Abstract
Background
Chronic kidney disease (CKD) increases cardiovascular disease (CVD) risk. However, the association of mildly reduced kidney function with CVD risk is unclear.
Methods and Results
This study investigated the association of estimated glomerular filtration rate (eGFR) with prevalent CVDs, 10‐year Framingham risk for coronary heart disease (CHD), and 10‐year risk of atherosclerotic cardiovascular diseases (ASCVD) in 239 832 participants from the baseline of the Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study. With an interviewer‐assisted questionnaire, we collected information on CVD, including reported CHD, stroke, or myocardial infarction. Chronic Kidney Disease–Epidemiology Collaboration (CKD‐EPI) equation was used to calculate eGFR. Compared with individuals with normal eGFR (≥90 mL/min per 1.73 m2), those with decreased eGFR (75–89, 60–74, and <60 mL/min per 1.73 m2) had higher risk of prevalent obesity, diabetes mellitus, hypertension, and dyslipidemia in both men and women (P for trend all <0.001). Moreover, a significantly higher 10‐year Framingham risk for CHD and 10‐year risk for ASCVD was observed in both men and women with mildly decreased eGFR (60–89 mL/min per 1.73 m2).
Conclusions
Even mildly reduced eGFR (under 90 mL/min per 1.73 m2) is associated with elevated 10‐year Framingham risk for CHD and 10‐year ASCVD risk among Chinese adults.
Keywords: atherosclerotic cardiovascular diseases, cardiovascular diseases, estimated glomerular filtration rate, Framingham Risk Score, reduced kidney function
Subject Categories: Cardiovascular Disease, Epidemiology, Lifestyle, Risk Factors
Introduction
Chronic kidney disease (CKD) increases cardiovascular morbidity and mortality. Studies have found a significant association between the severity of CKD (stage III–V) and cardiovascular disease (CVD) risk among the general population1, 2, 3 and among high‐risk populations with a history of hypertension, diabetes mellitus, or CVD.4 However, the association of mildly reduced kidney function with the risk of coronary heart disease (CHD) and stroke is unclear. Some studies suggested that subjects with mildly decreased estimated glomerular filtration rate (eGFR; 60–89 mL/min per 1.73 m2) were at a significantly higher risk of CHD and stroke.5 In contrast, a recent collaborative meta‐analysis of general‐population–based cohort studies suggested that cardiovascular mortality risk was relatively constant at eGFR 75 to 105 mL/min per 1.73 m2.6
Lifetime risk of CVD is substantial, and the condition is often silent or may occur without warning, underscoring the importance of prevention.7 Efforts to estimate absolute CVD risk of individuals have developed numerous risk prediction tools that synthesize vascular risk factors. Among them, the Framingham Risk Score (FRS) equation was widely accepted. The FRS was first developed based on data obtained from the Framingham Heart Study, to estimate the 10‐year risk of developing CHD.8, 9 On the other hand, the new Pooled Cohort Risk Assessment Equations that were developed to estimate 10‐year risk of atherosclerotic cardiovascular diseases (ASCVD) based on pooled data from 5 large National Institute of Health–funded cohorts, has drawn much debate.10, 11, 12 The equations were used in a recent American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults, which recommends that persons without known CVD should be treated with statins if their 10‐year risk of a cardiovascular event (myocardial infarction, stroke, or cardiovascular death) exceeds 7.5%.12, 13 In addition to traditional cardiovascular risk factors, including age, sex, high blood pressure, smoking, dyslipidemia, and diabetes mellitus, other factors, such as renal function, are under investigation in association with CVD. To date, few studies have examined whether mildly reduced kidney function was related with 10‐year risk for a first hard ASCVD event.
Accordingly, the objective of the present study was to evaluate associations between mildly reduced kidney function with cardiometabolic risk factors as well as prevalent and predicted risk of CVD in Chinese adults. This study is a cross‐sectional analysis of data collected at baseline in the Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study, which was a nation‐wide prospective observational cohort study launched in 2011.14, 15, 16 The present analysis examined associations of eGFR with CVD risk factors as well as prevalent CVDs, 10‐year risk for CHD (FRS) and 10‐year risk for a first hard ASCVD event in this large Chinese population age 40 years or older.
Methods
Study Population
The REACTION study was conducted among 259 657 adults, ages 40 years and older, in 25 communities across mainland China, from 2011 to 2012. The methodology of REACTION study has been described previously.14, 15, 16 Eligible study participants were identified from the local residence registration records and must be age 40 years or older. There was no restriction on sex or ethnicity. Each eligible subject was approached by trained local community workers using a door‐to‐door invitation method. Those who agreed to participate and signed informed consent were scheduled for a personal interview and a clinic visit within a week after the recruitment. The clinic visit took place at the local health stations or community clinics in the participants’ residential area. Participants were asked to keep fasting for 10 hours and to provide a first morning spot urine sample before coming to their clinic appointment. All examinations were scheduled in the morning. The personal interview was conducted by trained study personnel using a structured questionnaire. A total of 284 460 eligible subjects consented for the study and 260 738 (91.7%) completed a personal interview and 271 404 (95.4%) donated a blood sample. Among the 260 738 participants who completed the personal interview, 1081 people were later found to be younger than 40 years. After excluding these subjects, the cohort had a total of 259 657 participants with complete recruitment. Of these, we excluded subjects age 79 years or older (n=3891) and those with missing data on body mass index (BMI; n=5308), blood pressure (n=787), serum creatinine (n=1003), blood glucose (n=8808), cholesterol (n=7), or high‐density lipoprotein (HDL; n=21). Finally, 239 832 participants were included in the analysis.
The study was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao‐Tong University School of Medicine and conducted in accord with institutional guidelines. All study participants provided written informed consent.
Data Collection
Interviews about sociodemographic, medical history, family history, and lifestyle factors were conducted by trained personnel using the standard questionnaire. Body weight, height, waist circumference, and blood pressure (BP) were measured according to a standard protocol and were performed by experienced nurses. BMI was calculated as body weight in kilograms divided by body height squared in meters (kg/m2). Waist circumference was measured at umbilical level in a standing position. BP was measured with an automated electronic device (OMRON Model HEM‐752 FUZZY; Omron Company, Dalian, China) in a seated position 3 times consecutively with a 1‐minute interval each. The 3 readings of systolic BP (SBP) and diastolic BP (DBP) were averaged for analysis. Participants who smoked 1 cigarette per day or 7 per week regularly during the past 6 months were defined as current smokers. The type and frequency of alcohol consumption were recorded, and those who consumed alcohol once per week regularly during the past 6 months were defined as current drinkers. The International Physical Activity Questionnaire was used to estimate physical activity during work, transportation, and leisure time by collecting intensity, duration, and frequency of physical activity.
