Abstract
Background
Tricuspid annuloplasty is increasingly performed during left heart valve surgery, but the long‐term clinical outcome postoperatively is not satisfactory. The aim of this study was to determine whether residual pulmonary hypertension (PHT) contributes to the adverse outcome.
Methods and Results
One‐hundred thirty‐seven patients (age 61±11 years; men, 30%) who underwent tricuspid annuloplasty during left‐side valve surgery were enrolled. The mean pulmonary artery systolic pressure before surgery was 49±13 mm Hg and 32±15 mm Hg following surgery. Patients were divided into 3 groups according to postoperative pulmonary artery systolic pressure: no residual PHT (n=78, 57%), mild residual PHT (n=43, 31%), or significant residual PHT (n=16, 12%). A preoperative larger right ventricular (RV) geometry and tricuspid valve tethering area were associated with mild or significant residual PHT. A total of 24 adverse events (20 heart failures and 4 cardiovascular deaths) occurred during a median follow‐up of 25 months. Kaplan–Meier survival curve demonstrated that patients with significant residual PHT had the highest percentage of adverse events followed by those with mild residual PHT. Patients with no residual PHT had a very low risk of adverse events. Multivariable Cox regression analysis revealed that both mild (hazard ratio=4.94; 95% CI =1.34–18.16; P=0.02) and significant residual PHT (hazard ratio=8.67; 95% CI =2.43–30.98; P<0.01) were independent factors associated with adverse events.
Conclusions
The present study demonstrated that 43% of patients who underwent tricuspid annuloplasty had residual PHT. The presence of mild or significant residual PHT was associated with adverse events in these patients.
Keywords: pulmonary hypertension, tricuspid annuloplasty, valvular surgery
Subject Categories: Valvular Heart Disease, Pulmonary Hypertension, Echocardiography
Introduction
Tricuspid regurgitation (TR) secondary to left heart valve disease is associated with increased mortality and decreased functional outcome.1, 2 In order to reduce TR and improve clinical outcome, interest in tricuspid annuloplasty (TA) during left heart valve surgery has increased in recent years3, 4, 5 and the number of tricuspid procedures has doubled during the past decade.6 Although TA has shown a satisfactory perioperative and 30‐day result, 10‐year survival is limited to 50% to 74%.7, 8, 9, 10, 11 It is thus important to understand the mechanism that contributes to the high events rate in order to improve the postoperative clinical outcome in these patients.
The pathophysiology of TR in patients with left heart valve disease is multifactorial and includes the presence of pulmonary hypertension (PHT).12 In addition, TR may cause right ventricular (RV) failure and shift the interventricular septum, resulting in restricted left ventricular (LV) filling and subsequent increased LV diastolic and pulmonary artery systolic pressure (PASP).13 The presence of TR and PHT are thus closely associated, and one contributes to the other in patients with left heart valve disease. Despite correction of both TR and left heart valve status in patients who undergo concomitant TA during left heart valve surgery, PASP may remain high. Little is known about the prevalence of residual PHT and its prognostic implication in patients undergoing TA. The aim of the present study was to evaluate the prevalence and predictors of residual PHT, and determine whether it contributes to an adverse outcome in patients who undergo concomitant TA during left heart valve surgery.
Methods
Study Population
From January 2008 to February 2014, a total of 194 consecutive Chinese patients who underwent elective TA together with left‐side valve surgery at Queen Mary Hospital were prospectively recruited. Patients with a documented history of congenital heart disease (n=6) or implanted pacemaker (n=10) were excluded. Detailed echocardiographic assessment was performed before and at least 6 months following surgery (median 25 months). Patients with poor quality echocardiography images (n=25) that were not suitable for further measurement were also excluded. Adverse outcome was defined as the occurrence of heart failure requiring admission or cardiovascular mortality. All outcomes were retrieved from the interhospital system or by telephone interview. An additional 16 patients who experienced heart failure (median 1.5 months) before follow‐up echocardiography for evaluation of residual PHT were excluded. A final total of 137 patients (41 male; mean age 61 years with SD of 11) were included in this study. The study was part of the Chinese Valvular Heart Disease Study to evaluate Chinese patients with valvular heart disease in an attempt to evaluate the pattern of disease, pathophysiology, and their clinical outcome.14 The study was approved by the ethics committee of the West Cluster Hospital Authority of Hong Kong, and all subjects gave written informed consent.
Clinical Parameters
Clinical data on preoperative and postoperative variables were collected from patient records by 1 investigator. The etiology of valvular heart disease was recorded as chronic rheumatic heart disease or non–chronic rheumatic heart disease according to the predominant lesion of the valve. Combined valvular surgery with TA was also recorded. New York Heart Association classification was recorded as class I/II or class III/IV, and the status of valvular atrial fibrillation was also recorded for each subject. Conventional cardiovascular risk factors such as history of diabetes mellitus, hypercholesterolemia, hypertension, and smoking status were documented. Data on cardiovascular medication prescribed following TA were retrieved from Hospital Authority records.
