Abstract
Background
The risk of stroke imposed by atrial fibrillation (AF) is significantly greater in women than men; however, the mechanism remains elusive. We hypothesized that left atrial (LA) remodeling and poor contractile function of LA appendage (LAA) would be more predominant in women than men among AF patients.
Methods and Results
A total of 579 AF patients (216 women vs age‐, AF type–, and incidences of heart failure, hypertension, diabetes mellitus, stroke or transient ischemic attack, and vascular disease–matched 363 men, 61.3±10.2 years old, 70.1% paroxysmal AF) who underwent AF catheter ablation were included. Sex differences in LA volume index (LAVI) and LAA emptying flow velocity (FV) were analyzed in risk factor 0, 1, and ≥2 groups, according to their CHA 2 DS 2‐VASc scores beyond sex category. LAA‐FV was more significantly reduced in women with risk factor ≥2 than in men of the same risk group (P=0.022). Women showed greater LAVI than their male counterparts in the risk factor ≥2 group (P<0.001). The majority of female patients with a history of stroke had a large LAVI and low LAA‐FV (P<0.001); however, no such distribution was observed in men (P=0.596). LA volume index (odds ratio [OR], 1.038; 95% CI, 1.003–1.075, P=0.035) or LAA‐FV (OR, 0.976; 95% CI, 0.952–0.999; P=0.047) was significantly associated with a history of stroke in women.
Conclusions
More‐extensive LA remodeling and deterioration in LAA function were noted in women than in men with high calculated risk of stroke in AF.
Keywords: atrial fibrillation, remodeling, sex, stroke
Subject Categories: Atrial Fibrillation
Introduction
Atrial fibrillation (AF) is the most prevalent arrhythmia worldwide that is increasing in prevalence.1 Arrhythmia is also associated with increased morbidity and mortality; AF raises the risk of stroke by 4‐ to 5‐fold.2 The effect of arrhythmia, however, does not seem to be uniform across all patients given that many population‐based studies have pointed out that the risk of stroke imposed by AF is significantly greater in women.3, 4, 5 Recently, Emdin et al.6 showed that AF is linked with an almost 2‐fold higher risk of stroke in women compared to men through the meta‐analysis of 30 cohort studies. In fact, female sex is 1 component of the CHA2DS2‐VASc scoring system for assessing the risk of thromboembolism in nonvalvular AF. The mechanism behind the observed differences between men and women in AF‐related embolic risk is still unclear. Recently, it has been reported that women are more likely than men to have left ventricular (LV) diastolic dysfunction.7, 8, 9, 10 In previous studies, we have found that LV diastolic dysfunction is associated with the degree of left atrial (LA) electroanatomical remodeling and LA appendage (LAA) flow velocity (LAA‐FV), and that LA remodeling is associated with the risk and events of stroke in AF.11, 12 Based on these studies, we hypothesized that LA remodeling and poor contractile function of LAA are more predominant in women than men among AF patients with risk of stroke. The aim of this study was to demonstrate sex differences in LA electroanatomical remodeling in association with the number of risk factors for stroke.
Methods
Patient Selection
The study protocol adhered to the Declaration of Helsinki and was approved by the institutional review board of Severance Hospital of Yonsei University (Seoul, Korea). This study enrolled 579 (363 males, 216 females; 1.68 males for 1 female) of 1794 consecutive patients enlisted in the Yonsei AF Ablation Cohort (clinicaltrials.gov; NCT 02138695), who underwent radiofrequency catheter ablation (RFCA) for symptomatic and drug‐refractory nonvalvular AF. All patients provided written informed consent, and discontinued all antiarrhythmic drugs more than 5 half‐lives prior to RFCA. The 1794 patients in the cohort were first screened using the following exclusion criteria: (1) presence of LA or LAA thrombi on transesophageal echocardiogram; (2) no measurements of LA volume index (LAVI) by transthoracic echocardiography or LAA emptying flow velocity by transesopahgeal echocardiography; (3) significant structural heart disease other than LV hypertrophy: moderate‐to‐severe mitral regurgitation (≥grade 2), congenital heart disease, or cardiomyopathy screened by medical history and echocardiogram; and (4) a past history of AF ablation or cardiac surgery. We enrolled consecutive female patients in the Yonsei AF ablation cohort after applying exclusion criteria and matched them with male patients for age, type of AF, and incidences of congestive heart failure, hypertension, diabetes mellitus, stroke or transient ischemic attack (TIA), and vascular disease.13 A total of 216 female patients (61.9±9.9 years old, 71.3% paroxysmal type) were successfully matched with 363 male patients (61.0±10.4 years old, 69.4% paroxysmal type). Men and women were managed equally for prevalent diseases. Then, the CHA2DS2‐VASc score were calculated for each of 579 patients and each sex group was divided based on their scores into risk factor (beyond sex category) 0, 1, and ≥2 groups. Additionally, each sex group was divided based on the history of stroke or TIA for the analysis of sex difference in electroanatomical remodeling of LA according to history of stroke or TIA.
