Abstract
Background
Apheresis is an important treatment for reducing low‐density lipoprotein cholesterol (LDL‐C) in patients with familial hypercholesterolemia (FH). We systematically reviewed the current literature surrounding LDL‐C apheresis for FH.
Methods and Results
Electronic databases were searched for publications of LDL‐C apheresis in patients with FH. Inclusion criteria include articles in English published in 2000–2013 that provide descriptions of practice patterns, efficacy/effectiveness, and costs related to LDL‐C apheresis in patients with FH. Data were stratified by country and FH genotype where possible. Thirty‐eight studies met the inclusion criteria: 8 open‐label clinical trials, 11 observational studies, 17 reviews/guidelines, and 2 health technology assessments. The prevalence of FH was not well characterized by country, and underdiagnosis was a barrier to FH treatment. Treatment guidelines varied by country, with some guidelines recommending LDL‐C apheresis as first‐line treatment in patients with homozygous FH and after drug therapy failure in patients with heterozygous FH. Additionally, guidelines typically recommended weekly or biweekly LDL‐C apheresis treatments conducted at apheresis centers that may last 2 to >3 hours per session. Studies reported a range for mean LDL‐C reduction after apheresis: 57–75% for patients with homozygous FH and 58–63% for patients with heterozygous FH. Calculated annual costs (in US$2015) may reach US$66 374 to US$228 956 per patient for weekly treatment.
Conclusions
LDL‐C apheresis treatment may be necessary for patients with FH when drug therapy is inadequate in reducing LDL‐C to target levels. While apheresis reduces LDL‐C, high per‐session costs and the frequency of guideline‐recommended treatment result in substantial annual costs, which are barriers to the optimal treatment of FH.
Keywords: apheresis, familial hypercholesterolemia, low‐density lipoprotein cholesterol
Subject Categories: Health Services
Introduction
Familial hypercholesterolemia (FH) is an autosomal‐dominant genetic disease that is a cause of premature‐onset coronary heart disease.1, 2, 3 Patients with FH may be partially responsive or poorly responsive to statins because of homozygous (2 defective alleles) or heterozygous (1 defective allele) mutations of the genes involved in low‐density lipoprotein cholesterol (LDL‐C) metabolism, such as those encoding the LDL receptor apoprotein B100 or proprotein convertase subtilisin/kexin type 9.4 Given that the efficacy of statins depends in part on increasing LDL receptor activity, patients with homozygous FH (HoFH) and some patients with heterozygous FH (HeFH) with substantially reduced or absent LDL receptors may not experience adequate risk‐stratified reduction in LDL‐C levels with statin treatment.4, 5 These patients must rely on more aggressive forms of treatment, such as LDL‐C apheresis, to reduce LDL‐C to recommended levels.4, 6, 7
Apheresis is a procedure in which either the plasma is separated from red blood cells before the physical removal of LDL‐C or the LDL‐C is directly removed from whole blood.8 It is currently the treatment of choice for patients with FH whose LDL‐C levels are not able to be reduced to target levels with conventional lipid‐lowering drug therapy.8 Older studies have demonstrated that LDL‐C apheresis is effective in reducing LDL‐C levels and the incidence of cardiovascular events (CVEs).9, 10, 11, 12 However, the follow‐up periods of these studies are often too short to comprehensively determine the longer‐term clinical benefits of apheresis in the FH population. As pointed out in the recent scientific statement from the American Heart Association regarding the agenda for FH identification and management, evidence gaps exist regarding the treatment and research of FH.2 Further, the efficacy and treatment patterns of LDL‐C apheresis in patients with FH have not been recently aggregated and assessed.
In this systematic review, we evaluate the current literature surrounding LDL‐C apheresis treatment for patients with FH who are unable to manage LDL‐C with conventional lipid‐lowering drug therapies.
Methods
Relevant articles assessing LDL‐C apheresis in patients with HoFH and HeFH were searched by using PubMed (MEDLINE), EMBASE, and Cochrane Central Register of Controlled Trials. The literature search was performed using a combination of terms included in Table S1. Country‐specific health technology assessment (HTA) websites and the Centre for Reviews and Dissemination electronic bibliographic database were used to identify documents assessing LDL‐C apheresis in patients with FH. References mentioned in the review articles were searched for the original source. Titles and abstracts from the search were screened against the inclusion/exclusion criteria, and selections were reviewed by 2 reviewers; discrepancies were resolved by discussion. The inclusion criteria included articles written in English on LDL‐C apheresis in patients with FH published between January 1, 2000, to October 10, 2013, for PubMed, EMBASE, and Cochrane Central Register of Controlled Trials, while no date restrictions were placed for HTA searches. E‐publications (articles not yet published and only available online) were not searched. Articles meeting the criteria were selected for full text review, and relevant data were abstracted where appropriate. Articles reviewed include clinical studies, guidelines, reviews, and observational studies. Those articles addressing nonapheresis lipid‐lowering therapies, reporting studies assessing specific apheresis technology efficacy, and case studies were excluded. Results are reported by stratification of findings by FH type (ie, HoFH, HeFH, FH type unspecified) as reported in studies, wherever possible. Where other lipid‐lowering background therapies (if any) were used, within a study in combination with LDL‐C apheresis, the treatment effect attributable to LDL‐C apheresis was reported (if available). Costs included in this review are US$2015 unless otherwise noted. Institutional review board approval was not obtained as our systematic literature review involved the retrospective analyses of prior published deidentified human studies.
Results
The literature search returned 189 unique citations, and 38 articles were retained after full‐text review (Figure). Retained articles included 17 reviews and guidelines (44.7%), 11 observational studies (28.9%), 8 open‐label clinical trials (21.1%), and 2 HTA studies (5.3%).
Figure 1.
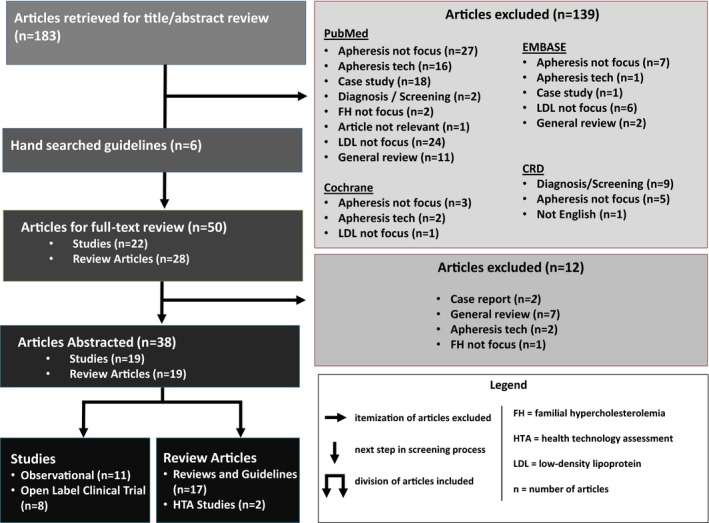
Itemization of articles excluded, right arrow. Next step in screening process, down arrow. Division of articles included, double down arrow. CRD indicates cardiac remodeling and dysfunction.