Conventional Echocardiography
Detailed transthoracic echocardiography was performed in all subjects before and after cardiac surgery. Patients were imaged in the left lateral decubitus position using a commercially available echocardiography system (Vingmed Vivid 7; General Electric Vingmed Ultrasound, Milwaukee, WI). A 3.5‐MHz transducer was used to obtain images that were digitally stored in cine‐loop format (5 cardiac cycles). Offline analysis was performed using EchoPAC version 112.0 (General Electric–Vingmed, Horten, Norway). The LV systolic and diastolic volume and ejection fraction were measured according to the modified biplane Simpson's rule.15
Right heart echocardiographic parameters were measured according to the current recommendations.15, 16 From the apical 4‐chamber views, the following RV parameters were measured: RV basal and midcavity diameter, and RV longitudinal dimension. The basal diameter was defined as the maximal short‐axis dimension in the basal one third of the RV seen on the 4‐chamber view. The midcavity diameter was measured in the middle third of the RV at the level of the LV papillary muscles. The longitudinal dimension is the distance from the plane of the tricuspid annulus to the RV apex. The RV spherical index was calculated from the following equation: (midcavity diameter×longitudinal dimension)/(basal diameter).17 From the same view, RV end‐diastolic area (RVEDA) and end‐systolic area (RVESA) were measured by manually tracing the RV endocardial border, and RV fractional area change (RVFAC) was calculated from the following equation: (RVEDA−RVESA)/RVEDA×100%.16 Minimal tricuspid valve (TV) annular dimension was measured at end diastole from the insertion of the septal leaflet to the insertion of the anterior leaflet. The TV tethering area was then measured by tracing the area between the atrial surface of the leaflets and the annulus plane at end systole. Finally, PASP was estimated by RV systolic pressure that was calculated from peak TR velocity by continuous‐wave Doppler using simplified Bernoulli equation and combining this value with the estimated right atrial pressure (RAP): PASP=4(V)2+RAP.16
PHT was graded according to the PASP value based on the results of the transthoracic echocardiography examination before and after TA surgery: No PHT was diagnosed if PASP <35 mm Hg, mild PHT with 35 ≤PASP <50 mm Hg, and significant PHT with PASP ≥50 mm Hg.18
Statistical Analysis
Data are expressed as mean±SD for continuous variables and frequencies or proportions for categorical variables. One‐way ANOVA with post‐hoc test by Bonferroni was used to examine the differences among groups. Unadjusted and adjusted logistic regression analysis was performed to determine the independent variables of preoperative RV parameters most associated with residual PHT following TA. Kaplan–Meier curve was constructed and the adverse outcomes among 3 groups were compared using the log‐rank test. Scaled Schoenfield residuals regressed on time were used to test for the proportional hazards assumption. Cox regression model adjusted for age, sex, and New York Heart Association class III/IV was performed to evaluate the impact of residual PHT on cardiovascular adverse events. The test of proportional hazards assumption was performed using Stata, Version 13.0 (StataCorp LP, College Station, TX), and all other statistical analyses were performed using the statistical package SPSS for windows (Version 17.0; SPSS, Chicago, IL) and P values reported are 2‐sided for consistency. A value of P<0.05 was considered statistically significant.
Results
Baseline Characteristics
The mean age of the study population was 61±11 years and 41 (30%) were male. The majority of patients had underlying atrial fibrillation and 99 (72%) had chronic rheumatic heart disease as the cause of valve pathology. The mean PASP before TA was 49±13 mm Hg (range, 20–85 mm Hg) and 66 (48%), 55 (40%), and 16 (12%) patients, respectively, showed significant PHT, mild PHT, and no PHT before TA.
Prevalence and residual PHT in patients following TA
Following TA, mean PASP was significantly reduced to 32±15 mm Hg (range, 10–66 mm Hg; P<0.01) and 78 (57%) patients had no residual PHT following TA. Nonetheless, mild residual PHT was present in 43 (31%) patients and significant residual PHT in 16 (12%). The detailed changes in the degree of PHT before and following TA are described in Figure 1.
Figure 1.
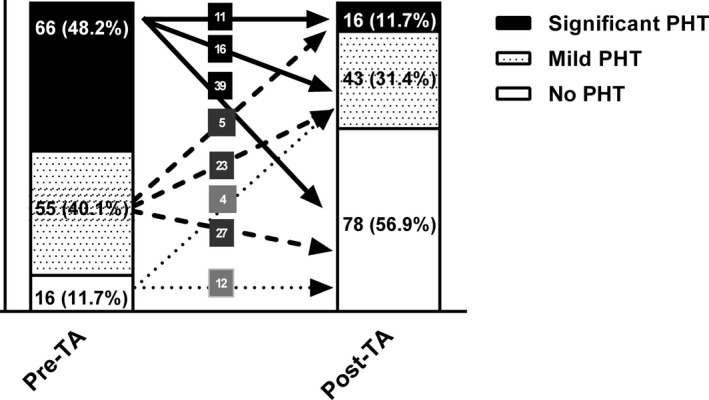
Percentage of patients with no pulmonary hypertension (PHT), mild PHT, and significant PHT before and after tricuspid annuloplasty (TA).