Echocardiographic Assessment and Three‐Dimensional Computed Tomography
Transthoracic echocardiography was conducted as recommended by the American Society of Echocardiography.14 From the apical window, a 1‐ to 2‐mm pulsed Doppler sample volume was placed at the mitral valve tip, and mitral flow velocities from 5 to 10 cardiac cycles were recorded to determine peak velocities of early diastolic mitral inflow (E), late diastolic mitral inflow, and deceleration time of E velocity. Mitral annular velocities were measured by tissue Doppler imaging using the pulsed‐wave mode. Early diastolic mitral annular velocities (Em) and late diastolic and systolic velocities of the mitral annulus were measured from the apical 4‐chamber view with a 2‐ to 5‐mm sample volume placed at the septal corner of the mitral annulus. Transesophageal echocardiography was performed using a 5‐MHz multi‐plane probe (Philips Medical Systems, Andover, MA) before RFCA procedures to exclude the presence of thrombi. All 4 pulmonary veins (PVs), when possible, were measured for absolute values of peak systolic and diastolic flow velocities. Peak LAA emptying flow velocity (FV) was measured by pulsed wave Doppler within the proximal third of the appendage15 for at least 3 consecutive beats. Three‐dimensional (3D) spiral computed tomography (CT; 64 Channel, Light Speed Volume CT; Philips, Brilliance 63, Amsterdam, the Netherlands) was performed in all patients and analyzed on an imaging processing workstation (Aquarius; TeraRecon, Inc., Foster City, CA). Each LA image was divided into 3 portions: the venous LA (posterior LA, including the antrum and posterior wall); the anterior LA (excluding LAA and venous LA); and the LAA.16 The absolute volumes of each portion were divided by body surface area to produce regional LA volume indices. All echocardiography data were analyzed by 2 expert cardiologists, and all CTs were measured by 2 expert radiologists. The correlation coefficients for inter‐ and intraobserver reliability were 0.92 and 0.95 for echocardiography and 0.96 and 0.97 for 3D CT analysis, respectively.
LA Electroanatomical Mapping
Intracardiac electrograms were recorded using the Prucka CardioLab electrophysiology system (General Electric Medical Systems Inc., Milwaukee, WI, USA), and RFCA procedures were performed using 3D electroanatomical mapping (NavX; St. Jude Medical Inc., Minnetonka, MN) merged with 3D CT. Because of frequently recurring AF at baseline, we acquired LA voltage after circumferential PV isolation and/or cardioversion. We generated 3D voltage maps by obtaining contact bipolar electrograms from 350 to 500 points on the LA endocardium during atrial pacing with a pacing cycle length of 500 ms. Bipolar electrograms were filtered at 32 to 300 Hz. Color‐coded voltage maps were generated by recording bipolar electrograms and measuring peak‐to‐peak voltage for 416 patients (153 women and 263 men), as previously described.16 If frequently recurring AF still persisted after 3 attempts of cardioversion, no further efforts were made to generate an LA voltage map.
Statistical Analyses
Continuous variables were presented as the mean±SD, and categorical variables were presented as absolute and relative frequencies. Data for sex groups were compared using the Student t test for continuous variables. Categorical variables were compared using the chi‐square test as well as Fisher's exact test, when necessary. Comparisons of the stroke event rates among 4 groups divided according to the averaged LAA‐FV and LA volume index were also made using the chi‐square test in each sex. Patients with missing data for a variable of interest were excluded from relevant analyses. In order to identify factors associated with events of stroke, uni‐ and multivariable logistic regression analyses were performed. To compare different effects of LA remodeling in men and women, a pooled analysis with statistical interactions was performed. Only those variables found to be significant in univariable analyses were included in the multivariable analysis. A P value <0.05 was regarded as statistically significant.
Results
Baseline Characteristics
Comparisons of baseline characteristics between women (n=216) and AF type–, age‐, and the incidences of heart failure, hypertension, diabetes mellitus, stroke/TIA, and vascular disease–matched men (n=363) are shown in Table 1. The average CHA2DS2‐VASc score was higher by ≈1 point for women (P<0.001), in part attributed to the sex category included in the risk scoring system. The average CHA2DS2‐VASc score for men and women in the risk factor ≥2 group was 3.1 and 4.1, respectively (P<0.001). Comparisons between women and men were then made after they were divided into risk factor 0, 1, and ≥2 groups based on CHA2DS2‐VASc scores beyond sex category (Table 2). In the risk factor 0 group, women (n=53) had a higher estimated glomerular filtration rate (eGFR; P=0.026) than men (n=95). In the risk factor 1 group (men, n=89; women, n=48), no differences in baseline characterisitcs were observed except for postablational prescription rate of angiotensin‐converting enzyme inhibitor (ACEi) or angiotensin II receptor blockers (ARB). In the risk factor ≥2 group, women (n=115) had a higher body mass index (BMI; P=0.012) than their male counterparts (n=179).