Participants without a known history of diabetes mellitus underwent the oral glucose tolerance test, and plasma glucose was obtained at 0 and 2 hours during the test. Plasma glucose concentrations were evaluated at local hospitals using the glucose oxidase or hexokinase method within 2 hours after blood sample collection under a stringent quality‐control mechanism. The Hemoglobin Capillary Collection System (Bio‐Rad Laboratories, Hercules, CA) was used to collect finger capillary whole blood from each participant and shipped to the central laboratory of the study. Sera were aliquoted into 0.5‐mL Eppendorf tubes within 2 hours and shipped by air in dry ice to the central laboratory of the study located at Shanghai Institute of Endocrine and Metabolic Diseases, which is certified by the College of American Pathologists. The level of hemoglobin A1c (HbA1c) was determined by using the method of high‐performance liquid chromatography (VARIANT II and D‐10 Systems; Bio‐Rad). Serum insulin, total cholesterol, low‐density lipoprotein (LDL) cholesterol (LDL‐C), HDL cholesterol (HDL‐C), triglycerides, and creatinine were measured using an autoanalyzer (ARCHITECT ci16200 analyzer; Abbott Laboratories, Abbott, IL). The index of homeostasis model assessment of insulin resistance (HOMA‐IR) was calculated according to the formula: HOMA‐IR=fasting insulin concentrations (mIU/L)×fasting plasma glucose concentrations (mmol/L)/22.5.
Classification and Definition
Kidney function was defined on the basis of eGFR, calculated using the Chronic Kidney Disease–Epidemiology Collaboration (CKD‐EPI) equation.17 Participants were divided into 4 groups according to eGFR levels of ≥90, 75–89, 60–74, and <60 mL/min per 1.73 m2.
General overweight was defined as BMI of 24.0 to ≤27.9 kg/m2 and obesity was defined as BMI of 28.0 kg/m2 or higher, according to World Health Organization definitions.
Diabetes mellitus was diagnosed as fasting plasma glucose ≥7.0 mmol/L, or postprandial plasma glucose ≥11.1 mmol/L, or self‐reported previous diagnosis of diabetes mellitus by physicians and taking antidiabetic medications. Hypertension was assessed by SBP ≥140 mm Hg, or DBP ≥90 mm Hg, or self‐reported previous diagnosis of hypertension by physicians and taking antihypertensive medications. Dyslipidemia was defined according to the modified National Cholesterol Education Program–Adult Treatment Panel III (NCEP‐ATP III) as: hypercholesterolemia, total cholesterol ≥6.22 mmol/L (240 mg/dL); hypertriglyceridemia, triglycerides ≥2.26 mmol/L (200 mg/dL), high LDL‐C, LDL‐C ≥4.14 mmol/L (160 mg/dL), and low HDL‐C, HDL‐C <1.04 mmol/L (40 mg/dL).
With an interviewer‐assisted questionnaire, we collected information on CVDs. The question was open‐ended: “Has a doctor or other health professional ever told you that you have CHD, stroke, or myocardial infarction?” We grouped CVDs (reported CHD, stroke, or myocardial infarction) in the analysis. Validation of the self‐reported CVD was performed in Shanghai Youyi Community, 1 of the 25 communities. The medical records from the relevant hospitalizations were reviewed by 2 physicians, who were blind to the self‐reported data, classified the cases as definite, questionable, or misdiagnosed, and the validation rate of CVD was 91.1%.16
The FRS was calculated according to the NCEP‐ATP III algorithm, based on coronary risk factors including age, sex, total cholesterol, HDL‐C, SBP, and smoking habit.18 The calculated total scores were used to estimate the 10‐year CHD risk in participants without previous CVD or diabetes mellitus. FRS >20% in men was considered as high for 10‐year CHD risk.18
Estimated 10‐year ASCVD risk score was calculated using the new Pooled Cohort Risk Assessment Equations developed from hazards models that included the covariates of sex, age, treated or untreated SBP level, total cholesterol and HDL‐C levels, current smoking status (yes or no), and history of diabetes mellitus (yes or no), according to the recommendation of ACC/AHA Task Force on Practice Guidelines,19 and ASCVD scores ≥7.5% were identified as high risk for 10‐year ASCVD in participants without previous CVD.
Statistical Analysis
All statistical analyses were performed with SAS software (version 9.3; SAS Institute Inc., Cary, NC). Analyses were performed for men and women separately. Participants were categorized into 4 groups according to eGFR levels of ≥90, 75–89, 60–74, or <60 mL/min per 1.73 m2. Cardiovascular risk factors are presented as medians (interquartiles) for continuous variables, or numbers (percentages) for categorical parameters. Logistic regression analysis was used to determine the relationships of kidney function with cardiometabolic risk factors, previous CVDs, 10‐year CHD risk, and 10‐year ASCVD risk. Two sets of models were used: model 1, age‐adjusted; model 2, adjusted for traditional risk factors of CVDs, including education attainment (high school education or above or not), current cigarette smoking (yes/no), current alcohol consumption (yes/no), metabolic equivalent of task minutes per week (MET‐min/week), HbA1c, SBP, DBP, dyslipidemia (yes/no), angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) use (yes/no), statin use (yes/no), and insulin use (yes/no). Results of logistic regression analysis are reported as odds ratios (ORs) and 95% CIs.
We then tested potential interactions of eGFR with age, sex, diabetes mellitus, and hypertension in association with CVD risk by adding interaction terms to the multivariate logistic regression models. Results of logistic regression analysis are reported as ORs and 95% CIs. P value for interaction was calculated by a likelihood ratio test comparing models with and without the interaction term. The statistical tests were 2‐sided, and P<0.05 was considered statistically significant.
Results
General characteristics of the study population by eGFR categories are presented in Table 1. The mean age (years) of participants with normal eGFR (≥90 mL/min per 1.73 m2) was 56 in men and 54 in women. Compared with individuals with normal eGFR, men and women with reduced kidney function or CKD were much older, having a lower proportion of current smoker, higher levels of fasting and postprandial blood glucose, HbA1c, HOMA‐IR, BP, serum cholesterol, triglyceride, and LDL and lower HDL level, as well as a larger waist circumference. Logistic regression analysis indicated that the reduced eGFR was associated with a higher prevalence of obesity, diabetes mellitus, hypertension, and dyslipidemia in both men and women (P for trend all <0.05; Figure 1; Table 2).
Table 1.