Clinical characteristics in patients with residual PHT
The baseline clinical characteristics of patients with no residual PHT, mild residual PHT, and significant residual PHT are shown in Table 1. Patients were older in those with mild residual PHT or significant residual PHT compared with those with no residual PHT. Nonetheless, other clinical parameters were similar among all 3 groups.
Table 1.
Clinical Characteristics of Patients Undergoing TA
| Variable | No Residual PHT (n=78) | Mild Residual PHT (n=43) | Significant Residual PHT (n=16) | P Value |
|---|---|---|---|---|
| Preoperative clinical characteristics | ||||
| Age, y | 58.2±11.0 | 65.2±9.7a | 66.6±9.1a | <0.01b |
| Male, n (%) | 28 (35.9) | 7 (16.3)a | 6 (37.5) | 0.06 |
| Hypertension, n (%) | 13 (16.7) | 6 (14.0) | 3 (18.8) | 0.88 |
| Diabetes mellitus, n (%) | 13 (16.7) | 5 (11.6) | 4 (25.0) | 0.45 |
| Hypercholesterolemia, n (%) | 19 (24.4) | 14 (32.6) | 5 (31.2) | 0.59 |
| Current smoker, n (%) | 8 (10.3) | 3 (7.0) | 1 (6.2) | 0.77 |
| Atrial fibrillation, n (%) | 62 (79.5) | 36 (83.7) | 15 (93.8) | 0.38 |
| Etiology, n (%) | ||||
| Non‐CRHD | 24 (30.8) | 11 (25.6) | 3 (18.8) | 0.58 |
| CRHD | 54 (69.2) | 32 (74.4) | 13 (81.2) | |
| New York Heart Association, n (%) | ||||
| Class I/II | 47 (60.3) | 26 (60.5) | 9 (56.2) | 0.95 |
| Class III/IV | 31 (39.7) | 17 (39.5) | 7 (43.8) | |
| Medications, n (%) | ||||
| ACEI/ARB | 56 (71.8) | 27 (62.8) | 10 (62.5) | 0.53 |
| β‐Blocker | 45 (57.7) | 25 (58.1) | 6 (37.5) | 0.31 |
| Statin | 27 (34.6) | 15 (34.9) | 8 (50.0) | 0.49 |
| Aspirin | 37 (47.4) | 21 (48.8) | 51 (31.2) | 0.45 |
| Surgical details | ||||
| Combined valvular surgery with TA, n (%) | ||||
| MV repair | 22 (28.2) | 15 (34.9) | 4 (25.0) | 0.95 |
| MVR | 34 (43.6) | 17 (39.5) | 7 (43.8) | |
| AVR | 2 (2.6) | 2 (4.7) | 1 (6.2) | |
| MV repair+AVR | 4 (5.1) | 2 (4.7) | 0 (0) | |
| DVR | 16 (20.5) | 7 (16.2) | 4 (25.0) | |
| Concomitant CABG with TA, n (%) | 3 (3.8) | 1 (2.3) | 0 (0) | 0.68 |
Values are mean±SD or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CRHD, chronic rheumatic heart disease; DVR, dual valvular replacement; MV, mitral valve; MVR, mitral valve replacement; PHT, pulmonary hypertension; TA, tricuspid annuloplasty.
P<0.05 compared with no residual PHT group.
P<0.01 by One‐way ANOVA among three groups.
Preoperative echocardiographic parameters
The preoperative echocardiographic parameters for the 3 groups are shown in Table 2. LV dimension and ejection fraction were similar among all 3 groups. The RV basal, midcavity diameters, and TV annulus diameter were larger in patients with significant residual PHT than in those with no residual PHT. Patients with mild or significant residual PHT had a higher prevalence of severe TR than those with no residual PHT. Nonetheless, the remaining RV echocardiographic parameters were similar for the 3 groups.
Table 2.
Preoperative Echocardiographic Parameters in Patients Undergoing TA
| Variable | No Residual PHT (n=78) | Mild Residual PHT (n=43) | Significant Residual PHT (n=16) | P Value |
|---|---|---|---|---|
| LV parameters | ||||
| LV end‐diastolic volume, mL | 111.9±52.2 | 99.8±41.3 | 101.2±34.9 | 0.36 |
| LV end‐systolic volume, mL | 53.8±29.9 | 45.8±22.7 | 47.8±21.6 | 0.27 |
| LV ejection fraction (%) | 51.4±13.0 | 54.9±9.8 | 53.4±10.2 | 0.30 |
| RV parameters | ||||
| RV basal diameter, cm | 3.9±0.9 | 4.2±0.9 | 4.5±0.9a | 0.02b |
| RV midcavity diameter, cm | 3.0±0.8 | 3.4±0.8 | 3.7±0.7a | <0.01b |
| RV longitudinal diameter, cm | 5.8±1.1 | 6.1±1.0 | 6.0±1.0 | 0.52 |
| RV spherical index | 4.6±1.1 | 4.9±1.2 | 4.9±0.9 | 0.27 |
| RV end‐diastolic area, cm2 | 17.2±6.5 | 18.3±5.3 | 19.9±6.1 | 0.25 |
| RV end‐systolic area, cm2 | 10.9±4.6 | 10.9±3.9 | 12.3±3.7 | 0.48 |
| RV fractional area change (%) | 36.4±12.1 | 40.5±12.8 | 37.5±11.0 | 0.23 |
| TV deformations | ||||
| TV annulus diameter, cm | 3.4±0.7 | 3.7±0.6 | 4.2±0.7a, c | <0.01b |
| TV tethering area, cm2 | 1.0±0.5 | 1.2±0.5 | 1.4±0.4 | 0.05 |
| PASP, mm Hg | 49.5±14.8 | 46.9±11.8 | 53.1±7.8 | 0.26 |
| TR degree, n (%) | ||||
| Mild‐moderate TR | 61 (78.2) | 18 (41.9)a | 7 (43.8)a | <0.01b |
| Severe TR | 17 (21.8) | 25 (58.1)a | 9 (56.2)a | |
Values are mean±SD or n (%). LV indicates left ventricular; PASP, pulmonary artery systolic pressure; PHT, pulmonary hypertension; RV, right ventricular; TA, tricuspid annuloplasty; TR, tricuspid regurgitation; TV, tricuspid valve.