Epidemiology
The prevalence of FH is not well characterized in many countries. Of the articles included in this review, 20 (52.6%) reported prevalence: 15 reported the same prevalence rate regardless of country, 2 reported country‐specific rates along with method of prevalence determination, and 3 reported country‐specific rates but did not mention how prevalence was determined. The majority of studies reported a prevalence rate of 1:1 million for HoFH and 1:500 for HeFH despite being set in different countries. These rates can be traced to 2 genetic studies published in the 1970s. The first study from Slack identified 7 HoFH UK patients and used the Hardy–Weinberg equation to estimate an HeFH prevalence of 1:500.13 The second study, by Goldstein and Brown, extrapolated the frequency of HeFH among myocardial infarction survivors to the general US population and calculated an HeFH prevalence of 1:500 with a range of 1:200 to 1:1000.14 Various nonapheresis studies (not included in this review) reported that higher rates of FH have been observed among genetically isolated subpopulations, ranging from 1:30 000 to 1:100 000 for HoFH and from 1:67 to 1:270 for HeFH.15
LDL‐C Apheresis Diagnostic Criteria
Of the articles yielded by the literature search, only 15 (39.5%) specified diagnostic criteria: 8 review articles/guidelines (2 UK,16, 17 2 US,5, 18 1 Australia,6 1 Japan,4 1 Spain,19 and 1 country not‐specified), 3 observational studies (France,20 Germany,21 and US22), 3 open‐label trials (Italy,23 Japan,24 and Turkey25), and 1 HTA (Canada26).
The most commonly referenced diagnostic criteria for diagnosis of familial hypercholestrolemia were the Simon Broome FH register and the Dutch Lipid Network criteria. The Broome criteria incorporate diagnostic components related to lipid levels, family history, clinical presentation, and DNA testing and are the primary criteria presented in the National Institute for Health and Care Excellence clinical guidelines.17 Separate lipid thresholds are incorporated for children and adults, although DNA evidence alone is sufficient to diagnose definite FH. The Dutch criteria include similar components in diagnosing adults but use a more granular point‐based system that allows for greater diagnostic sensitivity; they are the favored diagnostic criteria in Australia's clinical guidelines.6 Both the Broome and Dutch criteria are described as acceptable diagnostic criteria by the Ontario Medical Advisory Secretariat HTA.26 Neither the Simon Broome nor the Dutch criteria make a specific distinction between HoFH and HeFH diagnosis.
Of the articles included in this review, 9 (23.7%) expressed a need for better diagnosis with 5 articles stating that FH underdiagnosis is a problem.6, 23, 26, 27, 28 Australia's clinical guidelines reported that <10% of FH cases have been diagnosed,6 while the Ontario HTA stated that only 15% of the cases in Canada are accurately diagnosed.26 Another problem noted is late diagnosis. The literature suggests that effective treatment for FH requires early identification, yet patients are often diagnosed only after a coronary event.6, 27, 28 This suggests that increased awareness and earlier screening and diagnosis are needed to improve the prognosis of the pediatric and adolescent FH population. To better meet these unmet needs, multiple groups advocate the use of cascade screening.29
LDL‐C Apheresis Guidelines
The literature search identified 11 clinical guidelines. Six guidelines (Japan,4 Australia,6 National Institute for Health and Care Excellence/UK,17 Spain,19 and US18, 29) outlined FH treatment recommendations (Table S2), while 5 (Germany,30, 31 UK,32 and US5, 29, 33) described LDL‐C apheresis treatment for hypercholesterolemia (Table S3).
Although it is widely accepted that patients with HoFH usually require apheresis to reduce LDL‐C to target levels, country‐specific guidelines vary on when LDL‐C apheresis should be used in patients with FH.4, 5 Japanese guidelines were the most aggressive, recommending LDL‐C apheresis as first‐line treatment for all patients with HoFH.4 Other guidelines required that patients with HoFH exceed a specific LDL‐C threshold or are treatment refractory. All guidelines recommend concomitant lipid‐lowering therapy along with apheresis to reduce LDL‐C levels.
For patients with HeFH who are treatment refractory and have cardiovascular disease, all guidelines recommend LDL‐C apheresis in combination with lipid‐lowering therapies.
Across FH types, LDL‐C apheresis treatment eligibility, LDL‐C thresholds, target acute LDL‐C reduction (LDL‐C reduction immediately after an apheresis session), interval LDL‐C level (LDL‐C level between apheresis sessions), and time‐average LDL‐C reduction (average LDL‐C levels between 2 time points to account for LDL‐C rebound after apheresis treatment) were not consistently reported and varied by country when they were reported (Table 1).3, 4, 5, 6, 19, 29, 30, 31, 32
Table 1.
Comparison of Country‐Specific Apheresis/FH Guidelines
| Guideline Elements | Australia6 | Japan4 | UK32 | USA5, 29, 33 | Spain19 | Germany30, 31 |
|---|---|---|---|---|---|---|
| LDL‐C thresholds for apheresis treatment |
|
|
|
|
|
|
| Session intervals | Unspecified FH: Every 2–3 wk | HoFH: Every 1–2 wk on a permanent basis | HoFH: Every 1–2 wk on a permanent basis | 1–2 wk | Not specified | Not specified |
| Therapeutic targets |
|
|
|
|
|
|
FH indicates familial hypercholesterolemia; LDL‐C, low‐density lipoprotein cholesterol; HoFH, homozygous FH; HeFH, heterozygous FH; CAD, coronary artery disease; CHD, coronary heart disease; CVD, cardiovascular disease.
LDL‐C eligibility thresholds varied from >160 to ≥500 mg/dL for patients with HoFH and >160 to >300 mg/dL for treatment refractory patients with HeFH. Target acute LDL‐C reduction ranged from 55 to 70% across all FH types (HoFH, HeFH, and unspecified FH types). Target interval LDL‐C levels/time‐average reductions were defined as percent reductions or as absolute LDL‐C levels. Although there was no consensus on treatment targets, a need for generally accepted guidelines for LDL‐C apheresis treatment was recognized.8, 31
For all FH types, guidelines recommend initial concomitant lipid‐lowering therapy with statins (combined with ezetimibe in Japanese guidelines) along with apheresis to reduce LDL‐C levels. If patients are nonresponsive to statin treatment or require further LDL‐C reductions, additional treatment with ezetimibe, niacin, fibrates, or bile acid–binding resins is recommended. There was no evident stratification based on FH type of recommendations for concomitant lipid‐reducing medication.