Table 1.
Comparisons of Baseline Characteristics by Sex
| Men (n=363) | Women (n=216) | P Value | |
|---|---|---|---|
| Age, y | 61.0±10.4 | 61.9±9.9 | 0.325 |
| Paroxysmal AF, n (%) | 252 (69.4) | 154 (71.3) | 0.707 |
| AF duration, months | 67.3±77.5 | 58.1±80.7 | 0.441 |
| Body mass index, kg/m2 | 24.7±2.6 | 24.7±3.0 | 0.978 |
| eGFR, mL/min per 1.73 m2 | 77.7±17.3 | 78.6±19.2 | 0.531 |
| Risk factors | |||
| Congestive heart failure, n (%) | 33 (9.1) | 22 (10.2) | 0.663 |
| Hypertension, n (%) | 199 (54.8) | 115 (53.2) | 0.731 |
| Age ≥75, n (%) | 36 (9.9) | 22 (10.2) | 0.917 |
| Diabetes mellitus, n (%) | 53 (14.6) | 34 (15.7) | 0.719 |
| Stroke or TIA, n (%) | 59 (16.3) | 45 (20.8) | 0.180 |
| Vascular disease, n (%) | 60 (16.5) | 29 (13.4) | 0.342 |
| CHA2DS2‐VASc score | 1.8±1.6 | 2.9±1.6 | <0.001a |
| 0, n (%) | 95 (26.2) | ||
| 1, n (%) | 89 (24.5) | 53 (24.5) | |
| 2, n (%) | 73 (20.1) | 48 (22.2) | |
| ≥3, n (%) | 106 (29.2) | 115 (53.3) | |
| Medications | |||
| ACEi/ARB (%) | 140 (38.6) | 80 (37.2) | 0.791 |
| β‐blocker (%) | 108 (29.8) | 67 (31.2) | 0.779 |
| Statin (%) | 108 (29.8) | 77 (35.8) | 0.140 |
| Echocardiographic parameters | |||
| LA volume index, mL/m2 | 35.0±11.9 | 39.4±14.3 | <0.001a |
| LV ejection fraction, % | 62.5±8.9 | 65.0±7.3 | <0.001a |
| LV mass index, g/m2 | 94.8±22.2 | 92.1±22.7 | 0.207 |
| E/Em | 10.3±5.1 | 12.2±4.7 | <0.001a |
| LAA flow velocity, cm/s | 50.1±21.6 | 47.3±22.8 | 0.141 |
ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; E, mitral inflow early diastolic velocity; eGFR, estimated glomerular filtration rate; Em, mitral annulus early diastolic velocity; LA, left atrium; LAA, left atrial appendage; LV, left ventricle; TIA, transient ischemic attack.
P<0.05.
Table 2.
Comparisons of Baseline Characteristics by Stroke Risk and Sex
| Risk Factor 0 | Risk Factor 1 | Risk Factor ≥2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men (n=95) | Women (n=53) | P Value | Men (n=89) | Women (n=48) | P Value | Men (n=179) | Women (n=115) | P Value | |
| Age, y | 53.1±8.4 | 52.7±8.9 | 0.821 | 58.0±8.2 | 59.9±7.2 | 0.184 | 66.8±8.7 | 67.0±7.8 | 0.851 |
| Paroxysmal AF, n (%) | 73 (76.8) | 41 (77.4) | 0.943 | 60 (67.4) | 39 (81.2) | 0.110 | 119 (66.5) | 74 (64.3) | 0.708 |
| Body mass index, kg/m2 | 24.8±2.6 | 23.9±3.1 | 0.053 | 25.1±3.0 | 24.1±2.6 | 0.066 | 24.5±2.4 | 25.3±3.0 | 0.012a |
| eGFR, mL/min per 1.73 m2 | 83.8±12.1 | 88.8±14.8 | 0.026a | 81.5±15.0 | 80.9±19.1 | 0.829 | 72.5±19.1 | 73.0±19.1 | 0.839 |
| Risk factors | |||||||||
| Congestive heart failure, n (%) | 0 (0.0) | 0 (0.0) | — | 5 (5.6) | 5 (10.4) | 0.320 | 28 (15.6) | 17 (14.8) | 0.870 |
| Hypertension, n (%) | 0 (0.0) | 0 (0.0) | — | 61 (68.5) | 25 (52.1) | 0.066 | 138 (77.1) | 90 (78.3) | 0.886 |
| 65≤ Age <75, n (%) | 0 (0.0) | 0 (0.0) | — | 18 (20.2) | 16 (33.3) | 0.101 | 86 (48.0) | 54 (47.0) | 0.905 |
| Age ≥75, n (%) | 0 (0.0) | 0 (0.0) | — | 0 (0.0) | 0 (0.0) | — | 36 (20.1) | 22 (19.1) | 0.882 |
| Diabetes mellitus, n (%) | 0 (0.0) | 0 (0.0) | — | 2 (2.2) | 2 (4.2) | 0.612 | 51 (28.5) | 32 (27.8) | 0.902 |
| Stroke or TIA, n (%) | 0 (0.0) | 0 (0.0) | — | 0 (0.0) | 0 (0.0) | — | 59 (33.0) | 45 (39.1) | 0.318 |
| Vascular disease, n (%) | 0 (0.0) | 0 (0.0) | — | 3 (3.4) | 0 (0.0) | 0.552 | 57 (31.8) | 29 (25.2) | 0.239 |
| Medications | |||||||||
| ACEi/ARB (%) | 3 (3.2) | 3 (5.7) | 0.667 | 45 (50.6) | 15 (31.2) | 0.032a | 92 (51.4) | 62 (53.9) | 0.720 |
| β‐blocker (%) | 14 (14.7) | 13 (24.5) | 0.183 | 24 (27.0) | 12 (25.0) | 0.842 | 70 (39.1) | 42 (36.5) | 0.713 |
| Statin (%) | 9 (9.5) | 6 (11.3) | 0.779 | 19 (21.3) | 13 (27.1) | 0.527 | 80 (44.7) | 58 (50.4) | 0.341 |
ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack.