P<0.05 compared with no residual PHT group.
P<0.05 by One‐way ANOVA among three groups.
P<0.05 compared with mild residual PHT group.
Multivariable logistic regression adjusted for age, sex, and New York Heart Association class was performed for each preoperative right heart parameter to look for independent variables associated with residual PHT following TA (Table 3). Results showed that preoperative RV basal and midcavity diameter, TV annulus, TV tethering area, and TR degree were all independent parameters associated with mild or significant residual PHT in patients undergoing TA. No such association was found for other parameters.
Table 3.
Preoperative Echocardiographic Predictors of Residual PHT by Logistic Regression
| Variable | Logistic Regression Analysis | Mild Residual PHT | Significant Residual PHT | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| RV basal diameter | Unadjusted | 1.50 (0.95–2.36) | 0.08a | 2.25 (1.18–4.27) | 0.01a |
| Adjusted age+sex | 1.83 (1.09–3.05) | 0.02a | 2.43 (1.19–4.96) | 0.02a | |
| Adjusted age, sex+NYHA | 1.85 (1.10–3.11) | 0.02a | 2.55 (1.22–5.33) | 0.01a | |
| RV midcavity diameter | Unadjusted | 1.70 (1.04–2.76) | 0.03a | 2.59 (1.31–5.12) | <0.01a |
| Adjusted age+sex | 2.06 (1.20–3.55) | <0.01a | 2.96 (1.36–6.42) | <0.01a | |
| Adjusted age, sex+NYHA | 2.07 (1.20–3.57) | <0.01a | 2.97 (1.37–6.45) | <0.01a | |
| RV longitudinal diameter | Unadjusted | 1.22 (0.85–1.76) | 0.28 | 1.15 (0.70–1.92) | 0.58 |
| Adjusted age+sex | 1.50 (0.99–2.29) | 0.06 | 1.30 (0.72–2.36) | 0.39 | |
| Adjusted age, sex+NYHA | 1.50 (0.99–2.29) | 0.06 | 1.30 (0.72–2.36) | 0.39 | |
| RV spherical index | Unadjusted | 1.28 (0.91–1.81) | 0.16 | 1.36 (0.81–2.29) | 0.25 |
| Adjusted age+sex | 1.48 (1.00–2.19) | 0.05 | 1.54 (0.86–2.78) | 0.15 | |
| Adjusted age, sex+NYHA | 1.48 (1.00–2.19) | 0.05 | 1.56 (0.86–2.83) | 0.14 | |
| RV end‐diastolic area | Unadjusted | 1.03 (0.97–1.09) | 0.37 | 1.06 (0.98–1.14) | 0.14 |
| Adjusted age+sex | 1.06 (0.99–1.14) | 0.08 | 1.07 (0.99–1.17) | 0.10 | |
| Adjusted age, sex+NYHA | 1.06 (0.99–1.14) | 0.08 | 1.07 (0.99–1.17) | 0.10 | |
| RV end‐systolic area | Unadjusted | 1.00 (0.91–1.08) | 0.90 | 1.07 (0.95–1.19) | 0.28 |
| Adjusted age+sex | 1.06 (0.96–1.17) | 0.25 | 1.11 (0.98–1.26) | 0.11 | |
| Adjusted age, sex+NYHA | 1.06 (0.96–1.17) | 0.26 | 1.11 (0.98–1.26) | 0.11 | |
| RV fractional area change | Unadjusted | 1.03 (1.00–1.06) | 0.09a | 1.01 (0.96–1.06) | 0.73 |
| Adjusted age+sex | 1.01 (0.98–1.05) | 0.48 | 1.00 (0.95–1.04) | 0.85 | |
| Adjusted age, sex+NYHA | 1.01 (0.98–1.05) | 0.46 | 1.00 (0.95–1.04) | 0.84 | |
| TV annulus | Unadjusted | 1.71 (0.93–3.12) | 0.08a | 5.24 (1.97–13.95) | <0.01a |
| Adjusted age+sex | 2.15 (1.08–4.30) | 0.03a | 5.47 (1.77–16.90) | <0.01a | |
| Adjusted age, sex+NYHA | 2.21 (1.09–4.47) | 0.03a | 6.02 (1.90–19.03) | <0.01a | |
| TV tethering area | Unadjusted | 1.68 (0.75–3.75) | 0.21 | 3.32 (1.15–9.61) | 0.03a |
| Adjusted age+sex | 2.67 (1.05–6.78) | 0.04a | 4.58 (1.34–15.65) | 0.02a | |
| Adjusted age, sex+NYHA | 2.85 (1.09–7.46) | 0.03a | 4.79 (1.38–16.59) | 0.01a | |
| PASP | Unadjusted | 0.99 (0.96–1.01) | 0.32 | 1.02 (0.98–1.06) | 0.34 |
| Adjusted age+sex | 0.98 (0.95–1.01) | 0.15 | 1.02 (0.98–1.06) | 0.44 | |