Cardiovascular Risk Factors in Chinese Adults Ages ≥40 Years With Different Kidney Function Status (REACTION 2011–2012), (N=239 832)
| Variable | Kidney Function (eGFR) | P trend | |||
|---|---|---|---|---|---|
| Normal | Mildly Reduced | CKD | |||
| (≥90) | (75–89) | (60–74) | (<60) | ||
| Men | |||||
| No./% | 59 377/72.20 | 15 834/19.25 | 5215/6.34 | 1820/2.21 | |
| Age, y | 55.9 (48.6–61.7) | 64.5 (57.6–71.0) | 65.4 (59.5–71.5) | 68.1 (61.5–73.4) | <0.0001 |
| High school education or above, % | 39.97 | 44.30 | 44.91 | 40.77 | <0.0001 |
| Current smoker, % | 41.31 | 31.73 | 28.11 | 26.47 | <0.0001 |
| Current drinker, % | 28.33 | 20.51 | 16.60 | 12.93 | <0.0001 |
| Physically active during leisure time, % | 12.7 | 14.9 | 15.7 | 13.9 | <0.0001 |
| BMI, kg/m2 | 24.6 (22.4–26.8) | 24.7 (22.7–26.9) | 25.0 (23.0–27.1) | 24.9 (22.7–27.1) | <0.0001 |
| Waist circumference, cm | 86.5 (80.0–93.0) | 88.0 (81.5–93.8) | 88.0 (82.0–94.0) | 89.0 (82.0–95.0) | <0.0001 |
| SBP, mm Hg | 131 (120–145) | 136 (124–150) | 139 (126–152) | 143 (129–158) | <0.0001 |
| DBP, mm Hg | 80 (73–87) | 80 (72–87) | 80 (73–88) | 80 (73–89) | 0.69 |
| FBG, mmol/L | 5.6 (5.2–6.2) | 5.7 (5.2–6.4) | 5.8 (5.3–6.7) | 5.8 (5.3–6.8) | <0.0001 |
| PBG, mmol/L | 7.0 (5.6–9.3) | 7.7 (6.1–10.5) | 8.0 (6.2–10.9) | 8.6 (6.6–12.2) | <0.0001 |
| HbA1c, % | 5.8 (5.5–6.2) | 5.9 (5.6–6.3) | 5.9 (5.6–6.4) | 6.0 (5.6–6.6) | <0.0001 |
| Total cholesterol, mg/dL | 4.67 (4.00–5.38) | 4.85 (4.22–5.50) | 4.92 (4.26–5.60) | 4.82 (4.17–5.55) | <0.0001 |
| Triglyceride, mg/dL | 1.29 (0.90–1.94) | 1.36 (0.97–2.00) | 1.47 (1.04–2.13) | 1.49 (1.07–2.15) | <0.0001 |
| HDL‐C, mg/dL | 1.20 (1.00–1.45) | 1.21 (1.03–1.43) | 1.20 (1.02–1.40) | 1.16 (0.98–1.37) | 0.74 |
| LDL‐C, mg/dL | 2.68 (2.15–3.24) | 2.83 (2.33–3.37) | 2.90 (2.35–3.46) | 2.84 (2.25–3.39) | <0.0001 |
| HOMA‐IR | 1.51 (0.98–2.31) | 1.68 (1.12–2.55) | 1.90 (1.26–2.86) | 2.05 (1.34–3.18) | <0.0001 |
| Women | |||||
| No./% | 115 690/73.41 | 31 625/20.07 | 7579/4.81 | 2692/1.71 | |
| Age, y | 54.0 (48.0–59.2) | 63.4 (57.4–70.0) | 65.6 (59.3–71.7) | 67.0 (60.2–72.4) | <0.0001 |
| High school education or above, % | 34.83 | 28.66 | 25.41 | 23.60 | <0.0001 |
| Current smoker, % | 1.18 | 1.69 | 2.22 | 2.17 | <0.0001 |
| Current drinker, % | 1.65 | 1.82 | 1.60 | 1.66 | 0.33 |
| Physically active during leisure time, % | 11.8 | 13.4 | 13.0 | 11.9 | <0.0001 |
| BMI, kg/m2 | 24.1 (22.0–26.5) | 24.5 (22.3–26.9) | 24.8 (22.6–27.2) | 24.9 (22.5–27.4) | <0.0001 |
| Waist circumference, cm | 81.7 (76.0–88.0) | 84.0 (78.0–91.0) | 86.0 (79.0–92.0) | 86.8 (80–93.5) | <0.0001 |
| SBP, mm Hg | 127 (115–141) | 135 (121–150) | 137 (124–152) | 140 (125–154) | <0.0001 |
| DBP, mm Hg | 76 (70–83) | 76 (70–84) | 77 (70–85) | 77 (70–85) | <0.0001 |
| FBG, mmol/L | 5.4 (5.1–5.9) | 5.6 (5.2–6.3) | 5.7 (5.3–6.6) | 5.8 (5.3–6.8) | <0.0001 |
| PBG, mmol/L | 7.0 (5.9–8.8) | 7.7 (6.3–10.2) | 8.1 (6.5–11.0) | 8.5 (6.6–12.4) | <0.0001 |
| HbA1c, % | 5.8 (5.5–6.1) | 5.9 (5.6–6.3) | 6.0 (5.7–6.5) | 6.1 (5.7–6.7) | <0.0001 |
| Total cholesterol, mg/dL | 4.88 (4.17–5.61) | 5.27 (4.63–5.96) | 5.34 (4.67–6.05) | 5.28 (4.60–6.06) | <0.0001 |
| Triglyceride, mg/dL | 1.23 (0.89–1.75) | 1.48 (1.08–2.08) | 1.59 (1.15–2.24) | 1.69 (1.20–2.49) | <0.0001 |
| HDL‐C, mg/dL | 1.34 (1.12–1.58) | 1.36 (1.16–1.59) | 1.34 (1.15–1.57) | 1.28 (1.09–1.51) | <0.0001 |
| LDL‐C, mg/dL | 2.78 (2.24–3.37) | 3.06 (2.52–3.63) | 3.09 (2.54–3.67) | 3.00 (2.41–3.63) | <0.0001 |
| HOMA‐IR | 1.69 (1.18–2.44) | 1.93 (1.32–2.88) | 2.14 (1.45–3.22) | 2.31 (1.53–3.72) | <0.0001 |
Data are medians (interquartiles) for continuous variables, or percentages for categorical parameters. BMI indicates body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment‐estimated insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; PBG, postprandial blood glucose; REACTION, Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study; SBP, systolic blood pressure.
Figure 1.