LDL‐C Apheresis Treatment Patterns
Actual use of LDL‐C apheresis for the treatment of FH is not well described in the literature. Only 6 articles described LDL‐C apheresis treatment rates, although estimates were not stratified by FH genotype and may include patients with non–FH‐related hyperlipidemia.7, 8, 16, 27, 30, 34 Reported rates differed widely across countries, and there was ambiguity regarding the denominator (source population from which patients with FH receiving LDL‐C apheresis are identified) of these rates; thus, they were assumed to represent annual treatment rates in the general population. In Europe, reported LDL‐C apheresis rates were the highest in Germany (12 per 1 million persons), followed by Sweden (3 per 1 million), France and Italy (2 per 1 million), and the United Kingdom (0.6 per 1 million).32 In the United States, it was estimated that 1.3 per 1 million receive LDL‐C apheresis treatment.7, 16 Worldwide, it was estimated that there are ≈2500 patients receiving LDL‐C apheresis treatment, with 1400 to 1500 of them in Germany.30 Although only 37 patients in the United Kingdom were receiving LDL‐C apheresis treatment in 2007, it was estimated that 200 patients were eligible for treatment.16, 27, 34 LDL‐C apheresis treatment rates were not reported for other countries.
Treatment intervals were not stratified by FH type and were generally consistent between countries. The majority of studies (n=13, 68.4%) reported intervals of ≤2 weeks: 4 articles reported weekly LDL‐C apheresis,21, 35, 36, 37 4 articles reported treatment every 1 to 2 weeks,9, 23, 38, 39 and 5 articles reported a 2‐week interval.20, 27, 40, 41, 42 Of the studies that reported an interval >2 weeks, the majority reported a 3‐ to 4‐week interval and 1 observational study reported a treatment interval of 8 weeks.
Background lipid‐reducing therapy reported to be administered concomitantly with apheresis included statins (simvastatin, lovastatin, atorvastatin, pravastatin, and rosuvastatin), fibrates (gemfibrozil, bezafibrate, and fenofibrate), ezetimibe, and probucol. Background therapies were not stratified by FH type.
The literature suggests that certain treatment barriers may exist, including the invasiveness and duration of the procedure (ranging from 2 to >3 hours),7, 23 patient burden and quality of life,6, 8 and cost/resource utilization.6, 8, 23, 26, 28, 32 Three articles emphasized the disparity between the presumed number of patients who may benefit from LDL‐C apheresis and the number of apheresis centers, suggesting that apheresis may be unavailable for patients in need.7, 8, 27
Apheresis LDL‐C Reduction Efficacy
Acute LDL‐C reduction percentages are presented in Table 2.9, 20, 21, 22, 23, 24, 25, 27 Reductions were consistent across studies: 9 (64%) reported reductions between 60% and 79%, 4 (29%) reported reductions between 50% and 59%, and 1 (7%) reported an 82% reduction. Only 7 studies assessed patients with HoFH and patients with HeFH separately. While patients with HoFH had higher mean LDL‐C levels before and after LDL‐C apheresis compared with patients with HeFH, reduction percentages were similar for both patient groups: 57% and 75% and 58% and 63%, respectively. Studies of patients with HoFH reported post–LDL‐C apheresis LDL‐C levels that ranged from 72 to 148 mg/dL, which were higher than those in studies of patients with HeFH, with a range of 62 to 81 mg/dL.
Table 2.
LDL‐C Level and Percent Acute LDL‐C Reduction Preapheresis and Postapheresis Treatmenta
| Author | Country | Study Type | No. of FH Patients | Mean Age of FH Patients (Range) | Mean Years Receiving Apheresis Treatment | Mode of Apheresis | Mean Preapheresis LDL‐C Level (Range), mg/dL | Mean Postapheresis LDL‐C Level (Range), mg/dL | Mean Acute Reduction (Range) |
|---|---|---|---|---|---|---|---|---|---|
| Keller, 200921 | Germany | Retrospective observational study | HoFH: 12 | Not reported | Not reported | Dextran sulfate adsorption, DALI, and LF | Not reported | Not reported | 63–69% |
| Schamberger, 200038 | Germany | Open‐label clinical trial | HeFH: 22 | 52 y (34–75) | Not reported | Immunoadsorption, dextran sulfate adsorption, HELP, and LF | 166 | 62 | 62.6% |
| Bamabauer, 20039 | Germany | Prospective observational study |
HoFH: 4 HeFH: 41 |
Not reported | Not reported | Dextran sulfate adsorption, immunoadsorption, DALI, and immunoadsorption with special anti‐lipoprotein(a) columns | 297.6 | 131.3 | 55.9% |
| Julius, 201343 (UHD) | Germany | Prospective observational study | Unspecified: 64 | Not reported | Not reported | HELP, LF, DALI, dextran sulfate adsorption, and immunoadsorption | 123.7 | 38.7 | 69% (39–88%) |
| Julius, 201343 (other centers) | Germany | Prospective observational study |
HoFH: 1 Unspecified: 54 |
Not reported | Not reported | HELP, LF, MONET, DALI, dextran sulfate adsorption, and immunoadsorption | 139.2 | 42.5 | 69% (26–95%) |
| van Buuren, 201237 | Germany | Retrospective observational study | FH unspecified: 27 | 49 y (10–67) | Not reported | HELP | 223 | 83 | 63% |
| Masaki, 200524 | Japan | Open‐label clinical trial | HeFH: 18 | 48 y (35–73) | 9.8 y | Dextran sulfate adsorption | 157 | 66.3 | 57.8% |
| Archontakis, 200727 | UK | Retrospective observational study | HeFH: 7 | 56.3 y (35–66) | Not reported | Dextran sulfate adsorption | 212.3 (155.5–344.2)a | 81.2 (38.7–150.8)a | 62% (56–75%) |
| Graesda,l 2012b, 36 | Norway | Prospective observational study | HoFH: 8 | 30 (7–55) | 12.4 | Whole blood column and plasma filtration | 197 (174–282) | 85 (50–108) | 57% |
| Coker, 200925 | Turkey | Open‐label clinical trial | HoFH: 10 | 8.4 y (2–12) | Not reported | DFPP and dextran sulfate adsorption | 375.5 (193–643) | 147.5 (74–281) | 62.8% (43–73%) |
| Hudgins, 200822 | USA | Prospective observational study | HoFH: 29 | 15 y (5–27) | 6 y | Dextran sulfate adsorption | 521 (243–713) | Not reported | 75% (68–83%) |
| Opole, 200741 | USA | Open‐label clinical trial | FH unspecified: 13 | 56 y | Not reported | HELP and dextran sulfate adsorption | 208 | 99 | 52% |
| Blaha, 200944 | Czech Republic | Prospective observational study |
HoFH: 3 HeFH: 9 |
47 y (21–63) | 7.2 y | Immunoadsorption | Not reported | Not reported | 82% |
| Palcoux, 200820 | France | Prospective observational study |
HoFH: 11 HeFH: 13 non‐FH: 3 |
21.1 (9–29) | Not reported | DALI and dextran sulfate adsorption | 356 | 100 | 72% |
| Kolovou, 201223 | Italy | Open‐label clinical trial |
HoFH: 2 HeFH and non‐FH: 19 |
HoHF: 12 y (11–13) HeFH and non‐FH: 44 y |
3.9 y | DALI |
HoFH: 288 HeFH and non‐FH: 196 |
HoFH: 72 HeFH and non‐FH: 53 |
HoFH: 76% HeFH and non‐FH: 72% |
DALI indicates direct adsorption of lipoproteins; DFPP, double filtration plasma apheresis; FH, familial hypercholesterolemia; HeFH, heterozygous FH; HELP, heparin‐induced extracorporeal lipoprotein precipitation; HoFH, homozygous FH; LDL‐C, low‐density lipoprotein cholesterol; LF, lipid filtration; MONET, membrane filtration optimized novel extracorporeal treatment; UHD, University Hospital Carl Gustav Carus in Dresden.