| Adjusted age, sex+NYHA | 0.98 (0.95–1.01) | 0.15 | 1.02 (0.98–1.06) | 0.44 | |
| TR degree (severe/mild‐moderate) | Unadjusted | 4.98 (2.22–11.20) | <0.01a | 4.61 (1.50–14.20) | <0.01a |
| Adjusted age+sex | 4.98 (2.05–12.10) | <0.01a | 4.01 (1.23–13.04) | 0.02a | |
| Adjusted age, sex+NYHA | 5.09 (2.08–12.43) | <0.01a | 4.01 (1.23–13.03) | 0.02a | |
NYHA indicates New York Heart Association; OR, odds ratio; PASP, pulmonary artery systolic pressure; PHT, pulmonary hypertension; RV, right ventricular; TR, tricuspid regurgitation; TV, tricuspid valve.
P<0.1 in univariate logistic regression and was entered into multivariate logistic regression; and P<0.05 for multivariate logistic regression.
Postoperative echocardiographic parameters
The postoperative echocardiographic characteristics of patients following TA are shown in Table 4. The RV geometry including the RV basal diameter, RVEDA, RVESA, and TV tethering area were larger in patients with significant residual PHT than in those with mild residual PHT or no residual PHT. Furthermore, severe TR following TA was more common in patients with significant residual PHT compared with the other 2 groups. No difference was evident in other postoperative echocardiographic parameters among the 3 groups. All patients had an improved LV and RV geometry and function following TA (Table S1). Furthermore, patients with no residual PHT had significant improvement in both LV and RV dimension and function, whereas the improvement of LV function became insignificant in patients with mild residual PHT. Importantly, the RVEDA and RVFAC were not significantly improved in patients with significant residual PHT.
Table 4.
Postoperative Echocardiographic Characteristics of Patients Who Underwent TA
| Variable | No Residual PHT (n=78) | Mild Residual PHT (n=43) | Significant Residual PHT (n=16) | P Value |
|---|---|---|---|---|
| LV parameters | ||||
| LV end‐diastolic volume, mL | 86.6±27.3 | 78.2±27.1 | 92.8±50.0 | 0.20 |
| LV end‐systolic volume, mL | 36.9±19.2 | 34.7±18.3 | 41.8±40.3 | 0.56 |
| LV ejection fraction (%) | 59.2±8.8 | 57.1±10.5 | 57.2±11.0 | 0.46 |
| RV parameters | ||||
| RV basal diameter, cm | 3.5±0.6 | 3.6±0.6 | 4.0±0.6a | 0.02b |
| RV midcavity diameter, cm | 2.7±0.5 | 2.7±0.5 | 3.0±0.6 | 0.10 |
| RV longitudinal diameter, cm | 5.6±0.9 | 5.8±0.9 | 5.7±0.7 | 0.58 |
| RV spherical index | 4.3±0.9 | 4.2±1.0 | 4.3±0.7 | 0.92 |
| RV end‐diastolic area, cm2 | 13.5±3.6 | 14.2±3.7 | 16.3±5.2a | 0.03b |
| RV end‐systolic area, cm2 | 7.3±2.5 | 7.4±2.2 | 9.2±3.4a | 0.03b |
| RV fractional area change (%) | 46.0±8.3 | 48.0±7.3 | 43.6±8.1 | 0.15 |
| TV deformations | ||||
| TV annulus diameter, cm | 2.7±0.5 | 2.8±0.5 | 3.0±0.6 | <0.05b |
| TV tethering area, cm2 | 0.67±0.21 | 0.78±0.27 | 0.93±0.40a | <0.01b |
| PASP, mm Hg | 22.4±10.2 | 40.8±4.4a | 57.6±5.3a, c | <0.01b |
| TR degree, n (%) | ||||
| Mild‐moderate TR | 78 (100) | 43 (100) | 15 (93.8) | 0.02b |
| Severe TR | 0 (0) | 0 (0) | 1 (6.2) | |
Values are the mean±SD or n (%). LV indicates left ventricular; PASP, pulmonary artery systolic pressure; PHT, pulmonary hypertension; RV, right ventricular; TA, tricuspid annuloplasty; TR, tricuspid regurgitation; TV, tricuspid valve.
P<0.05 compared with no residual PHT group.
P<0.05 by One‐way ANOVA among three groups.