Association between kidney function and cardiometabolic risk factors. Prevalence of obesity, diabetes mellitus, hypertension, and dyslipidemia in Chinese adults ages 40 to 79 (REACTION 2011–2012), stratified by estimated glomerular filtration rate (eGFR) status in men (A) and women (B). REACTION indicates Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study.
Table 2.
Association Between Kidney Function and Cardiovascular Risk Factors in Chinese Adults Ages ≥40 Years (REACTION 2011–2012), (N=239 832)
| Normal | Mildly Reduced Kidney Function | CKD | P trend | ||
|---|---|---|---|---|---|
| (eGFR ≥90) | (eGFR 75–89) | (eGFR 60–74) | (eGFR <60) | ||
| Obesity | |||||
| Men | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 1.23 (1.17–1.30)a | 1.39 (1.29–1.50)a | 1.34 (1.19–1.51)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)b | 1.00 | 1.17 (1.12–1.24)a | 1.26 (1.16–1.37)a | 1.10 (0.96–1.25) | <0.001 |
| Women | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 0.96 (0.92–0.99) | 1.15 (1.05–1.19)a | 1.21 (1.10–1.33)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)b | 1.00 | 0.96 (0.92–0.99) | 1.07 (1.00–1.14)a | 1.12 (1.01–1.24)a | 0.08 |
| Diabetes mellitus | |||||
| Men | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 1.10 (1.05–1.14)a | 1.28 (1.20–1.36)a | 1.74 (1.59–1.91)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)c | 1.00 | 1.06 (1.01–1.10)a | 1.12 (1.05–1.19)a | 1.47 (1.34–1.62)a | <0.001 |
| Women | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 1.10 (1.07–1.14)a | 1.26 (1.19–1.33)a | 1.69 (1.56–1.83)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)c | 1.00 | 1.11 (1.07–1.15)a | 1.22 (1.16–1.29)a | 1.58 (1.45–1.71)a | <0.001 |
| Hypertension | |||||
| Men | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 1.16 (1.12–1.21)a | 1.52 (1.43–1.62)a | 2.15 (1.95–2.38)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)d | 1.00 | 1.12 (1.07–1.16)a | 1.42 (1.34–1.52)a | 2.10 (1.88–2.34)a | <0.001 |
| Women | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 1.06 (1.03–1.09)a | 1.24 (1.18–1.31)a | 1.61 (1.48–1.75)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)d | 1.00 | 1.07 (1.04–1.11)a | 1.22 (1.16–1.29)a | 1.56 (1.43–1.70)a | <0.001 |
| Dyslipidemia | |||||
| Men | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 1.08 (1.04–1.12)a | 1.35 (1.27–1.43)a | 1.67 (1.53–1.82)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)e | 1.00 | 0.97 (0.93–1.01) | 1.16 (1.09–1.23)a | 1.43 (1.30–1.58)a | <0.001 |
| Women | |||||
| Age‐adjusted OR (95% CI) | 1.00 | 1.01 (0.98–1.04) | 1.13 (1.07–1.18)a | 1.38 (1.28–1.48)a | <0.001 |
| Multivariable‐adjusted OR (95% CI)e | 1.00 | 0.99 (0.96–1.01) | 1.06 (1.01–1.12)a | 1.28 (1.18–1.38)a | <0.001 |
CKD indicates chronic kidney disease; eGFR, estimated glomerular filtration rate; OR, odds ratio; REACTION, Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study.
Significant data.
Multivariable model adjusted for age, education attainment, current smoking status, current alcohol consumption, metabolic equivalent of task minutes per week (MET‐min/week), hemoglobin A1c (HbA1c), systolic blood pressure (SBP), diastolic blood pressure (DBP), and hyperlipidemia (yes/no).
Multivariable model adjusted for age, body mass index (BMI), education attainment, current smoking status, current alcohol consumption, MET‐min/week, SBP, DBP, and hyperlipidemia (yes/no).
Multivariable model adjusted for age, BMI, education attainment, current smoking status, current alcohol consumption, MET‐min/week, HbA1c, and hyperlipidemia (yes/no).
Multivariable model adjusted for age, BMI, education attainment, current smoking status, current alcohol consumption, MET‐min/week, HbA1c, SBP, and DBP.
The association between eGFR and prevalence of CVD was evaluated. A total of 5463 (6.64%) men and 9364 (5.94%) women had a history of CVD, including 3662 (4.77%) of CHD, 623 (0.81%) of myocardial infarction, and 1701 (2.22%) of stroke in men, and 7547 (5.13%) of CHD, 426 (0.29%) of myocardial infarction, and 1969 (1.34%) of stroke in women. A significantly increased prevalent CVD risk was observed in male and female participants with an eGFR under 60 mL/min per 1.73 m2. If stratified by eGFR (≥90, 75–89, 60–74, and <60 mL/min per 1.73 m2), the prevalence of self‐reported CVD was 5.07%, 9.82%, 11.63%, and 15.99% in men, and 4.36%, 9.53%, 12.17%, and 14.38% in women, respectively (Table 3). Multivariable‐adjusted ORs (95% CI) of prevalent CVD risk associated with estimated GFR ≥90, 75–89, 60–74, and <60 mL/min per 1.73 m2 were 1.00, 1.02 (0.95–1.10), 1.06 (0.96–1.18), and 1.21 (1.05–1.40; P trend=0.02) for men, and 1.00, 0.95 (0.90–1.01), 1.02 (0.94–1.11), and 1.13 (1.00–1.28) (P trend=0.29) for women, respectively.
Table 3.