Acute LDL‐C reduction is defined by LDL‐C reduction immediately after an apheresis treatment session.
Conversion used, 1 mmol/L=38.66976 mg/dL.
Median LDL‐C levels presented.
Concomitant lipid‐lowering therapies included statins (simvastatin, lovastatin, atorvastatin, pravastatin, and rosuvastatin), fibrates (gemfibrozil, bezafibrate, and fenofibrate), ezetimibe, and probucol, and use of these therapies was not stratified by FH type.
Despite the efficacy of LDL‐C apheresis in lowering LDL‐C levels, some studies reported that cholesterol concentrations may gradually increase after LDL‐C apheresis treatment and reach pretreatment levels within 2 to 4 weeks.8, 34, 39 Only 4 studies reported mean interval LDL‐C levels (ranging between 102 and 272 mg/dL), with 2 studies reporting a percent interval reduction of nearly 50% (Table 3).22, 24, 36, 43 Additionally, 5 studies assessed the LDL‐C reduction during an extended follow‐up period (range 1–5 years) of LDL‐C apheresis treatments, which ranged between 22% and 36% compared with baseline LDL‐C levels (Table 4).22, 25, 27, 40, 44
Table 3.
Interval LDL‐C Reduction Between Apheresis Treatments
| Author | Country | Study Type | No. of FH Patients | Interval Between Apheresis Treatment Sessions, d | Mean Interval LDL‐C Level, mg/dL | Percent Interval Reduction From Baseline |
|---|---|---|---|---|---|---|
| Julius, 201343 (UHD) | Germany | Prospective observational study | Unspecified: 64 | Variablea | 101.7 | Not reported |
| Julius, 201343 (other centers) | Germany | Prospective observational study |
HoFH: 1 Unspecified: 54 |
Variableb | 112.5 | Not reported |
| Masaki, 200524 | Japan | Open‐label clinical trial | HeFH: 18 | 30.1 (mean) | 146 | 46% |
| Graesdal, 2012a, 36 | Norway | Prospective observational study | HoFH: 8 | 7 | 162 | Not reported |
| Hudgins, 200822 | USA | Prospective observational study | HoFH: 29 | 14–21 (mean) | 272 | 48% |
LDL‐C indicates low‐density lipoprotein cholesterol; FH, familial hypercholesterolemia; HoFH, homozygous FH; HeFH, heterozygous FH; UHD, University Hospital Carl Gustav Carus in Dresden.
Weekly (68.8%), biweekly (20.3%), once per month (9.4%), once in 8 wk (1.6%), twice per week (0%).
Weekly (74.5%), biweekly (18.2%), once per month (0%), once in 8 wk (0%), twice per week (7.3%).
Table 4.
Reduction of LDL‐C Over an Extended Follow‐up Period From First to Last Session of Apheresis
| Author | Country | Study Type | No. of FH Patients | Follow‐up Time | Mean LDL‐C Reduction Level (Range) mg/dL | Mean Percent LDL‐C Reduction |
|---|---|---|---|---|---|---|
| Archontakis 200727 | UK | Retrospective observational study | HeFH: 7 | 4 y 4 mo | 218.5 (159.7–323.3)a | 21.7% |
| Matsuzaki, 200240 | Japan | Open‐label clinical trial | HeFH: 19 | 1 y | 140 | 34.3% |
| Coker, 200925 | Turkey | Open‐label clinical trial | HoFH: 10 | 5 y | 238.8 | 36.4% |
| Hudgins, 200822 | USA | Prospective observational study | HoFH: 29 | Not reported | 341 | 34% |
| Blaha, 200944 | Czech Republic | Prospective observational study |
HoFH: 3 HeFH: 9 |
3 y | 201.1 | Not reported |
LDL‐C indicates low‐density lipoprotein cholesterol; FH indicates familial hypercholesterolemia; HeFH, heterozygous FH; HoFH, homozygous FH.
Conversion used, 1 mmol/L=38.66976 mg/dL.
There were 5 studies that included children under age 18 in their study population, and of those, 2 studies only included children. These studies reported similar LDL‐C reduction compared with studies of adults. Coker et al reported that the mean acute reduction of LDL‐C in children with HoFH was 63%.25 Palcoux et al reported that the mean acute reduction of LDL‐C in children with HoFH and HeFH was 72% (results were not stratified by FH type).20
Apheresis CVE Reduction
The risk of CVE was calculated in 4 studies with heterogeneous patient populations and varying follow‐up periods. Gordon conducted a retrospective analysis of patients of the US LDL Apheresis Registry and found that patients experienced a 48% decrease in the rate of CVE after apheresis treatment (9.14 CVE before apheresis and 4.72 CVE per 1000 patient‐months after apheresis treatment) but did not stratify by FH type.45 Kolovou et al reported the percentage of event‐free survival for 7 years was 67% but did not stratify the analysis on FH type either. In addition, the analysis contains non–familial hypercholesterolemia patients.23 Sachais et al reported that after treatment with apheresis for an average of 2.5 years, patients with HoFH had a 3.2‐fold decrease in CVEs and a >20‐fold decrease in cardiovascular interventions.42 Masaki et al reported that the event‐free percentages of major coronary events (cardiac death, myocardial infarction) for patients with HeFH were 94% and 89% at 6 and 10 years, respectively.24 In Australian guidelines, it is recognized that patients with HoFH should be classified as exceptionally high risk for CVEs because of increased native levels of LDL‐C.6
Studies that included only children reported progression of cardiovascular disease (CVD) during LDL‐C apheresis treatment. Coker et al reported that 4 of 10 children had coronary heart disease that persisted after apheresis treatment, with 2 patients dying from aortic stenosis.25 Palcoux et al reported that 5 of 27 children experienced CVEs during apheresis treatment: 1 patient with HoFH had an aortic valve replacement, 3 compound patients with HeFH (the presence of two recessive alleles at a particular gene locus that can cause genetic disease) had angina; and 1 patient who was not genetically screened for FH type required surgical management of aortic stenosis.20
Apheresis Safety
The general consensus across studies is that LDL apheresis is well‐tolerated and is a safe means of reducing LDL‐C. The most common side effects reported were hypotension and nausea/vomitting, which ranged between 0.73% and 5.8% and 1% and 2.6% of apheresis treatments, respectively.9, 22, 27, 42 Serious side effects such as serious hypotension and allergic reactions occur very rarely: 0.13–1.3% and 0.2–0.4% of apheresis treatments, respectively.5, 9, 21 Both hypotension and nausea/vomitting were reported at 0.2% of all treatments in a study that only included children with HoFH.25 Problems with access site such as central line infections, puncture difficulties, technical problems, and issues with vascular access have also been reported.23, 26, 27, 46
Cost of LDL‐C Apheresis
LDL‐C apheresis costs were reported for Australia, Germany, France, the United Kingdom, the United States, and Canada, but data sources and cost calculation methodology were often not cited in the articles (Table 5).5, 6, 8, 16, 32, 34, 47 LDL‐C apheresis costs per session typically ranged between $1617 and $2762 across countries, with no stratification by FH type. LDL‐C apheresis costs in Germany and France were the lowest ($1276–$1392).8 Lee et al reported a maximum cost of $4403 in the United States.16 Of the 7 non‐HTA articles that reported LDL‐C apheresis costs or methodology, only 2 reported a source for cost figures; thus, it is difficult to ascertain the accuracy of reported costs.7, 16 Nevertheless, estimates indicate that annual LDL‐C apheresis treatment costs may be substantial considering the frequency of required sessions. Assuming once‐weekly treatment, annual per‐person costs may be as low as $66 374 ($33 187 for biweekly treatment) in Germany and France and as high as $228 956 ($114 478 for biweekly treatment) in the United States.