P<0.05 compared with mild residual PHT group.
Adverse outcome
The median duration of follow‐up after postoperative echocardiography was 25 months (range 1–95 months). A total of 24 adverse events occurred during this time: 20 patients developed congestive heart failure and 4 cardiovascular mortality (2 heart failure–related, 1 sudden death, and 1 acute myocardial infarction). Kaplan–Meier survival curves according to the degree of residual PHT are shown in Figure 2, and the P for testing proportional hazards assumption was 0.64, indicating that the scaled Schoenfield residuals showed no association with time. Patients who underwent TA with significant residual PHT had the highest percentage of adverse events, followed by patients with mild residual PHT. Patients with no residual PHT had a very low risk of adverse events. To be specific, the rate of freedom from adverse events after TA at 1 and 3 years was 75±11% and 18±15% in patients with significant residual PHT, 92±4% and 75±8% in those with mild residual PHT, and 98±2% and 86±7% in patients with no residual PHT, respectively (P<0.01). After adjusting for age, sex, and New York Heart Association class, both mild and significant residual PHT were independent factors associated with adverse events in patients undergoing TA (Table 5).
Figure 2.
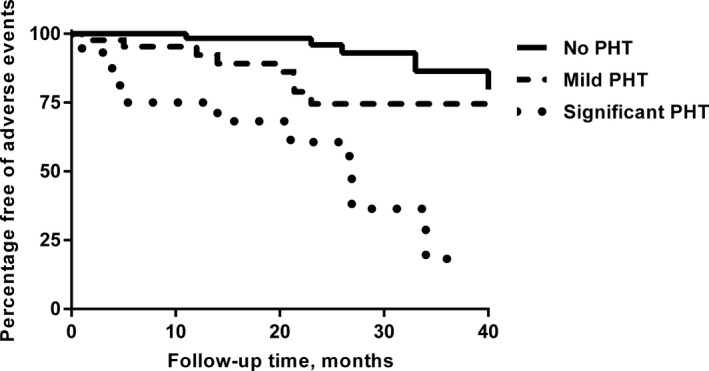
Kaplan–Meier analysis comparing incidence of adverse events in patients with no residual pulmonary hypertension (PHT), mild residual PHT,, and significant residual PHT who underwent tricuspid annuloplasty.
Table 5.
Cox Regression Analysis of Residual Pulmonary Hypertension (PHT) Following Tricuspid Annuloplasty (TA) in Association With Adverse Events
| Variable | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Mild residual PHT (35 mm Hg ≤PASP <50 mm Hg) | |||
| Unadjusted | 4.69 | 1.59 to 13.84 | <0.01a |
| Adjusted for age and sex | 4.74 | 1.32 to 16.99 | 0.02a |
| Adjusted for age, sex, and NYHA | 4.94 | 1.34 to 18.16 | 0.02a |
| Significant residual PHT (PASP ≥50 mm Hg) | |||
| Unadjusted | 13.72 | 4.21 to 44.65 | <0.01a |
| Adjusted for age and sex | 7.93 | 2.30 to 27.33 | <0.01a |
| Adjusted for age, sex, and NYHA | 8.67 | 2.43 to 30.98 | <0.01a |
NYHA indicates New York Heart Association; PASP, pulmonary artery systolic pressure.
P<0.05.
Discussion
The present study demonstrated that residual PHT occurred in 43% of patients who underwent TA. Preoperative enlarged RV geometry, namely, basal, midcavity diameters, TV annulus diameter, TV tethering area, and significant TR were associated with residual PHT. Patients with no and mild residual PHT had improved RV dimension and function but not in patients with significant residual PHT. Importantly, the presence of mild residual PHT had a 4.9‐fold risk and significant residual PHT had an 8.7‐fold risk of adverse events compared with no residual PHT. These results provide evidence that residual PHT in patients who undergo TA, a common condition, is an important factor that contributes to adverse events following surgery.
In the present study where patients required concomitant TA during left heart surgery, the prevalence of preoperative PHT was 88%. This high prevalence of preoperative PHT was expected, as the presence of significant TR may increase PASP irrespective of the left heart valve status. In the same context, prior reports have shown that the prevalence of postoperative residual PHT varies according to different types of valve surgery: 5% to 13% in patients who undergo mitral valve repair19, 20 and over 40% in those who undergo mitral valve replacement.21 Nonetheless, the prevalence of residual PHT in patients undergoing TA has not been studied. In the present study, 43% of patients had residual PHT and 27% had significant residual PHT (PASP ≥50 mm Hg). The underlying mechanism of residual PHT in these patients, even after correction of both left and right heart valvular status, remains uncertain. In patients with left heart valvular disease, the mechanism of PHT includes the following: (1) passive retrograde transmission of elevated left atrial pressures as a result of left heart valvular disease; (2) reactive pulmonary vasoconstriction; and (3) irreversible pulmonary vascular remodeling. Patients with left heart valvular disease who had concomitant TR had another factor that contributed to PHT: TR causing RV dilatation and dysfunction, shifting the interventricular septum towards the left ventricle, causing restricted LV filling and increased LV diastolic and pulmonary artery pressure, a phenomenon described as “restriction dilation syndrome.”13 By correcting both the left and right valvular status, the remaining patients with residual PHT are likely to be those with irreversible pulmonary vascular remodeling. This presumption should nonetheless be confirmed by future studies that determine the relationship of the reversibility of preoperative pulmonary vascular remodeling with residual PHT following surgery.