P<0.05.
Sex Differences in Electroanatomical Remodeling of LA at Different Stroke Risks
Using echocardiographic and LA voltage measurements, sex differences in electroanatomical remodeling were analyzed in each risk group. Although the risk factor 0 and 1 groups showed no differences in mechanical function of LAA between men and women, the risk factor ≥2 group showed significantly lower LAA‐FV in women (P=0.022; Figure 1A). A similar pattern was observed in anatomical remodeling of LA. LAVI was similar between sexes in risk factor 0 and 1 groups; however, LA was considerably larger in women in the risk factor ≥2 group (P<0.001; Figure 1B). When comparisons of LA endocardial voltage were made among patients whose voltage measurements were available (n=416), endocardial voltage of LA or LAA was lower in women compared to men regardless of risk factor numbers (Figure 1C and 1D). Overall, among patients at a high calculated risk of stroke, anatomical remodeling of LA and contractile dysfunction of LAA were more pronounced in women than in men.
Figure 1.
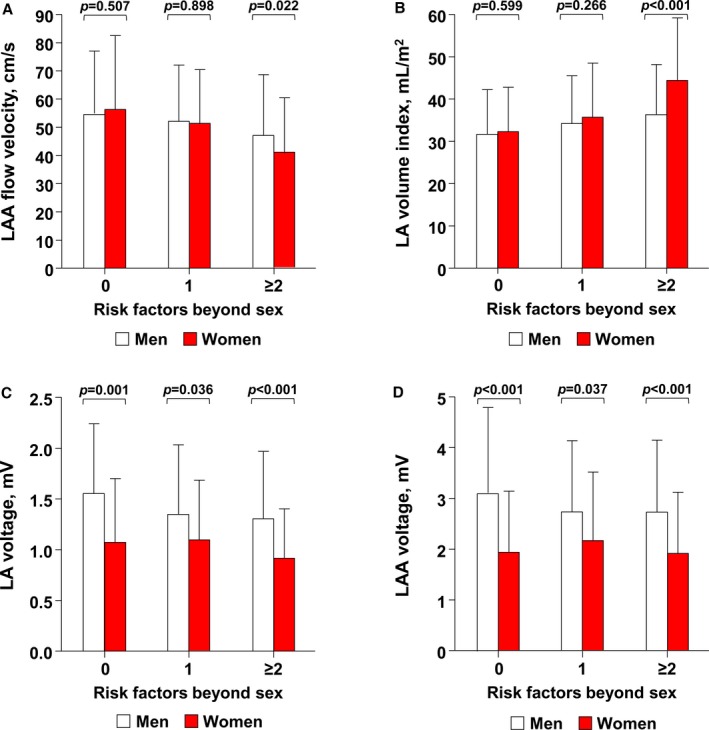
Comparisons of LAA mechanical function (A), LA volume index (B), mean LA endocardial voltage (C), and LAA voltage (D) between men and women of the same stroke risk (predicted by CHA 2 DS 2 VASc scores). Sex differences in LAA emptying flow velocity and LA volume index were prominent only among patients with risk factor ≥2. LA indicates left atrium; LAA, left atrial appendage; TIA, transient ischemic attack.