Table 5.
Costs of Apheresis Treatment, by Country
| Author | Country | FH Type | Cost Per Session (US$ 2015)a | Biweekly Treatment Annual Costs per Person (US$ 2015)b | Weekly Treatment Annual Costs per Person (US$ 2015)b |
|---|---|---|---|---|---|
| Thompson, 200832 | UK | Unspecified | $1617–$1940c | $42 035–$50 435 | $84 070–$100 869 |
| Archontakis, 200834 | UK | Unspecified | $1617–$1940c | $42 035–$50 435 | $84 070–$100 869 |
| Lee, 201116 | UK | Unspecified | $2424c | $63 034 | $126 068 |
| Lee, 201116 | USA | Unspecified | $2202–$4403 | $57 252–$114 478 | $114 504–$228 956 |
| Mehta, 200947 | USA | Unspecified | $2210–$2762 | $57 460–$71 812 | $114 920–$143 624 |
| Vella, 20015 | USA | Unspecified | $2677 | $69 602 | $139 204 |
| Watts, 20116 | Australia | Unspecified | $1723–$2037c | $44 804–$52 967 | $89 618–$105 933 |
| Thompson, 20108 | Germany and France | Unspecified | $1276–$1392c | $33 187–$36 184 | $66 374–$72 367 |
FH indicates familial hypercholesterolemia.
Inflated to 2015 costs using average 2015 US consumer price index, http://www.bls.gov/data/inflation_calculator.htm.
Calculated from costs per session.
Ex‐US$ 2015 calculated from reported currency and year using IRS yearly average exchange rates, https://www.irs.gov/Individuals/International-Taxpayers/Yearly-Average-Currency-Exchange-Rates.
In contrast, LDL‐C apheresis costs in Canada were well documented, and the methodology used to calculate costs was more transparent. The Canadian HTAs calculated costs and analyzed budget impact by considering equipment, maintenance, disposable materials, and physician/nurse costs.26, 28 Equipment costs were fixed over time and divided into annual costs over 10 years. Maintenance costs were reported annually. Disposable equipment supplies and physician/nurse costs were reported per session. For the 13 patients with HoFH and 115 patients with HeFH in Ontario, these translated into annual weekly LDL‐C apheresis treatment costs of $881 930 ($440 965 for biweekly treatment) and $7 875 587 ($3 937 793 for biweekly treatment), respectively. Treatment of the estimated 765 undiagnosed patients with HeFH cost $52 453 324 ($26 226 662 for biweekly treatment).
Discussion
LDL‐C apheresis remains a cornerstone in the treatment of patients with HoFH and more severe HeFH as conventional lipid‐lowering drug therapies are not adequate to control the high levels of LDL‐C in this patient population. However, LDL‐C apheresis treatment patterns are not well described by country, and the global disease burden of FH is unclear. This review demonstrates that (1) there is little consensus on LDL‐C apheresis treatment recommendations in patients with FH across countries, (2) there is a substantial unmet need in treating patients with FH, and (3) there are considerable barriers in accessing LDL‐C apheresis treatment, particularly with respect to cost and availability by geographic location.
Difficulties in characterizing FH disease burden is rooted in the lack of actual country‐specific prevalence estimates for HoFH and HeFH. The cardinal FH epidemiologic figures most often cited in different countries are population prevalence figures of 1:1 million for HoFH and 1:500 for HeFH. These figures are based on analyses of small subsets of patients and the application of the Hardy–Weinberg equation, which requires a large number of assumptions. Moreover, the source data for these prevalence estimates are from studies published before 1980 and may no longer be applicable. Deviations from conventional prevalence figures have been reported in a recent study of the Danish Civil Registration System.48 These investigators found that the prevalence of FH, as diagnosed according to the Dutch Lipid Network criteria, may be as high as 1:137 for definite and probable FH and 1:504 for definite FH alone. Similarly, a recent screening in a northern European population reported an HeFH prevalence of 1:200.1 Thus, the true country‐specific prevalence of FH may be higher than 1:500; this is certainly true for the Netherlands, South Africa, and Quebec, Canada. The study also reported that the available FH prevalence data are inaccurate (ranging from 1:200 to 1:2000) because of a reliance on hospital patients, registry samples, or calculations that used the Hardy–Weinberg equation to estimate prevalence figures. Other issues in characterizing the burden of FH revolve around the underdiagnosis of patients with FH. In Canada and Australia, it is estimated that as few as 10–15% of patients with FH are accurately diagnosed or are diagnosed at all.6, 26 Further, patients often remain undiagnosed until they experience a coronary event. This level of underdiagnosis may result in grave impacts on patient prognosis as guidelines estimate that the risk for developing coronary artery disease for untreated patients with FH is 20 times higher than that for treated patients.4
Despite the findings that apheresis is effective in reducing LDL‐C levels, methodological issues cause difficulties in drawing conclusions from the study results. One issue is that patients with HoFH and HeFH are often aggregated in analyses. Only 63% of the studies included in this review assessed LDL‐C reductions in patients with HoFH and HeFH separately. This lack of stratification by FH type limits the usefulness of study findings and may lead to increased variability in LDL‐C apheresis efficacy results. The results presenting the efficacy of apheresis in reducing LDL‐C in patients with FH may also be misleading. The studies included in this review indicate that while percent LDL‐C reductions for patients with HoFH or HeFH are similar, actual LDL‐C levels are still higher in patients with HoFH compared with patients with HeFH. Further, when interval LDL‐C reduction and LDL‐C reductions over an extended follow‐up period are taken into account, the LDL‐C reduction of apheresis becomes more modest, which is likely related to the rebound of LDL‐C levels after apheresis treatment.7, 8 While studies demonstrate that apheresis decreases the rate of CVE, the duration of follow‐up time reported by these studies may not be long enough to fully capture downstream CVE (range 1–5 years). This may introduce substantial bias and produce lower rates of CVE than if longer follow‐up periods were used. Additionally, reduction in CVE is not reported by using either a standard comparison or standard patient population, with 50% of studies reporting CVE reduction as determined by comparing CVE rates in patients with FH before and after apheresis, 25% reported as event‐free percentage of major coronary events in patients with FH, and 25% reported as event‐free survival with the analysis including patients with FH and those without FH,