Association Between Kidney Function and Cardiovascular Diseases in Chinese Adults Ages ≥40 Years (REACTION 2011–2012)
| Normal | Mildly Reduced Kidney Function | CKD | P trend | ||
|---|---|---|---|---|---|
| (eGFR ≥90) | (eGFR 75–89) | (eGFR 60–74) | (eGFR <60) | ||
| Cardiovascular diseasesa (n=289 832) | |||||
| Men (n=82 246) | |||||
| Case percentage (%) | 3010 (5.1) | 1555 (9.8) | 607 (11.6) | 291 (16.0) | |
| Age‐adjusted OR (95% CI) | 1.00 | 1.10 (1.03–1.18)b | 1.25 (1.14–1.38)b | 1.61 (1.40–1.85)b | <0.001 |
| Multivariable‐adjusted OR (95% CI)c | 1.00 | 1.02 (0.95–1.10) | 1.06 (0.96–1.18) | 1.21 (1.05–1.40)b | 0.02 |
| Women (n=157 586) | |||||
| Case percentage (%) | 5041 (4.4) | 3014 (9.5) | 922 (12.2) | 387 (14.4) | |
| Age‐adjusted OR (95% CI) | 1.00 | 0.98 (0.93–1.03) | 1.12 (1.03–1.21)b | 1.28 (1.13–1.44)b | 0.001 |
| Multivariable‐adjusted OR (95% CI)c | 1.00 | 0.95 (0.90–1.01) | 1.02 (0.94–1.11) | 1.13 (1.00–1.28)b | 0.22 |
| 10‐year High Framingham risk for CHDd (n=181 683) | |||||
| Men (n=59 360) | |||||
| Case percentage (%) | 1833 (4.1) | 1010 (9.6) | 411 (12.8) | 157 (16.6) | |
| Age‐adjusted OR (95% CI) | 1.00 | 1.31 (1.20–1.43)b | 1.73 (1.53–1.95)b | 2.03 (1.68–2.44)b | <0.001 |
| Multivariable‐adjusted OR (95% CI)e | 1.00 | 1.36 (1.23–1.52)b | 1.70 (1.47–1.97)b | 1.72 (1.36–2.17)b | <0.001 |
| Women (n=122 323) | |||||
| Case percentage (%) | 26 (0.0) | 206 (0.9) | 69 (1.5) | 35 (2.4) | |
| Age‐adjusted OR (95% CI) | 1.00 | 1.62 (1.05–2.50)b | 1.84 (1.14–2.98)b | 2.47 (1.43–4.26)b | <0.001 |
| Multivariable‐adjusted OR (95% CI)e | 1.00 | 1.49 (0.94–2.35) | 1.91 (1.15–3.17)b | 1.99 (1.11–3.58)b | <0.001 |
| 10‐year high risk for ASCVDf (n=225 005) | |||||
| Men (n=76 783) | |||||
| Case percentage (%) | 32 346 (57.4) | 11 890 (83.3) | 4062 (88.2) | 1402 (91.7) | |
| Age‐adjusted OR (95% CI) | 1.00 | 1.31 (1.23–1.39)b | 1.54 (1.37–1.73)b | 2.09 (1.65–2.65)b | <0.001 |
| Multivariable‐adjusted OR (95% CI)c | 1.00 | 1.57 (1.44–1.73)b | 1.60 (1.35–1.90)b | 1.41 (1.01–1.97)b | <0.001 |
| Women (n=148 222) | |||||
| Case percentage (%) | 12 793 (11.6) | 13 329 (46.6) | 3741 (56.2) | 1435 (62.3) | |
| Age‐adjusted OR (95% CI) | 1.00 | 1.26 (1.20–1.32)b | 1.54 (1.42–1.67)b | 2.06 (1.79–2.36)b | <0.001 |
| Multivariable‐adjusted OR (95% CI)c | 1.00 | 1.25 (1.17–1.33)b | 1.30 (1.16–1.45)b | 1.52 (1.25–1.84)b | <0.001 |
ASCVD indicates atherosclerotic cardiovascular diseases; CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular diseases; OR, odds ratio; REACTION, Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study.
Analysis was carried out in participants ages 40 to 79 years (n=289 832), and cardiovascular diseases (CVD) was defined as self‐reported CHD, stroke, or myocardial infarction.
Significant data.
Multivariable model adjusted for age, body mass index (BMI), education attainment, current smoking status, current alcohol consumption, metabolic equivalent of task minutes per week (MET‐min/week), hemoglobin A1c (HbA1c), systolic blood pressure (SBP), diastolic blood pressure (DBP), dyslipidemia, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) use (yes/no), statin use (yes/no), and insulin use (yes/no).
Analysis was carried out in participants ages 40 to 79 years, free of CVD and diabetes (n=181 683), and Framingham Risk Score >20% were identified as at high risks for 10‐year CHD.
Multivariable model adjusted for age, BMI, education attainment, current smoking status, current alcohol consumption, MET‐min/week, HbA1c, SBP, DBP, dyslipidemia, ACEI/ARB use (yes/no), and statin use (yes/no).
Analysis was carried out in participants ages 40 to 79 years, free of CVD (n=225 005), and individuals with ASCVD score ≥7.5% were identified as at high risks for 10‐year ASCVD.
We further calculated estimated 10‐year Framingham risk for CHD in participants who were free of CVD and diabetes mellitus (n=181 683) and estimated the 10‐year risk for a first hard ASCVD event in the CVD‐free Chinese adults (n=225 005; Table 3; Figure 2). FRS and 10‐year ASCVD risk was much higher in men than in women. In this study population, the mean (±SD) FRS was 10.77% (±6.54%) in men and 2.17% (±2.72%) in women. Furthermore, 5.75% of men and 0.27% of women had 10‐year Framingham risk for CHD >20%. Mean (±SD) ASCVD risk score was 14.92% (±12.42%) in men and 5.45% (±7.57%) in women. A total of 64.7% men and 21.1% women had ASCVD risk ≥7.5% in this population.
Figure 2.
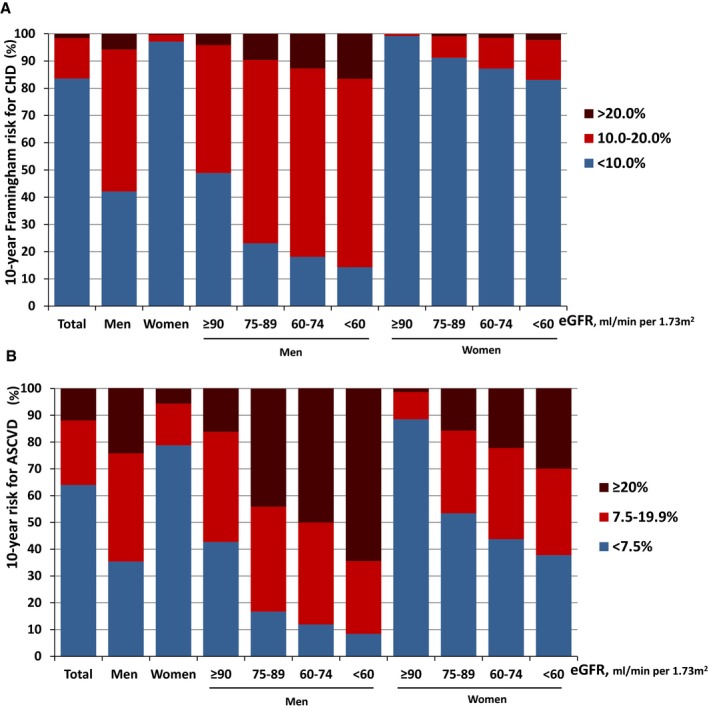
A, Distribution of estimated 10‐year Framingham risk for coronary heart disease (CHD) in the CVD and diabetes mellitus–free Chinese adults ages 40 to 79 (REACTION 2011–2012), stratified by sex and estimated glomerular filtration rate (eGFR) groups (N=181 683). (B) Distribution of estimated 10‐year risk for a first hard atherosclerotic cardiovascular diseases (ASCVD) event in the CVD‐free Chinese adults ages 40 to 79 (REACTION 2011–2012), stratified by sex and eGFR groups (N=225 005). REACTION indicates Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study.