Atrial Remodeling and LV Function Depending on a History of Stroke or TIA
When each sex group was divided based on history of stroke or TIA, considerably advanced remodeling was observed in women with a history of stroke or TIA (Table 3). Women with a history of stroke or TIA had a significantly lower LAA‐FV (P<0.001) and LV ejection fraction (P=0.003) in comparison with women without as well as men with a history of ischemic cerebrovascular events (P=0.015 for LAA‐FV; P=0.441 for LV ejection fraction). Likewise, LAVI (P<0.001) and E/Em (P=0.008) were greater in women with a history of stroke or TIA than in women without as well as men with a history of the disease (P<0.001 for LAVI; P=0.103 for E/Em). However, no significant differences in these parameters were observed between men with and without a history of stroke. Once patients with a history of stroke or TIA were plotted on a graph based on their LAVI and LAA‐FV, the majority of female patients had a large LAVI and low LAA‐FV (P<0.001, Figure 2A); however, no such distribution was observed in men (P=0.596, Figure 2B). Furthermore, a multivariable logistic regression analysis using interaction term between sex and LAVI (female sex×LAVI) or LAA‐FV (female sex×LAA‐FV) showed that a history of stroke or TIA has significant associations with LA volume index (adjusted odds ratio [OR], 1.038; 95% CI, 1.003–1.075; P=0.035) or LAA‐FV (adjusted OR, 0.976; 95% CI, 0.952–0.999; P=0.047) among women than among men (Table 4). Usage of anticoagulant at the time of stroke or TIA event was comparable between men and women (6.8% vs 4.4%; P=0.999).
Table 3.
Comparisons of Electroanatomical Remodeling of LA Between Patients With and Without History of Stroke or TIA
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n=363) | Stroke/TIA (n=59) | No Stroke (n=304) | P Value | Overall (n=216) | Stroke/TIA (n=45) | No Stroke (n=171) | P Value | |
| Transthoracic echocardiography (n=579) | ||||||||
| LA dimension, mm | 41.8±5.6 | 43.0±4.8 | 41.5±5.8 | 0.072 | 41.0±6.0 | 43.5±5.9 | 40.4±5.9a | 0.002b |
| LA volume index, mL/m2 | 35.0±11.9 | 36.4±12.2 | 34.7±11.8 | 0.340 | 39.4±14.3 | 47.3±16.1c | 37.3±13.1a | <0.001b |
| LVEDD, mm | 50.2±4.4 | 49.5±4.5 | 50.3±4.4 | 0.203 | 48.3±4.6 | 47.7±5.8 | 48.5±4.2a | 0.427 |
| LVESD, mm | 34.2±4.9 | 33.4±4.9 | 34.3±4.8 | 0.172 | 32.0±4.4 | 32.8±6.3 | 31.8±3.7a | 0.313 |
| LV ejection fraction, % | 62.5±8.9 | 63.6±9.5 | 62.2±8.8 | 0.281 | 65.0±7.3 | 62.1±9.8 | 65.8±6.3a | 0.003b |
| LV mass index, g/m2 | 94.8±22.2 | 94.9±18.1 | 94.7±23.0 | 0.966 | 92.1±22.7 | 93.4±25.0 | 91.9±22.1 | 0.723 |
| E/Em | 10.3±5.1 | 11.5±8.0 | 10.0±4.2 | 0.188 | 12.2±4.7 | 13.9±6.1 | 11.8±4.2a | 0.008b |
| Transesophageal echocardiography (n=579) | ||||||||
| LAA flow velocity, cm/s | 50.1±21.6 | 46.1±21.0 | 50.8±21.6 | 0.126 | 47.3±22.8 | 36.5±18.2c | 50.1±23.0 | <0.001b |
| LA voltage map, mV (n=416) | ||||||||
| Entire LA | 1.4±0.7 | 1.4±0.6 | 1.4±0.7 | 0.952 | 1.0±0.5 | 0.9±0.5c | 1.0±0.6a | 0.178 |
| Anterior LA | 1.2±0.6 | 1.2±0.5 | 1.2±0.6 | 0.632 | 0.9±0.5 | 0.7±0.4c | 0.9±0.5a | 0.127 |
| Venous LA | 1.2±0.9 | 1.2±1.0 | 1.2±0.9 | 0.990 | 0.9±0.8 | 0.8±0.8c | 1.0±0.7a | 0.243 |
| LAA | 2.9±1.5 | 2.8±1.3 | 2.9±1.5 | 0.951 | 2.0±1.2 | 1.8±1.3c | 2.0±1.2a | 0.428 |
E indicates mitral inflow early diastolic velocity; Em, mitral annulus early diastolic velocity; LA, left atrium; LAA, left atrial appendage; LV, left ventricle; LVEDD, left ventricular end‐diastolic dimension; LVESD, left ventricular end‐systolic dimension; TIA, transient ischemic attack.
P<0.05 versus men with no history of stroke/TIA.
P<0.05.
P<0.05 versus men with history of stroke/TIA.
Figure 2.