The rebound of LDL‐C after apheresis treatments may require weekly or biweekly apheresis treatments to maintain reduced levels of LDL‐C. The frequency of LDL‐C apheresis treatments required for patients with FH is a significant barrier given the invasiveness and cost of treatment, which may impact patient quality of life, reduce access to LDL‐C apheresis, and thus compound the CVD risk in these patients.6, 7, 8, 23 LDL‐C apheresis is an invasive procedure that spans the length of ≥2 hours, which may be undesirable or inconvenient for patients with FH. Patients may also have difficulty accessing LDL‐C apheresis treatment as the availability of LDL‐C apheresis is restricted to specialized centers in many countries. LDL‐C apheresis equipment, disposable supplies, and requirement of intensive medical staff capacity contribute to the often prohibitive cost of LDL‐C apheresis. Because of low availability and high cost, LDL‐C apheresis is often restricted to patients with the most severe FH, typically patients with HoFH or severe HeFH who are treatment refractory. Consequently, it may be unethical to withhold LDL‐C apheresis treatment from patients with FH who are at high risk of CVD and may benefit from LDL‐C apheresis but are ineligible for or cannot gain access to LDL‐C apheresis treatment.28 Given the necessity for lipid‐lowering treatments beyond conventional statins in patients with FH, it is crucial that these areas of unmet need be filled to appropriately treat patients with FH. There is a need for conducting more economic analyses (eg, cost benefit, cost effectiveness, health‐related quality of life analysis) to compare the long‐term costs and tradeoffs associated with LDL‐C apheresis versus other available pharmacological treatments for FH. In addition, communicating and disseminating such evidence effectively would lead to better‐informed patients and improved patient‐centered clinical judgment of their physicians.
A potential limitation of this review is that changes in inflammatory and oxidative status and other lipid parameters (eg, apo‐B, Lp[a]) resulting from apheresis were not included in this review. The largest meta‐analysis of the association between Lp(a) and CVE risk found that for every 3.5‐fold higher Lp(a) concentration, the risk of coronary heart disease increases by 13% after adjusting for age, sex, systolic blood pressure, smoking, history of diabetes, and total cholesterol.49 Although Lp(a) reduction appears to be associated with CVE risk based on meta‐analyses of observational research, the causal link between Lp(a) reduction and CVD prevention and treatment requires evidence from randomized controlled trials.50 To date, there have been no randomized controlled trials s published that assessed the effect of reducing Lp(a) on CVE risk or CV mortality.51, 52, 53 Another issue is that Lp(a) levels vary widely across gender and ethnicities.54 Thus, it is difficult to interpret the results of Lp(a) reduction studies without better characterization of Lp(a) levels and CVE risk across different ethnicities.50, 54 Additionally, efficacy and safety outcomes of FH apheresis studies were not generally stratified by FH type (Ho, He, unspecified), and further exploration of FH apheresis outcomes by FH type may be required. Future research should build on this systematic literature review and include a meta‐analysis to quantitatively summarize the evidence of LDL‐C apheresis for the treatment of FH.
During the timeframe in which this review was conducted, country‐specific guidelines contained varied recommendations for apheresis treatment thresholds, intervals, and LDL‐C targets. Fortunately, recently published European consensus papers and an international FH consensus paper provide a foundation for standardization of FH apheresis treatment across countries.1, 3, 55 However, as mentioned in the American Heart Association's scientific statement regarding the agenda for FH, limitations exists in current available diagnostic schemas; country‐specific models of care for FH are required to increase FH awareness and treatment.2 In addition, several unmet needs remain to be addressed. While observational and non‐randomized trials have shown that LDL‐C reduction with apheresis treatment is associated with decreased CVE, randomized controlled trials are needed to confirm these findings. CVEs may have been triggers for the initiation of LDL‐C apheresis in some patients; therefore the apheresis CVE reduction comparison summarized in this study may not be representative of the actual CVE reduction efficacy of apheresis. Observational and non‐randomized trials also typically have short follow‐up periods so studies with longer follow‐up periods (ie, more than 10 years) will need to be conducted to assess the long‐term efficacy and confirm the safety of apheresis treatment for FH. Finally, other treatment modalities for FH should be explored as the costs, availability, and duration of sessions may be substantial barriers for some patients. Novel LDL‐C lowering treatments may also be needed as many patients on apheresis and standard lipid‐lowering drugs continue to experience progressing CVD.
Sources of Funding
This work is funded by Amgen Inc.
Disclosures
Quek and Gandra are employees and stockholders of Amgen Inc. Richhariya was an employee and current stockholder of Amgen Inc. Calimlim is an employee of ICON plc which was funded by Amgen Inc. to carry out this work. Nordyke, Wang and Kim were employees of ICON Plc which was funded by Amgen Inc. to carry out this work. Toth receives consultancy fees from Amgen Inc.
Supporting information
Table S1. Search Terms for the PubMed Database
Table S2. Familial Hypercholesterolemia Treatment Guideline Recommendations
Table S3. Apheresis Treatment Guidelines Recommendations
Acknowledgments
The authors would like to express their sincere appreciation to Michael L Friedman, MA, (Los Angeles, CA, United States) for his editorial assistance in preparation of this manuscript.