The distribution of the 2 estimated risk scores, stratified by sex and eGFR groups, is illustrated in Figure 2. The 10‐year Framingham risk for CHD and 10‐year ASCVD risk increased with reduction of kidney function in both men and women (P trend<0.001; Figure 2). Multivariable‐adjusted ORs (95% CI) of 10‐year high Framingham risk for CHD associated with eGFR ≥90, 75–89, 60–74, and <60 mL/min per 1.73 m2 were 1.00, 1.36 (1.23–1.52), 1.70 (1.47–1.97), and 1.72 (1.36–2.17; P trend<0.001) for men, and 1.00, 1.49 (0.94–2.35), 1.91 (1.15–3.17), and 1.99 (1.11–3.58; P trend<0.001) for women, respectively (Table 3). The multivariable‐adjusted ORs (95% CI) of 10‐year high ASCVD risk associated with eGFR (≥90, 75–89, 60–74, and <60 mL/min per 1.73 m2) were 1.00, 1.57 (1.44–1.73), 1.60 (1.35–1.90), and 1.41 (1.01–1.97; P trend<0.001) for men, and 1.00, 1.25 (1.17–1.33), 1.30 (1.16–1.45), and 1.52 (1.25–1.84; P trend<0.001) for women, respectively (Table 3).
Next, we analyzed the potential interactions of eGFR with age, sex, diabetes mellitus, and hypertension in modifying CVD risk. As shown in Tables S1 through S3, there are significant interactions of eGFR with age and hypertension in modifying the risk of CVD as measured by self‐reported CVD prevalence, 10‐year Framingham risk for CHD, and 10‐year ASCVD risk. A significant interaction was observed for diabetes mellitus in self‐reported CVD and 10‐year ASCVD risk. Sex showed an interaction with eGFR in modifying the risk of 10‐year CHD only.
Discussion
This study demonstrated that CKD (eGFR <60 mL/min per 1.73 m2) and even mildly reduced eGFR (60–89 mL/min per 1.73 m2) was associated with an increased prevalence of cardiometabolic risk factors, 10‐year high Framingham risk for CHD, and 10‐year high risk for ASCVD. CKD (eGFR <60 mL/min per 1.73 m2) was also associated with a significantly higher prevalence of previous CVD. To the best of our knowledge, this is the first nation‐wide epidemiology study conducted on a community‐based population in mainland China to investigate the association between eGFR, cardiometabolic risk factors, and 10‐year ASCVD risk.
Numerous studies on the epidemiology of CVD in patients with end‐stage renal disease have shown the significant burden of CVD. However, there are conflicting findings from community‐based studies on the association between mildly reduced kidney function and CVD. Some studies demonstrated that mildly renal insufficiency was an independent predictor for association of increased risk and mortality of CVD in the general population.20, 21 In a cohort study of 11 940 Caucasians and 16 451 African‐American type 2 diabetes mellitus patients with 6.1 to 6.8 years of follow‐up, mildly reduced eGFR at baseline (under 75 mL/min per 1.73 m2) or during follow‐up (under 90 mL/min per 1.73 m2) were both associated with increased risk of incident CHD and stroke.5 In contrast, the Framingham Heart Study, during an average of 15 years of follow‐up of 6233 subjects in the US general population, suggested that the association between mild renal insufficiency and CVD is not independent, and appears to be attributed to the co‐occurrence of traditional CVD risk factors with CKD.22 However, for the composite outcome after adding all‐cause mortality to the previous CVD outcomes, the association became significant (hazard ratio, 1.19; 95% CI, 1.07–1.32). The findings from our current study provide additional support for the hypothesis that mild‐to‐moderate renal insufficiency could be an independent risk factor for CVD. The inconsistent findings could be explained by differences in the study populations and methods of eGFR calculation. Most of these studies included a heterogeneous patient proportion with established CVD, hypertension, and diabetes mellitus, both of which may affect the association between eGFR and cardiovascular mortality. In addition, most studies estimated eGFR using a Modification of Diet in Renal Disease equation that is less precise, especially in subjects with normal or mildly reduced renal function, compared with the recently developed CKD‐EPI formula, which can better predict CVD risk.17
Although the mechanism linking the association between impaired kidney function and CVD risk is not well established, several mechanisms have been proposed. Traditional CVD risk factors, such as older age, smoking, diabetes mellitus, hypertension, and dyslipidemia, often coexist with CKD,23, 24 whereas healthy lifestyle habits (normal body weight, not smoking, regular exercise, moderate alcohol intake, consumption of breakfast cereals, and consumption of fruits and vegetables) were individually and jointly associated with a lower lifetime risk of incident CVD.25, 26 Atherosclerosis could be another important factor linking impaired kidney function and the risk of incident CVD. Elevated asymmetric dimethyl arginine, reduced nitric oxide bioavailability, and endothelial dysfunction in renal disease, which are associated with atherosclerosis, could also contribute to incident CVD.27, 28 Experimental studies suggest that the renin‐angiotensin system (RAS) and its primary effector peptide, angiotensin II, are involved in the pathophysiology of cardiac hypertrophy and failure.29, 30 Activation of the RAS, which may partly depend on the adaptation to loss of renal mass that results in changes in renal hemodynamics, frequently occurs in CKD. Furthermore, inflammatory markers, such as leukocyte count, serum amyloid A, C‐reactive protein, fibrinogen, interleukin‐6, tumor necrosis factor‐α, D‐dimer, and the adhesion molecule, E‐selection, are often elevated, and these factors may alter the progression of atherosclerosis through their contribution to the production of reactive oxygen species.31, 32
There are several strengths in our study, including the large sample size, representativeness of Chinese adults, and diagnosis of diabetes mellitus based on fasting and postprandial glucose level. Our study has several potential limitations that merit comment. First, because of the cross‐sectional nature of the current study, our CVD outcome was prevalent CVD; thus, no causal inference can be drawn. Prospective studies with incident CVD outcomes are needed to clarify their precise inter‐relationship. Second, we may have over‐ (or under)‐estimated 10‐year ASCVD risk in the Chinese population using the ASCVD risk score, which was established in American populations by the ACC/AHA. The ASCVD risk equations used in this study may need validation in Chinese populations. As mentioned earlier, we observed a higher CVD risk when using the ASCVD score compared to that using the FRS. Third, collection of CHD, stroke, and myocardial infarction status was based on self‐reported questionnaire, and the duration of diseases was not available for further analysis. We have previously reported a validation rate of 91.1% for self‐reported CHD, stroke, and myocardial infarction events in 1 of the communities from the REACTION study.16 Thus, the recall bias from self‐reported events could not be completely excluded. Fourth, the use of eGFR to assess renal function in healthy individuals could be prone to measurement bias.33 Insulin or iothalamate clearances are invasive and cumbersome, and 24‐hour urine collections are very unreliable.34 Nevertheless, eGFR has been used extensively and routinely in large epidemiological studies. Fifth, we did not have information on albuminuria, which may be an independent risk factor for CVD outcomes.35, 36, 37 Finally, although our analyses were adjusted for an extensive set of confounding factors, residual confounding attributed to the measurement error in the assessment of confounding factors, unmeasured factors, such as dietary factors, and family history of diabetes mellitus, hypertension, CVD, and other chronic diseases, cannot be excluded.