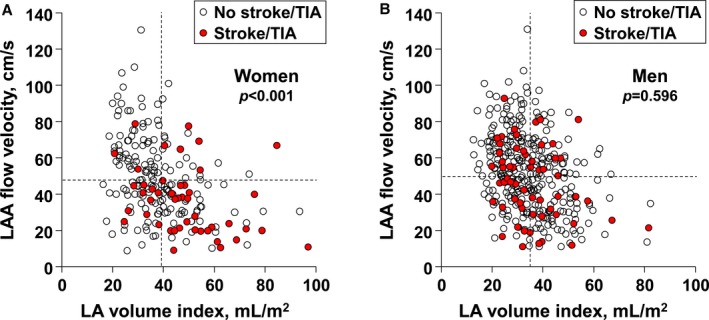
Scatterplot of patients with a history of stroke or TIA based on their LAA mechanical function and LA volume index in each sex. A, In women, the majority of patients with a history of cerebrovascular accidents showed advanced electroanatomical remodeling of LA (mean value of LA volume index: 39.4 mL/m2; LAA‐FV [flow velocity]: 47.3 cm/s). B, In men, however, no such distribution was observed (mean value of LA volume index: 35.0 mL/m2; LAA‐FV: 50.1 cm/s). LA indicates left atrium; LAA, left atrial appendage; TIA, transient ischemic attack.
Table 4.
A Logistic Regression Analysis of Factors Associated With the History of Stroke or TIA
| Variables | Univariable Analysis | Multivariable Analysis (Model 1)a | Multivariable Analysis (Model 2)b | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.034 (1.011–1.057) | 0.004 | ||||
| Female sex | 1.356 (0.881–2.086) | 0.166 | ||||
| Paroxysmal AF | 0.899 (0.569–1.421) | 0.649 | ||||
| AF duration | 0.996 (0.989–1.002) | 0.191 | ||||
| Body mass index | 1.012 (0.938–1.092) | 0.751 | ||||
| eGFR | 0.987 (0.976–0.998) | 0.020c | 1.003 (0.977–1.029) | 0.849 | 0.992 (0.980–1.004) | 0.211 |
| Congestive heart failure | 1.017 (0.494–2.090) | 0.964 | ||||
| Hypertension | 1.245 (0.810–1.913) | 0.318 | ||||
| Diabetes mellitus | 2.103 (1.246–3.548) | 0.005c | 1.781 (1.019–3.111) | 0.043c | 1.636 (0.932–2.874) | 0.087 |
| LV ejection fraction | 0.993 (0.969–1.017) | 0.559 | ||||
| E/Em | 1.065 (1.023–1.109) | 0.002c | 1.033 (0.989–1.079) | 0.143 | 1.037 (0.995–1.081) | 0.085 |
| Entire LA voltage | 0.819 (0.557–1.204) | 0.309 | ||||
| Anterior LA voltage | 0.820 (0.514–1.306) | 0.403 | ||||
| Venous LA voltage | 0.897 (0.668–1.205) | 0.471 | ||||
| LAA voltage | 0.940 (0.792–1.114) | 0.473 | ||||
| LA volume index×Female sexd | 1.035 (1.002–1.069) | 0.038c | 1.038 (1.003–1.075) | 0.035c | ||
| LAA flow velocity×Female sexe | 0.977 (0.954–0.999) | 0.045c | 0.976 (0.952–0.999) | 0.047c | ||
AF indicates atrial fibrillation; E, mitral inflow early diastolic velocity; eGFR, estimated glomerular filtration rate; Em, mitral annulus early diastolic velocity; LA, left atrium; LAA, left atrial appendage; LV, left ventricle; OR, odds ratio.
Adjusted for eGFR, diabetes mellitus, E/Em, female sex, and LA volume index.
Adjusted for eGFR, diabetes mellitus, E/Em, female sex, and LAA flow velocity.
P<0.05.
Adjusted for the main effect (female sex and LA volume index).
Adjusted for the main effect (female sex and LAA flow velocity).
Discussion
Our findings indicate that anatomical remodeling of LA and contractile dysfunction of LAA were more advanced in women than men among AF patients with high calculated risk of stroke (risk factor ≥2). These sex‐based differences appeared to be associated with comorbidites found in patients with severe risk of ischemic stroke. The mechanistic linkage of LV diastolic function, LA structural change, and LAA contractile function may be the cause behind the differences highlighted in this study.
Anatomical Remodeling of LA and Sex‐Based Differences
Fibrillating LA is known to undergo electroanatomical remodeling, but the mechanisms remain to be elucidated. From what has been discovered, sustained high‐frequency excitation of atria is believed to trigger events like oxidative stress, calcium overload, inflammation, and myofibroblast activation. Subsequently, these events lead to remodeling of extracellular matrix and electrophysiology of the atria.17 One of these events, inflammation, may be more severe in women than in men. The Women Health Study highlighted that inflammatory markers like C‐reactive protein showed correlations with nonvalvular AF in women, and that brain natriuretic peptide levels were also higher in women.18 Whether these differences have led to differences in electroanatomical remodeling between the sexes has been a controversial topic.19, 20 Electroanatomical remodeling of atria remains an important area of study in AF because atrial dilatation, extent of atrial fibrosis, and diminished endocardial voltage have all been reported to be related to stroke,11, 16, 21 a common, potentially detrimental complication of the arrhythmia that disproportionately affect women over men among AF patients.3, 4 Avgil Tsadok et al.22 reported that the incidence rate of stroke as a complication of AF was 2.02 per 100 person‐years (95% CI, 1.95–2.10) in women in comparison to 1.61 per 100 person‐years (95% CI, 1.54–1.69) in men (P<0.001).