(J Am Heart Assoc.2016;5:e003294 doi: 10.1161/JAHA.116.003294)
References
- 1. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg‐Hansen A; European Atherosclerosis Society Consensus P . Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gidding SS, Ann Champagne M, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes‐Virella M, Watts GF, Wierzbicki AS. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–2192. [DOI] [PubMed] [Google Scholar]
- 3. Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, Bruckert E, Defesche J, Lin KK, Livingston M, Mata P, Parhofer KG, Raal FJ, Santos RD, Sijbrands EJ, Simpson WG, Sullivan DR, Susekov AV, Tomlinson B, Wiegman A, Yamashita S, Kastelein JJ. Integrated guidance on the care of familial hypercholesterolemia from the International FH Foundation. J Clin Lipidol. 2014;8:148–172. [DOI] [PubMed] [Google Scholar]
- 4. Harada‐Shiba M, Arai H, Oikawa S, Ohta T, Okada T, Okamura T, Nohara A, Bujo H, Yokote K, Wakatsuki A, Ishibashi S, Yamashita S. Guidelines for the management of familial hypercholesterolemia. J Atheroscler Thromb. 2012;19:1043–1060. [DOI] [PubMed] [Google Scholar]
- 5. Vella A, Pineda AA, O'Brien T. Low‐density lipoprotein apheresis for the treatment of refractory hyperlipidemia. Mayo Clin Proc. 2001;76:1039–1046. [DOI] [PubMed] [Google Scholar]
- 6. Watts GF, Sullivan DR, Poplawski N, van Bockxmeer F, Hamilton‐Craig I, Clifton PM, O'Brien R, Bishop W, George P, Barter PJ, Bates T, Burnett JR, Coakley J, Davidson P, Emery J, Martin A, Farid W, Freeman L, Geelhoed E, Juniper A, Kidd A, Kostner K, Krass I, Livingston M, Maxwell S, O'Leary P, Owaimrin A, Redgrave TG, Reid N, Southwell L, Suthers G, Tonkin A, Towler S, Trent R. Familial hypercholesterolaemia: a model of care for Australasia. Atheroscler Suppl. 2011;12:221–263. [DOI] [PubMed] [Google Scholar]
- 7. Robinson JG. Management of familial hypercholesterolemia: a review of the recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Manag Care Pharm. 2013;19:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson GR, Catapano A, Saheb S, Atassi‐Dumont M, Barbir M, Eriksson M, Paulweber B, Sijbrands E, Stalenhoef AF, Parhofer KG. Severe hypercholesterolaemia: therapeutic goals and eligibility criteria for LDL apheresis in Europe. Curr Opin Lipidol. 2010;21:492–498. [DOI] [PubMed] [Google Scholar]
- 9. Bambauer R, Schiel R, Latza R. Low‐density lipoprotein apheresis: an overview. Ther Apher Dial. 2003;7:382–390. [DOI] [PubMed] [Google Scholar]
- 10. Gordon B, Kelsey SF, Dau PC, Gotto AM Jr, Graham K, Illingworth DR, Isaacsohn J, Jones PH, Leitman SF, Saal SD, Stein EA, Stern TN, Troendle A, Zwiener RJ. Long‐term effects of low‐density lipoprotein apheresis using an automated dextran sulfate cellulose adsorption system. Liposorber Study Group. Am J Cardiol. 1998;81:407–411. [DOI] [PubMed] [Google Scholar]
- 11. Mabuchi H, Koizumi J, Shimizu M, Kajinami K, Miyamoto S, Ueda K, Takegoshi T; Hokuriku‐FH‐LDL‐Apheresis Study Group . Long‐term efficacy of low‐density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Am J Cardiol. 1998;82:1489–1495. [DOI] [PubMed] [Google Scholar]
- 12. Richter WO, Donner MG, Hofling B, Schwandt P. Long‐term effect of low‐density lipoprotein apheresis on plasma lipoproteins and coronary heart disease in native vessels and coronary bypass in severe heterozygous familial hypercholesterolemia. Metabolism. 1998;47:863–868. [DOI] [PubMed] [Google Scholar]
- 13. Slack J. Inheritance of familial hypercholesterolemia. Atheroscler Rev. 1979;5:35–66. [Google Scholar]
- 14. Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci USA. 1973;70:2804–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldstein J, Hobbs H, Brown M. Lipids: familial hypercholesterolemia In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis S, Ballabio A, Gibson K, Mitchell G, eds. Scriver's Online Metabolic and Molecular Bases of Inherited Disease. McGraw Hill: New York, New York; Available at: http://ommbid.mhmedical.com/content.aspx?bookid=971§ionid=62637931. Accessed March 17, 2015. [Google Scholar]
- 16. Lee WP, Datta BN, Ong BB, Rees A, Halcox J. Defining the role of lipoprotein apheresis in the management of familial hypercholesterolemia. Am J Cardiovasc Drugs. 2011;11:363–370. [DOI] [PubMed] [Google Scholar]
- 17. National Institute for Health and Care Excellence . Indentification and management of familial hypercholesterolaemia: NICE clinical guideline. 2008.
- 18. Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J. Cardiovascular risk reduction in high‐risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. [DOI] [PubMed] [Google Scholar]
- 19. Civeira F. Guidelines for the diagnosis and management of heterozygous familial hypercholesterolemia. Atherosclerosis. 2004;173:55–68. [DOI] [PubMed] [Google Scholar]
- 20. Palcoux JB, Atassi‐Dumont M, Lefevre P, Hequet O, Schlienger JL, Brignon P, Roussel B. Low‐density lipoprotein apheresis in children with familial hypercholesterolemia: follow‐up to 21 years. Ther Apher Dial. 2008;12:195–201. [DOI] [PubMed] [Google Scholar]
- 21. Keller C. LDL‐apheresis in homozygous LDL‐receptor‐defective familial hypercholesterolemia: the Munich experience. Atheroscler Suppl. 2009;10:21–26. [DOI] [PubMed] [Google Scholar]
- 22. Hudgins LC, Kleinman B, Scheuer A, White S, Gordon BR. Long‐term safety and efficacy of low‐density lipoprotein apheresis in childhood for homozygous familial hypercholesterolemia. Am J Cardiol. 2008;102:1199–1204. [DOI] [PubMed] [Google Scholar]
- 23. Kolovou G, Hatzigeorgiou G, Mihas C, Gontoras N, Litras P, Devekousos D, Kontodima P, Sorontila C, Bilianou H, Mavrogeni S. Changes in lipids and lipoproteins after selective LDL apheresis (7‐year experience). Cholesterol. 2012;2012:976578 doi: 10.1155/2012/976578. Epub January 24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masaki N, Tatami R, Kumamoto T, Izawa A, Shimada Y, Takamatsu T, Katsushika S, Ishise S, Maruyama Y, Yoshimoto N. Ten‐year follow‐up of familial hypercholesterolemia patients after intensive cholesterol‐lowering therapy. Int Heart J. 2005;46:833–843. [DOI] [PubMed] [Google Scholar]
- 25. Coker M, Ucar SK, Simsek DG, Darcan S, Bak M, Can S. Low density lipoprotein apheresis in pediatric patients with homozygous familial hypercholesterolemia. Ther Apher Dial. 2009;13:121–128. [DOI] [PubMed] [Google Scholar]
- 26. Health Quality Ontario . Low density lipoprotein aphereis: an evidence‐based analysis. Ontario Health Technology Assessment Series. 2007;5:1–101. [PMC free article] [PubMed] [Google Scholar]
- 27. Archontakis S, Pottle A, Hakim N, Ilsley C, Barbir M. LDL‐apheresis: indications and clinical experience in a tertiary cardiac centre. Int J Clin Pract. 2007;61:1834–1842. [DOI] [PubMed] [Google Scholar]