The present study demonstrated that older age and the presence of severe TR were associated with residual PHT following TA. Nonetheless, previous studies have not evaluated the preoperative RV geometry in relation to the development of residual PHT in patients who undergo valve surgery. The current study provides firm evidence that RV geometry, including RV basal, midcavity diameter, TV annulus diameter, and TV tethering area, are associated with residual PHT following TA. These results suggest that the presence of RV adverse remodeling, representing long‐standing disease duration, is closely associated with an irreversible component of PHT. As a result, early surgical correction of valvular status, before the development of RV geometry enlargement and possibly irreversible pulmonary vascular remodeling, may reduce the chance of developing residual PHT.
Although there is evidence that concomitant TA is a low‐risk procedure with no increase in perioperative mortality and morbidity,22, 23 these patients had a high mortality rate late after surgery. In the present study, the development of both mild and significant residual PHT was independently associated with adverse outcome. This could be explained by the presence of residual PHT and consequent increased RV afterload with ultimate progression to chamber dilatation and RV failure.24 This is further confirmed by the fact that patients with significant residual PHT had no improvement of RV function and dimension. The prognostic value of PHT has also been reported in patients who undergo left heart valve surgery without concomitant TA.25, 26, 27, 28 The present study thus emphasizes that residual PHT is a key mechanism that could explain the high adverse outcome in these patients.
Clinical Implications
The present study demonstrates that mild and significant residual PHT carries a 4.9‐ and 8.7‐fold increased risk of adverse events, respectively. Indeed, 25% and 82% of patients with significant residual PHT will develop adverse events after 1 and 3 years, respectively. Routine postoperative echocardiography to identify the presence of residual PHT in patients who undergo TA is thus essential to identify those at high risk. In addition, the present study also demonstrated that patients with preoperative RV geometry dilatation and a large TV tethering area were likely to develop residual PHT. This further supports our prior observation that early surgical correction, before alteration of RV geometry and TV tethering area, improves clinical outcome possibly by preventing the development of residual PHT.14
Limitations
The present study was not able to determine the presence of irreversible pulmonary vascular remodeling that can only be assessed by invasive right heart catheterization and reversibility testing. Due to the limited sample size, some factors such as sex, TV tethering area, RV end‐systolic area, and RVEDA were not very strong but might have provided some evidence of making a difference. Future study including such assessment may provide additional insight into the underlying pathophysiology of residual PHT. Furthermore, only patients who underwent TA together with left‐side heart valve surgery were studied; results should be verified in patients with isolated TR.
Conclusions
This study demonstrated that residual PHT occurred in 43% of patients who underwent TA and was associated with a preoperative enlarged RV geometry and TV tethering area. Importantly, the presence of mild residual PHT had a 4.9‐fold risk and significant residual PHT had an 8.7‐fold risk of adverse events compared with those with no residual PHT. This provides evidence that residual PHT is an important factor that contributes to adverse events in patients undergoing TA.
Disclosures
None.
Supporting information
Table S1. Pre‐ and Postoperative Echocardiographic Characteristics of Patients Who Underwent TA
(J Am Heart Assoc. 2016;5:e003353 doi: 10.1161/JAHA.116.003353)
References
- 1. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long‐term survival. J Am Coll Cardiol. 2004;43:405–409. [DOI] [PubMed] [Google Scholar]
- 2. Groves PH, Lewis NP, Ikram S, Maire R, Hall RJ. Reduced exercise capacity in patients with tricuspid regurgitation after successful mitral valve replacement for rheumatic mitral valve disease. Br Heart J. 1991;66:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. [DOI] [PubMed] [Google Scholar]
- 4. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers H‐J, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Popescu BA, Von Segesser L, Badano LP, Bunc M, Claeys MJ, Drinkovic N, Filippatos G, Habib G, Kappetein AP, Kassab R, Lip GYH, Moat N, Nickenig G, Otto CM, Pepper J, Piazza N, Pieper PG, Rosenhek R, Shuka N, Schwammenthal E, Schwitter J, Mas PT, Trindade PT, Walther T. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 6. Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Tricuspid valve surgery: the past 10 years from the nationwide inpatient sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–1049. [DOI] [PubMed] [Google Scholar]