On the other hand, recent experiences of an Asian cohort have shown that there was no sex difference in thromboembolic events.23, 24, 25 Asian cohort studies on nonvalvular AF patients without any antithrombotic therapy in Taiwan, China, and Hong Kong showed that female sex is not a risk factor for stroke (OR, 0.94 for female to male in Taiwan [n=7920]; 95% CI, 0.79–1.13; P=0.51; hazard ratio [HR], 1.92 in China [n=885]; 95% CI, 0.66–5.58; P=0.23; HR, 1.03 in Hong Kong [n=3881]; 95% CI, 0.88–1.20; P=0.72), suggesting racial differences in the incidence of thromboembolism between the Eastern and Western AF patients. Further evidence regarding this important issue is needed.
Differential Mechanisms of Stroke Generation in AF
Studies have provided possible reasons for the discrepancy in the rates of stroke between men and women with AF. One possible reason may be that women tend to be older and later in the course of the disease when they are referred for ablation.26 Another reason may be that women with AF tend to have a higher prevalence of comorbid conditions like hypertension and diabetes mellitus.27 However, the differences in stroke rates persisted even after controlling for comorbidity and age.22 In our current study, we propose that increased susceptibility to atrial remodeling attributed to more‐severe diastolic dysfunction of LV additionally exposes women to the risk of stroke in comparison with men. We have recently reported that degree of LA remodeling and LAA mechanical function in AF are closely related to LV diastolic dysfunction.12 Other studies have demonstrated that LV diastolic function is more likely to be found in women7 because of their tendency toward central aortic stiffness and increased susceptibility to load‐dependent LV diastolic dysfunction.9 Furthermore, the severity of LV diastolic dysfunction was found to be associated with increased risk of stroke as calculated by the CHADS2 scoring system.28 Therefore, we divided female patients and their age‐, AF type–, and components of CHA2DS2‐VASc scoring system‐matched male counterpart into 3 groups according to their stroke risk scores after excluding the sex category and analyzed electroanatomical remodeling of LA in different stroke risk groups. Sex differences in atrial dilatation and LAA contractile function were absent among patients with risk factor 0 and 1 groups. However, as their stroke risk scores increased, LAVI was larger and LAA‐FV was lower in women. Obesity is known to be associated with increased LA size,29 and female patients with high risk had higher BMI than their male counterpart in our study. However, in overall patients with risk factor ≥2, female sex was significantly associated with increased LAVI after controlling for age, type of AF, and BMI. Although the reason for sex differences in LAVI and LAA‐FV in only risk factor ≥2 group is unclear, we speculate that hypertension and diastolic dysfunction accelerate LA remodeling and LAA dysfunction in women with a relatively thin LA wall earlier with a higher CHA2DS2‐VASc score. Our results are also consistent with mechanisms for thrombus formation in AF, which include endothelial dysfunction and hemodynamic stasis.30
Clinical Implications
Current guidelines on anticoagulation for AF patients recommend that patients with CHA2DS2‐VASc score 1 or above be started on anticoagulation, unless patients with score 1 earned the point for being a female.31 The results of our study support the current guidelines given that LV diastolic dysfunction as well as electroanatomical remodeling appear to be more severe in women only when other comorbidity is present. Given that the mechanistic linkage of LV diastolic function, LA structural change, and LAA contractile function seems to increase the risk for stroke, regular examinations of LA structure, LAA function, and LV diastolic function may be valuable in AF patients to better prevent AF progression and stroke events. Whether anticoagulation prescriptions should be filled based on extensive electroanatomical remodeling alone, however, needs to be further studied.
Study Limitations
Despite that we provided the observation of LA remodeling and LAA contractile dysfunction between sex, the causal relationship remained to be determined. Given that the sex differences could be reflected by using the CHA2DS2‐VASc score, the finding of the present study did not provide more‐diagnostic value. The study population consisted of a highly selected group of patients who were deemed appropriate for rhythm control. Hence, there might have been a selection bias that resulted in the exclusion of patients with severe anatomical remodeling of the atria. Measurements of endocardial voltage were available in 71.8% of patients included. However, there was no difference in inclusion rate of voltage analysis between sex (153 of 216 [70.8%] for women and 263 of 363 [72.4%] for men).
Conclusion
Among AF patients with high calculated risks of stroke, anatomical remodeling of LA, and contractile dysfunction of LAA were more advanced in women. Sex differences in anatomical remodeling may additionally expose women to a risk of stroke in AF.