- 28. Moga C, Harstall C. Low Density Lipoprotein Apheresis for the Treatment of Familial Hypercholesterolemia. Edmonton, AB: Alberta Heritage Foundation for Medical Research; 2004. [Google Scholar]
- 29. Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, Daniels SR, Gidding SS, de Ferranti SD, Ito MK, McGowan MP, Moriarty PM, Cromwell WC, Ross JL, Ziajka PE; National Lipid Association Expert Panel on Familial Hypercholesterolemia . Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1–S8. [DOI] [PubMed] [Google Scholar]
- 30. Heigl F, Hettich R, Eder B, Arendt R. Lipoprotein apheresis standard for apheresis competence centers—an updated synthesis and amendment to pre‐existing standards. Atheroscler Suppl. 2013;14:57–65. [DOI] [PubMed] [Google Scholar]
- 31. Schettler V, Neumann CL, Hulpke‐Wette M, Hagenah GC, Schulz EG, Wieland E. Current view: indications for extracorporeal lipid apheresis treatment. Clin Res Cardiol Suppl. 2012;7:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson GR. Recommendations for the use of LDL apheresis. Atherosclerosis. 2008;198:247–255. [DOI] [PubMed] [Google Scholar]
- 33. Szczepiorkowski ZM, Winters JL, Bandarenko N, Kim HC, Linenberger ML, Marques MB, Sarode R, Schwartz J, Weinstein R, Shaz BH. Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the apheresis applications committee of the American Society for Apheresis. J Clin Apher. 2010;25:83–177. [DOI] [PubMed] [Google Scholar]
- 34. Archontakis S, Pottle A, Barbir M. Low‐density lipoprotein‐apheresis: an update. Br J Cardiol. 2008;15:83–85. [Google Scholar]
- 35. Goldammer A, Wiltschnig S, Heinz G, Jansen M, Stulnig T, Horl WH, Derfler K. Atorvastatin in low‐density lipoprotein apheresis‐treated patients with homozygous and heterozygous familial hypercholesterolemia. Metabolism. 2002;51:976–980. [DOI] [PubMed] [Google Scholar]
- 36. Graesdal A, Bogsrud MP, Holven KB, Nenseter MS, Narverud I, Langslet G, Brekke M, Retterstol K, Arnesen KE, Ose L. Apheresis in homozygous familial hypercholesterolemia: the results of a follow‐up of all Norwegian patients with homozygous familial hypercholesterolemia. J Clin Lipidol. 2012;6:331–339. [DOI] [PubMed] [Google Scholar]
- 37. van Buuren F, Kreickmann S, Horstkotte D, Kottmann T, Mellwig KP. HELP apheresis in hypercholesterolemia and cardiovascular disease: efficacy and adverse events after 8,500 procedures. Clin Res Cardiol Suppl. 2012;7:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schamberger BM, Geiss HC, Ritter MM, Schwandt P, Parhofer KG. Influence of LDL apheresis on LDL subtypes in patients with coronary heart disease and severe hyperlipoproteinemia. J Lipid Res. 2000;41:727–733. [PubMed] [Google Scholar]
- 39. Yamamoto A, Harada‐Shiba M, Kawaguchi A, Oi K, Kubo H, Sakai S, Mikami Y, Imai T, Ito T, Kato H, Endo M, Sato I, Suzuki Y, Hori H. The effect of atorvastatin on serum lipids and lipoproteins in patients with homozyous familial hypercholesterolemia undergoing LDL‐apheresis therapy. Atherosclerosis. 2000;153:89–98. [DOI] [PubMed] [Google Scholar]
- 40. Matsuzaki M, Hiramori K, Imaizumi T, Kitabatake A, Hishida H, Nomura M, Fujii T, Sakuma I, Fukami K, Honda T, Ogawa H, Yamagishi M. Intravascular ultrasound evaluation of coronary plaque regression by low density lipoprotein‐apheresis in familial hypercholesterolemia: the low density lipoprotein‐apheresis coronary morphology and reserve trial (LACMART). J Am Coll Cardiol. 2002;40:220–227. [DOI] [PubMed] [Google Scholar]
- 41. Opole IO, Belmont JM, Kumar A, Moriarty PM. Effect of low‐density lipoprotein apheresis on inflammatory and noninflammatory high‐density lipoprotein cholesterol. Am J Cardiol. 2007;100:1416–1418. [DOI] [PubMed] [Google Scholar]
- 42. Sachais BS, Katz J, Ross J, Rader DJ. Long‐term effects of LDL apheresis in patients with severe hypercholesterolemia. J Clin Apher. 2005;20:252–255. [DOI] [PubMed] [Google Scholar]
- 43. Julius U, Taseva K, Fischer S, Passauer J, Bornstein SR. Current situation of lipoprotein apheresis in Saxony. Atheroscler Suppl. 2013;14:51–55. [DOI] [PubMed] [Google Scholar]
- 44. Blaha M, Zadak Z, Blaha V, Andrys C, Havel E, Vyroubal P, Blazek M, Filip S, Lanska M, Maly J. Extracorporeal LDL cholesterol elimination (25 years of experience in CZ). Atheroscler Suppl. 2009;10:17–20. [DOI] [PubMed] [Google Scholar]
- 45. Gordon BR. Incorporation of low‐density lipoprotein apheresis into the treatment program of patients with severe hypercholesterolemia. Curr Atheroscler Rep. 2000;2:308–313. [DOI] [PubMed] [Google Scholar]
- 46. American Society for Apheresis . Procedure: LDL‐apheresis 2011. Available at: http://www.apheresis.org/resource/resmgr/fact_sheets_file/ldl_apheresis.pdf. Accessed March 18, 2015.
- 47. Mehta PK, Baer J, Nell C, Sperling LS. Low‐density lipoprotein apheresis as a treatment option for hyperlipidemia. Curr Treat Options Cardiovasc Med. 2009;11:279–288. [DOI] [PubMed] [Google Scholar]
- 48. Benn M, Watts GF, Tybjaerg‐Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol‐lowering medication. J Clin Endocrinol Metab. 2012;97:3956–3964. [DOI] [PubMed] [Google Scholar]
- 49. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. J Am Med Assoc. 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg‐Hansen A. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Phan BAP, Toth PP. Lipoprotein(a): epidemiology, atherogenic activity and impact on cardiovascular risk. Clin Lipidol. 2013;8:195–203. [Google Scholar]
- 52. Allian‐Sauer MU, Falko JM. Role of apheresis in the management of familial hypercholesterolemia and elevated Lp(a) levels. Clin Lipidol. 2011;6:523–538. [Google Scholar]
- 53. Orso E, Ahrens N, Kilalic D, Schmitz G. Familial hypercholesterolemia and lipoprotein(a) hyperlipidemia as independent and combined cardiovascular risk factors. Atheroscler Suppl. 2009;10:74–78. [DOI] [PubMed] [Google Scholar]
- 54. Joshi PH, Krivitsky E, Qian Z, Vazquez G, Voros S, Miller J. Do we know when and how to lower lipoprotein(a)? Curr Treat Options Cardiovasc Med. 2010;12:396–407. [DOI] [PubMed] [Google Scholar]
- 55. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen‐Thiessen E, Tybjaerg‐Hansen A, Watts GF, Averna M, Boileau C, Boren J, Catapano AL, Defesche JC, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Ray KK, Stalenhoef AF, Stroes E, Taskinen MR, Wiegman A, Wiklund O, Chapman MJ. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Terms for the PubMed Database
Table S2. Familial Hypercholesterolemia Treatment Guideline Recommendations
Table S3. Apheresis Treatment Guidelines Recommendations


