraria from Merck and Co (no compensation), Amorcyte, The Medicines Co, Medscape, Bayer, Daiichi Sankyo, and Menarini International. Dr De Ferrari reports personal fees from Merck and Amgen. Dr Lewis reports grant support and personal fees from Amgen, and personal fees from Merck Sharp & Dohme. Ms Murphy is a member of the TIMI Study Group and has received honoraria from Merck and Co. Dr Scirica is a member of the TIMI Study Group and has received consulting fees from AstraZeneca, GE Healthcare, Gilead, Lexicon, Arena, Eisai, St. Jude's Medical, Forest Pharmaceuticals, Bristol‐Myers Squibb, Boston Clinical Research Institute, Covance, University of Calgary, and Elsevier Practice Update Cardiology. Dr White received support as a member of the steering committee from Merck and Co. Dr Morrow is a member of the TIMI Study Group and reports consulting fees from Abbott Laboratories, AstraZeneca, Eli Lilly, Gilead, Instrumentation Laboratory, Konica Minolta, Merck and Co, Novartis, and Roche Diagnostics. The other authors report no conflicts.
(J Am Heart Assoc. 2016;5:e003237 doi: 10.1161/JAHA.116.003237)
References
- 1. Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KAA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJO, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 2. Magnani G, Bonaca MP, Braunwald E, Dalby AJ, Fox KAA, Murphy SA, Nicolau JC, Oude Ophuis T, Scirica BM, Spinar J, Theroux P, Morrow DA. Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc. 2015;4:e001505 doi: 10.1161/JAHA.114.001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F‐J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 4. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. [DOI] [PubMed] [Google Scholar]
- 5. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 6. Morrow DA, Scirica BM, Fox KA, Berman G, Strony J, Veltri E, Bonaca MP, Fish P, McCabe CH, Braunwald E. Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the Thrombin‐Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2P). Am Heart J. 2009;158:335–341. [DOI] [PubMed] [Google Scholar]
- 7. Bonaca MP, Scirica BM, Braunwald E, Wiviott SD, O'Donoghue ML, Murphy SA, Morrow DA. Coronary stent thrombosis with vorapaxar versus placebo results from the TRA 2°P‐TIMI 50 trial. J Am Coll Cardiol. 2014;64:2309–2317. [DOI] [PubMed] [Google Scholar]
- 8. Bonaca MP, Wiviott SD, Braunwald E, Murphy SA, Ruff CT, Antman EM, Morrow DA. American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON‐TIMI 38. Circulation. 2012;125:577–583. [DOI] [PubMed] [Google Scholar]
- 9. Scirica B, Morrow D, Antman E, Bonaca M, Murphy S, Braunwald E, Wiviott S. Timing and clinincal setting of cardiovascular death or myocardial infarction following PCI for ACS—observations from the Triton‐TIMI 38 trial. J Am Coll Cardiol. 2012;59:E340–E340. [Google Scholar]
- 10. Cavender MA, Gibson CM, Braunwald E, Wiviott SD, Murphy SA, Toda Kato E, Plotnikov AN, Amuchástegui M, Oude Ophuis T, van Hessen M, Mega JL. The effect of rivaroxaban on myocardial infarction in the ATLAS ACS 2—TIMI 51 trial. Eur Heart J Acute Cardiovasc Care. 2014;4:468–474. [DOI] [PubMed] [Google Scholar]
- 11. Leonardi S, Tricoci P, White HD, Armstrong PW, Huang Z, Wallentin L, Aylward PE, Moliterno DJ, Van de Werf F, Chen E, Providencia L, Nordrehaug JE, Held C, Strony J, Rorick TL, Harrington RA, Mahaffey KW. Effect of vorapaxar on myocardial infarction in the thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (TRA·CER) trial. Eur Heart J. 2013;34:1723–1731. [DOI] [PubMed] [Google Scholar]
- 12. Sandoval Y, Smith SW, Thordsen SE, Apple FS. Supply/demand type 2 myocardial infarction: should we be paying more attention? J Am Coll Cardiol. 2014;63:2079–2087. [DOI] [PubMed] [Google Scholar]
- 13. Morrow DA, Wiviot SD, White HD, Nicolau JC, Bramucci E, Murphy SA, Bonaca MP, Ruff CT, Scirica BM, McCab CH, Antman EM, Braunwald E. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel‐Thrombolysis in Myocardial Infarction 38. Circulation. 2009;19:2758–2764. [DOI] [PubMed] [Google Scholar]
- 14. Alpert JS, Thygesen KA, White HD, Jaffe AS. Diagnostic and therapeutic implications of type 2 myocardial infarction: review and commentary. Am J Med. 2014;127:105–108. [DOI] [PubMed] [Google Scholar]
- 15. Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd‐Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 16. Leger AJ, Covic L, Kuliopulos A. Protease‐activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. [DOI] [PubMed] [Google Scholar]