Sources of Funding
This work was supported by a grant (A085136) from the Korea Health 21 R&D Project, Ministry of Health and Welfare and a grant (NRF‐2013R1A2A2A01014634) from the Basic Science Research Program run by the National Research Foundation of Korea (NRF) which is funded by the Ministry of Science, ICT & Future Planning (MSIP).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003361 doi: 10.1161/JAHA.116.003361)
References
- 1. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 2. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 4. Friberg J, Scharling H, Gadsboll N, Truelsen T, Jensen GB; Copenhagen City Heart S . Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol. 2004;94:889–894. [DOI] [PubMed] [Google Scholar]
- 5. Dagres N, Nieuwlaat R, Vardas PE, Andresen D, Levy S, Cobbe S, Kremastinos DT, Breithardt G, Cokkinos DV, Crijns HJ. Gender‐related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol. 2007;49:572–577. [DOI] [PubMed] [Google Scholar]
- 6. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta‐analysis of cohort studies. BMJ. 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 8. Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, Yamagishi H, Toda I, Yoshiyama M, Yoshikawa J, Yoshida K. Age‐ and gender‐specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging. 2009;2:41–46. [DOI] [PubMed] [Google Scholar]
- 9. Shim CY, Park S, Choi D, Yang WI, Cho IJ, Choi EY, Chung N, Ha JW. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57:1226–1233. [DOI] [PubMed] [Google Scholar]
- 10. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park JH, Joung B, Son NH, Shim JM, Lee MH, Hwang C, Pak HN. The electroanatomical remodelling of the left atrium is related to CHADS2/CHA2DS2VASc score and events of stroke in patients with atrial fibrillation. Europace. 2011;13:1541–1549. [DOI] [PubMed] [Google Scholar]
- 12. Lee JS, Shim CY, Wi J, Joung B, Ha JW, Lee MH, Pak HN. Left ventricular diastolic function is closely associated with mechanical function of the left atrium in patients with paroxysmal atrial fibrillation. Circ J. 2013;77:697–704. [DOI] [PubMed] [Google Scholar]
- 13. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 15. Agmon Y, Khandheria BK, Gentile F, Seward JB. Echocardiographic assessment of the left atrial appendage. J Am Coll Cardiol. 1999;34:1867–1877. [DOI] [PubMed] [Google Scholar]
- 16. Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, Choi JI, Hwang C, Kim YH. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: comparison of three‐dimensional computed tomographic images and voltage mapping. J Cardiovasc Electrophysiol. 2009;20:1349–1356. [DOI] [PubMed] [Google Scholar]
- 17. Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, Buring JE, Albert CM. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walters TE, Teh AW, Spence S, Morton JB, Kistler PM, Kalman JM. Absence of gender‐based differences in the atrial and pulmonary vein substrate: a detailed electroanatomic mapping study. J Cardiovasc Electrophysiol. 2014;25:1065–1070. [DOI] [PubMed] [Google Scholar]
- 20. Tsai WC, Chen YC, Lin YK, Chen SA, Chen YJ. Sex differences in the electrophysiological characteristics of pulmonary veins and left atrium and their clinical implication in atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:550–559. [DOI] [PubMed] [Google Scholar]
- 21. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 22. Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012;307:1952–1958. [DOI] [PubMed] [Google Scholar]
- 23. Lin LY, Lee CH, Yu CC, Tsai CT, Lai LP, Hwang JJ, Chen PC, Lin JL. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation—a nation wide database analysis. Atherosclerosis. 2011;217:292–295. [DOI] [PubMed] [Google Scholar]
- 24. Guo Y, Apostolakis S, Blann AD, Wang H, Zhao X, Zhang Y, Zhang D, Ma J, Wang Y, Lip GY. Validation of contemporary stroke and bleeding risk stratification scores in non‐anticoagulated Chinese patients with atrial fibrillation. Int J Cardiol. 2013;168:904–909. [DOI] [PubMed] [Google Scholar]
- 25. Siu CW, Lip GY, Lam KF, Tse HF. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm. 2014;11:1401–1408. [DOI] [PubMed] [Google Scholar]
- 26. Patel D, Mohanty P, Di Biase L, Sanchez JE, Shaheen MH, Burkhardt JD, Bassouni M, Cummings J, Wang Y, Lewis WR, Diaz A, Horton RP, Beheiry S, Hongo R, Gallinghouse GJ, Zagrodzky JD, Bailey SM, Al‐Ahmad A, Wang P, Schweikert RA, Natale A. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7:167–172. [DOI] [PubMed] [Google Scholar]
- 27. Potpara TS, Marinkovic JM, Polovina MM, Stankovic GR, Seferovic PM, Ostojic MC, Lip GY. Gender‐related differences in presentation, treatment and long‐term outcome in patients with first‐diagnosed atrial fibrillation and structurally normal heart: the Belgrade atrial fibrillation study. Int J Cardiol. 2012;161:39–44. [DOI] [PubMed] [Google Scholar]
- 28. Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman E, Patel I, Shi M, Mercuri M, Mitrovic V, Braunwald E, Solomon SD. Left atrial structure and function in atrial fibrillation: ENGAGE AF‐TIMI 48. Eur Heart J. 2014;35:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 30. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155–166. [DOI] [PubMed] [Google Scholar]
- 31. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; Guidelines ESCCfP . 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]