In conclusion, we found that even mildly reduced eGFR (<90 mL/min per 1.73 m2) was associated with 10‐year high ASCVD risk among Chinese adults. A prospective follow‐up of this nation‐wide representative population is ongoing and of great importance to further assess the predicting value of mildly reduced eGFR for future CVD risk.
Appendix
REACTION Study Group
Steering Committee: Guang Ning (Principle Investigator), National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Yiming Mu, Chinese People's Liberation Army General Hospital, Beijing, China; Jiajun Zhao, Shandong Provincial Hospital affiliated to Shandong University, Jinan, China; Weiqing Wang, National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Chao Liu, Jiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing, China; Yufang Bi, National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Donghui Li, Department of Gastrointestinal Medical Oncology, the University of Texas MD Anderson Cancer Center, Houston, Texas; and Shenghan Lai, Johns Hopkins University School of Medicine, Baltimore, Maryland; Zachary T. Bloomgarden, Mount Sinai School of Medicine, New York.
Working Group: Weiqing Wang, Yufang Bi, Jieli Lu, National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Yiming Mu, Chinese People's Liberation Army General Hospital, Beijing, China; Jiajun Zhao, Shandong Provincial Hospital affiliated to Shandong University, Jinan, China; Chao Liu, Jiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing, China; Lulu Chen, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Lixin Shi, Affiliated Hospital of Guiyang Medical College, Guiyang, China; Qiang Li, The Second Affiliated Hospital of Harbin Medical University, Harbin, China; Tao Yang, The First Affiliated Hospital with Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China; Li Yan, Sun Yat‐sen Memorial Hospital, Sun Yat‐sen University, Guangzhou, China; Qin Wan, The Affiliated Hospital of Luzhou Medical College, Luzhou, China; Shengli Wu, Karamay Municipal People's Hospital, Xinjiang, China; Guixia Wang, The First Hospital of Jilin University, Changchun, China; Zuojie Luo, The First Affiliated Hospital of Guangxi Medical University, Nanning, China; Xulei Tang, The First Hospital of Lanzhou University, Lanzhou, China; Gang Chen, Fujian Provincial Hospital, Fujian Medical University, Fuzhou, China; Yanan Huo, Jiangxi People's Hospital, Nanchang, China; Zhengnan Gao, Dalian Municipal Central Hospital, Dalian, China; Qing Su, Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China; Zhen Ye, Zhejiang Provincial Center for Disease Control and Prevention, Zhejiang,China; Youmin Wang, The First Affiliated Hospital of Anhui Medical University, Hefei, China; Guijun Qin, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China; Huacong Deng, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China; Xuefeng Yu, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Feixia Shen, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China; Li Chen, Qilu Hospital of Shandong University, Jinan, China.
Sources of Funding
This work is supported by the grants 2013BAI09B13 from the National Clinical Research Center for Metabolic Diseases, 201502007 from the Ministry of Health, 2015BAI12B14 from the Ministry of Science and Technology, 2015CB553601 from the National Basic Research Program of China (973 Program), and 81321001, 81390350, 81130016, and 81561128019 from the National Natural Science Foundation of China. Lu was supported by Chenxing Plan of Shanghai Jiaotong University, Shuguang Plan (15SG15), and Gaofeng Clinical Medicine Grant (20152202) from Shanghai Municipal Education Commission.
Disclosures
None.
Supporting information
Table S1. Association of Prevalent Self‐Reported CVD With eGFR Level in Different Subgroups. Regression models were adjusted for age, sex, BMI, drinking status, smoking status, physical activity, SBP, DBP, A1c, dyslipidemia (yes/no), ACEI/ARB use (yes/no), statin use (yes/no), and insulin use (yes/no) (except for the strata variables).
Table S2. Association of High Risks for 10‐Year CHD (Framingham Risk Score ≥20% in Men and ≥10% in Women) With eGFR Level in Different Subgroups. Regression models were adjusted for age, sex, BMI, drinking status, smoking status, physical activity, SBP, DBP, A1c, dyslipidemia (yes/no), ACEI/ARB use (yes/no), statin use (yes/no), and insulin use (Yes/No) (except for the strata variables).
Table S3. Association of High Risks for 10‐Year ASCVD (ASCVD Score ≥7.5%) With eGFR Level in Different Subgroups. Regression models were adjusted for age, sex, BMI, drinking status, smoking status, physical activity, SBP, DBP, A1c, dyslipidemia (yes/no), ACEI/ARB use (yes/no), statin use (yes/no), and insulin use (yes/no) (except for the strata variables).
Acknowledgments
We are deeply grateful for all the participants in the REACTION cohort for making this study possible.