- 7. Marquis‐Gravel G, Bouchard D, Perrault LP, Pagé P, Jeanmart H, Demers P, Carrier M, Cartier R, Poirier NC, Hébert Y, Pellerin M. Retrospective cohort analysis of 926 tricuspid valve surgeries: clinical and hemodynamic outcomes with propensity score analysis. Am Heart J. 2012;163:851–858. [DOI] [PubMed] [Google Scholar]
- 8. Moraca RJ, Moon MR, Lawton JS, Guthrie TJ, Aubuchon KA, Moazami N, Pasque MK, Damiano RJ Jr. Outcomes of tricuspid valve repair and replacement: a propensity analysis. Ann Thorac Surg. 2009;87:83–88. [DOI] [PubMed] [Google Scholar]
- 9. Van de Veire NR, Braun J, Delgado V, Versteegh MI, Dion RA, Klautz RJ, Bax JJ. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg. 2011;141:1431–1439. [DOI] [PubMed] [Google Scholar]
- 10. McCarthy PM, Bhudia SK, Rajeswaran J, Hoercher KJ, Lytle BW, Cosgrove DM, Blackstone EH. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674–685. [DOI] [PubMed] [Google Scholar]
- 11. Bernal JM, Pontón A, Diaz B, Llorca J, García I, Sarralde JA, Gutiérrez‐Morlote J, Pérez‐Negueruela C, Revuelta JM. Combined mitral and tricuspid valve repair in rheumatic valve disease: fewer reoperations with prosthetic ring annuloplasty. Circulation. 2010;121:1934–1940. [DOI] [PubMed] [Google Scholar]
- 12. Mutlak D, Aronson D, Lessick J, Reisner SA, Dabbah S, Agmon Y. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? Chest. 2009;135:115–121. [DOI] [PubMed] [Google Scholar]
- 13. Antunes MJ, Barlow JB. Management of tricuspid valve regurgitation. Heart. 2007;93:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yiu KH, Wong A, Pu L, Chiang MF, Sit KY, Chan D, Lee HY, Lam YM, Chen Y, Siu CW, Lau CP, Au WK, Tse HF. Prognostic value of preoperative right ventricular geometry and tricuspid valve tethering area in patients undergoing tricuspid annuloplasty. Circulation. 2014;129:87–92. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 16. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 17. Kwon DA, Park JS, Chang HJ, Kim YJ, Sohn DW, Kim KB, Ahn H, Oh BH, Park YB, Choi YS. Prediction of outcome in patients undergoing surgery for severe tricuspid regurgitation following mitral valve surgery and role of tricuspid annular systolic velocity. Am J Cardiol. 2006;98:659–661. [DOI] [PubMed] [Google Scholar]
- 18. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. [DOI] [PubMed] [Google Scholar]
- 19. Nozohoor S, Hyllén S, Meurling C, Wierup P, Sjögren J. Prognostic value of pulmonary hypertension in patients undergoing surgery for degenerative mitral valve disease with leaflet prolapse. J Card Surg. 2012;27:668–675. [DOI] [PubMed] [Google Scholar]
- 20. Murashita T, Okada Y, Kanemitsu H, Fukunaga N, Konishi Y, Nakamura K, Koyama T. The impact of preoperative and postoperative pulmonary hypertension on long‐term surgical outcome after mitral valve repair for degenerative mitral regurgitation. Ann Thorac Cardiovasc Surg. 2015;21:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Briongos Figuero S, Moya Mur JL, Garcia‐Lledo A, Centella T, Salido L, Acena Navarro A, Garcia Martin A, Garcia‐Andrade I, Oliva E, Zamorano JL. Predictors of persistent pulmonary hypertension after mitral valve replacement. Heart Vessels. 2016;31:1091–1099. [DOI] [PubMed] [Google Scholar]
- 22. Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127–132. [DOI] [PubMed] [Google Scholar]
- 23. Chan V, Burwash IG, Lam BK, Auyeung T, Tran A, Mesana TG, Ruel M. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg. 2009;88:1209–1215. [DOI] [PubMed] [Google Scholar]
- 24. Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle‐pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. [DOI] [PubMed] [Google Scholar]
- 25. Goldstone AB, Chikwe J, Pinney SP, Anyanwu AC, Funt SA, Polanco A, Adams DH. Incidence, epidemiology, and prognosis of residual pulmonary hypertension after mitral valve repair for degenerative mitral regurgitation. Am J Cardiol. 2011;107:755–760. [DOI] [PubMed] [Google Scholar]
- 26. Kainuma S, Taniguchi K, Daimon T, Sakaguchi T, Funatsu T, Kondoh H, Miyagawa S, Takeda K, Shudo Y, Masai T, Fujita S, Nishino M, Sawa Y. Does stringent restrictive annuloplasty for functional mitral regurgitation cause functional mitral stenosis and pulmonary hypertension? Circulation. 2011;124:S97–S106. [DOI] [PubMed] [Google Scholar]
- 27. Medvedofsky D, Klempfner R, Fefer P, Chernomordik F, Hamdan A, Hay I, Goldenberg I, Raanani E, Guetta V, Segev A. The significance of pulmonary arterial hypertension pre‐ and post‐transfemoral aortic valve implantation for severe aortic stenosis. J Cardiol. 2015;65:337–342. [DOI] [PubMed] [Google Scholar]
- 28. Sinning JM, Hammerstingl C, Chin D, Ghanem A, Schueler R, Sedaghat A, Bence J, Spyt T, Werner N, Kovac J, Grube E, Nickenig G, Vasa‐Nicotera M. Decrease of pulmonary hypertension impacts on prognosis after transcatheter aortic valve replacement. EuroIntervention. 2014;9:1042–1049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pre‐ and Postoperative Echocardiographic Characteristics of Patients Who Underwent TA