(J Am Heart Assoc. 2016;5:e003328 doi: 10.1161/JAHA.116.003328)
References
- 1. Kurth T, de Jong PE, Cook NR, Buring JE, Ridker PM. Kidney function and risk of cardiovascular disease and mortality in women: a prospective cohort study. BMJ. 2009;338:b2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 3. Anand S, Shivashankar R, Ali MK, Kondal D, Binukumar B, Montez‐Rath ME, Ajay VS, Pradeepa R, Deepa M, Gupta R, Mohan V, Venkat Narayan KM, Tandon N, Chertow GM, Prabhakaran D. Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney Int. 2015;88:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis C , van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El‐Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T. Lower estimated glomerular filtration rate and higher albuminuria are associated with all‐cause and cardiovascular mortality. A collaborative meta‐analysis of high‐risk population cohorts. Kidney Int. 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Katzmarzyk PT, Horswell R, Zhao W, Johnson J, Hu G. Kidney function and the risk of cardiovascular disease in patients with type 2 diabetes. Kidney Int. 2014;85:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chronic Kidney Disease Prognosis C , Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 8. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 9. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; Group CHDRP . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 10. Cook NR, Ridker PM. Response to comment on the reports of over‐estimation of ASCVD risk using the 2013 AHA/ACC risk equation. Circulation. 2014;129:268–269. [DOI] [PubMed] [Google Scholar]
- 11. Muntner P, Safford MM, Cushman M, Howard G. Comment on the reports of over‐estimation of ASCVD risk using the 2013 AHA/ACC risk equation. Circulation. 2014;129:266–267. [DOI] [PubMed] [Google Scholar]
- 12. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. [DOI] [PubMed] [Google Scholar]
- 14. Bi Y, Lu J, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Wan Q, Wu S, Yang T, Yan L, Liu Y, Wang G, Luo Z, Tang X, Chen G, Huo Y, Gao Z, Su Q, Ye Z, Wang Y, Qin G, Deng H, Yu X, Shen F, Chen L, Zhao L, Zhang J, Sun J, Dai M, Xu M, Xu Y, Chen Y, Lai S, Bloomgarden ZT, Li D, Ning G. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2014;6:147–157. [DOI] [PubMed] [Google Scholar]
- 15. Ning G; Reaction Study G . Risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2012;4:172–173. [DOI] [PubMed] [Google Scholar]
- 16. Lu J, Bi Y, Wang T, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Wan Q, Wu S, Qin G, Yang T, Yan L, Liu Y, Wang G, Luo Z, Tang X, Chen G, Huo Y, Gao Z, Su Q, Ye Z, Wang Y, Deng H, Yu X, Shen F, Chen L, Zhao L, Dai M, Xu M, Xu Y, Chen Y, Lai S, Ning G. The relationship between insulin‐sensitive obesity and cardiovascular diseases in a Chinese population: results of the REACTION study. Int J Cardiol. 2014;172:388–394. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A . Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. [DOI] [PubMed] [Google Scholar]
- 20. Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn Study. Kidney Int. 2002;62:1402–1407. [DOI] [PubMed] [Google Scholar]
- 21. Ohsawa M, Fujioka T, Ogasawara K, Tanno K, Okamura T, Turin TC, Itai K, Ogawa A, Yoshida Y, Omama S, Onoda T, Nakamura M, Makita S, Ishibashi Y, Tanaka F, Kuribayashi T, Ohta M, Sakata K, Okayama A. High risks of all‐cause and cardiovascular deaths in apparently healthy middle‐aged people with preserved glomerular filtration rate and albuminuria: a prospective cohort study. Int J Cardiol. 2013;170:167–172. [DOI] [PubMed] [Google Scholar]
- 22. Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community‐based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. [DOI] [PubMed] [Google Scholar]
- 23. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease HBPRCC, Epidemiology, Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes A . Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Lifestyle factors and antihypertensive treatment on the risks of ischemic and hemorrhagic stroke. Hypertension. 2012;60:906–912. [DOI] [PubMed] [Google Scholar]
- 26. Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kielstein JT, Boger RH, Bode‐Boger SM, Frolich JC, Haller H, Ritz E, Fliser D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002;13:170–176. [DOI] [PubMed] [Google Scholar]
- 28. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. [DOI] [PubMed] [Google Scholar]
- 29. Barnes GD, Alam S, Carter G, Pedersen CM, Lee KM, Hubbard TJ, Veitch S, Jeong H, White A, Cruden NL, Huson L, Japp AG, Newby DE. Sustained cardiovascular actions of APJ agonism during renin‐angiotensin system activation and in patients with heart failure. Circ Heart Fail. 2013;6:482–491. [DOI] [PubMed] [Google Scholar]
- 30. Levy BI. Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin‐angiotensin system. Circulation. 2004;109:8–13. [DOI] [PubMed] [Google Scholar]
- 31. Wilson PW; CDC, AHA . CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: ability of inflammatory markers to predict disease in asymptomatic patients: a background paper. Circulation. 2004;110:e568–e571. [DOI] [PubMed] [Google Scholar]
- 32. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. [DOI] [PubMed] [Google Scholar]
- 33. Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. [DOI] [PubMed] [Google Scholar]
- 34. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int. 2002;62:997–1004. [DOI] [PubMed] [Google Scholar]
- 35. Lee ET, Howard BV, Wang W, Welty TK, Galloway JM, Best LG, Fabsitz RR, Zhang Y, Yeh J, Devereux RB. Prediction of coronary heart disease in a population with high prevalence of diabetes and albuminuria: the Strong Heart Study. Circulation. 2006;113:2897–2905. [DOI] [PubMed] [Google Scholar]
- 36. Muntner P, Bowling CB, Gao L, Rizk D, Judd S, Tanner RM, McClellan W, Warnock DG. Age‐specific association of reduced estimated glomerular filtration rate and albuminuria with all‐cause mortality. Clin J Am Soc Nephrol. 2011;6:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouchi R, Babazono T, Yoshida N, Nyumura I, Toya K, Hayashi T, Hanai K, Tanaka N, Ishii A, Iwamoto Y. Association of albuminuria and reduced estimated glomerular filtration rate with incident stroke and coronary artery disease in patients with type 2 diabetes. Hypertens Res. 2010;33:1298–1304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association of Prevalent Self‐Reported CVD With eGFR Level in Different Subgroups. Regression models were adjusted for age, sex, BMI, drinking status, smoking status, physical activity, SBP, DBP, A1c, dyslipidemia (yes/no), ACEI/ARB use (yes/no), statin use (yes/no), and insulin use (yes/no) (except for the strata variables).
Table S2. Association of High Risks for 10‐Year CHD (Framingham Risk Score ≥20% in Men and ≥10% in Women) With eGFR Level in Different Subgroups. Regression models were adjusted for age, sex, BMI, drinking status, smoking status, physical activity, SBP, DBP, A1c, dyslipidemia (yes/no), ACEI/ARB use (yes/no), statin use (yes/no), and insulin use (Yes/No) (except for the strata variables).
Table S3. Association of High Risks for 10‐Year ASCVD (ASCVD Score ≥7.5%) With eGFR Level in Different Subgroups. Regression models were adjusted for age, sex, BMI, drinking status, smoking status, physical activity, SBP, DBP, A1c, dyslipidemia (yes/no), ACEI/ARB use (yes/no), statin use (yes/no), and insulin use (yes/no) (except for the strata variables).


